Abstract
AIM: To distinguish upper from lower gastrointestinal (GI) bleeding.
METHODS: Patient records between April 2011 and March 2014 were analyzed retrospectively (3296 upper endoscopy, and 1520 colonoscopy). Seventy-six patients had upper GI bleeding (Upper group) and 65 had lower GI bleeding (Lower group). Variables were compared between the groups using one-way analysis of variance. Logistic regression was performed to identify variables significantly associated with the diagnosis of upper vs lower GI bleeding. Receiver-operator characteristic (ROC) analysis was performed to determine the threshold value that could distinguish upper from lower GI bleeding.
RESULTS: Hemoglobin (P = 0.023), total protein (P = 0.0002), and lactate dehydrogenase (P = 0.009) were significantly lower in the Upper group than in the Lower group. Blood urea nitrogen (BUN) was higher in the Upper group than in the Lower group (P = 0.0065). Logistic regression analysis revealed that BUN was most strongly associated with the diagnosis of upper vs lower GI bleeding. ROC analysis revealed a threshold BUN value of 21.0 mg/dL, with a specificity of 93.0%.
CONCLUSION: The threshold BUN value for distinguishing upper from lower GI bleeding was 21.0 mg/dL.
Keywords: Logistic regression analysis, Likelihood analysis, Receiver-operator characteristic analysis, Blood urine nitrogen, Hemoglobin
Core tip: Differentiation of upper vs lower gastrointestinal (GI) bleeding is crucial. Laboratory test variables were investigated for their ability to distinguish upper from lower GI bleeding from retrospective analysis. Total protein, hemoglobin, and lactate dehydrogenase were lower and blood urea nitrogen (BUN) was higher in patients with upper GI bleeding. The threshold BUN value for distinguishing upper from lower GI bleeding was 21.0 mg/dL, with a specificity of 93.0%.
INTRODUCTION
Upper gastrointestinal (GI) bleeding is defined as bleeding that occurs proximal to the Treitz ligament, and lower GI bleeding occurs distal to the Treitz ligament. Causes of upper GI bleeding include gastric ulcers, duodenal ulcers, and gastric cancer[1]. Causes of lower GI bleeding include diverticula, angiodysplasia, and colorectal cancer[2]. The mortality rate of upper GI bleeding ranges from 3.5% to 7.4%[3,4] and that of lower GI bleeding is 1.9%[5,6]. Lower GI bleeding is less severe than upper GI bleeding[7]. Upper GI bleeding is diagnosed and treated with endoscopy, using methods such as clipping and bipolar electrocoagulation[8]. When patients do not respond to these therapies, arteriography with embolization is performed[9,10]. Lower GI bleeding ceases spontaneously[7], although 8%-37% of patients with lower GI bleeding are treated endoscopically with methods such as bipolar electrocoagulation and argon-plasma coagulation[11].
The mortality rate is 40% for patients with GI bleeding who are hemodynamically unstable[12]. An accurate diagnosis of upper or lower GI bleeding is important because early endoscopy significantly reduces mortality[13]. When patients present with hematemesis, the diagnosis of upper GI bleeding is readily apparent. Patients presenting with melena (tarry stools) likely have upper GI bleeding, while hematochezia suggests lower GI bleeding[14]. When patients do not display hematemesis, melena, or hematochezia, it is difficult to diagnose upper vs lower GI bleeding.
Blood testing is recommended before upper GI endoscopy or colonoscopy is performed because of its low cost and few complications[15]. Therefore, we sought to identify blood test parameters that could be useful in predicting upper vs lower GI bleeding.
