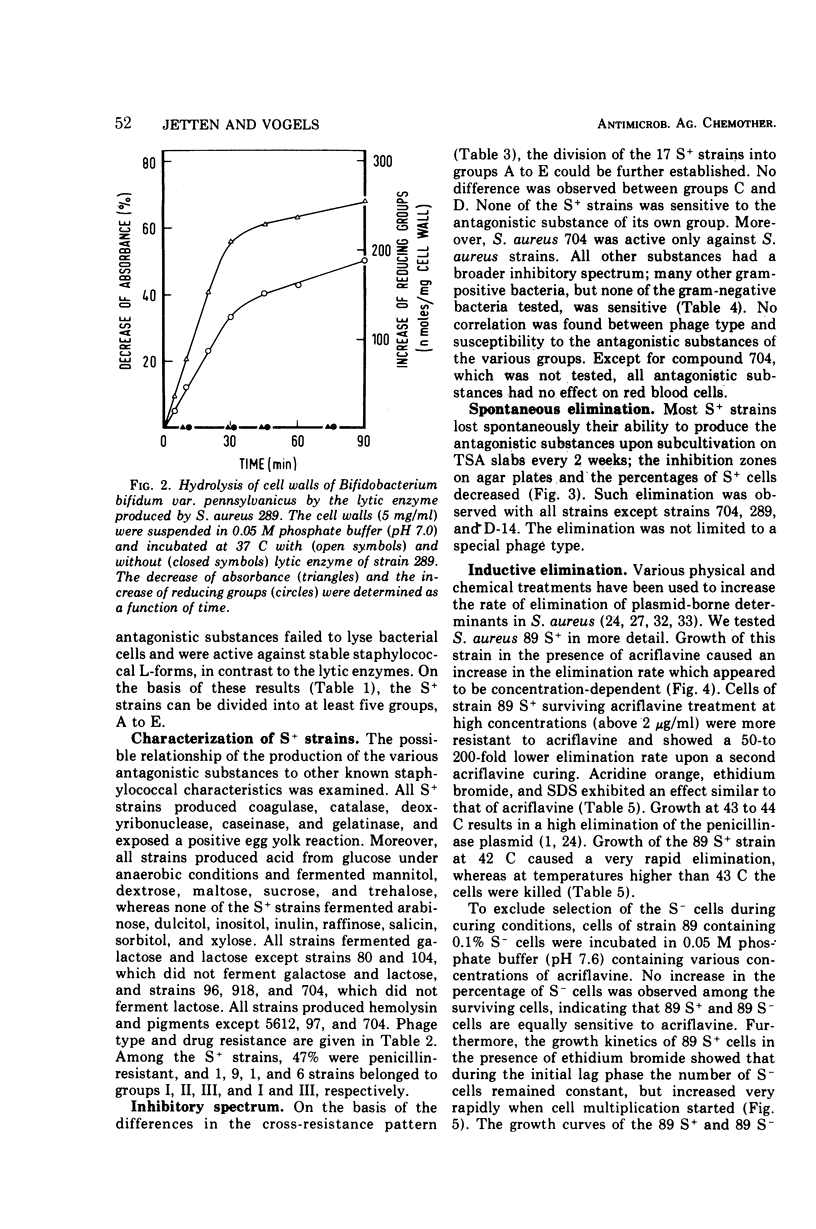
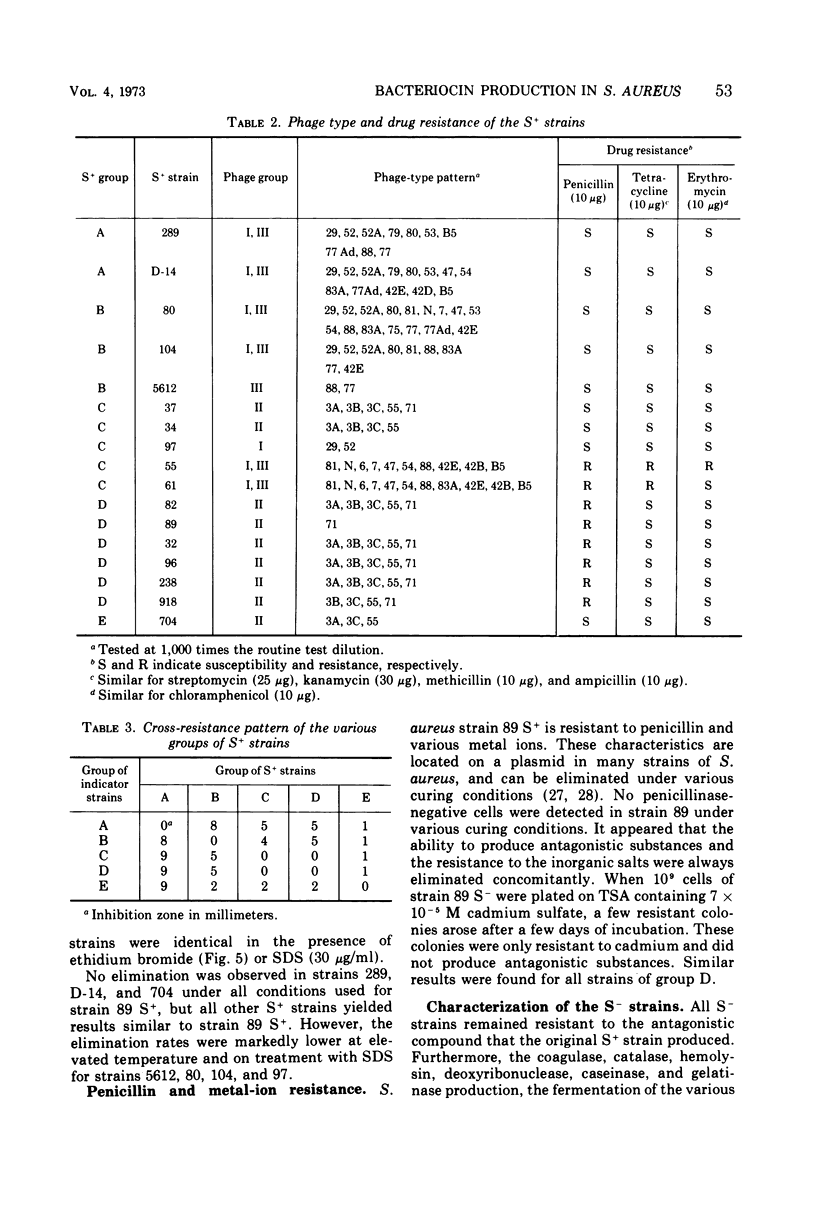
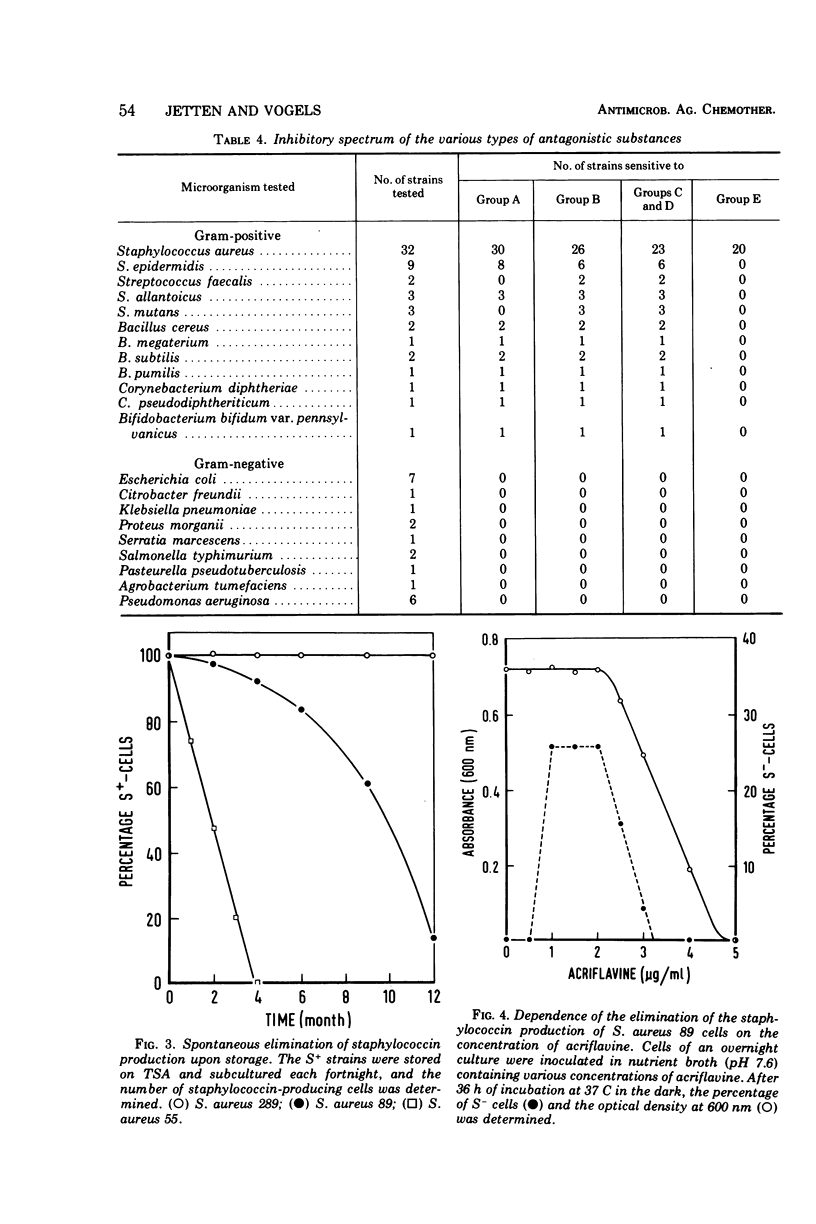
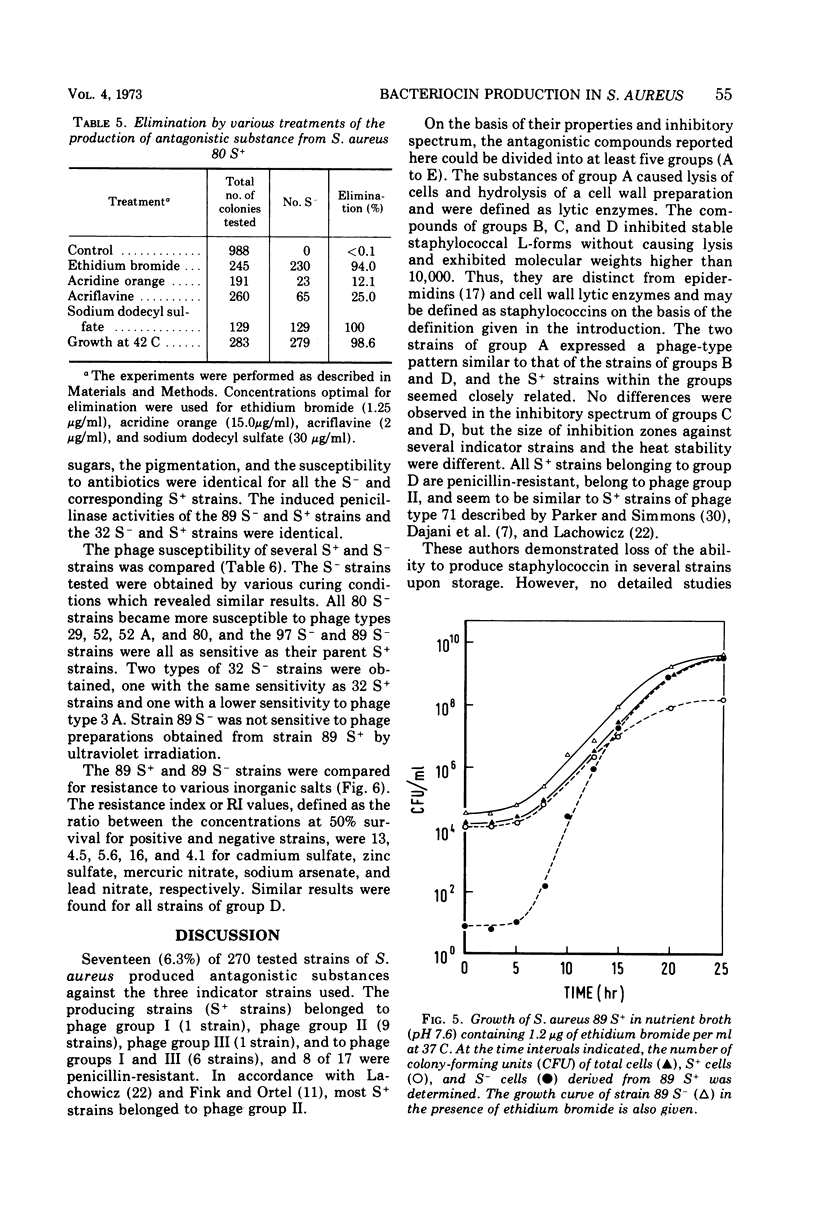
Abstract
About 6% of the tested strains of Staphylococcus aureus produced antagonistic substances against staphylococcal indicator strains. The production was low in liquid cultures and could not be induced by ultraviolet irradiation or by treatment with mitomycin C. The antagonistic substances could be classified into at least five groups on the basis of their properties and cross-resistance pattern. One group consisted of lytic enzymes and the four others of staphylococcins. One of the four types of staphylococcins was active against S. aureus only, and the three other types had a broader inhibitory spectrum against gram-positive organisms but not against gram-negative bacteria. A relationship was found between some groups of producing strains and their phage type. The ability to produce staphylococcins was eliminated spontaneously upon storage and more rapidly by treatment with ethidium bromide, acriflavine, acridine orange, sodium dodecyl sulfate, and growth at 42 C. The resistance to several inorganic salts was co-eliminated. No co-elimination of penicillinase production was observed. Selective effects during elimination were ruled out, and the results suggest that the genes for staphylococcin production are plasmid-borne determinants.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asheshov E. H. Loss of antibiotic resistance in Staphylococcus aureus resulting from growth at high temperature. J Gen Microbiol. 1966 Mar;42(3):403–410. doi: 10.1099/00221287-42-3-403. [DOI] [PubMed] [Google Scholar]
