Abstract
Sinusoidal obstruction syndrome (SOS), previously known as hepatic veno-occlusive disease, is a rare disorder in solid organ transplant patients, and is an uncommon complication after liver transplantation. Severe SOS with hepatic failure causes considerable mortality. Tacrolimus has been reported to be an offending agent, which potentially plays a role in the pathophysiological process of SOS. SOS due to tacrolimus has been reported in lung and pancreatic transplantations, but has never been described in a liver transplant recipient. Herein, we present a case of SOS after liver transplantation, which was possibly related to tacrolimus. A 27-year-old man developed typical symptoms of SOS with painful hepatomegaly, ascites and jaundice after liver transplantation, which regressed following withdrawal of tacrolimus. By excluding other possible predisposing factors, we concluded that tacrolimus was the most likely cause of SOS.
Keywords: Liver transplantation, Sinusoidal obstruction syndrome, Veno-occlusive disease, Tacrolimus, Predisposing factor
Core tip: We describe a rare case of sinusoidal obstruction syndrome following liver transplantation, which was possibly related to tacrolimus. We believe that this condition is uncommon and has rarely been reported in liver transplant recipients.
INTRODUCTION
Sinusoidal obstruction syndrome (SOS), previously known as hepatic veno-occlusive disease, is a rare disorder with the unique etiopathogenesis of toxic injury to hepatic sinusoids, which induces progressive fibrotic obliteration of centrilobular veins. Painful hepatomegaly, ascites and jaundice are typical symptoms of SOS[1-3]. In general, SOS is a difficult condition, in which 16%-50% of patients are likely to develop irreversible illness, and have a fatal outcome due to hepatic failure. Severe SOS causes mortality in approximately 84%-90% of the patients[2,4].
SOS can occur in post-transplant patients, and the majority of research has been carried out in post-hematopoietic stem cell transplantation (HSCT) patients related to preconditioning treatment[1,2,4]. A limited number of cases of SOS have been reported after renal, lung, pancreatic and liver transplantations[5-8]. In liver transplantation, SOS is unusual, and azathioprine therapy or acute rejection is considered the most common etiology[8-10]. Tacrolimus may be another possible and rare pathogenic agent as it has potential cytotoxicity to endothelial cells and precipitates their dysregulation[11]. To the best of our knowledge, SOS due to tacrolimus has been reported in lung and pancreatic transplantations, but has never been described after liver transplantation[6,7]. Herein, we present a case of SOS following liver transplantation, who achieved complete clinical remission after discontinuation of tacrolimus.
CASE REPORT
A 27-year-old man underwent an ABO-identical liver transplantation for acute hepatic failure due to hepatitis B. The graft was obtained from a donor after cardiac death with a warm ischemia time of 5 min and cold ischemia time of 8 h. No specific pathology was observed on biopsy of the donated graft at the time of transplantation (Figure 1A). Operation time was 6 h with satisfactory reconstruction of vessels and biliary duct. Early post-operative recovery period was uneventful. Piperacillin-tazobactam, fluconazole and ganciclovir were administered as prophylaxis against infection. Entecavir and hepatitis B immunoglobulin were administered as prophylaxis against hepatitis B virus recurrence. A routine immunosuppressive regimen consisting of tapering prednisone, tacrolimus and mycophenolate mofetil was applied. Remission of hepatic function and coagulation was achieved one week after transplantation. The patient was discharged on normal graft function with excellent flow in the hepatic veins, portal vein and hepatic artery on day 20 (Figure 2A and B).
Figure 1.
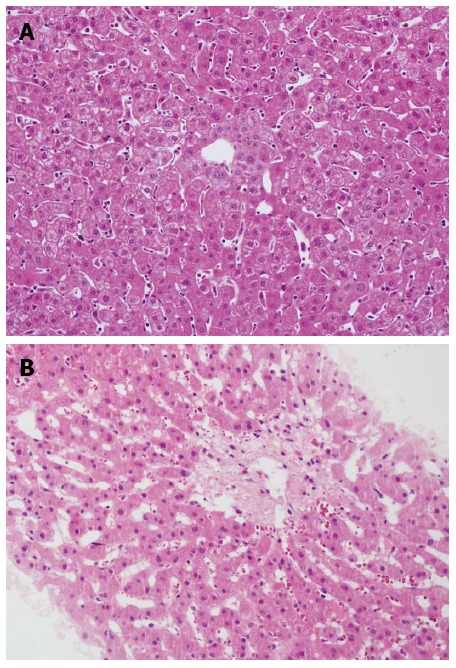
Biopsy of the donated graft at the time of transplantation was normal (HE, × 200) (A) and liver biopsy showed sinusoidal congestion and fibrosis of centrilobular veins at 88 d after transplantation (HE, × 200) (B).