MATERIALS AND METHODS
Patients
Patient records between April 2011 and March 2014 were analyzed retrospectively. During this time, 3296 patients underwent upper endoscopy and 1520 underwent colonoscopy. The group with upper GI bleeding (Upper group) comprised 50 male (69.2 ± 13.2 years) and 26 female (72.3 ± 10.2 years) patients. The group with lower GI bleeding (Lower group) comprised 35 male (69.4 ± 12.7 years) and 30 female (73.9 ± 10.4 years) patients. The diseases that caused upper GI bleeding are listed in Table 1. Bleeding from a gastric or duodenal ulcer was restricted to a spurting vessel (1a), an oozing vessel (1b), a visible vessel (2a), or a clot (2b), according to the Forrest classification system[16]. Diseases that caused lower GI bleeding are listed in Table 2. Laboratory test parameters were the focus of the present study. Clinical comments are not presented. Our study was reviewed and approved by the National Hospital Organization Shimoshizu Hospital Ethics Committee and was not designated as a clinical trial because it was performed as part of routine clinical practice. Patient anonymity was maintained. All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Table 1.
Diseases that caused upper gastrointestinal bleeding
| Diseases | No. of patients |
| Gastric ulcer | 31 |
| Gastric cancer | 28 |
| Duodenal ulcer | 7 |
| Acute gastric mucosal lesion | 4 |
| Esophageal ulcer | 2 |
| Esophageal varix | 2 |
| Esophagitis | 1 |
| Gastric invasion of pancreatic cancer | 1 |
| Total | 76 |
Table 2.
Diseases that caused lower gastrointestinal bleeding
| Diseases | No. of patients |
| Colorectal cancer | 47 |
| Ulcerative colitis | 6 |
| Colitis | 4 |
| Ulcer | 4 |
| Diverticulum | 1 |
| Hemorrhoid | 1 |
| Proctitis | 1 |
| Unknown | 1 |
| Total | 65 |
Upper GI endoscopy and colonoscopy
Patients received upper GI endoscopy for screening, examination of abdominal symptoms, or anemia. The endoscopic devices used were the GIF-N260H, GIF-XP260NS, GIF-PG260, GIF-XQ260, and GIF-Q260 (Olympus, Tokyo, Japan). Colonoscopy was performed in patients with abdominal symptoms, anemia, or a positive fecal occult blood test, or for screening purposes. The devices used were the CF-Q260 and PCF-Q260AI (Olympus).
Blood test variables
The blood test variables analyzed were white blood cell (WBC) count, hemoglobin, C-reactive protein (CRP), platelet count, total protein (TP), albumin, total bilirubin, alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, γ-glutamyl transpeptidase, lactate dehydrogenase (LDH), uric acid, blood urea nitrogen (BUN), creatinine, total cholesterol, triglycerides, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, blood glucose, hemoglobin A1c, body mass index, carcinoembryonic antigen, and carbohydrate antigen 19-9.
Statistical analysis
One-way analysis of variance (ANOVA) was performed to reveal differences in the variables between the Upper and Lower groups. Fisher’s exact test was used to compare the percentage of patients with cancer in the Upper and Lower groups. Logistic regression analysis was performed to reveal variables that were significantly associated with the diagnosis of upper vs lower GI bleeding. Receiver-operator characteristic (ROC) analysis was applied to determine the threshold value that can differentiate between upper and lower GI bleeding. P < 0.05 was used to indicate statistical significance. JMP version 10.0.2 (SAS Institute, Cary, NC, United States) was used for statistical analyses. The statistical methods of this study were reviewed by Yasufumi Motoyoshi from National Hospital Organization Shimoshizu Hospital.
RESULTS
To reveal differences in variables between the Upper and Lower groups, ANOVA was performed (Table 3). Hemoglobin (P = 0.023), TP (P = 0.0002), and LDH (P = 0.009) were significantly lower in the Upper group than in the Lower group. BUN was significantly higher in the Upper group than in the Lower group (P = 0.0065). Thus, hemoglobin, TP, LDH, and BUN were useful in the diagnosis of upper vs lower GI bleeding. Consequently, further analyses focused on these four variables.
Table 3.