- Bentley D. W., Haque R. U., Murphy R. A., Lepper M. H. Biotyping, and epidemiological tool for coagulase-negative staphylococci. Antimicrob Agents Chemother (Bethesda) 1967;7:54–59. [PubMed] [Google Scholar]
- Dajani A. S., Gray E. D., Wannamaker L. W. Effect of Bactericidal Substance from Staphylococcus aureus on Group A Streptococci I. Biochemical Alterations. Infect Immun. 1970 May;1(5):485–490. doi: 10.1128/iai.1.5.485-490.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajani A. S., Wannamaker L. W. Demonstration of a bactericidal substance against beta-hemolytic streptococci in supernatant fluids of staphylococcal cultures. J Bacteriol. 1969 Mar;97(3):985–991. doi: 10.1128/jb.97.3.985-991.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval-Iflah Y. Lysogenic conversion of the lipase in Staphylococcus pyogenes group 3 strains. Can J Microbiol. 1972 Sep;18(9):1491–1497. doi: 10.1139/m72-228. [DOI] [PubMed] [Google Scholar]
- Farmer J. J., 3rd Epidemiological differentiation of Serratia marcescens: typing by bacteriocin production. Appl Microbiol. 1972 Feb;23(2):218–225. doi: 10.1128/am.23.2.218-225.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer J. J., 3rd, Herman L. G. Epidemiological fingerprinting of Pseudomonas aeruginosa by the production of and sensitivity of pyocin and bacteriophage. Appl Microbiol. 1969 Nov;18(5):760–765. doi: 10.1128/am.18.5.760-765.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink H., Ortel S. Untersuchungen über Staphylococcine. I. Isolierung staphylococcinbildender Stämme, Herkunft, Lysisbild und Antibiotikaresistenz. Zentralbl Bakteriol Orig. 1969;211(1):39–47. [PubMed] [Google Scholar]
