Abstract
This study was carried out to investigate whether the pulmonary C-fiber hypersensitivity induced by hyperthermia is altered by prostaglandin E2 (PGE2). Single-unit afferent activities of pulmonary C-fibers were recorded in anesthetized, artificially ventilated rats when the intrathoracic temperature (Tit) was maintained at normal (N; ~36°C) and hyperthermia levels (H; ~41°C) by perfusion of heated saline into the thoracic chamber for 3 min. After ~20 min of recovery, the fiber activities were recorded again during infusion of PGE2 at both N and H levels of Tit. Our study showed: 1) The baseline fiber activity and responses to lung inflation, right atrial injection of capsaicin and adenosine were all increased by increasing Tit from N to H, and these hyperthermia-induced increases in sensitivities were also significantly augmented by PGE2. 2) These enhanced sensitivities induced by PGE2 were abolished by pretreatment with AH6809 and AH23848, selective antagonists of EP2 and EP4 prostanoid receptors, respectively. In conclusion, the hyperthermia-induced hypersensitivity of vagal pulmonary C-fibers is potentiated by PGE2, and this effect is mediated through activation of EP2 and EP4 prostanoid receptors.
Keywords: Prostaglandin E2, Hyperthermia, Pulmonary C fibers, Airway inflammation
1. Introduction
Tissue inflammation is known to lead to local hyperemia and an increase in temperature in the inflamed area (Gourine et al., 2001; Planas et al., 1995). Indeed, a higher tissue temperature in the airways of asthmatic patients has been recently reported (Paredi et al., 2002). A recent study in our laboratory has demonstrated that an increase in the intrathoracic temperature to 41°C, a body temperature frequently found during strenuous exercise or in patients with severe fever, elevated the baseline activity of vagal pulmonary C-fiber endings and the sensitivities of these afferents to chemical stimulants and to lung inflation in anesthetized rats (Ruan et al., 2005). These nonmyelinated pulmonary afferents are known to play an important role in protecting the lung under normal and pathophysiological conditions. Stimulation of these afferents can elicit extensive centrally-mediated reflex responses, including apnea, rapid shallow breathing, hypotension and bradycardia, bronchoconstriction, hypersecretion of mucus and cough (Coleridge and Coleridge, 1984; Lee and Pisarri, 2001).
Prostaglandin E2 (PGE2), an arachidonic acid cyclooxygenase metabolite, is locally released in the lung tissues during various airway inflammatory reactions (Holtzman, 1991) and has potent effects on the functions of a number of cells in the respiratory tract (Eglin and Whiting, 1988; Narumiya et al., 1999; Talpain et al., 1995). PGE2 is known to induce airway hypersensitivity; for example, inhalation of aerosolized PGE2 elicits cough and retrosternal soreness (Costello et al., 1985), and enhances the sensitivity of the cough reflex response to inhaled capsaicin in humans (Chaudry et al., 1989). Indeed, an earlier study in our lab has shown that PGE2 can elevate the sensitivity of pulmonary C-fiber afferents (Ho et al., 2000). Studies in an isolated pulmonary nodose/jugular neuron preparation further revealed that the sensitizing effect involves the intracellular cAMP/PKA signaling pathway, which is presumably triggered by activation of EP2 and EP4 prostanoid receptors expressed in these neurons (Kwong and Lee, 2002; Gu et al., 2003).
In view of the fact that both tissue hyperthermia and local release of PGE2 can occur concurrently during airway inflammation, this study was aimed to investigate: 1) if there is an interaction between these two factors in modulating the activity and sensitivity of pulmonary C-fiber afferents; 2) if so, whether the effect of PGE2 is mediated through activation of EP2 and EP4 prostanoid receptors.
2. Methods
The procedures described below were performed in accordance with the recommendations in the Guide for the Care and Use of Laboratory, published by the National Institutes of Health, USA, and were also approved by the University of Kentucky Institutional Animal Care and Use Committee.
