Abstract
AIM: To investigate the significance of the preoperative neutrophil-to-lymphocyte ratio (NLR) in the prognosis of patients with gastric cancer (GC).
METHODS: The clinical data of 291 GC patients were analysed retrospectively; these patients were divided into two groups according to their preoperative NLR: a high-NLR group (NLR ≥ 3.5, 131 cases) and a low-NLR group (NLR < 3.5, 160 cases). The clinicopathological characteristics and five-year survival rates of the two groups were compared. The NLR and other clinicopathological factors were subjected to univariate and multivariate survival analysis to evaluate the effects of the NLR on the prognosis of GC patients.
RESULTS: The lowest preoperative NLR among the 291 patients was 0.56, whereas the highest preoperative NLR was 74.5. The mean preoperative NLR was 5.99 ± 8.98. Age, tumour size, T staging, tumour-node-metastasis (TNM) staging and platelet count were significantly different between the high- and low-NLR groups (P < 0.05). The five-year survival rate of the high-NLR group was 17.0%, which was significantly lower than that of the low-NLR group (43.6%; 17.0% vs 43.6%, P < 0.05). The univariate analysis results showed that the five-year survival rate was related to age, tumour size, T staging, N staging, TNM staging, carcinoembryonic antigen value and NLR (P < 0.05). Multivariate analysis results showed that the NLR was an independent risk factor that likely affected the five-year survival rate of GC patients (P = 0.003, HR = 0.626, 95%CI: 0.460-0.852).
CONCLUSION: The preoperative NLR could be used as a prognostic factor for GC patients; in particular, a high NLR corresponded to poor prognosis of GC patients.
Keywords: Gastric cancer, Neutrophil-to-lymphocyte ratio, Prognosis, Inflammation, Survival rate
Core tip: This research preliminarily investigated the relationship between the preoperative neutrophil-to-lymphocyte ratio (NLR) and gastric cancer. The results revealed that a high NLR corresponded to poor prognosis of gastric cancer patients. Furthermore, preoperative NLR could be used as a prognostic factor for these patients.
INTRODUCTION
Gastric cancer (GC) is one of the most common types of gastrointestinal cancer; the mortality of GC ranks second among all malignancies[1]. Although the incidence of GC declined in recent years, prognosis has not greatly improved, and the five-year accumulative survival rate remains at approximately 25%[2]. GC is mainly treated by radical surgery; thus, factors associated with the prognosis of GC should be determined to effectively assist intervention therapy and to improve patient outcomes. The body’s inflammatory response plays an important role in tumour occurrence and development[3]. Inflammatory responses can inhibit apoptosis, promote angiogenesis and damage DNA, thereby promoting tumour growth and proliferation[4,5]. In cancer patients who are in the aggressive phase, inflammatory response indicators, such as C-reactive protein levels and platelet count, are usually higher and are related to poor prognosis[6,7]. Similarly, the body’s inflammatory response can cause changes in the peripheral white blood cell count, which is reflected as an increased neutrophil count and reduced lymphocyte count[8]. Therefore, NLR could be used as a good indicator of the systemic inflammatory state of cancer patients. NLR is closely related to the prognosis of various malignant tumours, such as liver cancer, colorectal cancer, breast cancer, bladder cancer and non-small cell lung cancer[9-14]. However, few studies have investigated the relationships of NLR and prognosis of GC patients[15-17]. This study aimed to investigate the effects of preoperative NLR in the prognosis of GC patients; our study also provided a reference for diagnostic and treatment strategies for GC.
MATERIALS AND METHODS
General information
A total of 291 GC cases treated and subjected to radical surgery in the Department of General Surgery, Nanjing First Hospital, China, from January 2005 to December 2009 were selected. These patients were not subjected to preoperative chemotherapy and were not affected by infectious diseases. The intraoperative situation confirmed that no distant metastasis was present. The patients’ clinical and pathological data were collected (Table 1). Postoperative regular telephone or outpatient follow up was performed for six months to five years; the follow-up rate was 91.1%. The clinicopathological staging of this research was in accordance with the criteria of American Joint Committee on Cancer Staging (7th edition)[18].
