Abstract
AIM: To detect the expression of COX-2 and HER-2 in colorectal cancer and to analyze their correlation and clinical significance.
METHODS: A total of 1026 colorectal cancer surgical specimens were collected from patients treated from December 2002 to December 2007 at the First Affiliated Hospital of Anhui Medical University. All specimens were made into 4-μm slices. The expression of COX-2 and HER-2 were detected by immunohistochemistry using the streptavidin-biotin-peroxidase method. The correlations between COX-2 and HER-2 expression and colorectal cancer clinical features were analyzed.
RESULTS: The positive rates of COX-2 and HER-2 expression in colorectal cancer were 77.97% (800/1026) and 46.20% (474/1026), respectively. There was a significant correlation between COX-2 and HER-2 expression in colorectal cancer (P < 0.05). In patients with tumor size ≥ 5 cm, the positive rates of COX-2 and HER-2 expression were 81.48% (308/378) and 57.94% (219/378), respectively. In patients with serosal invasion, the positive COX-2 and HER-2 expression rates were 80.53% (612/760) and 49.21% (374/760), respectively. In patients with lymph node metastasis, the positive expression rates were 85.04% (506/595) and 54.62% (325/595), respectively, and the positive expression rates differed significantly between patients with lymph node metastasis and those without (P < 0.05). In patients with Duke’s C and D colorectal cancer, the positive COX-2 and HER-2 expression rates were 82.80% (443/535) and 57.94% (310/535), respectively. In patients with poorly differentiated colorectal cancer, the positive expression rates were 74.49% (210/282) and 52.84% (149/282), respectively (P < 0.05). In patients with distant metastasis, the positive expression rates were 82.27% (116/141) and 53.90% (76/141), respectively (P < 0.05). These findings suggest that COX-2 and HER-2 have synergistic effects in colorectal cancer. COX-2 and HER-2 expression had no significant correlation with sex, age, or tumor location.
CONCLUSION: COX-2 and HER-2 are important markers for invasion and metastasis of colorectal cancer, and they act together to regulate the invasion and metastasis of colorectal cancer.
Keywords: Colorectal cancer, Correlation, COX-2, HER-2, Immunohistochemistry, Survival rate
Core tip: The relationship between and expression of COX-2 and HER-2 in colorectal cancer have not been fully elucidated. In this study, the expression of COX-2 and HER-2 was assessed in colorectal cancer tissues by immunohistochemistry, and the correlations between COX-2 and HER-2 expression and colorectal cancer clinical features were evaluated. Results demonstrated that COX-2 and HER-2 expression are significantly associated with serosal invasion, lymph node metastasis, Duke’s stage, and poorly differentiated cancer. COX-2 and HER-2 have synergistic effects in colorectal cancer. Both COX-2 and HER-2 are important markers for invasion and metastasis of colorectal cancer.
INTRODUCTION
Colorectal cancer is a very common malignant tumor of the digestive tract, with about 1.2 million new cases and 600000 deaths worldwide each year[1]. The incidence of colorectal cancer ranks second among various malignances in the Western developed countries. Although both the incidence and mortality of colorectal cancer rank between third and fifth in China, respectively, the incidence of colorectal cancer ranks second or third in large cities. Thus, colorectal cancer poses a serious threat to human health. As colorectal cancer has a complicated biologic behavior and is easy to relapse, metastasize, and develop resistance to chemotherapy drugs, the clarification of the mechanisms responsible for the development and progression of colorectal cancer and the development of early and effective diagnostic strategies and reasonable treatment strategies have always been focuses of research in the field of colorectal cancer[2].
Invasion and metastasis are the main biologic characteristics of malignant tumors. The poor therapeutic effects in colorectal cancer are often associated with tumor invasion and metastasis. Therefore, there is an emerging need to understand how to predict tumor invasion and metastasis and conduct early comprehensive therapy for tumors clinically[3]. In the present study, an immunohistochemical method was used to detect the expression of COX-2 and HER-2 in colorectal cancer, and the relationship between COX-2 and HER-2 expression and prognosis of colorectal cancer was analyzed, with an aim to provide a theoretical basis of pathologic diagnosis, prognosis evaluation, and treatment of this malignancy.
MATERIALS AND METHODS
Specimens
A total of 1026 colorectal cancer surgical specimens were collected from patients treated between December 2002 and December 2007 at the First Affiliated Hospital of Anhui Medical University. The patients ranged in age from 14 years to 88 years, with an average age of 57 years. There were 484 cases of colon cancer and 542 cases of rectal cancer. In terms of differentiation degree, 155 cases were well differentiated, 589 were moderately differentiated, and 282 were poorly differentiated. All specimens were fixed in 10% formalin, embedded in paraffin, and sectioned into 4-μm slices. Tissues 5 cm or above from the resection margin were used as controls. None of the patients received any radiotherapy, chemotherapy, or immunotherapy before surgery, and were confirmed pathologically after surgery. This study was approved by the Ethical Committee of Anhui Medical University.
Reagents
Rabbit anti-human COX-2 monoclonal antibody (SP21), rabbit anti-human HER-2 polyclonal antibody, and a color development kit were purchased from Zhongshan Golden Bridge Biotechnology (Beijing, China).
Immunostaining
Immunohistochemical staining was performed using the streptavidin-biotin-peroxidase method according to the manufacturer’s instructions. For the negative control, the primary antibody was replaced with PBS. Tissues known to express the antigens of interest were used as positive controls.
