Abstract
Production of blood stem cells from reprogrammed adult cells is notoriously difficult. It emerges that a supportive microenvironment may be crucial for their efficient generation
Bone-marrow transplants can be life-saving, but a large proportion of patients who are in need of a transplant — particularly those from ethnic minorities — lack suitable donors. Blood-cell precursors called haematopoietic stem cells are the basis of transplants because, when they are injected intravenously, they can migrate and engraft into the bone marrow, regenerating every blood-cell lineage. One way to combat the donor deficit, therefore, would be to generate patient-derived haematopoietic stem cells. However, this strategy has been hampered by problems with engrafting engineered stem cells, and by difficulties with maintaining haematopoietic 'stemness' in laboratory-cultured cells. On page 312 of this issue, Sandler et al.1 describe an approach for generating haematopoietic stem cells that circumvents these problems.
In their seminal experiment2, the stem-cell biologists Shinya Yamanaka and Kazutoshi Takahashi reprogrammed skin fibroblast cells into a 'reset' state. Starting with a series of candidate transcription factors, the researchers defined a combination of four factors that induce complete cellular dedifferentiation. The reprogrammed cells, called induced pluripotent stem (iPS) cells, can theoretically differentiate into any cell type in the body. However, differentiation of iPS cells into functional adult tissues has proved to be a challenge, owing to our lack of understanding about the complex cues required to program cells in vitro. As such, differentiation protocols for haematopoietic stem cells (HSCs) tend to yield embryonic-like blood cells that do not engraft efficiently into bone marrow3.
An alternative strategy is the direct reprogramming of adult cells into another lineage, without going through a pluripotent-cell stage. Adult fibroblasts have been successfully reprogrammed into several cell types, including neurons, cardiomyocytes and hepatocytes4. Last year5, four transcription factors (Gata2, cFos, Gfi1b and Etv6) were used to reprogram mouse fibroblasts into cells that expressed HSC surface markers and differentiated into blood-cell progenitors in vitro (Fig. 1). However, the reprogrammed cells could not robustly engraft into bone marrow after transplantation.
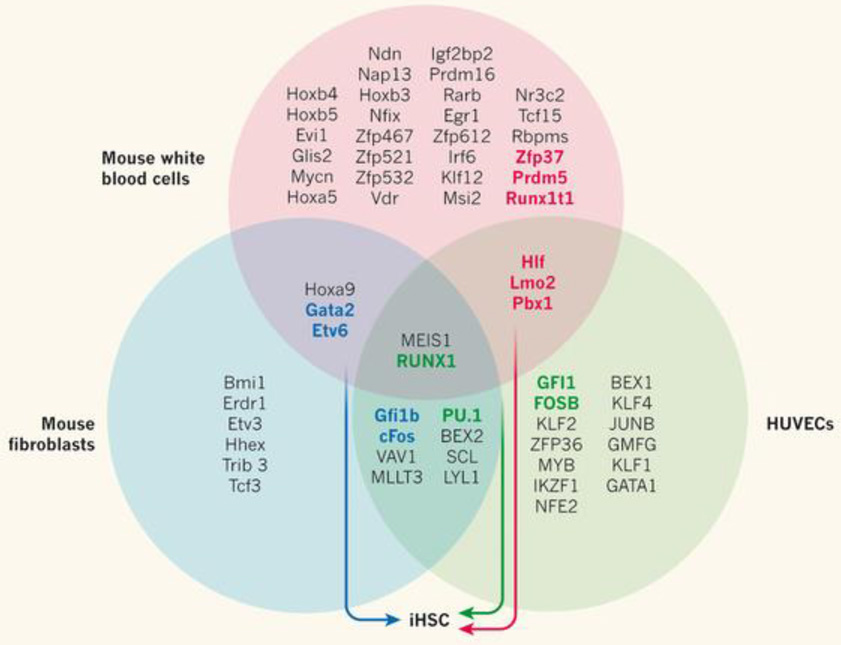
Figure 1.
In search of factors that induce haematopoietic stem cells. A Venn diagram illustrates the overlapping groups of transcription factors that have been tested in the quest to generate induced haematopoietic stem cells (iHSCs) from three adult cell types — mouse fibroblasts5 (the transcription-factor pool tested is represented by the blue circle), mouse white blood cells7 (pink) and human umbilical vein endothelial cells (HUVECs, green)1. Stepwise elimination of the transcription factors that were unnecessary for each protocol led to the identification of distinct groups that could generate iHSCs (bold text, colour indicates which transcription-factor combinations successfully reprogrammed each cell type). Sandler et al.1 report that the production of iHSCs from HUVECs requires four transcription factors: FOSB, GFI1, RUNX1 and PU.1.
During embryonic development, HSCs arise from vascular cells that line the aorta, and the cells continue to require signals from the vascular bed, or niche, for their maintenance and function throughout their lives. Sandler et al. reasoned that they could enhance the efficiency of direct reprogramming and maintain the self-renewing abilities of the induced HSCs (iHSCs) by starting with a cell type with a similar developmental origin to HSCs, and growing the cells in a microenvironment comparable to their in vivo niche.
