Abstract
Lentiviral vectors designed for the treatment of the hemoglobinopathies require the inclusion of regulatory and strong enhancer elements to achieve sufficient expression of the β-globin transgene. Despite the inclusion of these elements, the efficacy of these vectors may be limited by transgene silencing due to the genomic environment surrounding the integration site. Barrier insulators can be used to give more consistent expression and resist silencing even with lower vector copies. Here, the barrier activity of an insulator element from the human ankyrin-1 gene was analyzed in a lentiviral vector carrying an antisickling human β-globin gene. Inclusion of a single copy of the Ankyrin insulator did not affect viral titer, and improved the consistency of expression from the vector in murine erythroleukemia cells. The presence of the Ankyrin insulator element did not change transgene expression in human hematopoietic cells in short-term erythroid culture or in vivo in primary murine transplants. However, analysis in secondary recipients showed that the lentiviral vector with the Ankyrin element preserved transgene expression, whereas expression from the vector lacking the Ankyrin insulator decreased in secondary recipients. These studies demonstrate that the Ankyrin insulator may improve long-term β-globin expression in hematopoietic stem cells for gene therapy of hemoglobinopathies.
Introduction
Sickle cell disease (SCD) is one of the most common monogenic disorders, caused by a point mutation in the sixth codon of the human β-globin gene.1 This multisystem disease is associated with severe episodes of acute illness and progressive organ damage.2 While allogeneic hematopoietic stem cell transplantation (HSCT) may ameliorate SCD, it requires identification of an HLA-compatible donor and carries the risks of immunological complications of graft rejection and graft-versus-host disease.3 Autologous stem cell gene therapy is an alternative treatment without the limitations of allogeneic HSCT.
To date, several lentiviral vectors (LV) have been designed and successfully used to target β-hemoglobinopathies in ex vivo modified hematopoietic stem cells (HSC) showing phenotypic correction of in vitro preclinical human models4–6 and in vivo murine models.4,7–12 However, LV developed for the treatment of β-hemoglobinopathies require the presence of the β-globin introns,13,14 as well as the DNasel hypersensitive sites (HS) of the locus control region (LCR),15,16 which contain strong enhancers and regulatory elements to achieve sufficient expression of the β-globin transgene. The combination of these elements yields a complex vector payload that may have a deleterious effect on the titer and transduction efficiency of the LV.
Moreover, the efficacy of these vectors may be still limited by transgene silencing due to DNA methylation and heterochromatinization, as a consequence of chromosomal positional effects, which causes a lack of uniform and stable transgene expression. There are silencer sequences in the LV themselves, mainly located in the long terminal repeats (LTR), which also contribute to transgene silencing. Part of this problem was overcome by the deletions made in the 3′LTR of the self-inactivating (SIN)-LV;17 however, there still may be residual silencing even from SIN-LTR.18,19
The inclusion of insulator sequences in LV has improved the expression problems. The addition of the 5′DNase I HS 4 of the chicken β-globin locus (cHS4, 1.2 kb) in the LTR of a β-globin LV was able to rescue chromosomal position effect, as shown by correction of the thalassemia phenotype in in vivo murine models.20 However, the strategy of placing relatively long insulator elements in both LTR still adversely affects titers and stability of the LV.21,22 Alternatively to the prototypic cHS4, other insulator elements with barrier activity have been identified, such as the DNase I HS immediately 5′ to the promoter of the human ankyrin-1 gene (ANK1). Gallagher et al.23 demonstrated that this promoter region had structural and functional characteristics of a barrier element in erythroid cells, and was able to prevent gene silencing in vivo.
Here we analyzed the potential barrier activity of a 159 bp fragment of the 5′HS promoter region23 of the ANK1 (described from now on as “Ank insulator”) when placed in different orientations and sites within the previously described CCL-βAS3-FB LV.6 This promoter region included a point mutation in GATA-1 (ΔGATA-1) binding site to remove the enhancer activity; this point mutation was not expected to affect the barrier activity as described by Gallagher et al.23 In vitro clonal analysis of these constructs showed that a vector with a single copy of the Ank insulator in reverse orientation with respect to the LV genome (Ank-R) was shielded from chromosomal position effect as effectively as by the cHS4 insulator. This Ank-R LV construct was then compared in vivo with the parental vector, showing a more consistent expression of the transgene in clonal assays and a significantly higher expression of the transgene and lack of silencing in long-term vector-transduced murine HSC.
Results
Ankyrin-insulated lentiviral vector designs
We have shown that the CCL-AS3-FB LV (modified from CCL-βAS3, Figure 1a) is capable of efficient transduction of human SCD bone marrow (BM) CD34+ progenitor cells, and provides consistent expression of an antisickling β-globin gene reducing the percentage of sickling red blood cells (RBCs).6
Figure 1.
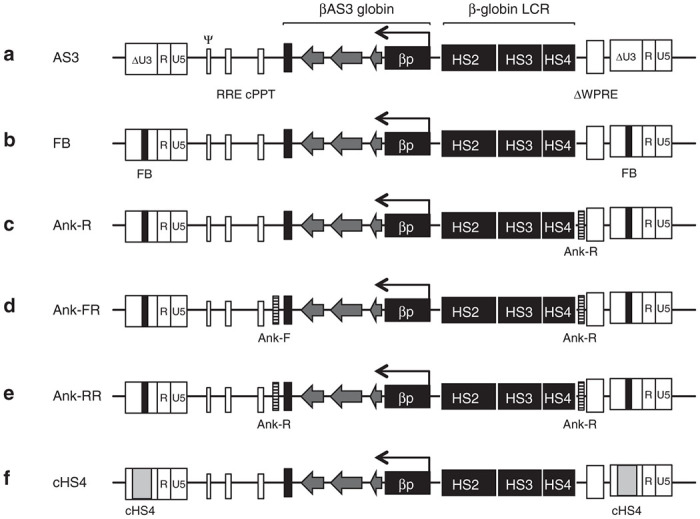
The proviral maps of the βAS3-globin vectors. The AS3 provirus has the βAS3-globin expression cassette including the human β-globin gene exons (arrow heads) with three amino acid substitutions to encode the βAS3-globin protein, introns and 3′ and 5′ flanking regions, the β-globin mini-LCR with hypersensitive sites 2–4, the mutated Woodchuck hepatitis virus post-transcriptional regulatory element (ΔWPRE) and the 3′SIN-LTR containing the FB insulator which is copied into the 5’LTR after reverse transcription. (a) AS3 LV, noninsulated version lacking the FB element; (b) CCL-βAS3-FB parental vector (FB LV); (c) Ank-R LV includes one copy of the Ank insulator in the reverse orientation; (d) Ank-FR LV with two copies of the Ank insulator element flanking the expression cassette, one in forward and one in reverse orientation; (e) Ank-RR LV with two copies of the Ank insulator flanking the expression cassette, both in reverse orientation (all of the Ank-insulated LV retain the FB element); and (f) a positive control vector that includes a full-length copy (1.2 kb) of the cHS4 insulator in the 3′LTR that is copied to 5’LTR during reverse transcription.
To test the potential barrier activity of the 159 bp 5′HS region of the promoter of the ANK1 gene, the parental CCL-βAS3-FB LV (FB LV) (Figure 1b) was modified to include the Ank element. All of the constructs designed contained one copy of the synthetic FB insulator placed in the 3′LTR of the parental FB LV. The FB element has been shown to exhibit the CTCF binding6 and functional activity of an enhancer-blocking insulator element, but does not have the barrier activity.24 One construct was designed to carry one copy of the Ank sequence upstream of the β-globin LCR cassette (near the 3′ end of the vector), which is in reverse orientation relative to LV transcription (Figure 1c) (Ank-R LV). In other constructs, a second copy of the Ank sequence was placed downstream of the βAS3-globin cassette (near the 5′ end of the vector), either in forward (Figure 1d) or reverse orientation (Figure 1e), (Ank-FR and Ank-RR LV, respectively). The noninsulated version of the CCL-βAS3-FB LV was used as a negative control (Figure 1a) (AS3 LV) and a sixth vector including the large prototypic cHS4 insulator (Figure 1e) (cHS4 LV) was used as a positive control for a known insulator barrier activity.