Immunostaining evaluation
Immunostaining evaluation was performed using a semi-quantitative scoring system by estimating the percentage of cells stained and staining intensity. Immunohistochemically stained sections were evaluated independently by two experienced pathologists, with five high-power visual fields observed in each section. The percentage of cells stained was scored as follows: 0 = 0%-5%; 1 = 6%-25%; 2 = 26%-50%; 3 = 51%-75%; 4 = 76%-100%. Staining intensity was scored as follows: 1 = faintly yellow; 2 = brownish yellow; 3 = brown. A combined score was calculated as the sum of staining intensity and percentage of stained cells, and immunostaining was scored as negative (-; combined score = 0-1); positive (+; combined score = 2-3); moderately positive (++; combined score = 4-5); strongly positive (+++; combined score = 6-7).
Statistical analysis
Statistical analyses of the immunohistochemical staining were performed using SPSS16.0 software (SPSS Inc., Chicago, IL, United States). Differences were tested for statistical significance using the Mann-Whitney U test. Survival analysis was conducted using the Kaplan-Meier method. P < 0.05 was considered statistically significant.
RESULTS
COX-2 expression in colorectal cancer
COX-2-positive cells showed brownish yellow granules in the cytoplasm (Figure 1A). The positive rate of COX-2 expression was 77.97% (800/1026) in all the specimens. In patients with a tumor size ≥ 5 cm, the positive rate of COX-2 expression was 81.48% (308/378). In patients with serosal invasion, the positive expression rate was 80.53% (612/760). In patients with Duke’s C and D colorectal cancer, the positive expression rate was 82.80% (443/535). In patients with lymph node metastasis, the positive expression rate was 85.04% (506/595), and the positive expression rate differed significantly between patients with lymph node metastasis and those without (χ2 = 41.213; P < 0.05). High COX-2 protein expression was significantly correlated with tumor size, infiltration depth, Duke’s stage, tumor differentiation, distant metastasis, and lymph node metastasis (P < 0.05), but not with sex, age, or tumor location (Table 1).
Figure 1.
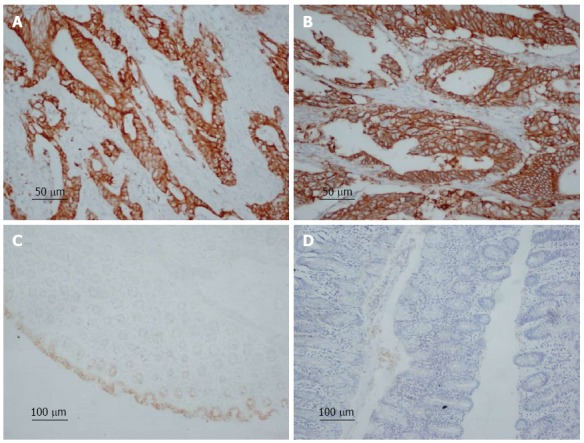
Immunohistochemical staining of COX-2 and HER-2 in colorectal cancer and normal colorectal tissue. A: COX-2-positive expression in colorectal cancer, magnification × 200; B: HER-2 positive expression in colorectal cancer, magnification × 200; C: COX-2-negative expression in normal colorectal tissue, magnification × 100; D: HER-2-negative expression in normal colorectal tissue, magnification × 100.
Table 1.
Relationship between COX-2/HER-2 expression and clinicopathologic factors n (%)
| Variable | n |
COX-2 |
HER-2 |
||||||
| Positive | Negative | χ2 | P value | Positive | Negative | χ2 | P value | ||
| Sex | 2.300 | 0.129 | 2.769 | 0.096 | |||||
| Male | 595 | 454 (76.3) | 62 (20.0) | 288 (48.4) | 307 (51.6) | ||||
| Female | 431 | 346 (80.2) | 32 (15.1) | 186 (43.2) | 245 (56.8) | ||||
| Age (yr) | 2.011 | 0.156 | 1.496 | 0.221 | |||||
| < 60 | 493 | 375 (76.1) | 40 (16.3) | 218 (44.2) | 275 (55.8) | ||||
| ≥ 60 | 533 | 425 (79.7) | 54 (19.6) | 256 (48.0) | 277 (52.0) | ||||
| Tumor site | 2.007 | 0.157 | 2.499 | 0.114 | |||||
| Rectum | 542 | 432 (79.7) | 45 (16.3) | 263 (48.5) | 279 (51.5) | ||||
| Colon | 484 | 368 (76.0) | 49 (19.9) | 211 (43.6) | 273 (56.4) | ||||
| Tumor size | 4.988 | 0.026 | 33.175 | 0.000 | |||||
| < 5 cm | 648 | 489 (75.5) | 159 (24.5) | 255 (39.4) | 393 (60.6) | ||||
| ≥ 5 cm | 378 | 308 (81.5) | 70 (18.5) | 219 (57.9) | 159 (42.1) | ||||
| Serosal invasion | 11.130 | 0.001 | 10.697 | 0.001 | |||||
| No | 266 | 188 (70.7) | 78 (29.3) | 100 (37.6) | 166 (62.4) | ||||
| Yes | 760 | 612 (80.5) | 148 (19.5) | 374 (49.2) | 386 (50.8) | ||||