Figure 2.
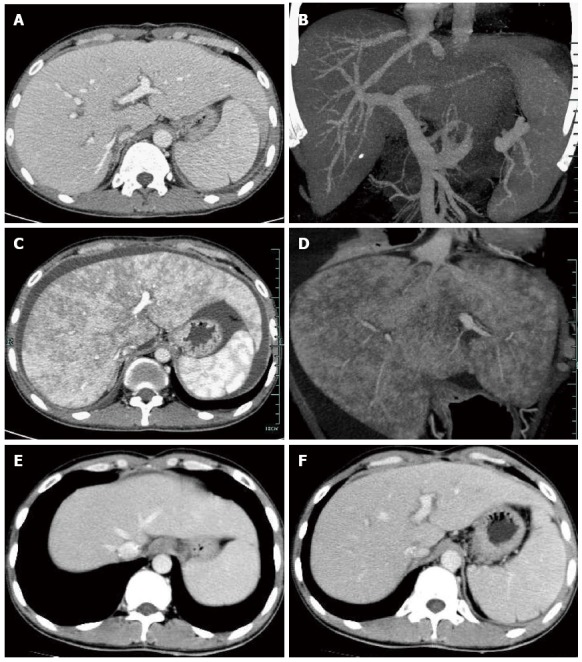
Computed tomography revealed excellent reconstructed blood flow in the hepatic veins, portal vein and hepatic artery at 14 d after transplantation (A, B), enlarged liver with patchy enhancement, obscure hepatic veins and massive ascites at 80 d (C, D), and normal radiologic presentations with resolved ascites and patchy enhancement, and recovered hepatic vein flow at 120 d (E, F).
The patient remained stable on tacrolimus (trough level 7-10 ng/mL), mycophenolate mofetil and entecavir for two months, but was hospitalized on day 80 due to anorexia, abdominal pain and polypnea. No natural remedies before the current admission were used. Physical examination revealed palpable liver 3 cm below the ribcage and positive shifting dullness. Serological tests, including liver function, renal function, routine blood examination, coagulation and tacrolimus concentration (trough level of tacrolimus was 8.9 ng/mL), were normal. Serological markers of viral infection such as hepatitis A, B, C, D and E, cytomegalovirus and Epstein-Barr virus were negative. Ultrasonography demonstrated ascites and enlarged liver with regular blood flow. Computed tomography (CT) showed enlarged liver with patchy enhancement, thin hepatic veins and massive ascites (Figure 2C and D). Initial medical treatment with a diuretic was ineffective. The patient’s condition continued to deteriorate, accompanied by weight gain of 3 kg above his baseline, obvious abdominal distention and high bilirubin (57 μmol/L on day 87). Faint yellow ascites and pleural effusion were drained to alleviate the symptoms. A percutaneous liver biopsy was obtained under ultrasound guidance after complete drainage of ascites on day 88, which showed the pathological findings of sinusoidal congestion and fibrosis of centrilobular veins (Figure 1B). Moreover, no evidence of acute rejection or viral hepatitis was found on pathology. All the findings supported the diagnosis of SOS, and excluded the possibility of chylous fistula, obstruction of outflow, hepatitis recurrence or rejection. He was started on a regimen of defibrotide for 2 wk, which was then stopped due to lack of efficacy, with increased bilirubin (76 μmol/L on day 104), rapidly expanding ascites and obvious weight gain. Tacrolimus concentration was controlled at 5-7 ng/mL and was subsequently discontinued as it was considered the only offending drug and was replaced by cyclosporine A at a concentration of 150 ng/mL. Complete resolution of clinical manifestations such as ascites and graft dysfunction was observed, with normalized radiologic presentations (Figure 2E and F) during the 2-wk period after tacrolimus discontinuation. The patient has remained asymptomatic on cyclosporine A, mycophenolate mofetil and entecavir for 6 mo. The clinical data including graft function, coagulation, immunosuppressant level and body weight are presented in Table 1.