2.1 Animal preparation
Male Sprague-Dawley rats (320-460 g, n = 36) were initially anesthetized with intraperitoneal injection of α-chloralose (100 mg/kg) and urethane (500 mg/kg) dissolved in a 2% borax solution; smaller (~1/10 of the initial dose) supplemental doses of the same anesthetics were given intravenously, whenever necessary, to maintain abolition of pain reflex elicited by paw pinch. Right femoral artery and vein were cannulated for recording arterial blood pressure (ABP) and for administration of PGE2, respectively. The left jugular vein was cannulated with the tip of the catheter advanced to slightly above the right atrium for bolus injections of chemical stimulants. The trachea was cannulated, and tracheal pressure (Pt) was measured (MP 45-28; Validyne, Northridge, CA, USA) via a side port of the tracheal cannula. The rats were artificially ventilated with a respirator (model 7025; UGO Basile, Comerio-Varese, Italy); tidal volume (VT) and respiratory frequency were set at 8 to 10 ml/kg and 50 breaths/min, respectively. Body temperature was maintained at ~36°C throughout the experiment by a heating pad placed under the animal lying in a supine position. Animals were killed at the end of the experiments by an intravenous injection of potassium chloride (200 mg/kg).
2.2 Isolated perfused thoracic chamber
To elevate and maintain the intrathoracic temperature (Tit) at a constant level, a thoracic chamber was prepared and perfused by isotonic saline that was kept at a constant temperature in a water bath (model 281; Precision, Winchester, VA, USA). After a midline thoracotomy, the inlet of the perfusion circuit, the tip of a PE-190 catheter, was sutured to the interior dorsal wall (bottom) of the thoracic cage that was then partially closed by sutures to form a chamber; the tip of the outlet catheter was positioned at the opening (top) of the thoracic chamber and connected to a suction pump. The perfusion was driven by the peristaltic pumps (model 3389; Control Company, Friendswood, TX, USA) and maintained at a rate of ~150 ml/min. A miniature temperature probe (BS4 52-1732; Physitemp Instruments Inc., Clifton, NJ, USA) was sutured to the interior wall of the thoracic cage to measure the Tit; another probe (BS4 52-1583, Physitemp Instrument Inc.) was inserted in the rectum to monitor animal's core temperature. The expiratory outlet of the respirator was placed under 3 cm H2O pressure to maintain a near-normal functional residual capacity.
2.3 Recording of single-unit pulmonary C-fiber activity
The conventional method for identifying and recording single-unit pulmonary C-fiber activity, as previously described (Ho et al., 2001), was used in this study. Briefly, the right cervical vagus nerve was separated from the carotid artery and sectioned rostrally. The caudal end of the cut vagus nerve was placed on a small dissection platform and desheathed; a thin filament was teased away from the nerve trunk and placed on a platinum-iridium hook electrode. Action potentials were amplified (P511K; Grass Instruments Co., Quincy, MA, USA), monitored by an audio monitor (AM8RS; Grass Instruments Co., Quincy, MA, USA), and displayed on an oscilloscope (model 2211; Tektronix Inc., Wilsonville, OR, USA). The thin filament was further split until the afferent activity from a single unit was electrically isolated. Both vagi were ligated just above the diaphragm to eliminate the electrical signals arising from abdominal visceras. The afferent activity of a single unit was first searched for by hyperinflation (3-4 × VT), and then identified by the immediate (delay < 1 sec) response to bolus injection of capsaicin (0.5-1.0 μg/kg) into the right atrium. Finally, the general locations of pulmonary C fibers were identified by their responses to the gentle pressing of the lungs with a blunt-ended glass rod. The signals of the afferent activities, Pt (MP 45-28; Validyne Engineering, Northridge, CA, USA) and ABP (P23AA; Statham, Spectramed, CA, USA) were recorded on a Gould Thermal Writer (TW11; Gould Instrument Systems Inc., Valley View, OH, USA) and on a videocassette recorder (SLVN900; Sony Electronics, Inc., Park Ridge, NJ, USA). Fiber activity (FA) was sampled at 3,000 Hz and analyzed continuously by an on-line computer (TS-100; Biocybernetics, Taipei, Taiwan) for each 0.5-sec interval.