Comparison of variables between patients with upper and lower gastrointestinal bleeding
| Characteristics | Upper group | Lower group | P value |
| Age (yr) | 70.4 ± 12.3 | 71.5 ± 11.9 | 0.6016 |
| WBC count (102/μL) | 8.792 ± 11.751 | 7.033 ± 3.438 | 0.2773 |
| Hb (g/dL) | 10.1 ± 3.2 | 11.3 ± 2.6 | 0.0230 |
| CRP (mg/dL) | 1.4 ± 1.5 | 2.0 ± 4.0 | 0.3220 |
| Plt (104/μL) | 27.4 ± 11.2 | 26.5 ± 11.1 | 0.6633 |
| TP (g/dL) | 6.0 ± 0.85 | 6.7 ± 0.8 | 0.0002 |
| Alb (g/dL) | 3.4 ± 0.6 | 3.5 ± 0.9 | 0.5958 |
| T-Bil (mg/dL) | 0.69 ± 0.57 | 0.70 ± 0.38 | 0.9236 |
| ALP (IU/L) | 236 ± 138 | 306 ± 218 | 0.2074 |
| AST (IU/L) | 25.3 ± 20.7 | 22.3 ± 10.0 | 0.3515 |
| ALT (IU/L) | 20.2 ± 18.2 | 16.9 ± 10.9 | 0.2422 |
| γ-GTP (IU/L) | 56.3 ± 88.1 | 32.1 ± 20.8 | 0.319 |
| LDH (IU/L) | 189.2 ± 51.7 | 243.7 ± 114.3 | 0.009 |
| UA (mg/dL) | 5.3 ± 2.0 | 5.8 ± 1.5 | 0.3541 |
| BUN (mg/dL) | 21.1 ± 15.8 | 14.1 ± 5.5 | 0.0065 |
| Cre (mg/dL) | 0.93 ± 0.35 | 0.84 ± 0.25 | 0.1153 |
| T-Chol (mg/dL) | 158.4 ± 45.8 | 175.4 ± 41.9 | 0.1966 |
| TG (mg/dL) | 100.7 ± 49.6 | 127.0 ± 79.7 | 0.4095 |
| HDL (mg/dL) | 45.4 ± 10.0 | 55.7 ± 18.2 | 0.1404 |
| LDL (mg/dL) | 90.6 ± 32.2 | 116.9 ± 40.7 | 0.1094 |
| BS (mg/dL) | 132.8 ± 57.5 | 117 ± 26.9 | 0.1979 |
| HbA1c (%) | 5.8 ± 0.8 | 6.6 ± 0.7 | 0.0379 |
| BMI | 21.6 ± 4.2 | 21.4 ± 3.4 | 0.9126 |
| CEA (ng/mL) | 13.6 ± 25.9 | 217.5 ± 753.5 | 0.2884 |
| CA19-9 (U/mL) | 784 ± 3.017 | 159.9 ± 450.5 | 0.3429 |
Plt: Platelet; TP: Total protein; Alb: Albumin; T-Bil: Total bilirubin; ALP: Alkaline phosphatase; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; BG: Blood glucose; BMI: Body mass index; BUN: Blood urea nitrogen; CA19-9: Carbohydrate antigen 19-9; CEA: Carcinoembryonic antigen; Cre: Creatinine; CRP: C-reactive protein; γ-GTP: γ-glutamyl transpeptidase; Hb: Hemoglobin; HbA1c: Hemoglobin A1c; HDL: High-density lipoprotein cholesterol; LDH: Lactate dehydrogenase; LDL: Low-density lipoprotein cholesterol; T-Chol: Total cholesterol; TG: Triglyceride; UA: Uric acid; WBC: White blood cell count.
To clarify the strength of the association between the difference in the blood test parameters and diagnosis of upper vs lower GI bleeding, logistic regression analysis was performed (Table 4). BUN had the largest χ2 value and the smallest P value, suggesting that BUN was the variable most strongly associated with the diagnosis of upper vs lower GI bleeding.
Table 4.