Table 1.
Comparison of clinicopathological characteristics between high- and low-neutrophil-to-lymphocyte groups n (%)
| Clinicopathological feature | n | High-NLR group | Low-NLR group | χ2 | P value |
| Gender | 0.163 | 0.686 | |||
| Male | 210 | 93 (44.3) | 117 (55.7) | ||
| Female | 81 | 38 (46.9) | 43 (53.1) | ||
| Age | 12.377 | 0.000 | |||
| < 65 yr | 142 | 49 (34.5) | 93 (65.5) | ||
| ≥ 65 yr | 149 | 82 (55.0) | 67 (45.0) | ||
| Tumor size | 20.852 | 0.000 | |||
| < 5 cm | 143 | 45 (31.5) | 98 (68.5) | ||
| ≥ 5 cm | 148 | 86 (58.1) | 62 (41.9) | ||
| Differentiation degree | |||||
| Middle and high differentiation | 130 | 59 (45.4) | 71 (54.6) | 0.013 | 0.910 |
| Low differentiation | 161 | 72 (44.7) | 89 (55.3) | ||
| T staging | |||||
| T1 | 20 | 2 (10.0) | 18 (90.0) | 20.731 | 0.000 |
| T2 | 29 | 13 (41.4) | 16 (58.6) | ||
| T3 | 177 | 74 (41.8) | 103 (58.2) | ||
| T4 | 65 | 42 (64.6) | 23 (35.4) | ||
| N staging | 4.185 | 0.242 | |||
| N0 | 55 | 18 (32.7) | 37 (67.3) | ||
| N1 | 127 | 60 (47.2) | 67 (52.8) | ||
| N2 | 78 | 38 (48.7) | 40 (51.3) | ||
| N3 | 31 | 15 (48.4) | 16 (51.6) | ||
| TNM staging | 11.363 | 0.003 | |||
| Stage I | 32 | 8 (25.0) | 24 (75.0) | ||
| Stage II | 123 | 49 (39.8) | 74 (60.2) | ||
| Stage III | 136 | 74 (54.4) | 62 (45.6) | ||
| platelet counting | 9.672 | 0.002 | |||
| < 300 × 109/L | 253 | 105 (41.5) | 148 (58.5) | ||
| ≥ 300 × 109/L | 38 | 26 (68.4) | 12 (31.6) | ||
| CEA | 2.972 | 0.085 | |||
| < 5 ng/mL | 178 | 73 (41.0) | 105 (59.0) | ||
| ≥ 5 ng/mL | 113 | 58 (51.3) | 55 (48.7) |
NLR: Neutrophil-to-lymphocyte ratio; CEA: Carcinoembryonic antigen.
Blood sampling
Neutrophil, lymphocyte and platelet counts and carcinoembryonic antigen (CEA) values of the patients were collected one week before these patients underwent surgery. NLR was then calculated, and 3.5 was set as a critical value. The patients were then divided into two groups: high-NLR group (NLR ≥ 3.5) with 131 cases and low-NLR group (NLR < 3.5) with 160 cases.
Statistical analysis
Data were statistically analysed using SPSS 20.0 statistical software. Counted data were subjected to a χ2 test. Variables likely to affect NLR were evaluated by logistic regression. The survival rate was calculated according to the Kaplan-Meier method. Survival rates were then compared by performing log-rank tests. Univariate and multivariate survival analyses were also conducted using a Cox proportional hazards model, in which P < 0.05 was considered statistically significant.