2.4 Experimental protocols
Two series of experiments were carried out. Study Series 1 was designed to determine whether intravenous infusion of PGE2 altered the potentiating effects of hyperthermia on the baseline activity and sensitivities to lung inflation and chemical stimulation in pulmonary C fibers. The baseline activity and sensitivities were determined in each fiber when Tit was maintained at two different levels: normal (N; ~36°C) and hyperthermia (H; ~41°C), each for 3 min; the N temperature was chosen because the normal body (core) temperature of the rats during sleep is ~36°C (Briese, 1998). Tit reached and remained at a steady state of 41°C in < 30 sec after the onset of perfusion, and returned rapidly (< 30 sec) to ~36°C upon perfusion with saline at body temperature. At least 20 min were allowed to elapse between temperature changes for a complete recovery. To test the fiber sensitivity to mechanical stimulation, lung inflation was applied by maintaining a constant Pt (15 or 30 cmH2O) for 10 sec. To test the fiber response to chemical stimulation, solution (volume: 0.15 ml) of capsaicin (0.5-1 μg/kg) or adenosine (170 μg/kg) was first slowly injected into the catheter (dead space: ~0.2 ml) and then flushed into the right atrium as a bolus with saline (0.4 ml). In each fiber, these responses were determined both at control and during intravenous infusion of PGE2 (3 μg/kg/min, 3 min), and then compared. Study Series 2 was carried out to investigate the possible role of EP2 and EP4 prostanoid receptors after the results of Study Series 1 indicated that the potentiating effects of hyperthermia were enhanced by PGE2. AH6809 (6-Isopropoxy-9-oxoxanthene-2-carboxylic acid) (50 μg/kg) and AH23848 (4Z-7-[(rel-1S,2S,5R)-5-((1,1′-Biphenyl-4-yl)methoxy)-2-(4-morpholinyl)-3-oxocyclopentyl]-4-heptenoic acid hemicalcium salt) (50 μg/kg), selective antagonists of EP2 and EP4 prostanoid receptors respectively (Abramovitz et al., 2000; Norel et al., 1999), were administered in combination 5 min before the PGE2 infusion, and the responses were then compared between PGE2 alone and PGE2 after pretreatment with the EP2 and EP4 antagonists. In a separate group of pulmonary C fibers, the same protocol as described above was carried out, except that AH6809 and AH23848 were replaced by their vehicles (DMSO; 0.3 ml, ~2.7% V/V in saline).
2.5 Materials
A mixture of 2% α-chloralose and 10% urethane was dissolved in a 2% borax solution. Capsaicin was dissolved in a stock solution at 250 μg/ml in a vehicle of 10% Tween 80, 10% ethanol and 80% isotonic saline. PGE2 (Sigma Chemicals; St. Louis, MO, USA) was dissolved in ethanol (2.5 mg/ml) and stored at −80°C. Adenosine was dissolved in saline (10 mg/ml) and stored at −20°C. AH6809 and AH23848 were purchased from Sigma (Sigma Chemicals; St. Louis, MO, USA) and dissolved in dimethyl sulfoxide (DMSO); these stock solutions (5 mg/ml) were divided into small aliquots and kept at −20°C. The solutions of these chemicals at desired concentrations were prepared daily by dilution with saline.
2.6 Statistical analysis
The change in FA (ΔFA) in response to a stimulus was calculated as the difference between the peak FA and the baseline FA (60-sec average) in each fiber. The peak response of FA was averaged over 2-sec intervals after the injection of capsaicin and 10-sec intervals after the injection of adenosine because the fiber discharge evoked by the latter lasted for a substantially longer duration. In the response to lung inflation, FA was averaged over the 10-sec duration of inflation. Data were then analyzed with a two-way repeated-measure ANOVA, unless mentioned otherwise. When the ANOVA showed a significant interaction, pair-wise comparisons were made with a post hoc analysis (Fisher's least significant difference). A P value < 0.05 was considered significant. Data are reported as mean ± S.E.M.