Table 1.
Clinical characteristics during disease course
| Days after LT | 50 | 80 | 87 | 104 | 111 | 118 |
| Clinical course | Current admission | Before defibrotide | After defibrotide/TAC withdrawal | Resolution | ||
| ALT (U/L) | 17 | 35 | 39 | 43 | 26 | 23 |
| TB (μmol/L) | 16 | 20 | 57 | 76 | 42 | 13 |
| Platelet (109/mL) | 156 | 171 | 183 | 175 | 158 | 173 |
| PT (s) | 11.5 | 11.3 | 12.5 | 12.7 | 11.5 | 12.2 |
| D-dimer (mg/L) | 0.27 | 0.34 | 0.52 | 0.41 | 0.32 | 0.33 |
| TAC (ng/mL) | 7.9 | 8.9 | 7.2 | 5.6 | ||
| CSA (ng/mL) | 126 | 165 | ||||
| Wt (kg) | 64.5 | 66.0 | 67.9 | 69.5 | 67.5 | 64.8 |
LT: Liver transplantation; ALT: Alanine aminotransferase; TB: Total bilirubin; PT: Prothrombin time; TAC: Tacrolimus; CSA: Cyclosporine A; Wt: Body weight.
DISCUSSION
Certain toxic drugs containing pyrrolizidine alkaloids, chemo-irradiation preconditioning regimens in stem cell transplantation and immunosuppressive therapy with azathioprine are common predisposing factors for SOS[4,5,12,13].
SOS is a rare finding after liver transplantation, but can be an infrequent cause of graft dysfunction. According to a review article, approximately 1.9% of cadaveric liver transplant recipients suffered from SOS after transplantation[10]. Another investigation of 1346 biopsy samples obtained after liver transplantation showed an SOS incidence of 2.3%[8]. Clinical manifestations such as body weight gain, ascites, hepatomegaly and jaundice are recognized as diagnostic clues for SOS[2]. It is important that common post-operative complications such as acute rejection, obstruction of outflow (anastomotic stenosis or twisting of the hepatic vein), biliary complications, and viral hepatitis should be excluded in post-liver transplant individuals. Unique pathologic findings characterized by fibrosis and obliteration of hepatic centrilobular veins, hemorrhagic centrilobular necrosis, and sinusoidal congestion are suggestive of the diagnosis of SOS, which ultimately result in outflow obstruction, portal hypertension and hepatic injury[1]. Enlarged liver with patchy enhancement, ascites, usually accompanied by pleural effusion, and obscure main hepatic veins due to congestive liver, are the most typical presentations on CT or magnetic resonance imaging. In particular, the area of the liver where veno-occlusion occurs may show relatively lower enhancement and form a patchy enhancement sign, which is rare in other liver disorders. Therefore, it is the most valuable radiologic feature in diagnosing SOS, and the grade of patchy enhancement is also associated with clinical severity[12]. Although the mortality rate for SOS is unclear, the deteriorating manifestations of ascites and graft failure could be a threat to survival, making re-transplantation inevitable[8].
Azathioprine was implicated as the main predisposing factor for onset of SOS in post-liver transplant patients when it was widely used in the last century, due to its vascular hepatotoxicity[9]. In addition, an immunological reaction has been proven to participate in the pathophysiological process of SOS after liver transplantation, and it is suggested that SOS is part of the presentation of rejection with endothelial predilection[8,10]. Therefore, the disease could be reversed by intensive immunosuppressive treatment with corticosteroids or intervention for antibody-mediated rejection in liver transplant recipients[14]. However, the exact causative factor is still undetermined[8]. It should be emphasized that the determination of SOS etiology is important as withdrawal of the offending drug plays a key role in the treatment of SOS following liver transplantation.
Tacrolimus, one of the most widely used calcineurin inhibitors, is safe and efficient in the prophylaxis and treatment of acute rejection in organ transplantation. However, as tacrolimus has potential cytotoxicity to endothelial cells and precipitates their dysregulation, it is suspected to contribute to the onset of SOS in some cases, although the pathogenic mechanism remains to be elucidated[11].