| Differentiation | 6.553 | 0.038 | 6.911 | 0.032 | |||||
| Well | 155 | 114 (73.5) | 41 (26.5) | 67 (43.2) | 88 (56.8) | ||||
| Moderate | 589 | 476 (80.8) | 113 (19.2) | 258 (43.8) | 331 (56.2) | ||||
| Poor | 282 | 210 (74.5) | 72 (25.5) | 149 (52.8) | 133 (47.2) | ||||
| Duke’s stage | 15.191 | 0.000 | 62.045 | 0.000 | |||||
| A + B | 491 | 357 (72.7) | 134 (27.3) | 164 (33.4) | 327 (66.6) | ||||
| C + D | 535 | 443 (82.8) | 92 (17.2) | 310 (57.9) | 225 (42.1) | ||||
| Lymph node metastasis | 14.865 | 0.000 | 40.430 | 0.000 | |||||
| Yes | 595 | 506 (85.0) | 89 (15.0) | 325 (54.6) | 270 (45.4) | ||||
| No | 431 | 294 (68.2) | 137 (31.8) | 149 (34.6) | 282 (65.4) | ||||
| Distal metastasis | 3.928 | 0.047 | 3.901 | 0.048 | |||||
| Yes | 141 | 119 (84.4) | 22 (15.6) | 76 (53.9) | 65 (46.1) | ||||
| No | 885 | 681 (76.9) | 204 (23.1) | 398 (45.0) | 487 (55.0) | ||||
HER-2 expression in colorectal cancer
HER-2 was localized mainly on the membrane of cancer cells, and HER-2-positive cells showed brownish yellow granules on the membrane (Figure 1B). The positive rate of HER-2 expression in colorectal cancer was 46.20% (474/1026) in all the specimens. In patients with a tumor size ≥ 5 cm, the positive rate of HER-2 expression rate was 57.94% (219/378). In patients with serosal invasion, the positive expression rate was 49.21% (374/760). In patients with Duke’s C and D colorectal cancer, the positive expression rate was 57.94% (310/535). In colorectal adenocarcinoma patients with lymph node metastasis, the positive expression rate was 54.62% (325/595), and the positive expression rate differed significantly between patients with lymph node metastasis and those without (χ2 = 40.430; P < 0.05). High HER-2 protein expression was significantly correlated with tumor size, invasion depth, Duke’s stage, tumor differentiation, distant metastasis, and lymph node metastasis (P < 0.05), but not with sex, age, or tumor location (Table 1).
Correlation between COX-2 and HER-2 expression in colorectal cancer
Of 800 COX-2 positive specimens, 350 were positive for HER-2 and 450 were negative. Of 226 COX-2 negative specimens, 124 were positive for HER-2 and 102 were negative. There was a significant positive correlation between COX-2 and HER-2 expression in colorectal cancer (χ2 = 8.762; P < 0.05) (Table 2).
Table 2.
Relationship between COX-2 and HER-2 expression
| COX-2 expression |
HER-2 expression |
Total | χ2 | P value | |
| Positive | Negative | ||||
| Positive | 350 | 450 | 800 | 8.762 | 0.003 |
| Negative | 124 | 102 | 226 | ||
| Total | 474 | 552 | 1026 | ||
COX-2 and HER-2 expression in normal colorectal tissues
A total of 50 tumor-adjacent colorectal tissues were used as normal colorectal tissues. Of these tissues, 6/50 (12.00%) showed positive COX-2 expression, and 1/50 (2.00%) showed positive HER-2 expression. Most of the normal tissues showed negative expression of COX-2 and HER-2 (Figure 1C and 1D).
Survival analysis
By February 28, 2011, 210/1026 (20.47%) patients were lost to follow-up, and 816/1026 (79.53%) had complete follow-up data. The follow-up duration ranged from 3 years to 5 years. The survival curves for COX-2 and HER-2 positive and negative patients are shown in Figure 2A and 2B, respectively. The survival curves for patients positive for both COX-2 and HER-2, those positive for either of them, and those negative for both are shown in Figure 3. These results showed that the survival time of COX-2 and HER-2 positive patients was significantly lower than that of COX-2 and HER-2 negative ones (P < 0.05).
Figure 2.
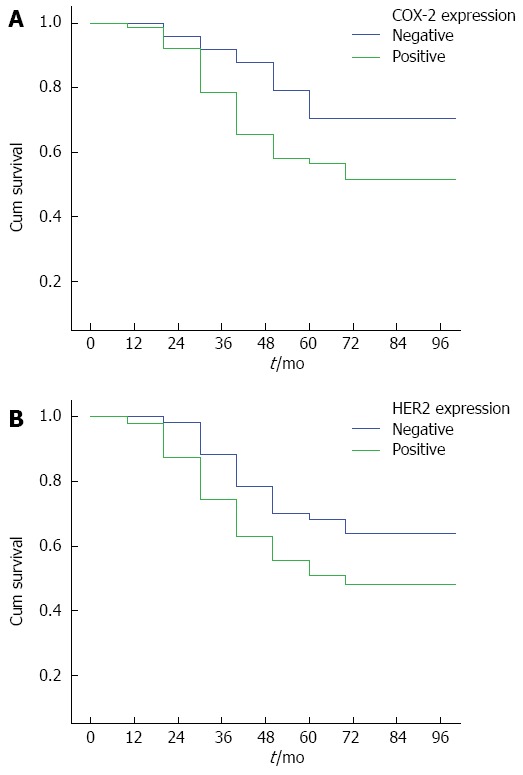
Survival curves for patients with colorectal cancer. A: Patients with positive and negative COX-2 expression; B: Patients with positive and negative HER-2 expression.