Results of logistic regression analysis
| χ2 | Odds | Odds (95%CI) | P value | |
| Hb | 0.04 | 1.045994 | 0.669624-1.717491 | 0.8446 |
| TP | 1.03 | 2.221154 | 0.514904-13.65946 | 0.3100 |
| LDH | 1.05 | 1.015110 | 0.987842-1.049002 | 0.3057 |
| BUN | 2.38 | 0.879782 | 0.697744-0.980732 | 0.1232 |
Hb: Hemoglobin; TP: Total protein; LDH: Lactate dehydrogenase; BUN: Blood urea nitrogen.
A likelihood analysis was performed to confirm that BUN had the strongest association with the differentiation between upper and lower GI bleeding (Table 5). BUN was the only variable that had P < 0.05. These data suggest that BUN was the most useful parameter to distinguish upper from lower GI bleeding.
Table 5.
Results of the likelihood ratio test
| Likelihood χ2 | P value | |
| Hb | 0.03883460 | 0.8438 |
| TP | 1.12378238 | 0.2891 |
| LDH | 1.13905685 | 0.2859 |
| BUN | 6.62036996 | 0.0101 |
Hb: Hemoglobin; TP: Total protein; LDH: Lactate dehydrogenase; BUN: Blood urea nitrogen.
Threshold values are useful to diagnose upper vs lower GI bleeding using blood test parameters. Therefore, ROC analysis was performed to determine the threshold values (Figure 1). The area under the ROC curve (AUC) seemed relatively large for TP and LDH.
Figure 1.
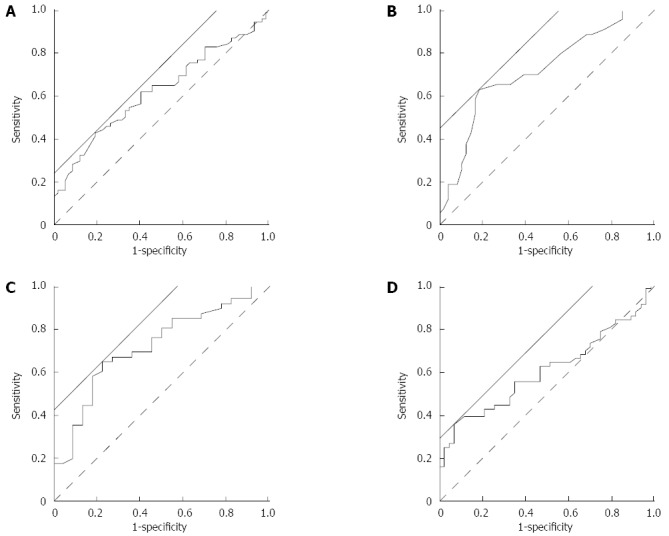
Receiver-operator characteristic analysis. Receiver-operator characteristic analysis was performed to determine the threshold value of hemoglobin (A), total protein (B), lactate dehydrogenase (C), and blood urea nitrogen (D) that could differentiate between upper and lower gastrointestinal bleeding. Solid straight line: a line with a slope of 45° used to calculate the threshold by JMP10.0.2 software, dashed line: reference line.
The AUC, threshold value, and sensitivity and specificity at the threshold value were calculated and are displayed in Table 6. The sensitivity of each variable was relatively low. The specificity of BUN was 93.0% at the threshold value of 21.0 mg/dL.
Table 6.
Results of receiver-operator characteristic analysis
| AUC | Threshold value | Sensitivity | Specificity | |
| Hb | 0.61894 | 8.7 (g/dL) | 42.7% | 80.7% |
| TP | 0.71197 | 6.3 (g/dL) | 62.8% | 81.3% |
| LDH | 0.71515 | 191 (IU/L) | 64.4% | 77.32% |
| BUN | 0.61459 | 21 (mg/dL) | 36.4% | 93.0% |
Hb: Hemoglobin; TP: Total protein; LDH: Lactate dehydrogenase; BUN: Blood urea nitrogen.
Logistic regression and likelihood analyses revealed that BUN had the strongest association with the diagnosis of upper vs lower GI bleeding. Thus, we conclude that BUN was the most useful variable for diagnosing upper vs lower GI bleeding, and the threshold value was 21.0 mg/dL, with a specificity of 93.0%.