- GHUYSEN J. M., STROMINGER J. L. STRUCTURE OF THE CELL WALL OF STAPHYLOCOCCUS AUREUS, STRAIN COPENHAGEN. I. PREPARATION OF FRAGMENTS BY ENZYMATIC HYDROLYSIS. Biochemistry. 1963 Sep-Oct;2:1110–1119. doi: 10.1021/bi00905a035. [DOI] [PubMed] [Google Scholar]
- Gagliano V. J., Hinsdill R. D. Characterization of a Staphylococcus aureus bacteriocin. J Bacteriol. 1970 Oct;104(1):117–125. doi: 10.1128/jb.104.1.117-125.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALBERT S. P., SWICK L., SONN C. Characteristics of antibiotic-producing strains of the ocular bacterial flora. J Immunol. 1953 Apr;70(4):400–410. [PubMed] [Google Scholar]
- HAMON Y., PERON Y. QUELQUES REMARQUES SUR LES BACT'ERIOCINES PRODUITES PAR LES MICROBES GRAM-POSITIFS. C R Hebd Seances Acad Sci. 1963 Jul 29;257:1191–1193. [PubMed] [Google Scholar]
- Hsu C., Wiseman G. M. The nature of epidermidins, new antibiotics from staphylococci. Can J Microbiol. 1972 Feb;18(2):121–125. doi: 10.1139/m72-021. [DOI] [PubMed] [Google Scholar]
- JEFFRIES C. D., HOLTMAN D. F., GUSE D. G. Rapid method for determining the activity of microorganisms on nucleic acids. J Bacteriol. 1957 Apr;73(4):590–591. doi: 10.1128/jb.73.4.590-591.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetten A. M., Vogels G. D. Mode of action of a Staphylococcus epidermidis bacteriocin. Antimicrob Agents Chemother. 1972 Dec;2(6):456–463. doi: 10.1128/aac.2.6.