Figure 3.
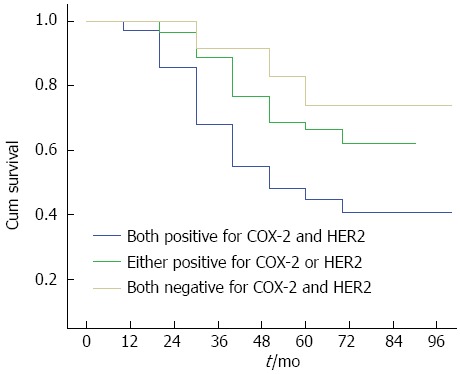
Survival curves of colorectal cancer patients positive for both COX-2 and HER-2, positive for either of them, and negative for both. Compared with patients positive for both markers, patients negative for both had better survival.
DISCUSSION
According to the results of this study, the positive rate of COX-2 expression in colorectal cancer is 77.97%, significantly higher than in normal colorectal tissues. COX-2 expression was significantly associated with lymph node metastasis[4]. This may be because COX-2 can: (1) increase the production of prostaglandins and inhibit the body’s immune response; (2) inhibit tumor cell apoptosis and promote cell proliferation; (3) regulate cell cycle progression; (4) promote tumor angiogenesis; (5) increase the expression of matrix metalloproteinases in tumor cells; and (6) induce activation of precursors of carcinogenic substances. As high COX-2 expression exists in precancerous lesions and carcinoma in situ and is significantly higher than in the normal tissue, it is generally believed that high COX-2 expression is an early event in tumorigenesis.
COX-2 is not or is lowly expressed in normal tissues; however, COX-2 expression is increased in inflammatory and tumor tissues and plays an important role in inflammation, cell proliferation, and differentiation, suggesting that COX-2 is involved in the initiation and progression of cancer. Brown et al[4] believed that most colorectal cancers have COX-2 overexpression, which can induce tumor angiogenesis, damage the immune system, and promote tumor invasion. Tuynman et al[5] incubated lymphocytes with COX-2-positive or -negative colorectal cancer cells and found that lymphocyte proliferation index was significantly reduced in COX-2-positive colorectal cancer cells. They also found that this effect could be inhibited by the COX-2 inhibitor NS-398, suggesting that COX-2 expression in colorectal cancer can inhibit the proliferation of lymphocytes to make the tumor evade the host immune response.
Elzagheid et al[6] performed an immunohistochemical analysis of 145 stage I-IV colorectal cancer specimens collected from 1981 to 1990 at Finland Turku University Hospital and found that patients with higher TNM stage (P < 0.06) and those with higher Duke’s stage (P < 0.045) had higher levels of COX-2 expression, though COX2 expression was not significantly associated with age, sex, tumor histologic grade, or lymph node metastasis. Research shows that regulation of COX-2 expression is a key step in colorectal carcinogenesis. As COX-2 expression is significantly associated with tumor stage, it is considered a prognostic factor for colorectal cancer. The increase in the activity of COX-2 promotes the progression of colorectal cancer.
Peng et al[7] conducted a meta-analysis of 23 studies involving 4567 colorectal cancer patients that evaluated the relationship between COX-2 expression detected by immunohistochemistry and patient survival, and they found that high expression of COX-2 was associated with slightly poorer survival. The present study draws a similar conclusion. Of 1026 colorectal cancer tissues, the positive rate of COX-2 expression is 77.97%. In colorectal adenocarcinoma patients with lymph node metastasis, the positive expression rate is 85.04%, significantly higher than in patients without lymph node metastasis. In addition, COX-2 protein expression is significantly associated with tumor size, infiltration depth, tissue differentiation, Duke’s stage, and distant metastasis, confirming that high COX-2 protein expression in colorectal cancer tissue may enhance the ability of tumor growth. This result is similar to many previous studies reporting that COX-2 overexpression in colorectal cancer tissue is closely related to tumor invasion, lymph node metastasis, and poor prognosis.
HER-2 is a protein with tyrosine kinase activity encoded by the cellular oncogene ERBB2, which is located on chromosome 17q21[8]. Under normal circumstances, the oncogene is inactive and is the normal component of the cellular genome, participating in the regulation of cell growth, differentiation, and division. The main forms of ERBB2 activation are abnormal gene amplification, abnormal transcription regulation, and mRNA overexpression, leading to excessive expression of its protein product and producing tumor transformation activity[9]. Numerous studies have shown that its overexpression is associated with pathologic morphology and biologic behavior in a wide variety of tumors, especially breast, gastric, ovarian, and colorectal cancers. Studies confirm that high expression of this protein in breast cancer is closely related to high S-phase fraction, high mitosis index, high thymine labeling index, and DNA heteroploidy.