The inclusion of the FB insulator in the 3′LTR did not modify the titer when compared with its noninsulated counterpart,6 neither did the addition of only one copy of the Ank insulator in reverse orientation (Ank-R). Instead, the inclusion of two copies of the Ank insulator, independently of their orientation (Ank-FR and Ank-RR), had a deleterious effect on the LV titers. As expected, the insertion of the full-length cHS4 insulator made the titers drop more than 10-fold21 (Supplementary Figure S1).
In vitro assessment of the insulator activity in clonal erythroid cells
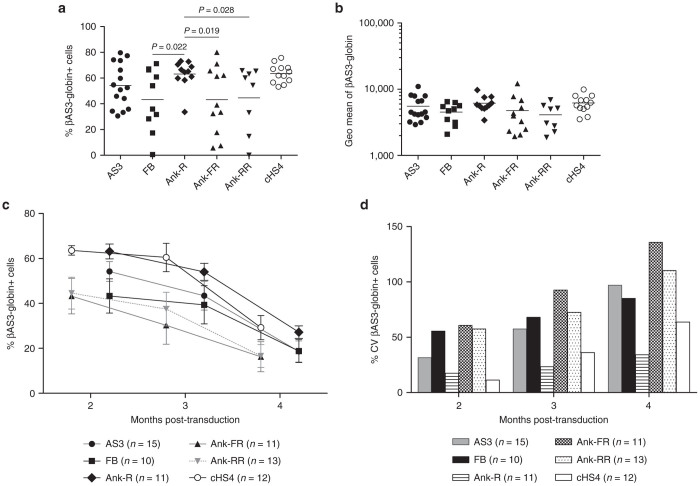
To test for protection against chromosomal-position effects, individual clones carrying only one copy of the vector were generated from pools of murine erythroleukemia (MEL) cells with each of the LV previously described. The parental CCL-βAS3-globin vector showed substantial variegation of expression, with 54.19 ± 17.02% of cells expressing the vector (Figure 2a). The clones carrying the Ank-R LV had a significantly higher percentage of cells expressing the βAS3-globin cassette (63.14 ± 10.99%) than: (i) those transduced with LV carrying two copies of the Ank insulator independently of their orientation (Ank-R versus Ank-FR, P = 0.019) (Ank-R versus Ank-RR, P = 0.028) (one-way analysis of the variance (ANOVA)) or (ii) those transduced with the parental FB LV (P = 0.022) (one-way ANOVA). The average percentage of expressing clones transduced with the Ank-R LV carrying one copy of the Ank insulator was equivalent to the positive-control clones transduced with the cHS4 insulator. The differences in expression between clones transduced with the LV insulated by the FB and noninsulated LV were not significant (one-way ANOVA).
Figure 2.
Consistent expression of the βAS3-globin cassette in MEL cell clones carrying one copy of the Ank insulator. (a) The percentage of cells expressing βAS3-globin transgene was analyzed by flow cytometry after in vitro erythroid differentiation 2 months post-transduction. Each point represents one single vector copy/cell clone (AS3, n = 15; FB, n = 10; Ank-R, n = 11; Ank-FR, n = 11; Ank-RR, n = 8; and cHS4, n = 12). Bars represent average values for each group. One-way ANOVA with pairwise comparisons was used to compare the percentage of cells expressing βAS3-globin transgene between groups. The significance threshold used for P-value was 0.05. (b) Geometric mean of βAS3-globin expression of the samples shown in (a), and at the same time point. Bars represent average values for each group. No significant increase in the level of expression of the βAS3-globin transgene was observed due to the addition of the Ank insulator (one-way ANOVA with multiple comparisons, P > 0.05). (c) Mean values of the percentage of cells expressing βAS3-globin transgene. The average and SE of the mean (SEM) of the percentage of cells expressing βAS3-globin transgene measured in each group of clones at 2, 3, and 4 months post-transduction are summarized. No significant differences were observed among groups over time (repeated-measure ANOVA). (d) Percentage of the CV (%CV) of βAS3-globin expressing MEL cell clones over time. The %CV at each time point was calculated as the ratio of the SD to the average percentage of expressing cells of the clones of each group at each time point.
No significant increase in the level of expression of the βAS3-globin transgene was observed, when the Ank-insulated LV were compared with the AS3, FB, and cHS4 LV, (Figure 2b) (one-way ANOVA with multiple comparisons); the Ank element was expected to protect from chromosomal-position effects, but not to enhance the level of transgene expression. Further analyses were performed 3 and 4 months post-transduction showing that, overall, a general silencing was observed in the clones of all the groups including the positive control (cHS4LV) and no significant differences were observed among groups (Figure 2c) (repeated-measure ANOVA).
The coefficient of variation (CV) of the βAS3-globin expression reflects the degree of expressing cells among clones with different integrations, and a lower CV indicates protection from chromosomal-position effect. A higher percentage of expressing cells along with more homogenous expressions was observed in the clones transduced with the Ank-R LV and cHS4 LV, as indicated by their lower CV after 2 months in culture (Figure 2d). Moreover, the Ank-R LV maintained a lower CV with respect to all of the other LV analyzed, including the cHS4 LV (Figure 2d) demonstrating the barrier activity of the Ank insulator.
The instability of insulator elements in LV vectors has been previously reported;21 to address this possible concern and evaluate the Ank-insulator stability, PCR in 10 MEL cell clones with each of the LV constructs carrying this element were performed. In the case of the Ank-FR and Ank-RR, 30–50% of the clones analyzed did not show amplification for one or both copies of the Ank insulator (top and middle rows in Supplementary Figure S2a). However, when only one copy of the Ank insulator was present, it was transferred intact in all of the clones screened (bottom row in Supplementary Figure S2a). All the clones analyzed were positive for the PCR used to measure transduction, amplifying the packaging signal sequence (Psi) region common for all the constructs (Supplementary Figure S2b). The cHS4-insulator stability was analyzed in both LTR, showing that 20% of the clones analyzed did not display amplification of the cHS4 insulator in the 5′LTR (first panel in Supplementary Figure S2c).
In vitro assessment of the Ank-R LV activity in primary human CD34+ cells
The effect of the Ank insulator on βAS3-globin expression from the LV was examined in transduced primary human SCD BM CD34+ HSC differentiated into mature erythrocytes.6,25 BM-derived CD34+ cells from multiple SCD donors were either mock-transduced or transduced with 2 × 107 TU/ml (multiplicity of infection (MOI) of 40) of the FB, Ank-R, and cHS4 LV. The average vector copy number (VCN) was comparable between the FB (1.2 ± 0.5) and Ank-R LV (1.0 ± 0.5) (P >0.01); however, the VCN was significantly reduced in cells transduced with the cHS4 LV (0.1 ± 0.1; P ≤ 0.02), (Supplementary Figure S3a). The average percentage of βAS3-globin mRNA per VCN was equivalent between the three LV (P > 0.05) (Supplementary Figure S3b). These data revealed no evidence of the Ank-element barrier activity in a bulk population of primary human cells in a short-term (3 weeks) in vitro culture.