RESULTS
Relationships of preoperative NLR and other clinicopathological factors
The lowest preoperative NLR of the 291 patients was 0.56, whereas the highest NLR was 74.5. The mean NLR was 5.99 ± 8.98. The distributions of NLR were listed as follows: NLR < 1.5, 35 cases; 1.5 ≤ NLR < 2.5, 27 cases; 2.5 ≤ NLR < 3.5, 53 cases; 3.5 ≤ NLR < 4.5, 42 cases; 4.5 ≤ NLR < 5.5, 15 cases; and NLR ≥ 5.5, 74 cases. The compared P values among different survival-rate groups were as follows (Figure 1A): P = 0.953 (NLR < 1.5 and 1.5 ≤ NLR < 2.5); P = 0.066 (1.5 ≤ NLR < 2.5 and 2.5 ≤ NLR < 3.5); P = 0.010 (2.5 ≤ NLR < 3.5 and 3.5 ≤ NLR < 4.5); P = 0.703 (3.5 ≤ NLR < 4.5 and 4.5 ≤ NLR < 5.5); and P = 0.852 (4.5 ≤ NLR < 5.5 and NLR ≥ 5.5). On the basis of these results (P = 0.010; 2.5 ≤ NLR < 3.5 and 3.5 ≤ NLR < 4.5), we selected NLR = 3.5 as the threshold. The patients were then divided into a high-NLR group (NLR ≥ 3.5) and a low-NLR group (NLR < 3.5).
Figure 1.
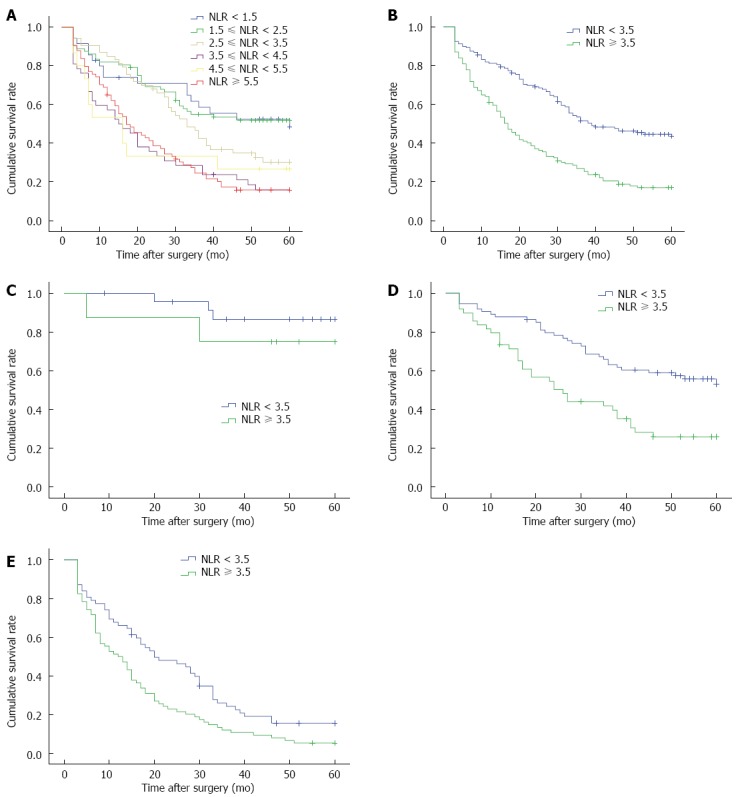
Five-year survival curves. A: All patients; B: High- and low-neutrophil-to-lymphocyte ratio groups; C: Stage I patients; D: Stage II patients; E: Stage III patients. NLR: Neutrophil-to-lymphocyte ratio.
Age, tumour size, T staging, tumour-node-metastasis (TNM) staging and platelet count significantly differed between high- and low-NLR groups (P < 0.05). By contrast, gender, differentiation degree, N staging and CEA values were not significantly different (P > 0.05). As the tumour invasion depth increased and clinicopathological staging progressed, the proportion of patients with high NLR correspondingly increased. The patients in the high-NLR group were older and exhibited larger tumours and high platelet counts (Table 1).
Logistic regression analysis was performed to evaluate the clinicopathological factors that likely caused the increased NLR. The results showed that age and tumour size were independent risk factors that possibly increased the NLR (P < 0.05; Table 2).
Table 2.