According to the published literature, there have only been two cases of SOS described after lung and pancreatic transplantation, which were clinically proven to be induced by tacrolimus[6,7]. The present case is the first to show a definite association between tacrolimus and SOS after liver transplantation. Our patient developed refractory ascites which was the most obvious complaint other than slightly increased jaundice. This probably indicated that sinusoidal injury had a significant effect on hepatic outflow and contributed to portal hypertension, but had a mild influence on hepatic metabolism of bilirubin in this case. Although recommended in reported guidelines[2], defibrotide therapy for this patient lacked efficacy. At the onset of the disease, it was difficult to confirm the predisposing factor. Considering that the patient had never been exposed to azathioprine or other specific suspicious drugs, with the exception of tacrolimus, and there was no evidence of acute rejection, tacrolimus was considered the offending drug, and was therefore discontinued. Clinical and radiological regression was observed following withdrawal of tacrolimus.
In conclusion, we describe a patient with SOS following liver transplantation. The possible causative drug was tacrolimus as complete recovery was observed after its withdrawal. Transplant surgeons should be aware of this rare condition following liver transplantation, and tacrolimus should be considered as a possible causative agent.
COMMENTS
Case characteristics
A 27-year-old man presented with anorexia, abdominal pain, jaundice and body weight gain after liver transplantation.
Clinical diagnosis
Sinusoidal obstruction syndrome which led to ascites, hepatomegaly and graft dysfunction.
Differential diagnosis
Acute rejection, hepatitis B recurrence, anastomotic stenosis of the hepatic vein.
Laboratory diagnosis
Alanine aminotransferase (43 U/L), total bilirubin (76 μmol/L), tacrolimus (5.6 ng/mL), platelet count (175 × 109/mL), D-dimer (0.41 mg/L), hepatitis B surface antigen, hepatitis B virus - DNA, anti- hepatitis A virus IgM/IgG, hepatitis C virus - RNA, hepatitis D virus - RNA, anti- hepatitis E virus IgM/IgG, cytomegalovirus pp65 and epstein-Barr virus - DNA were all negative.
Imaging diagnosis
Computed tomography showed enlarged liver with patchy enhancement, thin hepatic veins and massive ascites.
Pathological diagnosis
Biopsy of the graft revealed the pathological findings of sinusoidal congestion and fibrosis of centrilobular veins.
Treatment
Tacrolimus was discontinued as the only suspicious offending drug and replaced by cyclosporine A.
Related reports
Sinusoidal obstruction syndrome (SOS) due to tacrolimus has been reported in lung and pancreatic transplantations, but has never been described after liver transplantation.
Term explanation
SOS is an unusual clinical syndrome characterized by hepatomegaly, ascites, and jaundice, with the unique pathological findings of fibrosis and obliteration of hepatic centrilobular veins, hemorrhagic centrilobular necrosis, and sinusoidal congestion, due to injury of sinusoidal endothelial cells.
Experiences and lessons
Transplant surgeons should be aware of this rare condition following liver transplantation, and tacrolimus should be considered as a possible causative agent.
Peer-review
The authors report a case of patient with sinusoidal obstruction syndrome following liver transplantation due to hepatitis B-related acute hepatic failure. It is the first reported case of tacrolimus-related sinusoidal obstruction syndrome in liver transplant recipient. The paper is well structured and except some minor language issues well written.
Footnotes
Supported by National Natural Science Foundation of China, No. 81373160.
Ethics approval: The study was reviewed and approved by the Zhejiang University Institutional Review Board.