Transgene expression in clonal progeny from murine HSC
In vivo colony-forming unit from spleen assay (CFU-S) was performed to examine the expression of βAS3-globin transgene at a clonal level. BM from long-term primary recipients (at least five different mice per vector) were transplanted into each secondary recipient mouse (FB LV, n = 19; Ank-R LV, n = 11; and Mock, n = 5) for CFU-S analysis. CFU-S were harvested 10 days post-transplant (FB LV, 13 mice-produced colonies: n = 47; Ank-R LV, 7 mice-produced colonies: n = 36; and Mock, 2 mice-produced colonies: n = 5) for the VCN and gene-expression assessment. In the total secondary CFU-S analyzed, 91.5% (43/47colonies) contained the FB LV sequences and 97.2% (35/36 colonies) were positive for the Ank-R LV. The FB LV-treated colonies showed a greater variability and almost a twofold higher CV (62.9%) compared with the Ank-R colonies (36.7%), which showed a more homogeneous and consistent expression of the βAS3-globin transgene (Figure 3 and Table 1). For a further analysis of the clonal progeny, BM cells from long-term secondary mice were transplanted into tertiary recipients (FB LV, n = 14; Ank-R LV, n = 15; and Mock, n = 1). Tertiary CFU-S were harvested 10 days post-transplant (FB LV, 9 mice-produced colonies: n = 47; Ank-R LV, 15 mice-produced colonies, n = 42; and Mock, 1 mouse-produced colony, n = 5), and samples were analyzed for VCN and βAS3-globin mRNA production. In the colonies analyzed from the tertiary transplant recipients, 70.21 (33/47 colonies) and 66.67% (28/42 colonies) were positive for the FB and Ank-R LV proviruses, respectively.
Figure 3.
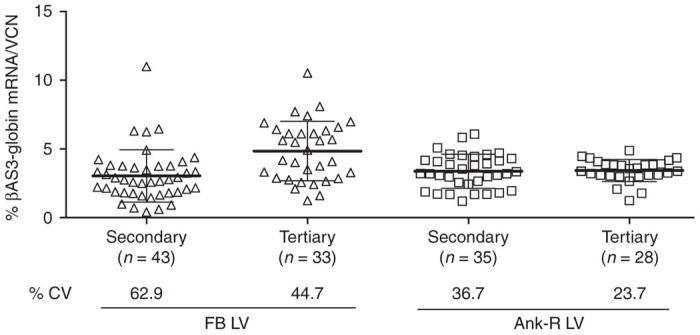
Transgene expression in clonal progeny from murine HSC. Percentage of βAS3-globin mRNA normalized per VCN from CFU-S in secondary and tertiary recipients harvested 10 days post-transplant. Bars represent average values for each group and SD. The percentage of CV (%CV) shown at the bottom of the graph was calculated as the ratio of the SD to the mean of βAS3-globin levels per VCN from the clones in each group.
Table 1. Summary of the percentage of expression of βAS3-globin mRNA per VCN in clonal progeny of secondary and tertiary mice shown in Figure 3.
|
FB LV |
Ank-R LV |
|||
|---|---|---|---|---|
| Secondary | Tertiary | Secondary | Tertiary | |
| Average % βAS3 | 3.04 | 4.82 | 3.38 | 3.43 |
| SD | 1.9 | 2.16 | 1.24 | 0.81 |
| %CV | 62.9 | 44.7 | 36.7 | 23.7 |
Both LV presented a lower CV in tertiary CFU-S compared with the secondary ones: for the FB LV, the CV decreased from 62.9% in the secondary to 44.7% in the tertiary mice; and for the Ank-R LV, the CV dropped from 36.7% in the secondary mice to 23.7% in the tertiary mice (Figure 3 and Table 1). However, as with the secondary CFU-S, the tertiary CFU-S from the FB LV-treated mice had a wider distribution of the percentage of transgene expression, resulting in more than a twofold higher SD, and therefore a CV that was 1.8 times greater than in the Ank-R LV mice. These results demonstrate the propensity of the Ank-R LV to protect from position-effect variation, providing a more stable and consistent expression of the βAS3-globin transgene.
In vivo expression of the βAS3-globin transgene by FB versus Ank-R LV
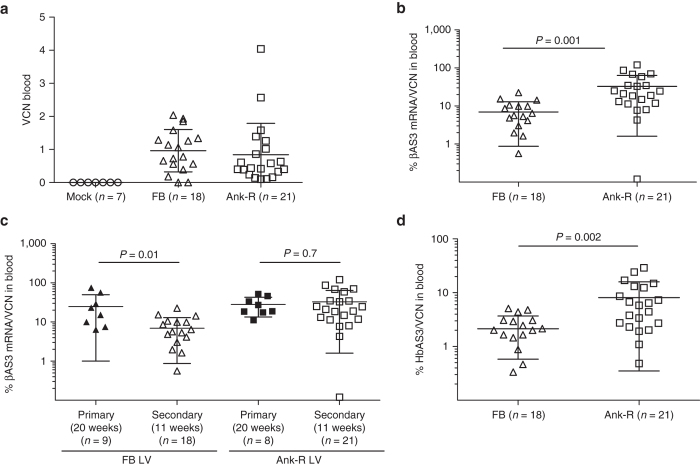
With the aim of analyzing gene expression in long-term murine HSC, primary and secondary transplants were carried out. Lineage negative cells from BM from CD45.1 (+) B6.SJL-Ptprca Pep3b/BoyJ male donor mice were transduced with the Ank-R LV and transplanted into congenic CD45.2 (+) C57bl/6J female mice and compared with mice transplanted with the cells transduced with the parental FB LV. VCN from transduced BM cells cultured in vitro for 2 weeks was 2.4 ± 0.2 for the FB LV and 2.6 ± 0.1 for the Ank-R LV, from two independent transductions.
Engraftment in blood was above 90% at 10 weeks post-transplant in all primary mice (Supplementary Figure S4a). VCN in blood samples was measured periodically every 3 weeks up to 20 weeks, showing stable and efficient gene transfer, without differences between vectors (repeated-measure ANOVA) (Supplementary Figure S5a). βAS3-globin mRNA expression was analyzed simultaneously from blood samples obtained for VCN analysis. The percentage of the βAS3-globin mRNA transcripts relative to the murine β-globin-like mRNA transcripts (“percentage of the βAS3-globin mRNA”) per VCN in blood of the primary recipients did not differ between groups (Supplementary Figure S5b) at any of the time points analyzed (repeated-measure ANOVA). Primary mice were euthanized 20 weeks post-transplant to perform secondary transplants. No differences were observed in engraftment, VCN, and βAS3-globin mRNA expression in the BM from the primary mice, as was observed in blood samples. The engraftment of donor cells in BM from primary mice ranged from 70 to 99% (Supplementary Figure S4b), and the VCN values were 2.03 ± 1.03 and 2.09 ± 1.14 for the FB LV and Ank-R LV, respectively; and the average expression of the βAS3-globin mRNA normalized to the VCN was 3.45 ± 1.47% and 4.85 ± 1.97% for the FB-LV and Ank-R LV, respectively (Supplementary Figure S6).