Multivariate analysis of neutrophil-to-lymphocyte ratio-associated risk factors
| Clinicopathological feature | HR | 95%CI | P value |
| Gender (M/F) | 1.219 | 0.693-2.146 | 0.492 |
| Age (yr) | 1.036 | 1.014-1.059 | 0.002 |
| Tumor size | 2.690 | 1.584-4.565 | 0.000 |
| Differentiation degree | 0.966 | 0.575-1.622 | 0.895 |
| T staging | 1.269 | 0.705-2.287 | 0.427 |
| N staging | 0.743 | 0.453-1.219 | 0.239 |
| TNM staging | 1.732 | 0.680-4.409 | 0.249 |
| Platelet counting | 0.999 | 0.996-1.002 | 0.395 |
| CEA | 1.001 | 0.998-1.003 | 0.630 |
M/F: Male/female; CEA: Carcinoembryonic antigen; HR: Hazard ratio.
Effects of NLR on the prognosis of GC patients
The five-year survival rate of the high-NLR group was 17.0%, which was significantly lower than that of the low-NLR group (43.6%; χ2 = 32.818, P < 0.001, Figure 1B). The univariate analysis results showed that the five-year survival rate was related to age, tumour size, T staging, N staging, TNM staging, CEA value and NLR (P < 0.001). These parameters were then subjected to multivariate analysis. The results showed that TNM staging and NLR were independent prognostic factors for the five-year survival rate of patients (P < 0.05; Table 3).
Table 3.
Univariate and multivariate survival analysis results
| Clinicopathological feature | Univariate-analyzed P value |
Multivariate analysis |
|
| P value | HR (95%CI) | ||
| Gender | 0.611 | ||
| Male | |||
| Female | |||
| Age | 0.000 | 0.096 | |
| < 65 yr | 0.774 (0.573-1.046) | ||
| ≥ 65 yr | 1.000 | ||
| Tumor size | 0.000 | 0.122 | |
| < 5 cm | 0.784 (0.576-1.067) | ||
| ≥ 5 cm | 1.000 | ||
| Differentiation degree | 0.108 | ||
| Middle and high differentiation | |||
| Low differentiation | |||
| T staging | 0.000 | 0.583 | |
| T1 | 0.898 | 0.879 (0.122-6.338) | |
| T2 | 0.579 | 0.797 (0.358-1.776) | |
| T3 | 0.170 | 0.739 (0.480-1.138) | |
| T4 | 1.000 | ||
| N staging | 0.000 | 0.080 | |
| N0 | 0.303 | 0.622 (0.252-1.535) | |
| N1 | 0.910 | 1.037 (0.556-1.934) | |
| N2 | 0.091 | 0.678 (0.433-1.063) | |
| N3 | 1.000 | ||
| TNM staging | 0.000 | 0.008 | |
| Stage I | 0.030 | 0.134 (0.022-0.822) | |
| Stage II | 0.004 | 0.387 (0.204-0.735) | |
| Stage III | 1.000 | ||
| Platelet counting | 0.382 | ||
| < 300 × 109/L | |||
| ≥ 300 × 109/L | |||
| CEA | 0.000 | 0.547 | |
| < 5 ng/mL | 0.912 (0.675-1.231) | ||
| ≥ 5 ng/mL | 1.000 | ||
| NLR | 0.000 | 0.003 | |
| < 3.5 | 0.626 (0.460-0.852) | ||
| ≥ 3.5 | 1.000 | ||
NLR: Neutrophil-to-lymphocyte ratio; CEA: Carcinoembryonic antigen; HR: Hazard ratio.
Our data were subjected to further stratification analysis. Our results showed that the five-year survival rates of high- and low-NLR groups of stage I patients were not significantly different (χ2 = 0.732, P = 0.392; Figure 1C). By contrast, the five-year survival rate of the high-NLR group of stage II and stage III patients was significantly lower than that of the low-NLR group (χ2 = 12.299, P < 0.001; χ2 = 7.507, P = 0.006; Figure 1D and E).