Informed consent: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest: The authors declare that there is no conflict of interest in this study.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: January 20, 2015
First decision: February 10, 2015
Article in press: April 9, 2015
P- Reviewer: Hassan Z S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
References
- 1.Helmy A. Review article: updates in the pathogenesis and therapy of hepatic sinusoidal obstruction syndrome. Aliment Pharmacol Ther. 2006;23:11–25. doi: 10.1111/j.1365-2036.2006.02742.x. [DOI] [PubMed] [Google Scholar]
- 2.Dignan FL, Wynn RF, Hadzic N, Karani J, Quaglia A, Pagliuca A, Veys P, Potter MN. BCSH/BSBMT guideline: diagnosis and management of veno-occlusive disease (sinusoidal obstruction syndrome) following haematopoietic stem cell transplantation. Br J Haematol. 2013;163:444–457. doi: 10.1111/bjh.12558. [DOI] [PubMed] [Google Scholar]
- 3.Senzolo M, Germani G, Cholongitas E, Burra P, Burroughs AK. Veno occlusive disease: update on clinical management. World J Gastroenterol. 2007;13:3918–3924. doi: 10.3748/wjg.v13.i29.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsirigotis PD, Resnick IB, Avni B, Grisariu S, Stepensky P, Or R, Shapira MY. Incidence and risk factors for moderate-to-severe veno-occlusive disease of the liver after allogeneic stem cell transplantation using a reduced intensity conditioning regimen. Bone Marrow Transplant. 2014;49:1389–1392. doi: 10.1038/bmt.2014.168. [DOI] [PubMed] [Google Scholar]
- 5.Azoulay D, Castaing D, Lemoine A, Samuel D, Majno P, Reynes M, Charpentier B, Bismuth H. Successful treatment of severe azathioprine-induced hepatic veno-occlusive disease in a kidney-transplanted patient with transjugular intrahepatic portosystemic shunt. Clin Nephrol. 1998;50:118–122. [PubMed] [Google Scholar]
- 6.Shah S, Budev M, Blazey H, Fairbanks K, Mehta A. Hepatic veno-occlusive disease due to tacrolimus in a single-lung transplant patient. Eur Respir J. 2006;27:1066–1068. doi: 10.1183/09031936.06.00048505. [DOI] [PubMed] [Google Scholar]
- 7.Wang SE, Shyr YM, Lee RC. Hepatic veno-occlusive disease related to tacrolimus after pancreas transplantation. J Chin Med Assoc. 2013;76:358–360. doi: 10.1016/j.jcma.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Sebagh M, Azoulay D, Roche B, Hoti E, Karam V, Teicher E, Bonhomme-Faivre L, Saliba F, Duclos-Vallée JC, Samuel D. Significance of isolated hepatic veno-occlusive disease/sinusoidal obstruction syndrome after liver transplantation. Liver Transpl. 2011;17:798–808. doi: 10.1002/lt.22282. [DOI] [PubMed] [Google Scholar]
- 9.Mion F, Cloix P, Boillot O, Gille D, Bouvier R, Paliard P, Berger F. [Veno-occlusive disease after liver transplantation. Association of acute cellular rejection and toxicity of azathioprine] Gastroenterol Clin Biol. 1993;17:863–867. [PubMed] [Google Scholar]
- 10.Sebagh M, Debette M, Samuel D, Emile JF, Falissard B, Cailliez V, Shouval D, Bismuth H, Reynès M. “Silent” presentation of veno-occlusive disease after liver transplantation as part of the process of cellular rejection with endothelial predilection. Hepatology. 1999;30:1144–1150. doi: 10.1002/hep.510300514. [DOI] [PubMed] [Google Scholar]
- 11.Ikezoe T, Yang J, Nishioka C, Honda G, Furihata M, Yokoyama A. Thrombomodulin protects endothelial cells from a calcineurin inhibitor-induced cytotoxicity by upregulation of extracellular signal-regulated kinase/myeloid leukemia cell-1 signaling. Arterioscler Thromb Vasc Biol. 2012;32:2259–2270. doi: 10.1161/ATVBAHA.112.251157. [DOI] [PubMed] [Google Scholar]
- 12.Wu XW, Wang WQ, Liu B, Xu JM, Yu YQ, Zhang S, Shen Y. Hepatic veno-occlusive disease after taking Gynura Rhizome: The value of multidetector computed tomography in diagnosing the disease and evaluating the clinical therapeutic effect. Hepatol Res. 2012;42:304–309. doi: 10.1111/j.1872-034X.2011.00918.x. [DOI] [PubMed] [Google Scholar]
- 13.Praprotnik S, Hocevar A, Ferlan-Marolt V, Tomsic M. Azathioprine induced hepatic veno-occlusive disease in systemic lupus erythematosus. Lupus. 2005;14:493–494. doi: 10.1191/0961203305lu2143xx. [DOI] [PubMed] [Google Scholar]
- 14.Yamada N, Urahashi T, Ihara Y, Sanada Y, Wakiya T, Okada N, Mizuta K. Veno-occlusive disease/sinusoidal obstruction syndrome associated with potential antibody-mediated rejection after pediatric living donor liver transplantation: a case report. Transplant Proc. 2012;44:810–813. doi: 10.1016/j.transproceed.2012.01.008. [DOI] [PubMed] [Google Scholar]