Secondary mice were euthanized 11 weeks post-transplant. High levels of engraftment were observed in blood and BM in the three groups of mice (Supplementary Figure S7). The blood samples were analyzed to determine gene marking in the two groups FB LV (0.96 ± 0.64) and Ank-R LV (0.84 ± 0.95) and no difference was seen in the two groups (P >0.05, two-sample t-test) (Figure 4a). However, the gene-expression values (βAS3-globin mRNA/VCN) were statistically higher in the Ank-R LV group compared with the FB LV group (P = 0.001, two-sample t-test) (Figure 4b). We observed that the βAS3-globin transgene expression per VCN declined in the FB LV group significantly (P = 0.01, two-sample t-test), from 24.76 ± 25.30% βAS3-globin mRNA/VCN in primary mice to 6.92 ± 6.05% βAS3-globin mRNA/VCN in secondary mice. In contrast, no significant differences were observed in the βAS3-globin transgene expression per VCN between primary (28.13 ± 14.64% βAS3-globin mRNA/VCN) and secondary mice (32.68 ± 31.09% βAS3-globin mRNA/VCN) for the Ank-R LV-treated group (P = 0.7, two-sample t-test). These results suggest that the inclusion of the Ank insulator protected long-term expression of the βAS3-globin cassette from silencing (Figure 4c).
Figure 4.
Long-term assessment of VCN, βAS3-globin mRNA, and HbAS3 expression in peripheral blood from secondary mice. (a) VCN analyzed in blood samples 11 weeks post-transplant. No differences in VCN were found between mice treated with the two different LV (P = 0.65, two-sample t-test). (b) Percentage of βAS3-globin mRNA per VCN from the samples shown in (a). Expression values were higher in the group of mice treated with Ank-R LV (P = 0.001, two-sample t-test). (c) The percentages of βAS3-globin mRNA per VCN, expressed in the primary and secondary mice at week 20 and 11 post-transplant, respectively, were analyzed for each LV (two-sample t-test). FB LV: primary mice, n = 9 and secondary, n = 18; for Ank-R LV-treated mice: primary, n = 8 and secondary, n = 21. The data shown in this graph at week 20 of the primary mice correspond to the average values shown in Supplementary Figure S5b; and the data shown at week 11 for the secondary mice are the same graphed in this panel in 4(b). (d) HPLC of blood samples from secondary recipients at week 11 post-transplant for hemoglobin tetramers analysis. The relative percentage of HbAS3 produced for each sample was calculated based on the sum total of areas under the curve for each of the primary hemoglobin peaks which included βmajor and βminor. Protein production was higher in the group of mice treated with Ank-R LV (P = 0.002, two-sample t-test). Bars represent average values and SD.
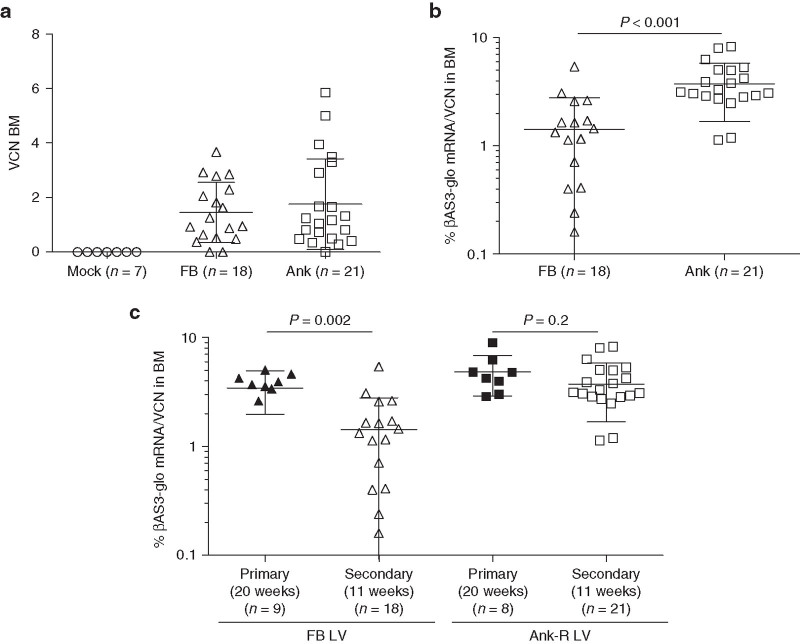
Similar results were obtained with BM samples from the secondary mice. There were no differences in gene transfer between the FB and Ank-R LV-treated groups (Figure 5a). However, the expression levels of the FB LV were significantly lower than the expression levels of the Ank-R LV (P < 0.001, two-sample t-test) (Figure 5b). When the transgene expression was compared between BM of primary and secondary mice, the same pattern as in blood was observed. The expression of the βAS3-globin transgene was maintained between primary (4.85 ± 1.97% βAS3-globin mRNA/VCN) and secondary mice (3.74 ± 2.06% βAS3-globin mRNA/VCN) in the Ank-R LV group (showing no significant differences P = 0.2, two-sample t-test), but was significantly lower in the secondary mice of the FB LV group (1.43 ± 1.36% βAS3-globin mRNA/VCN), compared with the primary mice (3.45 ± 1.47% βAS3-globin mRNA/VCN) (P = 0.002, two-sample t-test) (Figure 5c).
Figure 5.
Long-term assessment of VCN and βAS3-globin mRNA expression in BM from secondary mice. (a) VCN analyzed in BM samples 11 weeks post-transplant. No differences in VCN were found between mice treated with the two different LV (P = 0.50, two-sample t-test). (b) Percentage of βAS3-globin mRNA per VCN from the samples shown in (a). Expression values were higher in the group of mice treated with Ank-R LV (P < 0.001, two-samples t-test). (c) The percentages of βAS3-globin mRNA expressed in the primary and secondary mice at week 20 and 11 post-transplant, respectively, were analyzed for each LV (two-sample t-test). FB-LV treated mice: primary mice, n = 9 and secondary mice, n = 18; Ank-R LV-treated mice: primary, n = 8 and secondary, n = 21. The data shown in this graph at week 20 for the primary mice correspond to the average values shown in Supplementary Figure S6, (right y-axis); and the data shown at week 11 for the secondary mice are the same as graphed in this panel in 5(b). Bars represent average and SD values.
The expression of the vectors was also analyzed at the protein level by measuring AS3 hemoglobin (HbAS3) production in blood, by high-performance liquid chromatography (HPLC). The higher percentage of βAS3-globin mRNA produced per VCN by the Ank-R LV corresponded with a significantly higher production of HbAS3 protein per VCN (P = 0.002, two-sample t-test) (8.12 ± 7.77% HbAS3), compared with FB LV samples (2.13 ± 1.55% HbAS3) (Figure 4d). These results confirm that one copy of the Ank insulator in reverse orientation in the CCL-βAS3-FB LV protects the expression of the transgene from silencing in the long-term transduced HCS.
Discussion
Chromosomal-position effects on gene expression have a critical impact in LV-based gene therapy due to the semirandom pattern of integration of these vectors. If the integration of the vectors happens within or near heterochromatin areas, the level and pattern of expression of the transgene of interest can be negatively affected.26 Barrier insulators are defined as heterochromatin boundaries as they block the spread of the heterochromatin; addition of barrier elements to LV constructs may protect from heterochromatin formation in the provirus, leading to a more stable and consistent expression.
We have analyzed the potential capacity of the insulator from the 5’HS promoter region of the human ANK1 gene to provide a more stable and homogenous expression from the parental CCL-βAS3-FB LV previously described by our group.6 Breda et al.27 showed that in the context of a β-globin LV, a 190 bp fragment of the ANK1 promoter, when included in both LTR, was able to maintain phenotypic correction in β-thalassemic mice, at least to the same levels as its counterpart noninsulated LV. However, in all of the new constructs we designed, the Ank insulator was placed within the βAS3-globin cassette with the aim of keeping the Ank element flanked by the enhancer-blocking FB insulator, included in both LTR of the provirus. With this design, the genes surrounding the proviral integration site would be protected by the FB insulator from any residual enhancer activity from the Ank fragment. The stability of the Ank sequence in the different constructs was analyzed, indicating that the presence of more than one copy of the insulator led to the loss of one or both of these sequences. A potential explanation for the loss of the insulator would be deletion during reverse transcription or homologous recombination after the reverse transcription; however further analysis would be necessary to fully understand these events.