Ma et al[10] found that the peptide fragments of HER-2 after enzymatic digestion were almost completely consistent with those of the epidermal growth factor receptor (EGFR). Even in the absence of ligands, HER-2 can still lead to a sustainable activation of EGFR protein kinase, make the cells grow out of control, and result in tumor occurrence. At present, HER-2-targeted therapy has been widely used for the treatment of breast cancer; however, its role in colorectal cancer is rarely reported. The present study shows that the positive rate of HER-2 expression in colorectal cancer is 46.20%, suggesting that HER-2 has a very important role in the growth, invasion, and metastasis of colorectal cancer. Theoretically, HER-2-targeted drugs can also restrain invasion and metastasis of colorectal cancer in which HER-2 is overexpressed. A better understanding of the biologic characteristics of colorectal cancer may open a new avenue for targeted therapy of colorectal cancer[11].
This study was performed in two stages. In the first stage, 522 specimens were collected from December 2005 to December 2007, and the experiments were performed between March 2010 and September 2010. In the second stage, 504 specimens were collected from December 2002 to November 2002, and the experiments were performed between September 2010 and March 2010. When comparing the results between the two stages, the positive rates of COX-2 and HER-2 expression were slightly lower in the second stage. This might be due to prolonged specimen storage and the increase in the number of specimens. According to the pathologic reports, the 1026 tumors were roughly classified into the following histologic types: papillary adenocarcinoma, tubular adenocarcinoma, mucous adenocarcinoma, signet ring cell carcinoma, undifferentiated carcinoma, adenosquamous carcinoma, squamous cell carcinoma, carcinoid, and small cell carcinoma. In addition, there is currently controversy over whether cancer nodules should be classified as regional lymph node metastasis or distant metastasis and whether liver metastasis should be regarded as direct invasion or distant metastasis. Upon consideration, we finally decided to classify papillary adenocarcinoma and carcinoid as well-differentiated tumors, and classify mucous adenocarcinoma, signet ring cell carcinoma, undifferentiated carcinoma, and neuroendocrine carcinoma as poorly differentiated tumors. Liver metastases of hepatic flexure colon cancer were regarded as direct invasion of adjacent organs, while other liver metastases of colon cancer were regarded as distant metastasis. For Duke’s staging, TxN0M0 was regarded as A + B, and N1-3 or M1 as C + D. In terms of tumor differentiation, moderately or poorly differentiated adenocarcinoma was classified as moderately differentiated adenocarcinoma in the first stage, but was later classified as poorly differentiated adenocarcinoma in the second stage after consultation with several experienced pathologists. In terms of distant metastasis, cancer nodules were initially staged as N1, but later adjusted to M1 after literature search. These made the P values in preliminary experimental results in both groups > 0.05, which showed a slight difference from the results obtained using large samples. After adjustment, the P values became < 0.05 in both groups. This is consistent with multiple previous reports.
Many genes are activated in tumor development and progression, and these genes interact with each other to promote the growth and malignant transformation of tumors. There is a large body of evidence that upregulation of inducible COX-2 may promote the occurrence of colorectal cancer, but the mechanism responsible for regulating its expression is not clear. Previous studies show that there are two possible growth factor signaling pathways associated with COX-2 expression: HER-2/neu and transforming growth factor-β/Smad. Kiguchi et al[12] found that overexpression of HER-2/neu in the bile duct epithelium of transgenic mice induced upregulation of COX-2 expression. Vadlamudi et al[13] found that in human colorectal cancer cell lines, HER-2/neu activated factors promoting COX-2, causing COX-2 mRNA and protein expression and the accumulation of prostaglandin E2. Lucarelli et al[14] discovered that the positive rates of COX-2 in invasive breast cancer, ductal carcinoma in situ, and normal breast epithelial cells were 87%, 85%, and 75%, respectively, and those of HER-2 in invasive breast cancer and ductal carcinoma in situ detection were both 34%, suggesting that HER-2 and COX-2 may regulate each other.
Hirokazu et al[15] showed that the positive rate of COX-2 expression in gastric cancer was 54.8% and that of HER-2 was 86.4%. The present study examined the correlation between COX-2 and HER-2 expression in colorectal cancer. The results show that both COX-2 and HER-2 are highly expressed in colorectal cancer, and there is a significant correlation between the expression of COX-2 and HER-2. In addition, the positive rate of COX-2 expression in HER-2 positive patients is significantly higher than in HER-2 negative ones. Taken together, these findings suggest that HER-2 may upregulate the expression of COX-2. Studies have shown that the HER-2 inhibitor trastuzumab significantly prolongs survival of patients with colorectal cancer. Thus, whether inhibition of COX-2, a downstream protein of HER-2, could improve the therapeutic effect of HER-2 inhibitor deserves further research.
Studies have shown that high expression of both COX-2 and HER-2 increase the expression of vascular endothelial growth factor C (VEGF-C) in tumor cells. Su et al[16] discovered that transfection with COX-2 gene or exposure to prostaglandin E2 in lung adenocarcinoma cells significantly upregulated the expression of VEGF-C protein, whereas COX-2-specific inhibitors reduced the expression of endogenous VEGF-C. The authors suggested that COX-2 upregulated VEGF-C through prostaglandin receptor EP1 and HER-2. The present study found that COX-2 and HER-2 expression have a significant correlation, and both are significantly associated with lymph node metastasis, tumor infiltration depth, and Duke’s stage. We speculate that upregulation of COX-2 expression may promote the secretion of VEGF-C by tumor cells, thus promoting lymphangiogenesis via the VEGF-C/VEGFR-3 signaling pathway, and lead to lymphatic spread of tumor cells.