The authors isolated human umbilical-vein endothelial cells (HUVECs, readily available cells that line the umbilical vein), and forced them to express 26 transcription factors that are enriched in HSCs, but not in HUVECs. The researchers maintained the cultured cells in a medium that lacked serum, which can impair HSC maintenance (serum is normally included in culture media because it contains growth factors that promote cell proliferation). Sandler and colleagues kept the cells on a feeder-cell layer; this underlying cell monolayer released factors that made the culture conditions similar to the microenvironment of the HSC niche. The feeder cells, called E4ECs, were endothelial cells engineered to overexpress an adenoviral gene, E4ORF1, that promotes their survival, but not their proliferation, thereby maintaining a state that mimics the niche6.
When HUVECs were cultured in these conditions, Sandler and colleagues found that a small subset could form haematopoietic colonies. Systematic elimination of transcription factors that were unnecessary for reprogramming revealed that a combination of 4 of the 26 factors — FOSB, GFI1, RUNX1 and PU.1 — could reprogram HUVECs (Fig. 1). To be successful, reprogramming must simultaneously suppress the original cellular identity and confer a new one. The authors speculate that PU.1 combined with GFI1 downregulated vascular genes, possibly in combination with FOSB, and that PU.1 and RUNX1 upregulated haematopoietic-specifying genes. Reprogrammed HUVECs became self-renewing HSCs that could serially engraft into the bone marrow of immunodeficient mice and differentiate into mature blood cells.
Earlier this year7, another group reported the transformation of mature white blood cells from mice into engraftable HSCs that can form all blood-cell lineages. Reprogramming was accomplished with six transcription factors (Runx1t1, Hlf, Lmo2, Prdm5, Pbx1 and Zfp37), and the cells were matured in vivo to generate iHSCs (Fig. 1). It is probable, although it has not yet been demonstrated, that maturation in native HSC niches promoted cell survival and provided cues for iHSC generation.
Surprisingly, comparison of Sandler and colleagues' protocol with those used to reprogram mouse white blood cells7 or fibroblasts5 reveals that each method used a different transcription-factor cocktail to generate iHSCs. This may result from species differences, from the ability of each cell type to respond to different transcription factors or from the different epigenetic state of each cell type — that is, genomic modifications that affect gene expression without changing DNA sequence. Consistent with this possible role for epigenetic state, Sandler and colleagues' transcription-factor cocktail could not reprogram endothelial cells derived from embryonic stem cells, but could reprogram adult dermal microvascular endothelial cells.
It is worth mentioning that the surprisingly limited overlap in reprogramming factors between the three studies1, 5, 7 is representative of the differing starting pools of transcription factors used. Indeed, even though each was chosen on the basis of selective expression in HSCs, only two factors (RUNX1 and MEIS1) were included in the initial pool of every study. Thus, definitive conclusions about the cell-specificity and transcription-factor requirements for generating iHSCs await further analyses. The fact that the same result can be achieved with three different molecular combinations suggests a multiplicity of options for generating iHSCs.
The ability to reprogram adult endothelial cells has exciting implications for gene editing and cell therapy for blood diseases. Although HSCs have always been a desirable target for gene therapy, the difficulties of maintaining them in culture have limited their use. As adult endothelial cells can be cultured for several days without apparent loss of reprogramming efficiency, one can predict that patient-specific endothelial cells could be purified, genetically corrected, selected and then reprogrammed to deliver functional iHSCs.
As with all stem cells reprogrammed in culture, the risk of cancerous transformation remains. Although Sandler and colleagues found no signs of transformation 10 months after transplanting the iHSCs into mice, most of the factors used in iHSC generation are also associated with the development of leukaemia. This highlights the thin line between promoting self-renewal of healthy HSCs and potentiating cancerous transformation. A greater understanding of the reprogramming mechanisms at play may overcome this potential problem. Furthermore, such understanding will produce much-needed insight into the signals that trigger HSC emergence, and the molecular networks that instruct HSC programming.
Contributor Information
Daniel Lucas, Department of Cell and Developmental Biology, University of Michigan Medical School, Ann Arbor, Michigan 48109, USA.
Paul S. Frenette, Ruth L. and David S. Gottesman Institute for Stem Cell and Regenerative Medicine Research, Albert Einstein College of Medicine, New York, New York 10461, USA. paul.frenette@einstein.yu.edu
References
- 1.Sandler VM, et al. Nature. 2014;511:312–318. doi: 10.1038/nature13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takahashi K, Yamanaka S. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Sturgeon CM, Ditadi A, Clarke RL, Keller G. Nature Biotechnol. 2013;31:416–418. doi: 10.1038/nbt.2571. [DOI] [PubMed] [Google Scholar]
- 4.Pfaff N, Cantz T. Cell Stem Cell. 2013;13:131–133. doi: 10.1016/j.stem.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Pereira C-F, et al. Cell Stem Cell. 2013;13:205–218. doi: 10.1016/j.stem.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butler JM, et al. Blood. 2012;120:1344–1347. doi: 10.1182/blood-2011-12-398115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riddell J, et al. Cell. 2014;157:549–564. doi: 10.1016/j.cell.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]