Protection against position-effect variegation was observed in MEL clones only when a single copy of the Ank insulator was used. But, after 4 months in culture, the clones from all of the LV analyzed, including the ones containing the full cHS4 insulator, showed a dramatic loss of expression. This could be a result of the loss of differentiation capacity of the erythroleukemia cell line used for this assay after extended culture. Therefore, the Ank-R LV was compared with the FB and cHS4 LV in human primary cells. However, the in vitro assessment of the barrier activity of the Ank-R LV in cells from SCD BM donors was not conclusive, as a result of the short duration of the erythroid differentiation of the bulk population analyzed.
Chromosomal-position-effect variegation therefore was evaluated in vivo in CFU-S from secondary and tertiary mice, which provides a more stringent assay for expression in the products of long-term HSC than short-term cultures and primary transplants do. The consistent level of expression of the βAS3-globin transgene per VCN in the Ank-R LV group, compared with the wide range of expression of the transgene per VCN in the FB LV group, indicates that the Ank insulator is able to provide a more position-independent expression of the βAS3-globin transgene. The HS3 fragment of the β-globin gene LCR has been shown to be able to provide an open chromatin environment appropriate to confer copy number-dependent gene expression in transgenic mice;28,29 however, only the whole LCR is able to recruit the erythroid-specific transcription factors and histone-modifying complexes necessary to confer a full barrier activity.16 Thus, the current mini-LCR used in globin vectors may not be able to provide the optimal chromatin architecture necessary to confer position independence. Therefore, the addition of the Ank sequence upstream of the mini-LCR in the FB-LV might be responsible for recruiting transcription factors such as USF1/2 (ref. 23) that directly interacts with HAT and HMT maintaining high levels of H3 acetylation and K4 methylation as shown with the cHS4 insulator.30
The protection against position-effect variegation observed at a clonal level in the Ank-R LV group correlated with a lack of silencing in long-term vector-transduced HSC in serial transplants in the Ank-R LV-treated mice. The main difference between the vectors with and without the Ank insulator was observed in the transgene expression in blood in secondary mice, where the Ank-R LV provided a significantly higher percentage of expression of the βAS3-globin transgene per VCN, at both the mRNA and protein levels. Additionally, the average percentages of βAS3-globin mRNA per VCN in both primary and secondary mice from the Ank-R LV group were very consistent and sustained, showing no silencing of the βAS3-globin cassette. In contrast, the expression levels of the βAS3-globin transgene in the secondary mice treated with the FB LV were significantly lower compared with the primary mice of this group. The same pattern of mRNA expression per VCN was observed in the BM samples from secondary mice.
When initially describing the Ank element, Gallagher et al.23 demonstrated the enrichment for USF1/2 transcription factors and histone markers associated with open chromatin, thus following a model for barrier insulator activity in which proteins are recruited to block the spread of heterochromatin. Our data follow this model as the addition of the Ank insulator to the CCL-AS3-FB LV promoted in vitro and in vivo protection from chromosomal-effect variegation with a lower CV of the βAS3-globin transgene expression per VCN. In addition, the Ank insulator helped to maintain the expression of the βAS3-globin transgene per VCN at the RNA level, which was correlated with higher HbAS3 protein production in long-term secondary mice.
These data together indicate that the addition of the Ank insulator protects the βAS3-globin expression cassette from positional-effect variegation and long-term silencing after transduction of primitive murine HSC. Further analyses in murine SCD models will allow assessment of whether the addition of the Ank element to the CCL-AS3-FB LV significantly improves inhibition of sickling in a clinically beneficial manner.
Materials and Methods
βAS3-globin lentiviral vectors construct, packaging, and titer determination
The CCL-βAS3, CCL-βAS3-FB, and CCL-βAS3-cHS4 LV were previously described.6 Briefly, the LV carrying the Ank insulator were cloned as follows. Ank-dGATA1 top and bot deoxyoligonucleotides (see sequences below) were annealed and made double stranded with Pfu Ultra II HS Polymerase (Agilent, Santa Clara, CA). For Ank insertion at the 3′ end of the globin cassette next to the 3′ genomic enhancer, pCCLc-AS3-dWPRE-FB was linearized with EcoRV and dephosphorylated with Antarctic phosphatase (New England Biolabs, Ipswich, MA). Blunt-end ligation was carried out with this linearized vector and the annealed, double-stranded Ank cassette, and clones were screened for single-cassette insertion and proper orientation. For Ank insertion at the 5′ end of the globin cassette next to the HS4 sequence of the mini-LCR, pCCLc-AS3-dWPRE-FB was cut with MfeI (New England Biolabs, Ipswich, MA), and the large and small fragments were gel purified. The large fragment was religated, and the resulting plasmid was then digested with EcoRI (New England Biolabs), blunted, and dephosphorylated. The blunt-end ligation was carried out with this linear vector and the Ank cassette, and clones were screened for single-cassette insertion and proper orientation. The smallest of the gel-purified MfeI fragments was then reinserted to generate the full AS3 vector with desired Ank sequences. Ank-dGATA1-top /5Phos/TGGGGAGCGGGGCCTCCTGGGGTTGGGGGAGGAGGTGCTCTTGTAATC TGCGGTCCCCAGGCGGGCGCCACCCCTCCGCCCGCCCGTGCC Ank-dGATA1-bot /5Phos/GACGTGCGGGCCAGGCCCCCGAGGGCCTTAACGGCCCCAGAGGCGCTTGCTGTCGGGCCGGGCGCTCCCGGCACG GGCGGGCGGAGGGGT.
Small-scale production of the different LV and titering were performed as previously described.6 Large-scale viral preparations were produced and concentrated using tangential flow filtration and titered by qPCR as described previously.31
MEL cells culture, erythroid differentiation, and intracellular βAS3-globin staining for flow cytometry
MEL cells (ATCC number TIB-56, Manassas, VA) were cultured in Dulbecco’s Modified Eagle Medium (DMEM) (Mediatech, Herndon, VA) containing 1× glutamine, penicillin, and streptomycin (both from Cellgro, Manassas, VA) and 10% fetal bovine serum (FBS) (Gemini Bio-Products, West Sacramento, CA). Single-vector copy MEL cell clones were generated for subsequent expression analyses by transducing with each LV at low MOI (from 0.2 to 2). One week post-transduction, single cells were sorted from the pools using the fluorescence-activated cell sorting (FACS)-automated cell deposition unit (ACDU) technique into wells containing DMEM-10% FBS. After sorting, the MEL cell clones were expanded and characterized for the presence or absence of vector by PCR with primers amplifying the Psi region common to all the vectors. The PCR reaction was started at 94°C for 1 minute, followed by 35 cycles of 98°C for 5 seconds, 62°C for 5 seconds, and 72°C for 5 seconds, using an Eppendorf Mastercycler Pro S (Eppendorf, Hamburg, Germany). The resulting PCR product was loaded in a 2% agarose (BioExpress, Kaysville, UT) gel and run in 1× TAE (Cellgro, Manassas, VA) for a qualitative analysis of transduced MEL cell clones. Four weeks post-transduction, cells from each MEL cell clone containing at least one copy of an LV (as determined by PCR for Psi) were harvested for genomic DNA isolation using the PureLink Genomic DNA Mini Kit (Invitrogen, Carlsbad, CA) to identify those clones containing single-vector copies. The average VCN was determined by two separate qPCR reactions: one specific for the HIV-1 Psi in the LV provirus, and the other specific for the housekeeping gene UC483 present at consistent levels in the MEL cell line. The Psi signal was normalized to the amount of UC483 amplified to calculate the average number of VCN per cell in each transduced MEL cell clone based on a standard curve method described by Cooper et al.31
For in vitro erythroid differentiation, 2.5 × 105 MEL cells/ml were cultured in DMEM with 20% FBS, supplemented with 5 mmol/l N,N’-hexamethylene bisacetamide (HMBA) (Sigma-Aldrich, St. Louis, MO). After 5 days of differentiation, 1–3 × 106 MEL cells were harvested for intracellular staining with an anti-human β-globin antibody to assess the level of βAS3-globin expression. The cells were stained with the viability dye Aqua Dead Cell Stain (Life Technologies, Grand Island, NY) for 15 minutes, prior to fixation with 4% paraformaldehyde (USB Corporation, Cleveland, OH) for 1 hour, and subsequently with 100% methanol for 5 minutes (Sigma-Aldrich). Following fixation, the cells were blocked with 5% non-fat milk (Santa Cruz Biotechnology, Dallas, TX), and then stained with a PE-labeled anti-human β-globin antibody (Hemoglobin β(38-7) sc-21757, Santa Cruz Biotechnology, Santa Cruz, CA) in the presence of Fix & Perm Solution B (Life Technologies, Carlsbad, CA) for 30 minutes. All of the flow cytometry analyses were performed on an LSR Fortessa cell analyzer (BD Biosciences, San Jose, CA).