COX-2 expression in tumor tissue is correlated with prognosis. Smakman et al[17] found that in colon cancer cells with high COX-2 expression, the adhesion of the cells to matrix and the potential of cancer cells to metastasize increase, both of which are conducive to the development, progression, and metastasis of cancer. Zhang et al[18] detected the expression of COX-2 in 64 normal mucosal specimens, 116 primary colon cancer specimens, and 16 colon cancer metastases, and they found that the positive rate of COX-2 expression was 12% in normal mucosal tissues, 72% in primary tumors, and 100% in colon cancer metastases. The present study shows that the two- and four-year survival rates are significantly lower in the COX-2-positive group than in the COX-2-negative group, indicating that COX-2 overexpression is positively correlated with the recurrence and metastasis of colorectal cancer, and negatively with the prognosis of colorectal cancer.
Al-Maghrabi et al[19] found that 56% of patients with colorectal cancer showed positive cytoplasmic expression of COX-2, and COX-2 expression was positively associated with lymph node involvement and distant metastasis. In addition, high COX-2 expression was associated with a higher rate of tumor recurrence, suggesting that COX-2 expression provides useful prognostic information in colorectal cancer and may help screen patients with a high risk of recurrence. The present study indicates that the survival time of COX-2-negative patients is significantly higher than of COX-2-positive ones, further confirming that COX-2 expression is associated with a poor prognosis.
Dixon et al[20] showed that high COX-2 expression is an important factor contributing to colorectal carcinogenesis. COX-2 inhibitors (e.g., celecoxib) can reduce the risk of relapse of colorectal adenomas. Nonsteroidal anti-inflammatory drugs are known to reduce the risk and mortality of colorectal cancer by inhibiting cyclooxygenases. Kasper et al[21] performed an immunohistochemical analysis of COX-2 expression in colorectal cancer and liver metastases in 57 patients and found that COX-2 was consistently involved in the occurrence of metastatic colorectal cancer. COX-2 inhibitors exert their antitumor effects possibly by altering the signaling pathways related to cell sensitivity and apoptosis.
Rahman et al[22] analyzed 130 cases of colorectal cancer and found that high COX-2 expression was associated with resistance to chemotherapy drugs and faster tumor cell growth. If 5-fluorouracil is given together with celecoxib, the latter may inhibit multidrug resistance and improve the chemotherapy sensitivity of drug-resistant cells. Thus, COX-2 inhibitors combined with currently used 5-fluorouracil-based regimens may have potentially positive benefits. Roelofs et al[23] found high COX-2 mRNA expression in almost 80% of colorectal cancer cases, suggesting that COX-2 is a potential biomarker of cancer risk, and COX-2 inhibitors may prevent colon cancer. Kraus et al[24] conducted many animal experiments showing that COX-2 inhibitors prevent the formation of adenomas or delay their development, and reduce the morbidity and mortality of colorectal cancer. However, cancer prevention is still being ignored in the cancer research field.
HER-2 has malignant transforming activity, and overexpression of HER-2 often suggests high degree of malignancy and poor prognosis. Researchers from both China and other countries have reported that colorectal cancer patients with high HER-2 expression had earlier lymph node metastasis, poorer prognosis, and shorter survival[25,26]. The five-year survival rate of patients with HER-2 overexpression was significantly lower than those with negative HER-2 expression[27], and HER-2 overexpression can be used as a reliable indicator of colorectal cancer prognosis. However, Li et al[28] performed a meta-analysis and found that HER-2 overexpression may have little impact on survival of patients with colorectal cancer. Lu et al[29] believed that EGFR and HER2 may be used as potential biomarkers for lymph node metastasis and prognosis of colorectal cancer. EGFR or HER2 is a potential predictor of poor clinical prognosis of colorectal cancer. The present study also found that the survival of HER-2-positive patients is significantly lower than of HER-2-negative ones.
In conclusion, the findings presented here suggest that there is a positive correlation between COX-2 and HER-2 expression, and simultaneous expression of COX-2 and HER-2 can enhance the metastasis and invasion ability of colorectal cancer, and suggests poor prognosis in patients with colorectal cancer. Joint detection of COX-2 and HER-2 expression in colorectal cancer tissue can be used an effective index for evaluating prognosis and screening patients with a high risk of metastasis.
COX-2- and HER-2-targeted drugs provide another effective, comprehensive, individualized treatment option for patients with colorectal cancer. However, the following problems need to be solved before they can be used clinically: (1) the relationship between targeted drug therapy and expression of COX-2 and HER-2 in colorectal cancer needs to be further defined. It remains to be investigated how to formulate standardized indications for use of targeted drug therapy in colorectal cancer based on the expression of COX-2 and HER-2. This requires a multi-disciplinary approach; (2) the timing and dose of targeted drug therapy for colorectal cancer should be determined; and (3) the combined use of targeted drugs with surgery, chemotherapy, and radiotherapy, as well as the joint use of multiple molecular-targeted drugs should be explored. The better understanding of the biologic characteristics of colorectal cancer and the further research of targeted drug therapy will provide a new avenue for the treatment of colorectal cancer.
As COX-2 and HER-2 expression was detected using the immunohistochemical method in the present study, the results may be influenced by the quality of antibody reagents and the skill of operators. Also, quantitative detection could not be performed, though the method is simple. In addition, due to the large number of specimens and the long time span of the study, laboratory conditions such as temperature and humidity were different. As a result, the positive rates of COX-2 and HER-2 expression were slightly different from those reported in the literature. More stable detection methods, such as fluorescence in situ hybridization and quantitative PCR, and standardized reagents (e.g., fixed manufacturers) should be used in the future to overcome the above-listed problems.