DISCUSSION
BUN increases after ingestion of a large amount of protein or blood[17]. Thus, it is reasonable to expect that BUN increases following massive upper GI bleeding. The ratio of BUN to creatinine has been used to predict upper GI bleeding. A BUN/creatinine ratio > 30 and hemoglobin level < 8.0 g/dL indicate severe upper GI bleeding[18]. A BUN/creatinine ratio > 36 distinguishes upper from lower GI bleeding[19]. Al-Naamani et al[20] reported that BUN alone predicts the severity of upper GI bleeding. All the above-mentioned reports focus on upper GI bleeding. There are no reports on using BUN alone to differentiate between upper and lower GI bleeding. In our study, BUN was able to distinguish upper from lower GI bleeding. This may be explained by blood from upper GI bleeding being digested in the intestine, thereby increasing BUN, with blood in the colon or rectum due to lower GI bleeding not being digested[17].
In our study, the threshold value of BUN that distinguished upper from lower GI bleeding was 21.0 mg/dL. The sensitivity of the value was low. It is speculated that patients with BUN < 21.0 mg/dL were those with less severe upper GI bleeding or lower GI bleeding. Patients with severe upper GI bleeding had BUN > 21.0 mg/dL. BUN did not increase in patients with lower GI bleeding. Therefore, BUN > 21.0 mg/dL might be specific to patients with upper GI bleeding. When BUN is > 21.0 mg/dL, a clinician would predict upper GI bleeding with sensitivity of 36.4% and specificity of 93.0%. One limitation of the present study was that differentiation would be hard between upper and lower GI bleeding when BUN was < 21.0 mg/dL.
The present study found that hemoglobin, TP, and LDH were lower in patients with upper GI bleeding. Upper GI bleeding is more severe than lower GI bleeding[7]. Hemoglobin clearly decreases in patients with upper GI bleeding[21]. These facts indicate that lower hemoglobin indicates hemodynamic instability. It is reasonable to expect therefore that hemoglobin would be lower in such patients. The reasons for TP and LDH being lower in patients with upper GI bleeding are not clear.
The present study mainly consisted of nonvariceal bleeding, although it included two patients with variceal bleeding. Upper GI bleeding is mainly seen in patients with nonvariceal bleeding[22]. It is recommended that variceal or nonvariceal bleeding be considered regarding management of upper GI bleeding because management of variceal or nonvariceal bleeding is different[8,23].
In conclusion, TP, hemoglobin and LDH were lower, and BUN was higher in patients with upper GI bleeding. The threshold BUN value to distinguish upper from lower GI bleeding was 21.0 mg/dL.
COMMENTS
Background
Upper gastrointestinal (GI) bleeding is defined as bleeding that occurs proximal to the Treitz ligament, and lower GI bleeding occurs distal to the Treitz ligament. The mortality rate is 40% for patients with GI bleeding who are hemodynamically unstable. An accurate diagnosis of upper or lower GI bleeding is important because early endoscopy significantly reduces mortality.
Research frontiers
When patients present with hematemesis, the diagnosis of upper GI bleeding is readily apparent. Patients presenting with melena (tarry stools) likely have upper GI bleeding, while hematochezia suggests lower GI bleeding. When patients do not display hematemesis, melena, or hematochezia, it is difficult to diagnose upper vs lower GI bleeding.
Innovations and breakthroughs
Laboratory test variables were investigated for their ability to distinguish upper from lower GI bleeding. Hemoglobin (P = 0.023), total protein (P = 0.0002), and lactate dehydrogenase (P = 0.009) were significantly lower in patients with upper GI bleeding than those with lower. Blood urea nitrogen (BUN) was higher in patients with upper GI bleeding than those with in lower GI bleeding (P = 0.0065). Logistic regression analysis revealed that BUN was most strongly associated with the diagnosis of upper vs lower GI bleeding. Receiver-operator characteristics analysis revealed a threshold BUN value of 21.0 mg/dL, with a specificity of 93.0%.