The CV was calculated as the ratio of the SD to the average percentage of expressing cells of the clones of each group at each time point.
Stability of the insulators
The integrity of the Ank insulator was analyzed by PCR in MEL clones that were transduced with the three different LV carrying the Ank insulator in diverse positions and orientations. Genomic DNA isolation was performed using the PureLink Genomic DNA Mini Kit (Invitrogen). Two set of primers were designed to amplify the Ank sequence upstream from the β-globin LCR cassette (near the 5′ end of the vector), one set to amply the Ank insulator in forward orientation (Ank-F: Forward: 5′-AGT TTG GGT CGA GGA TTG GGG AGC-3′ and Reverse: 5′-GAA TTC CGA TCC GAT GAC GTG CGG G-3′, with an expected band of 190 bp) and a second set to amplify the Ank insulator in reverse orientation (Ank-R5’: Forward: 5′-GGG TCG AGG ATG ACG TGC GGG CCAG G 3′ and _Reverse: 5′-GAG AAT TCC GAT CCG ATT GGG GAG C-3′, with an expected band of 187 bp). A third set of primers was designed to amplify the Ank insulator located downstream of the β-globin LCR cassette (near the 3′ end of the vector), and placed in reverse orientation (Ank-R3′: _Forward: 5′-CGA GGG AAT TGA CGT GCG and Reverse: 5′- TTG ATT ATC GGA ATT TGG GGA GCG GGG C-3′, in this case the corresponding amplicon had a length of 183 bp). The stability of the cHS4 insulator was tested as well by PCR in MEL clones that were transduced with the LV carrying this insulator. In this case, the primers used were the following ones. To amplify the cHS4 sequence in the 5’LTR of the provirus: F-5LTR-FB: 5′-GGCTAATTCACTCCCAACGAAGACAAG-3′ and R-5LTR-FB: 5′-CTT CAG CAA GCC GAG TCC TGC-3′. To amplify the cHS4 sequence in the 3′LTR of the provirus: F- 3′LTRcHS4: 5′-CTG CAG ATA TCC ATC ACA CTG GCG G-3′ and R-3LTR-FB: 5′-CAG AGA GAC CCA GTA CAA GCA AAA AG-3′. PCR were executed using Taq DNA Polymerase, Native (Invitrogen) on an Eppendorf (Hamburg, Germany) thermocycler. The PCR products were visualized by GelGreen (Phenix Research Products, Candler, NC) on 2% agarose gels.
Human CD34+ cell isolation, transduction, erythroid differentiation, and VCN and mRNA analysis
Isolation of human CD34+ cells from BM, transduction, and in vitro erythroid differentiation were performed as described by Romero et al.6 Bone marrow aspirates from volunteer donors with SCD were obtained under UCLA IRB# 001399.
Isolation, transduction, and transplantation of murine HSCs
BM cells from femurs and tibias from untreated 8-week-old male CD45.1 (+) B6.SJL-Ptprca Pep3b/BoyJ (C57L/6 congenic) (Jackson Laboratories, Bar Harbor, ME) donor mice were harvested, lineage depleted using the Lineage Cell Depletion Kit (Miltenyi Biotech, Bergisch Gladbach, Germany) and linage negative (lin-)cells were cryopreserved. Forty hours prior to transplant, lin- cells were thawed and prestimulated in StemSpan SFEM serum-free expansion medium (STEMCELL Technologies, Vancouver, British Columbia, Canada) containing 50 ng/ml mSCF, 100 ng/ml human interleukin (IL)-11, 20 ng/ml mIL-3 (all PeproTech, Rocky Hill, NJ), 100 ng/ml hFlt3-L (Celldex Therapeutics, Needham, MA), and 1× glutamine, penicillin and streptomycin in plates coated with retronectin (20 μg/ml; Clontech, Mountain View, CA). After 16 hours of prestimulation, transductions with the FB and Ank-R LV were performed at the concentration of 2 × 107 TU/ml (MOI ~80). Tewnty-four hours post-transduction, the cells were harvested, counted, and 106 lin- cells were transplanted into 8-week-old female CD45.2 (+) C57bl/6J recipients (Jackson Laboratories) after total body irradiation (10.75 Gy split into two doses separated by 3 hours). Primary mice were maintained up to 4 months and retro-orbital bleeds were performed periodically for VCN and β-globin mRNA analysis.
Mice harvest and secondary transplants
Four months after transplants, BM cells from femurs and tibias of primary recipients were harvested. Portions of the marrow were used to determine engraftment by FACS, and for VCN and mRNA analyses. Prior to euthanasia, retro-orbital bleeds were performed as well for engraftment, VCN, and mRNA from blood analysis at that last time point. For long-term analyses of gene expression and silencing, 20 × 106 cells of whole BM cells were transplanted into each secondary recipient. Secondary mice were euthanized after 11 weeks for final analysis of engraftment, VCN, and βAS3-globin mRNA in blood and BM. All work with mice was done under protocols approved by the UCLA Animal Research Committee.
Spleen colony-forming units (CFU-S)
For vector expression analyses in clonal progeny from murine HSC, 1 × 106 whole BM cells from primary and secondary mice were transplanted in multiple CD45.2 female recipients. Individual CFU-S (secondary and tertiary) were isolated by dissection from spleens collected 10 days after transplants. Each CFU-S was divided in two halves, for genomic DNA isolation (PureLink Genomic DNA Mini Kit, Invitrogen) for VCN determination and for RNA isolation (RNeasy Plus Mini Kit, Qiagen, Valencia, CA) for βAS3-globin mRNA expression assay.
Engraftment assessment
Donor-recipient chimerism was determined in blood and BM samples from primary and secondary recipients by FACS. Samples were incubated at 4°C for 5 minutes with rat anti-mouse CD16/CD32 antibody to block Fc receptors, prior to addition of APC-conjugated mouse anti-mouse CD45.2 and V450-conjugated mouse anti-mouse CD45.1 (all antibodies from BD Biosciences) followed by incubation at 4°C for 30 minutes. Antibody excess was washed out with PBS and samples were analyzed with a LSR Fortessa cell analyzer (BD Biosciences). The percentage of engrafted donor cells was defined as follows: %CD45.1+/(%CD45.1+ + %CD45.2+).