DISCUSSION
Abnormal phenotypes of malignant cancer cells likely stimulate the accumulation of inflammatory cells and destroy the tumour-surrounding tissues, thereby causing a series of non-specific inflammatory responses. As a tumour grows, these inflammatory responses likely increase the peripheral blood neutrophil count and decrease the lymphocyte count; as a result, NLR increases. This result is consistent with those of previous studies[15,16,19]. Our study further found that the proportion of patients in the high-NLR group increased as the tumour invasion depth increased and the disease progressed; this finding is also consistent with those in previous studies. Shimada et al[15] performed a logistical regression analysis of clinicopathological factors that likely influence the increase in NLR and found that old age and high blood platelet count are independent risk factors of high NLR; the data of the present study showed that age and tumour size were independent risk factors that likely affected the increase in NLR.
High NLR is related to poor prognosis of patients with various malignant tumours[9,10,12,14]. Hirashima et al[17] revealed that NLR is related to the prognosis of patients with GC in the early stage; however, they did not further analyse whether NLR is an independent factor affecting the prognosis of GC patients. Jung et al[16] investigated patients with stage III and IV GC and found that the overall survival rate of the high-NLR group (≥ 2.0) was significantly lower than that of the low-NLR group. Indeed, NLR is an independent factor affecting patient’s overall survival rate. Shimada et al[15] studied 1028 GC cases subjected to radical surgery and found that the five-year survival rate of patients with high NLR (≥ 4.0) was significantly lower than that of patients with low NLR. Similarly, Shimada et al[15] found that NLR is an independent factor affecting patient’s five-year survival rate. Other scholars[20,21] also investigated patients with advanced GC treated with chemotherapy and found that high NLR is an independent risk factor influencing patient’s disease-free survival period and overall survival rate. In our study, the effect on five-year survival rate of the patients with NLR ≥ 3.5 was apparent compared with that of patients with NLR < 3.5 possibly because NLR was related to the development of GC. Multivariate analysis results showed that NLR was an independent factor that likely affected the patient’s five-year survival rate. Therefore, high preoperative NLR is an indicator of the poor prognosis of patients with GC.
Several explanations have been provided regarding the relationship of high NLR and poor prognosis. For instance, high NLR corresponds to an enhanced response of neutrophils to tumour inflammation; neutrophils secrete angiogenic factors, such as vascular endothelial growth factor, thereby stimulating angiogenesis and promoting tumour growth and metastasis[22]. Alternatively, peripheral blood lymphocytes are decreased, leading to reduced lymphocyte-mediated anti-tumour immune responses, which would accelerate disease progression. Furthermore, systemic inflammation is closely related to nutritional status and decreased organ function in cancer patients; thus, poor prognosis is observed[23].
High preoperative NLR indicated poor cancer prognosis; this result is very significant for cancer prevention and treatment. Moreover, the effects of anti-inflammatory drugs on tumour occurrence and development have been investigated extensively. For example, the prophylactic application of non-steroidal anti-inflammatory drugs (NSAIDs) can reduce the incidence of colon cancer by 40% to 50%; NSAIDs elicit the same preventive effects on lung cancer, oesophageal cancer and stomach cancer[24,25]. In addition, vaccination has been administered to promote an immune response of lymphocytes against tumours, thereby improving patient prognosis[26]. Indeed, patients with high preoperative NLR should be considered as high-risk patients who should be integrated with multi-mode anti-tumour therapies, such as chemotherapy, radiotherapy and immune therapy.
In summary, preoperative NLR was closely related to the prognosis of GC; in particular, a high NLR was an indicator that could be used to determine the poor prognosis of patients with GC. NLR could be determined using a simple, rapid and cost-effective detection technique; this technique could be applied efficiently to predict the prognosis of GC patients and to provide a reference for the integrated treatment of GC for broad applications.
COMMENTS
Background
Gastric cancer (GC) is one of the most common types of gastrointestinal cancers; however, the prognosis of GC is poor. The body’s inflammatory response plays an important role in tumour development. The neutrophil-to-lymphocyte ratio (NLR), which indicates the systemic inflammatory state of the body, is closely related to the prognosis of GC.
Research frontiers
NLR is closely related to the prognosis of various malignant tumours, such as liver cancer, colorectal cancer, breast cancer, bladder cancer and non-small cell lung cancer. However, few studies have investigated the relationships of NLR and prognosis of GC patients.