3. Results
When Tit was increased from N (average: 36.1°C) to H (average: 41.1°C), there was a small but significant increase (Δ = ~1.0°C) in the rectal temperature (Tr) toward the end of the 3-min period when the thoracic chamber was perfused with heated isotonic saline (P < 0.05; Table 1). During PGE2 infusion, there was an additional, very small but consistent increase in Tr (Δ = ~0.2°C). Both of these increases in Tr were reversible during recovery. During PGE2 infusion, mean arterial blood pressure (MABP) decreased significantly from 92.9 ± 3.6 mmHg at N to 80.2 ± 3.1 mmHg at H (n = 21, P < 0.05); in contrast, without PGE2 infusion (during control and recovery), there was no difference in MABP between N and H (Table 1). Heart rate increased significantly when Tit was raised from N to H, both with and without PGE2 infusion (P < 0.05), but the increase was more pronounced during PGE2 infusion (Table 1).
Table 1.
Systemic effects of hyperthermia and PGE2 infusion in anesthetized, open-chest and artificially ventilated rats.
| Tit (°C) | Tr (°C) | MABP (mmHg) | HR (beats/min) | ||
|---|---|---|---|---|---|
| Control | Normal | 36.1±0.0 | 35.5 ±0.1 | 94.5±2.8 | 368.5±7.3 |
| Hyperthermia | 41.0±0.1 | 36.6±0.2*† | 90.7±3.0 | 419.2±9.2* | |
| PGE2 infusion | Normal | 36.1±0.0 | 35.9±0.1 | 92.6±2.8 | 373.2±5.9 |
| Hyperthermia | 41.1±0.0 | 36.9±0.2*† | 80.1±2.8*† | 432.0±5.7*† | |
| Recovery | Normal | 36.2±0.0 | 36.0±0.1 | 100.4±2.1 | 380.6±7.7 |
| Hyperthermia | 41.2±0.0 | 36.9±0.2* | 99.3±2.5 | 415.4±8.4* |
Tit, intrathoracic temperature; Tr, rectal temperature; MABP, mean arterial blood pressure; HR, heart rate. Each level of Tit was maintained for 3 min, and at least 20 min elapsed between two experimental conditions for recovery. All variables were averaged over the last 30 sec under each condition.
significantly different (P < 0.05) from the corresponding data at normal Tit
significantly different (P ± 0.05) from the corresponding data at control (before PGE2 infusion). Data (mean ± S.E.M) are obtained from 23 rats (n=21 for Tr data) in Study Series 1.