DNA analysis
Genomic DNA isolations from blood samples and BM samples were performed using the NucleoSpin Tissue XS kit (Clontech) and the PureLink Genomic DNA Mini Kit (Invitrogen), respectively, and according to manufacturer’s instructions.
For VCN determination, primers and probe were designed to detect a conserved LV sequence (HIV-1 Psi region) (Forward-HIVU5: 5′-AAGTAGTGTGTGCCCGTCTG-3′; Reverse-HIV-Psi:5′-CCTCTGGTTTCCCTTTCGCT-3′ and probe: 5′-/5HEX/CCCTCAGAC/ZEN/CCTTTTAGTCAGTGTGGAAAATCTCTAG/3IABkFQ/-3′) and Y-chromosome-specific primers and probe (Mm00527143_cn) (purchased as a 20× premix of primers and FAM-MGBNFQ probe; Applied Biosystems, San Francisco, CA) were used for normalization. Reaction mixtures of 20 μl volume comprising 1× ddPCR Master Mix (Bio-Rad, Hercules, CA), primers, and probe (900 nmol/l and 250 nmol/l for Y-chromosome primers and probe respectively; 500 nmol/l and 100 nmol/l for Psi primers and probe, respectively) were used. Droplet generation was performed as described in Hindson et al.32 PCR amplification of droplet emulsion, droplet read, and ddPCR analysis were performed as previously described.6 VCN of donor cells was calculated by dividing the concentration (copies/μl) of Psi by the concentration of Y-chromosome.
RNA analysis
RNA from BM samples was extracted using the RNeasy Plus Mini Kit (Qiagen) according to the manufacturer’s instructions. Blood samples for RNA analysis were collected in RNAprotect Animal Blood Tubes (Qiagen) and incubated at room temperature for 2 hours, prior to RNA isolation with RNeasy Protect animal Blood Kit (Qiagen). In both cases, first-strand cDNA was synthesized using random primers, M-MLV reverse transcriptase, and RNAseOUT Recombinant Ribonuclease Inhibitor (all from Invitrogen) according to the manufacturer’s protocol.
To specifically detect βAS3-globin mRNA transcripts originating from the vector CCL-βAS3-FB, a set of primers and probe, previously described (HBBAS3),6 were used together with a second set of primers and probe designed to amplify the murine single haplotype β-like transcripts (Forward-murine β-glo 5′-TGCTGGTTGTCTACCCTTGGAC-3′; Reverse-murine β-glo 5′-TCGTTAAAGGCAGTTATCACTTTCTTGC-3′ and probe: 5′/5HEX/CCTATCCTC/ZEN/TGCCTCTGCTATCATGGGTAATGCCAAAGT/3IABκF) (all primers were used at a final concentration of 500 nmol/l and probes at 100 nmol/l). ddPCR was performed as described6 and the relative expression of βAS3-globin/murine β-globin-like was calculated by dividing the concentration (copies/μl) of βAS3-globin by the concentration of murine β-globin-like, and normalizing to the VC/cell measured for each sample.
High-performance liquid chromatography (HPLC)
Secondary mice were bled 11 weeks post-transplant. One microliter of peripheral blood per was lysed as follows. RBC were pelleted and lysed in Hemolysate reagent (Helena Laboratories, Beaumont, TX) for 5–10 minutes at room temperature. After centrifugation at 20,800g for 10 minutes at 4°C to remove cellular debris, RBC lysates were stored frozen at −80°C. Upon thawing, cell lysates were diluted 1:40 in mobile phase A and characterized by HPLC (Infinity 1260, Agilent) using a weak cation-exchange column (PolyCAT A, PolyLC, Coulmbia, MD). FASC Reference Material (Trinity Biotech, Wicklow, Ireland) was used to define the elution time of common human hemoglobin forms (HbF, HbA, HbS, and HbC). Analysis and peak integration were performed using OpenLAB CDS Chemstation software (Agilent, Santa Clara, CA). The relative percentage of HbAS3 produced for each sample was calculated based on the sum total of areas under the curve for each of the primary hemoglobin peaks, which included βmajor and βminor.
Statistical analysis
Descriptive statistics such as number of observations, mean and SD were reported and presented graphically for quantitative measurements. Normality assumption was checked for outcomes before statistical testing. For both primary and secondary mice, comparisons of expression (i.e., VCN, percentage of βAS3-globin mRNA/VCN, and percentage of HbAS3/VCN) and engraftment in BM and blood between experimental groups were performed by either two-sample t-tests (Figure 4a–d; Figure 5a–c and Supplementary Figure S6) or one-way ANOVA33 with pairwise comparisons (Figure 2a,b; Supplementary Figure S3a,b; Supplementary Figure S4a,b; Supplementary Figure S7a,b). Repeated-measure ANOVA34 was used to assess if there was treatment group difference in βAS3-globin expression in MEL cell clones (Figure 2c) or in blood over time (Supplementary Figure S5a,b). All hypothesis testing was two-sided and a significance threshold of 0.05 for P-value was used. Analyses were carried out using SAS version 9.3 (SAS Institute, Cary, NC).35
Acknowledgments
These studies were supported by a research grant from the National Institutes of Health (NHLBI PO1 HL073104) (D.B.K.), the Interdisciplinary Training In Virology and Gene Therapy award (5 T32 AI060567; M.D.H.), the Whitcome Pre-doctoral Training Program in the University of California, Los Angeles (UCLA) Molecular Biology Institute (M.D.H), the Ruth L. Kirschstein National Research Service Award (GM007185; A.R.C.), and the Initiative to Maximize Student Diversity (NIH GM55052; D.L.). The FACS Core of the Eli & Edythe Broad Center for Regenerative Medicine and Stem Cell Research was used for these studies. The authors would like to thank Patrick G. Gallagher for providing the sequence of human ANK1 gene used in these studies. Z.R. designed, performed, and analyzed experiments and wrote the manuscript. B.C.-F. and J.W. performed and analyzed experiments. M.L.K. performed all mouse transplants and harvests. F.U. performed HPLC. A.R.C, R.P.H., and S.S. designed, cloned, and produced LV. M.L.K., F.U., B.C.-F., M.D.H. and K.M.B. performed and collaborated with mouse design experiments. X.W. performed all statistical analysis. D.L. performed insulator stability PCR. D.B.K. provided intellectual contribution to project conception, experimental design, data analysis, and manuscript writing.
The authors have declared that no conflict of interest exists.