Innovations and breakthroughs
This study revealed that NLR was an independent risk factor that likely affected the five-year survival rate of GC patients.
Applications
A high NLR was one indicator that could be used to evaluate the poor prognosis of patients with GC. This finding suggested that NLR might provide a reference of the integrated treatment for patients with GC. NLR could be determined using a simple, rapid and cost-effective technique; thus, this technique could be used to predict the prognosis of patients with GC.
Terminology
The neutrophil-to-lymphocyte ratio, calculated as neutrophil counts divided by lymphocyte counts, is a possible marker of general immune responses to various stress stimuli.
Peer-review
This study investigated the significance of NLR retrospectively in patients who received surgical therapy to treat GC. The results are significant and applicable to clinical practices and studies.
Footnotes
Supported by Nanjing Science and Technology Project, No. 201106016.
Ethics approval: The study was reviewed and approved by the Nanjing First Hospital, Nanjing Medical University Institutional Review Board.
Informed consent: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest: The authors declare that there is no conflict of interest to disclose.
Data sharing: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: November 30, 2014
First decision: January 8, 2015
Article in press: March 19, 2015
P- Reviewer: Sumi K S- Editor: Yu J L- Editor: Stewart G E- Editor: Zhang DN
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 6.Shimada H, Nabeya Y, Okazumi S, Matsubara H, Shiratori T, Aoki T, Sugaya M, Miyazawa Y, Hayashi H, Miyazaki S, et al. Elevation of preoperative serum C-reactive protein level is related to poor prognosis in esophageal squamous cell carcinoma. J Surg Oncol. 2003;83:248–252. doi: 10.1002/jso.10275. [DOI] [PubMed] [Google Scholar]
- 7.Shimada H, Oohira G, Okazumi S, Matsubara H, Nabeya Y, Hayashi H, Takeda A, Gunji Y, Ochiai T. Thrombocytosis associated with poor prognosis in patients with esophageal carcinoma. J Am Coll Surg. 2004;198:737–741. doi: 10.1016/j.jamcollsurg.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 8.Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88:218–230. doi: 10.1016/j.critrevonc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Oh BS, Jang JW, Kwon JH, You CR, Chung KW, Kay CS, Jung HS, Lee S. Prognostic value of C-reactive protein and neutrophil-to-lymphocyte ratio in patients with hepatocellular carcinoma. BMC Cancer. 2013;13:78. doi: 10.1186/1471-2407-13-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Absenger G, Szkandera J, Pichler M, Stotz M, Arminger F, Weissmueller M, Schaberl-Moser R, Samonigg H, Stojakovic T, Gerger A. A derived neutrophil to lymphocyte ratio predicts clinical outcome in stage II and III colon cancer patients. Br J Cancer. 2013;109:395–400. doi: 10.1038/bjc.2013.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li MX, Liu XM, Zhang XF, Zhang JF, Wang WL, Zhu Y, Dong J, Cheng JW, Liu ZW, Ma L, et al. Prognostic role of neutrophil-to-lymphocyte ratio in colorectal cancer: a systematic review and meta-analysis. Int J Cancer. 2014;134:2403–2413. doi: 10.1002/ijc.28536. [DOI] [PubMed] [Google Scholar]
- 12.Azab B, Bhatt VR, Phookan J, Murukutla S, Kohn N, Terjanian T, Widmann WD. Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Ann Surg Oncol. 2012;19:217–224. doi: 10.1245/s10434-011-1814-0. [DOI] [PubMed] [Google Scholar]