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetten A. M., Vogels G. D. Nature and properties of a Staphylococcus epidermidis bacteriocin. J Bacteriol. 1972 Oct;112(1):243–250. doi: 10.1128/jb.112.1.243-250.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetten A. M., Vogels G. D., de Windt F. Production and purification of a Staphylococcus epidermidis bacteriocin. J Bacteriol. 1972 Oct;112(1):235–242. doi: 10.1128/jb.112.1.235-242.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAY J. W., HOUGHTON R. H., PERRET C. J. THE EFFECT OF GROWTH AT ELEVATED TEMPERATURES ON SOME HERITABLE PROPERTIES OF STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1964 Nov;37:157–169. doi: 10.1099/00221287-37-2-157. [DOI] [PubMed] [Google Scholar]
- McClung L. S., Toabe R. The Egg Yolk Plate Reaction for the Presumptive Diagnosis of Clostridium sporogenes and Certain Species of the Gangrene and Botulinum Groups. J Bacteriol. 1947 Feb;53(2):139–147. doi: 10.1128/jb.53.2.139-147.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrikin D. J., Terry C. S. Use of pyocin 78-C2 in the treatment of Pseudomonas aeruginosa infection in mice. Appl Microbiol. 1972 Jan;23(1):164–165. doi: 10.1128/am.23.1.164-165.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVICK R. P. Micro-iodometric assay for penicillinase. Biochem J. 1962 May;83:236–240. doi: 10.1042/bj0830236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVICK R. P., RICHMOND M. H. NATURE AND INTERACTIONS OF THE GENETIC ELEMENTS GOVERNING PENICILLINASE SYNTHESIS IN STAPHYLOCOCCUS AUREUS. J Bacteriol. 1965 Aug;90:467–480. doi: 10.1128/jb.90.2.467-480.