References
- Hoffman R, Benz EJ, Furie B, Shattil SJ.2009Hematology Churchill Livingstone; London, UK. pp. 1 [Google Scholar]
- Madigan C, Malik P. Pathophysiology and therapy for haemoglobinopathies. Part I: sickle cell disease. Expert Rev Mol Med. 2006;8:1–23. doi: 10.1017/S1462399406010659. [DOI] [PubMed] [Google Scholar]
- Bolaños-Meade J, Brodsky RA. Blood and marrow transplantation for sickle cell disease: overcoming barriers to success. Curr Opin Oncol. 2009;21:158–161. doi: 10.1097/CCO.0b013e328324ba04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthenveetil G, Scholes J, Carbonell D, Qureshi N, Xia P, Zeng L. Successful correction of the human beta-thalassemia major phenotype using a lentiviral vector. Blood. 2004;104:3445–3453. doi: 10.1182/blood-2004-04-1427. [DOI] [PubMed] [Google Scholar]
- Roselli EA, Mezzadra R, Frittoli MC, Maruggi G, Biral E, Mavilio F. Correction of beta-thalassemia major by gene transfer in haematopoietic progenitors of pediatric patients. EMBO Mol Med. 2010;2:315–328. doi: 10.1002/emmm.201000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero Z, Urbinati F, Geiger S, Cooper AR, Wherley J, Kaufman ML. -globin gene transfer to human bone marrow for sickle cell disease. J Clin Invest. 2013;123:3317–3330. doi: 10.1172/JCI67930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May C, Rivella S, Callegari J, Heller G, Gaensler KM, Luzzatto L. Therapeutic haemoglobin synthesis in beta-thalassaemic mice expressing lentivirus-encoded human beta-globin. Nature. 2000;406:82–86. doi: 10.1038/35017565. [DOI] [PubMed] [Google Scholar]
- Pawliuk R, Westerman KA, Fabry ME, Payen E, Tighe R, Bouhassira EE. Correction of sickle cell disease in transgenic mouse models by gene therapy. Science. 2001;294:2368–2371. doi: 10.1126/science.1065806. [DOI] [PubMed] [Google Scholar]
- Levasseur DN, Ryan TM, Pawlik KM, Townes TM. Correction of a mouse model of sickle cell disease: lentiviral/antisickling beta-globin gene transduction of unmobilized, purified hematopoietic stem cells. Blood. 2003;102:4312–4319. doi: 10.1182/blood-2003-04-1251. [DOI] [PubMed] [Google Scholar]
- Hanawa H, Hargrove PW, Kepes S, Srivastava DK, Nienhuis AW, Persons DA. Extended beta-globin locus control region elements promote consistent therapeutic expression of a gamma-globin lentiviral vector in murine beta-thalassemia. Blood. 2004;104:2281–2290. doi: 10.1182/blood-2004-03-0863. [DOI] [PubMed] [Google Scholar]
- Miccio A, Cesari R, Lotti F, Rossi C, Sanvito F, Ponzoni M. In vivo selection of genetically modified erythroblastic progenitors leads to long-term correction of beta-thalassemia. Proc Natl Acad Sci USA. 2008;105:10547–10552. doi: 10.1073/pnas.0711666105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestina TI, Hargrove PW, Jay D, Gray JT, Boyd KM, Persons DA. Correction of murine sickle cell disease using gamma-globin lentiviral vectors to mediate high-level expression of fetal hemoglobin. Mol Ther. 2009;17:245–252. doi: 10.1038/mt.2008.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniou M, Geraghty F, Hurst J, Grosveld F. Efficient 3′-end formation of human beta-globin mRNA in vivo requires sequences within the last intron but occurs independently of the splicing reaction. Nucleic Acids Res. 1998;26:721–729. doi: 10.1093/nar/26.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custódio N, Carmo-Fonseca M, Geraghty F, Pereira HS, Grosveld F, Antoniou M. Inefficient processing impairs release of RNA from the site of transcription. EMBO J. 1999;18:2855–2866. doi: 10.1093/emboj/18.10.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosveld F, de Boer E, Dillon N, Fraser P, Gribnau J, Milot E. The dynamics of globin gene expression and gene therapy vectors. Semin Hematol. 1998;35:105–111. [PubMed] [Google Scholar]
- Li Q, Peterson KR, Fang X, Stamatoyannopoulos G. Locus control regions. Blood. 2002;100:3077–3086. doi: 10.1182/blood-2002-04-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zufferey R, Dull T, Mandel RJ, Bukovsky A, Quiroz D, Naldini L. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol. 1998;72:9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J, Pannell D. The beta-globin locus control region versus gene therapy vectors: a struggle for expression. Clin Genet. 2001;59:17–24. doi: 10.1034/j.1399-0004.2001.590103.x. [DOI] [PubMed] [Google Scholar]
- Ellis J. Silencing and variegation of gammaretrovirus and lentivirus vectors. Hum Gene Ther. 2005;16:1241–1246. doi: 10.1089/hum.2005.16.1241. [DOI] [PubMed] [Google Scholar]
- Arumugam PI, Scholes J, Perelman N, Xia P, Yee JK, Malik P. Improved human beta-globin expression from self-inactivating lentiviral vectors carrying the chicken hypersensitive site-4 (cHS4) insulator element. Mol Ther. 2007;15:1863–1871. doi: 10.1038/sj.mt.6300259. [DOI] [PubMed] [Google Scholar]
- Urbinati F, Arumugam P, Higashimoto T, Perumbeti A, Mitts K, Xia P. Mechanism of reduction in titers from lentivirus vectors carrying large inserts in the 3′LTR. Mol Ther. 2009;17:1527–1536. doi: 10.1038/mt.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavazzana-Calvo M, Payen E, Negre O, Wang G, Hehir K, Fusil F. Transfusion independence and HMGA2 activation after gene therapy of human -thalassaemia. Nature. 2010;467:318–322. doi: 10.1038/nature09328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher PG, Steiner LA, Liem RI, Owen AN, Cline AP, Seidel NE. Mutation of a barrier insulator in the human ankyrin-1 gene is associated with hereditary spherocytosis. J Clin Invest. 2010;120:4453–4465. doi: 10.1172/JCI42240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramezani A, Hawley TS, Hawley RG. Combinatorial incorporation of enhancer-blocking components of the chicken beta-globin 5’HS4 and human T-cell receptor alpha/delta BEAD-1 insulators in self-inactivating retroviral vectors reduces their genotoxic potential. Stem Cells. 2008;26:3257–3266. doi: 10.1634/stemcells.2008-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douay L, Giarratana MC.2009. Ex vivo generation of human red blood cells: a new advance in stem cell engineering. In: Walker, JM, Audet, J and Stanford, WL (eds). Methods in Molecular Biology 482Humana Press; Totowa, NJ, 127–140. [DOI] [PubMed] [Google Scholar]
- Emery DW. The use of chromatin insulators to improve the expression and safety of integrating gene transfer vectors. Hum Gene Ther. 2011;22:761–774. doi: 10.1089/hum.2010.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breda L, Casu C, Gardenghi S, Bianchi N, Cartegni L, Narla M. Therapeutic hemoglobin levels after gene transfer in -thalassemia mice and in hematopoietic cells of -thalassemia and sickle cells disease patients. PLoS One. 2012;7:e32345. [Google Scholar]
- Li Q, Stamatoyannopoulos JA. Position independence and proper developmental control of gamma-globin gene expression require both a 5’ locus control region and a downstream sequence element. Mol Cell Biol. 1994;14:6087–6096. doi: 10.1128/mcb.14.9.6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J, Tan-Un KC, Harper A, Michalovich D, Yannoutsos N, Philipsen S. A dominant chromatin-opening activity in 5’ hypersensitive site 3 of the human beta-globin locus control region. EMBO J. 1996;15:562–568. [PMC free article] [PubMed] [Google Scholar]
- Huang S, Li X, Yusufzai TM, Qiu Y, Felsenfeld G. USF1 recruits histone modification complexes and is critical for maintenance of a chromatin barrier. Mol Cell Biol. 2007;27:7991–8002. doi: 10.1128/MCB.01326-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AR, Patel S, Senadheera S, Plath K, Kohn DB, Hollis RP. Highly efficient large-scale lentiviral vector concentration by tandem tangential flow filtration. J Virol Methods. 2011;177:1–9. doi: 10.1016/j.jviromet.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindson BJ, Ness KD, Masquelier DA, Belgrader P, Heredia NJ, Makarewicz AJ. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal Chem. 2011;83:8604–8610. doi: 10.1021/ac202028g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupert, G, Miller J.1997Beyond ANOVA CRC Press; Boca Raton, FL. pp. 1 [Google Scholar]
- Vonesh E, Chinchilli VM.1996Linear and Nonlinear Models for the Analysis of Repeated Measurements CRC Press; Boca Raton, FL. [Google Scholar]
- SAS Institute 2011SAS/STAT 9.3 User’s Guide SAS Institute; Cary, NC. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.