- 13.Gondo T, Nakashima J, Ohno Y, Choichiro O, Horiguchi Y, Namiki K, Yoshioka K, Ohori M, Hatano T, Tachibana M. Prognostic value of neutrophil-to-lymphocyte ratio and establishment of novel preoperative risk stratification model in bladder cancer patients treated with radical cystectomy. Urology. 2012;79:1085–1091. doi: 10.1016/j.urology.2011.11.070. [DOI] [PubMed] [Google Scholar]
- 14.Tomita M, Shimizu T, Ayabe T, Nakamura K, Onitsuka T. Elevated preoperative inflammatory markers based on neutrophil-to-lymphocyte ratio and C-reactive protein predict poor survival in resected non-small cell lung cancer. Anticancer Res. 2012;32:3535–3538. [PubMed] [Google Scholar]
- 15.Shimada H, Takiguchi N, Kainuma O, Soda H, Ikeda A, Cho A, Miyazaki A, Gunji H, Yamamoto H, Nagata M. High preoperative neutrophil-lymphocyte ratio predicts poor survival in patients with gastric cancer. Gastric Cancer. 2010;13:170–176. doi: 10.1007/s10120-010-0554-3. [DOI] [PubMed] [Google Scholar]
- 16.Jung MR, Park YK, Jeong O, Seon JW, Ryu SY, Kim DY, Kim YJ. Elevated preoperative neutrophil to lymphocyte ratio predicts poor survival following resection in late stage gastric cancer. J Surg Oncol. 2011;104:504–510. doi: 10.1002/jso.21986. [DOI] [PubMed] [Google Scholar]
- 17.Hirashima M, Higuchi S, Sakamoto K, Nishiyama T, Okada H. The ratio of neutrophils to lymphocytes and the phenotypes of neutrophils in patients with early gastric cancer. J Cancer Res Clin Oncol. 1998;124:329–334. doi: 10.1007/s004320050178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077–3079. doi: 10.1245/s10434-010-1362-z. [DOI] [PubMed] [Google Scholar]
- 19.Xu AM, Huang L, Zhu L, Wei ZJ. Significance of peripheral neutrophil-lymphocyte ratio among gastric cancer patients and construction of a treatment-predictive model: a study based on 1131 cases. Am J Cancer Res. 2014;4:189–195. [PMC free article] [PubMed] [Google Scholar]
- 20.Cho IR, Park JC, Park CH, Jo JH, Lee HJ, Kim S, Shim CN, Lee H, Shin SK, Lee SK, et al. Pre-treatment neutrophil to lymphocyte ratio as a prognostic marker to predict chemotherapeutic response and survival outcomes in metastatic advanced gastric cancer. Gastric Cancer. 2014;17:703–710. doi: 10.1007/s10120-013-0330-2. [DOI] [PubMed] [Google Scholar]
- 21.Lee S, Oh SY, Kim SH, Lee JH, Kim MC, Kim KH, Kim HJ. Prognostic significance of neutrophil lymphocyte ratio and platelet lymphocyte ratio in advanced gastric cancer patients treated with FOLFOX chemotherapy. BMC Cancer. 2013;13:350. doi: 10.1186/1471-2407-13-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Carlo E, Forni G, Musiani P. Neutrophils in the antitumoral immune response. Chem Immunol Allergy. 2003;83:182–203. doi: 10.1159/000071561. [DOI] [PubMed] [Google Scholar]
- 23.McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12:223–226. doi: 10.1097/MCO.0b013e32832a7902. [DOI] [PubMed] [Google Scholar]
- 24.Baron JA, Sandler RS. Nonsteroidal anti-inflammatory drugs and cancer prevention. Annu Rev Med. 2000;51:511–523. doi: 10.1146/annurev.med.51.1.511. [DOI] [PubMed] [Google Scholar]
- 25.García-Rodríguez LA, Huerta-Alvarez C. Reduced risk of colorectal cancer among long-term users of aspirin and nonaspirin nonsteroidal antiinflammatory drugs. Epidemiology. 2001;12:88–93. doi: 10.1097/00001648-200101000-00015. [DOI] [PubMed] [Google Scholar]
- 26.Lesterhuis WJ, de Vries IJ, Schuurhuis DH, Boullart AC, Jacobs JF, de Boer AJ, Scharenborg NM, Brouwer HM, van de Rakt MW, Figdor CG, et al. Vaccination of colorectal cancer patients with CEA-loaded dendritic cells: antigen-specific T cell responses in DTH skin tests. Ann Oncol. 2006;17:974–980. doi: 10.1093/annonc/mdl072. [DOI] [PubMed] [Google Scholar]