Applications
The threshold BUN value for distinguishing upper from lower GI bleeding was 21.0 mg/dL, with a sensitivity of 36.4% and a specificity of 93.0%.
Peer-review
It was a good idea to differentiate upper or lower GI bleeding with laboratory test variables. It seemed difficult to differentiate upper or lower GI bleeding when BUN < 21.0 mg/dL. Hb was lower in upper GI than lower. The lower Hb indicated severe bleeding, not the site of bleeding. Upper GI bleeding is mainly consisted of non-variceal bleeding. It would be recommended that variceal or non-varicela be considered regarding management of upper GI bleeding because management of variceal or non-variceal bleeding is different. Sensitivity of BUN was low to differentiate upper and lower GI bleeding.
Footnotes
Ethics approval: Judgment of the research proposal by Institutional Ethics Committee Principle Investigator, Minoru Tomizawa.
Informed consent: All study participants or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest: No potential conflicts of interest relevant to this article were reported.
Data sharing: No additional data available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: November 7, 2014
First decision: December 11, 2014
Article in press: January 21, 2015
P- Reviewer: De Silva AP, Stanciu C, Shehata MMM S- Editor: Qi Y L- Editor: Kerr C E- Editor: Zhang DN
References
- 1.Theocharis GJ, Thomopoulos KC, Sakellaropoulos G, Katsakoulis E, Nikolopoulou V. Changing trends in the epidemiology and clinical outcome of acute upper gastrointestinal bleeding in a defined geographical area in Greece. J Clin Gastroenterol. 2008;42:128–133. doi: 10.1097/01.mcg.0000248004.73075.ad. [DOI] [PubMed] [Google Scholar]
- 2.Marion Y, Lebreton G, Le Pennec V, Hourna E, Viennot S, Alves A. The management of lower gastrointestinal bleeding. J Visc Surg. 2014;151:191–201. doi: 10.1016/j.jviscsurg.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Hearnshaw SA, Logan RF, Lowe D, Travis SP, Murphy MF, Palmer KR. Acute upper gastrointestinal bleeding in the UK: patient characteristics, diagnoses and outcomes in the 2007 UK audit. Gut. 2011;60:1327–1335. doi: 10.1136/gut.2010.228437. [DOI] [PubMed] [Google Scholar]
- 4.Leontiadis GI, Molloy-Bland M, Moayyedi P, Howden CW. Effect of comorbidity on mortality in patients with peptic ulcer bleeding: systematic review and meta-analysis. Am J Gastroenterol. 2013;108:331–345; quiz 346. doi: 10.1038/ajg.2012.451. [DOI] [PubMed] [Google Scholar]
- 5.Venkatesh PG, Njei B, Sanaka MR, Navaneethan U. Risk of comorbidities and outcomes in patients with lower gastrointestinal bleeding - a nationwide study. Int J Colorectal Dis. 2014;29:953–960. doi: 10.1007/s00384-014-1915-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feinman M, Haut ER. Lower gastrointestinal bleeding. Surg Clin North Am. 2014;94:55–63. doi: 10.1016/j.suc.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Barnert J, Messmann H. Management of lower gastrointestinal tract bleeding. Best Pract Res Clin Gastroenterol. 2008;22:295–312. doi: 10.1016/j.bpg.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 8.Kim SY, Hyun JJ, Jung SW, Lee SW. Management of non-variceal upper gastrointestinal bleeding. Clin Endosc. 2012;45:220–223. doi: 10.5946/ce.2012.45.3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilkins T, Khan N, Nabh A, Schade RR. Diagnosis and management of upper gastrointestinal bleeding. Am Fam Physician. 2012;85:469–476. [PubMed] [Google Scholar]