Study Series 1
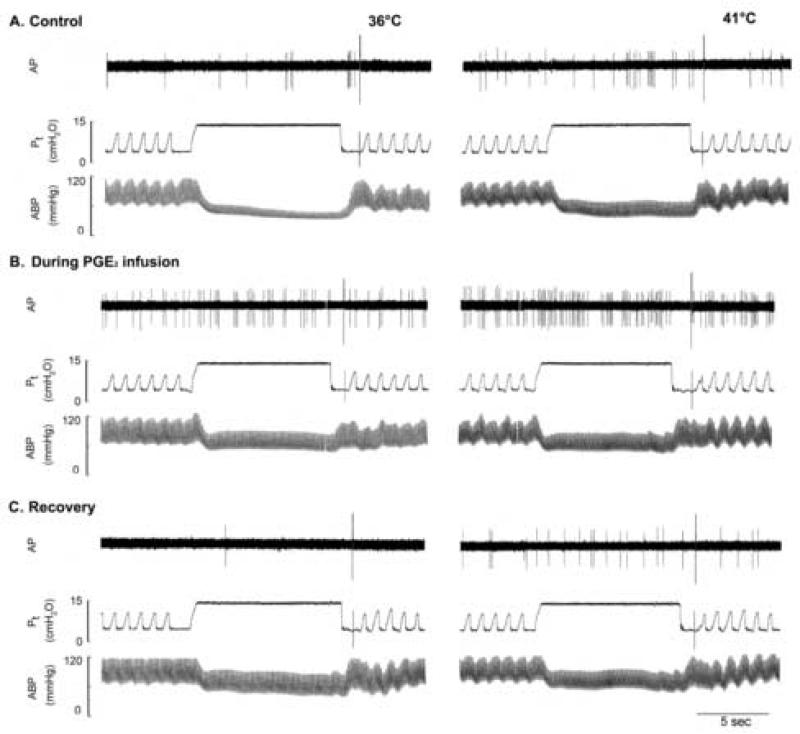
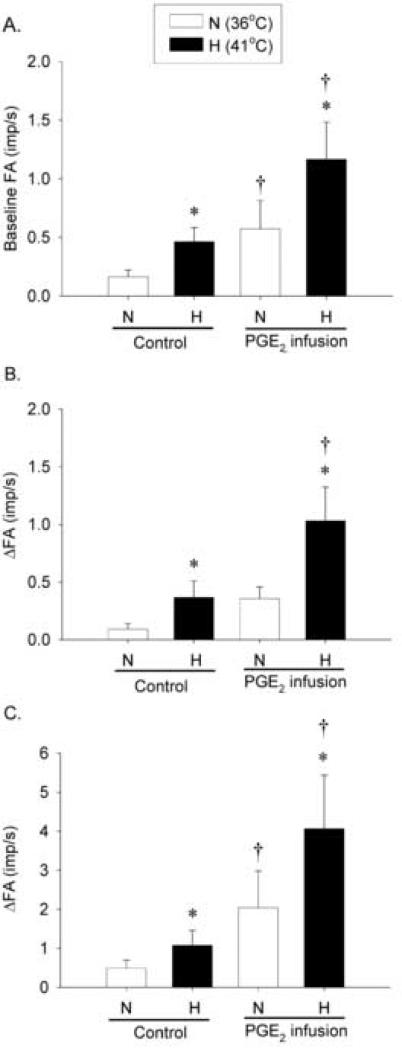
Similar to that reported previously (Ho et al., 2001), pulmonary C-fiber afferents usually have no or low baseline activity at control (e.g., Fig. 1). During PGE2 infusion, baseline FA was significantly higher than those at control at both N and H levels of Tit: at control (without PGE2), baseline FA were 0.16 ± 0.06 impulses/sec (imp/s) and 0.46 ± 0.12 imp/s at N and H, respectively; during PGE2 infusion, baseline FA increased to 0.57 ± 0.24 imp/s (n = 23, P < 0.05) and 1.20 ± 0.32 imp/s (n = 23, P < 0.05) at N and H, respectively (Fig. 1 and 2A). Thus, the hyperthermia-induced increase in baseline FA (Δ = 0.30 ± 0.09 imp/s at control, n = 23) was significantly augmented by PGE2 (Δ = 0.59 ± 0.15 imp/s during PGE2 infusion; n = 23, P < 0.05), suggesting a synergistic effect between PGE2 and hyperthermia. The hyperthermia-induced increase in baseline FA returned to control in 18 fibers tested 15-20 min after termination of the PGE2 infusion (e.g., Fig. 1).
Fig. 1. Experimental records illustrating the effects of PGE2 on the pulmonary C fiber responses to lung inflation in an anaesthetized, vagotomized and open-chest rat.
Left panel, responses to lung inflation (Pt =15 cmH2O for 10 sec) at Tit of 36°C; right panel, responses to lung inflation at Tit of 41°C. The responses to lung inflation were tested at 2nd minute during the 3-min constant perfusion of thoracic chamber with isotonic saline held at a constant temperature. A, B and C: before (control), during and 20 min after the intravenous PGE2 infusion (3 μg/kg/min for 3 min), respectively; 20 min were allowed to elapse between tests for a complete recovery. AP, action potential; Pt, tracheal pressure; ABP, arterial blood pressure. Receptor location, right lower lobe; rat body weight, 390 g. The large spike at the end of lung inflation in each panel was electronic noise generated by turning the respirator.