COMMENTS
Background
Colorectal cancer is a very common malignancy and the second leading cause of cancer deaths worldwide. Although significant advances have been made in the understanding and therapy of colorectal cancer in last decade, no effective targeted therapy drugs have been discovered. More attention should be focused on finding early diagnosis and prognosis markers of colorectal cancer. COX-2 and HER-2 are known to be involved in the progression of many cancers. However, the associations of COX-2 and HER-2 expression with the progression and prognosis of colorectal cancer have not been fully elucidated.
Research frontiers
In recent years, several interesting and promising molecular markers of colorectal cancer have been discovered, including the epidermal growth factor receptor and the vascular endothelial growth factor receptor. These molecules will help to identify early cancers, screening programs, and target therapies.
Innovations and breakthroughs
In this study, the authors assessed the expression of COX-2 and HER-2 in colorectal cancer tissues by immunohistochemistry, and subsequently analyzed the correlation of COX-2 and HER-2 expression with clinical features of colorectal cancer. They also found that COX-2 and HER-2 had synergistic effects in colorectal cancer.
Applications
Based on the finding that expression of COX-2 and HER-2 are significantly associated with clinical features of colorectal cancer, this study may trigger the interest of COX-2 and HER-2 as important prognostic markers and potential molecular targets in colorectal cancer therapy.
Peer-review
The authors conducted a descriptive study and revealed that the expression levels of the two molecules are associated with clinical features of colorectal cancer. The findings are expected to contribute to the development of molecularly targeted therapy for colorectal cancer.
Footnotes
Ethics approval: The study was reviewed and approved by the First Affiliated Hospital of Anhui Medical University Institutional Review Board.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: October 10, 2014
First decision: November 14, 2014
Article in press: March 31, 2015
P- Reviewer: Inauen W, Morita M S- Editor: Yu J L- Editor: A E- Editor: Zhang DN
References
- 1.Raskov H, Pommergaard HC, Burcharth J, Rosenberg J. Colorectal carcinogenesis--update and perspectives. World J Gastroenterol. 2014;20:18151–18164. doi: 10.3748/wjg.v20.i48.18151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sideris M, Papagrigoriadis S. Molecular biomarkers and classification models in the evaluation of the prognosis of colorectal cancer. Anticancer Res. 2014;34:2061–2068. [PubMed] [Google Scholar]
- 3.Almhanna K, El-Rayes B, Sethi S, Dyson G, Heilbrun L, Philip PA, Sarkar F. Association between COX-2 expression and effectiveness of COX-2 inhibitors in a phase II trial in patients with metastatic colorectal adenocarcinoma. Anticancer Res. 2012;32:3559–3563. [PMC free article] [PubMed] [Google Scholar]
- 4.Brown JR, DuBois RN. COX-2: a molecular target for colorectal cancer prevention. J Clin Oncol. 2005;23:2840–2855. doi: 10.1200/JCO.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 5.Tuynman JB, Hulscher JB, Steller EP, van Lanschot JJ, Richel DJ. [Cyclooxygenase(COX)-2-inhibition in the prevention and treatment of colorectal carcinoma] Ned Tijdschr Geneeskd. 2003;147:2207–2212. [PubMed] [Google Scholar]
- 6.Elzagheid A, Emaetig F, Alkikhia L, Buhmeida A, Syrjänen K, El-Faitori O, Latto M, Collan Y, Pyrhönen S. High cyclooxygenase-2 expression is associated with advanced stages in colorectal cancer. Anticancer Res. 2013;33:3137–3143. [PubMed] [Google Scholar]
- 7.Peng L, Zhou Y, Wang Y, Mou H, Zhao Q. Prognostic significance of COX-2 immunohistochemical expression in colorectal cancer: a meta-analysis of the literature. PLoS One. 2013;8:e58891. doi: 10.1371/journal.pone.0058891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang LX, Xiao YZ, Yin WD. Expression of c-erbB-2 and EGFR in colorectal carcinoma and their significance. Cancer Res Clin. 2003;15:314–316. [Google Scholar]
- 9.Sirica AE. Role of ErbB family receptor tyrosine kinases in intrahepatic cholangiocarcinoma. World J Gastroenterol. 2008;14:7033–7058. doi: 10.3748/wjg.14.7033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma WW, Adjei AA. Novel agents on the horizon for cancer therapy. CA Cancer J Clin. 2009;59:111–137. doi: 10.3322/caac.20003. [DOI] [PubMed] [Google Scholar]