- 10.Katano T, Mizoshita T, Senoo K, Sobue S, Takada H, Sakamoto T, Mochiduki H, Ozeki T, Kato A, Matsunami K, et al. The efficacy of transcatheter arterial embolization as the first-choice treatment after failure of endoscopic hemostasis and endoscopic treatment resistance factors. Dig Endosc. 2012;24:364–369. doi: 10.1111/j.1443-1661.2012.01285.x. [DOI] [PubMed] [Google Scholar]
- 11.Lhewa DY, Strate LL. Pros and cons of colonoscopy in management of acute lower gastrointestinal bleeding. World J Gastroenterol. 2012;18:1185–1190. doi: 10.3748/wjg.v18.i11.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walsh RM, Anain P, Geisinger M, Vogt D, Mayes J, Grundfest-Broniatowski S, Henderson JM. Role of angiography and embolization for massive gastroduodenal hemorrhage. J Gastrointest Surg. 1999;3:61–65; discussion 66. doi: 10.1016/s1091-255x(99)80010-9. [DOI] [PubMed] [Google Scholar]
- 13.Liu N, Liu L, Zhang H, Gyawali PC, Zhang D, Yao L, Yang Y, Wu K, Ding J, Fan D. Effect of intravenous proton pump inhibitor regimens and timing of endoscopy on clinical outcomes of peptic ulcer bleeding. J Gastroenterol Hepatol. 2012;27:1473–1479. doi: 10.1111/j.1440-1746.2012.07191.x. [DOI] [PubMed] [Google Scholar]
- 14.Peura DA, Lanza FL, Gostout CJ, Foutch PG. The American College of Gastroenterology Bleeding Registry: preliminary findings. Am J Gastroenterol. 1997;92:924–928. [PubMed] [Google Scholar]
- 15.Sonnenberg A. Test sequence in the management of gastrointestinal bleeding. Endoscopy. 2012;44:43–47. doi: 10.1055/s-0031-1291536. [DOI] [PubMed] [Google Scholar]
- 16.Forrest JA, Finlayson ND, Shearman DJ. Endoscopy in gastrointestinal bleeding. Lancet. 1974;2:394–397. doi: 10.1016/s0140-6736(74)91770-x. [DOI] [PubMed] [Google Scholar]
- 17.Cohn TD, Lane M, Zuckerman S, Messinger N, Griffith A. Induced azotemia in humans following massive protein and blood ingestion and the mechanism of azotemia in gastrointestinal hemorrhage. Am J Med Sci. 1956;231:394–401. doi: 10.1097/00000441-195604000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Srygley FD, Gerardo CJ, Tran T, Fisher DA. Does this patient have a severe upper gastrointestinal bleed? JAMA. 2012;307:1072–1079. doi: 10.1001/jama.2012.253. [DOI] [PubMed] [Google Scholar]
- 19.Richards RJ, Donica MB, Grayer D. Can the blood urea nitrogen/creatinine ratio distinguish upper from lower gastrointestinal bleeding? J Clin Gastroenterol. 1990;12:500–504. doi: 10.1097/00004836-199010000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Al-Naamani K, Alzadjali N, Barkun AN, Fallone CA. Does blood urea nitrogen level predict severity and high-risk endoscopic lesions in patients with nonvariceal upper gastrointestinal bleeding? Can J Gastroenterol. 2008;22:399–403. doi: 10.1155/2008/207850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomizawa M, Shinozaki F, Hasegawa R, Togawa A, Shirai Y, Ichiki N, Motoyoshi Y, Sugiyama T, Yamamoto S, Sueishi M. Reduced hemoglobin and increased C-reactive protein are associated with upper gastrointestinal bleeding. World J Gastroenterol. 2014;20:1311–1317. doi: 10.3748/wjg.v20.i5.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim KB, Yoon SM, Youn SJ. Endoscopy for nonvariceal upper gastrointestinal bleeding. Clin Endosc. 2014;47:315–319. doi: 10.5946/ce.2014.47.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu Y, Loffroy R, Lau JY, Barkun A. Multidisciplinary management strategies for acute non-variceal upper gastrointestinal bleeding. Br J Surg. 2014;101:e34–e50. doi: 10.1002/bjs.9351. [DOI] [PubMed] [Google Scholar]