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P. Extrachromosomal inheritance in bacteria. Bacteriol Rev. 1969 Jun;33(2):210–263. doi: 10.1128/br.33.2.210-263.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Roth C. Plasmid-linked resistance to inorganic salts in Staphylococcus aureus. J Bacteriol. 1968 Apr;95(4):1335–1342. doi: 10.1128/jb.95.4.1335-1342.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARKER M. T., SIMMONS L. E. The inhibition of Corynebacterium diphtheriae and other gram-positive organisms by Staphylococcus aureus. J Gen Microbiol. 1959 Oct;21:457–476. doi: 10.1099/00221287-21-2-457. [DOI] [PubMed] [Google Scholar]
- Rubin S. J., Rosenblum E. D. Effects of ethidium bromide on growth and on loss of the penicillinase plasmid of Staphylococcus aureus. J Bacteriol. 1971 Dec;108(3):1200–1204. doi: 10.1128/jb.108.3.1200-1204.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonstein S. A., Baldwin J. N. Loss of the penicillinase plasmid after treatment of Staphylococcus aureus with sodium dodecyl sulfate. J Bacteriol. 1972 Jan;109(1):262–265. doi: 10.1128/jb.109.1.262-265.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosti K. L. Production of and sensitivity to colicins among serologically classified strains of Escherichia coli. J Bacteriol. 1968 Dec;96(6):1947–1952. doi: 10.1128/jb.96.6.1947-1952.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAMENHOF S., GREER S. Heat as an agent producing high frequency of mutations and unstable genes in Escherichia coli. Nature. 1958 Aug 30;182(4635):611–613. doi: 10.1038/182611a0. [DOI] [PubMed] [Google Scholar]