- 11.Kavanagh DO, Chambers G, O’Grady L, Barry KM, Waldron RP, Bennani F, Eustace PW, Tobbia I. Is overexpression of HER-2 a predictor of prognosis in colorectal cancer? BMC Cancer. 2009;9:1. doi: 10.1186/1471-2407-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiguchi K, Carbajal S, Chan K, Beltrán L, Ruffino L, Shen J, Matsumoto T, Yoshimi N, DiGiovanni J. Constitutive expression of ErbB-2 in gallbladder epithelium results in development of adenocarcinoma. Cancer Res. 2001;61:6971–6976. [PubMed] [Google Scholar]
- 13.Vadlamudi R, Mandal M, Adam L, Steinbach G, Mendelsohn J, Kumar R. Regulation of cyclooxygenase-2 pathway by HER2 receptor. Oncogene. 1999;18:305–314. doi: 10.1038/sj.onc.1202307. [DOI] [PubMed] [Google Scholar]
- 14.Lucarelli AP, Martins MM, Montor W, Oliveira V, Galvão MA, Piato S. Cyclooxygenase-2 and human epidermal growth factor receptor type 2 (HER-2) expression simultaneously in invasive and in situ breast ductal carcinoma. Sao Paulo Med J. 2011;129:371–379. doi: 10.1590/S1516-31802011000600002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okano H, Shinohara H, Miyamoto A, Takaori K, Tanigawa N. Concomitant overexpression of cyclooxygenase-2 in HER-2-positive on Smad4-reduced human gastric carcinomas is associated with a poor patient outcome. Clin Cancer Res. 2004;10:6938–6945. doi: 10.1158/1078-0432.CCR-0731-03. [DOI] [PubMed] [Google Scholar]
- 16.Su JL, Shih JY, Yen ML, Jeng YM, Chang CC, Hsieh CY, Wei LH, Yang PC, Kuo ML. Cyclooxygenase-2 induces EP1- and HER-2/Neu-dependent vascular endothelial growth factor-C up-regulation: a novel mechanism of lymphangiogenesis in lung adenocarcinoma. Cancer Res. 2004;64:554–564. doi: 10.1158/0008-5472.can-03-1301. [DOI] [PubMed] [Google Scholar]
- 17.Smakman N, Kranenburg O, Vogten JM, Bloemendaal AL, van Diest P, Borel Rinkes IH. Cyclooxygenase-2 is a target of KRASD12, which facilitates the outgrowth of murine C26 colorectal liver metastases. Clin Cancer Res. 2005;11:41–48. [PubMed] [Google Scholar]
- 18.Zhang H, Sun XF. Overexpression of cyclooxygenase-2 correlates with advanced stages of colorectal cancer. Am J Gastroenterol. 2002;97:1037–1041. doi: 10.1111/j.1572-0241.2002.05625.x. [DOI] [PubMed] [Google Scholar]
- 19.Al-Maghrabi J, Buhmeida A, Emam E, Syrjänen K, Sibiany A, Al-Qahtani M, Al-Ahwal M. Cyclooxygenase-2 expression as a predictor of outcome in colorectal carcinoma. World J Gastroenterol. 2012;18:1793–1799. doi: 10.3748/wjg.v18.i15.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dixon DA, Blanco FF, Bruno A, Patrignani P. Mechanistic aspects of COX-2 expression in colorectal neoplasia. Recent Results Cancer Res. 2013;191:7–37. doi: 10.1007/978-3-642-30331-9_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kasper HU, Konze E, Dienes HP, Stippel DL, Schirmacher P, Kern M. COX-2 expression and effects of COX-2 inhibition in colorectal carcinomas and their liver metastases. Anticancer Res. 2010;30:2017–2023. [PubMed] [Google Scholar]
- 22.Rahman M, Selvarajan K, Hasan MR, Chan AP, Jin C, Kim J, Chan SK, Le ND, Kim YB, Tai IT. Inhibition of COX-2 in colon cancer modulates tumor growth and MDR-1 expression to enhance tumor regression in therapy-refractory cancers in vivo. Neoplasia. 2012;14:624–633. doi: 10.1593/neo.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roelofs HM, Te Morsche RH, van Heumen BW, Nagengast FM, Peters WH. Over-expression of COX-2 mRNA in colorectal cancer. BMC Gastroenterol. 2014;14:1. doi: 10.1186/1471-230X-14-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kraus S, Naumov I, Arber N. COX-2 active agents in the chemoprevention of colorectal cancer. Recent Results Cancer Res. 2013;191:95–103. doi: 10.1007/978-3-642-30331-9_5. [DOI] [PubMed] [Google Scholar]
- 25.Wu SW, Ma CC, Yang Y. The prognostic value of HER-2/neu overexpression in colorectal cancer: evidence from 16 studies. Tumour Biol. 2014;35:10799–10804. doi: 10.1007/s13277-014-2376-0. [DOI] [PubMed] [Google Scholar]
- 26.Jin W, Lin ZW, Gao MX, Yang DX. Relationship between the expression of p53, PCNA and c-erbB-2 proteins and biologic behaviors of human colorectal carcinoma. Tumor. 2002;22:406. [Google Scholar]
- 27.Xu TW, Chen DD, Zheng YB, Li YJ. Correlation between the Expression of C-erbB-2, nm23 Protein and Invasion, Lymph Node Metastasis of Human Colorectal Carcinoma. Huazhong Keji Daxue Xuebao. 2005;34:214. [Google Scholar]
- 28.Li C, Liu DR, Ye LY, Huang LN, Jaiswal S, Li XW, Wang HH, Chen L. HER-2 overexpression and survival in colorectal cancer: a meta-analysis. J Zhejiang Univ Sci B. 2014;15:582–589. doi: 10.1631/jzus.B1300258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu Y, Jingyan G, Baorong S, Peng J, Xu Y, Cai S. Expression of EGFR, Her2 predict lymph node metastasis (LNM)-associated metastasis in colorectal cancer. Cancer Biomark. 2012;11:219–226. doi: 10.3233/CBM-2012-00282. [DOI] [PubMed] [Google Scholar]