Fig. 2. Effects of PGE2 on the baseline activity and responses of pulmonary C fiber to lung inflation during hyperthermia.
A, average baseline FA (n = 23); B and C, average responses to lung inflation at Pt of 15 cmH2O and 30 cmH2O (n = 12), respectively (n = 12). Responses were tested at 2 different levels of Tit (N: 36°C, open bars; H: 41°C, closed bars) with or without PGE2 infusion (3 μg/kg/min for 3 min). Each level of Tit was maintained for 3 min, and 20 min were allowed to elapse between tests in each fiber. Baseline FA was averaged over 60 sec; ΔFA represents the difference between the peak FA (average over 10-sec interval for lung inflation) and the average baseline FA in each fiber. *, significantly different (P < 0.05) from the corresponding data at normal Tit (36°C); †, significantly different (P < 0.05) from the corresponding data at control (before PGE2 infusion). Data are mean ± S.E.M.
The fiber responses to lung inflations at Pt of both 15 and 30 cmH2O were elevated when Tit was raised to H level (Fig. 1, 2B and 2C). At control, the increases of FA in response to lung inflation at Pt of 30 cmH2O were 0.49 ± 0.21 imp/s and 1.07 ± 0.38 imp/s at N and H, respectively. In comparison, during PGE2 infusion, ΔFA increased to 2.04 ± 0.94 imp/s and 4.06 ± 1.37 imp/s at N and H, respectively (Fig. 2C). Thus, the hyperthermia-induced increase in ΔFA was significantly elevated by PGE2 (n = 12, P < 0.05). A similar potentiating effect of PGE2 was also found in the same fibers in their response to lung inflation at Pt of 15 cmH2O (Fig. 2B). The hyperthermia-induced increase in FA response to lung inflation returned to control when it was tested again in 9 fibers 15-20 min after termination of the PGE2 infusion (e.g., Fig. 1).
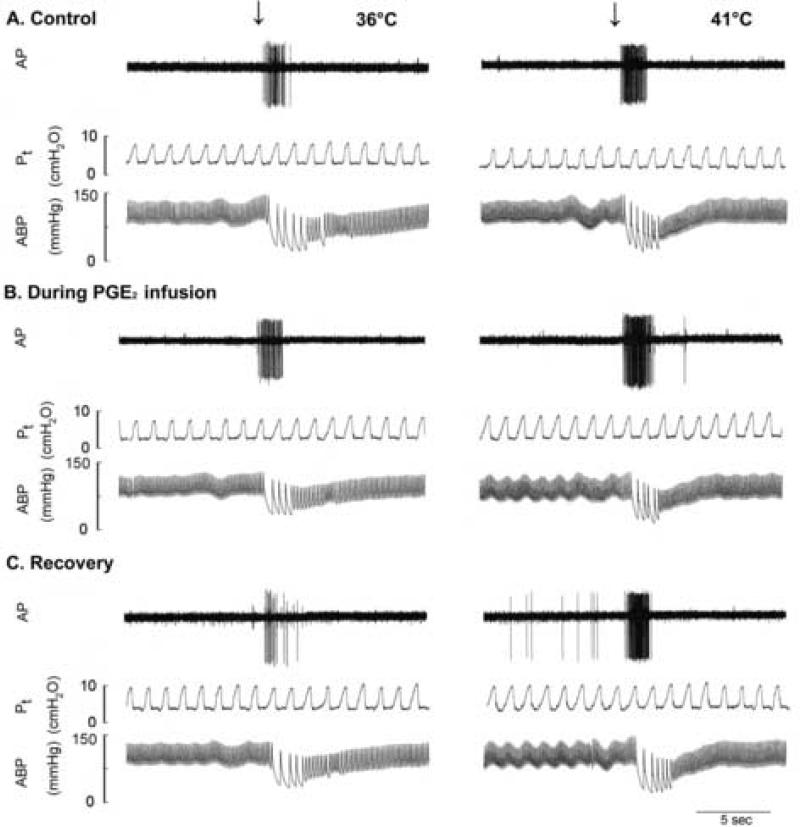
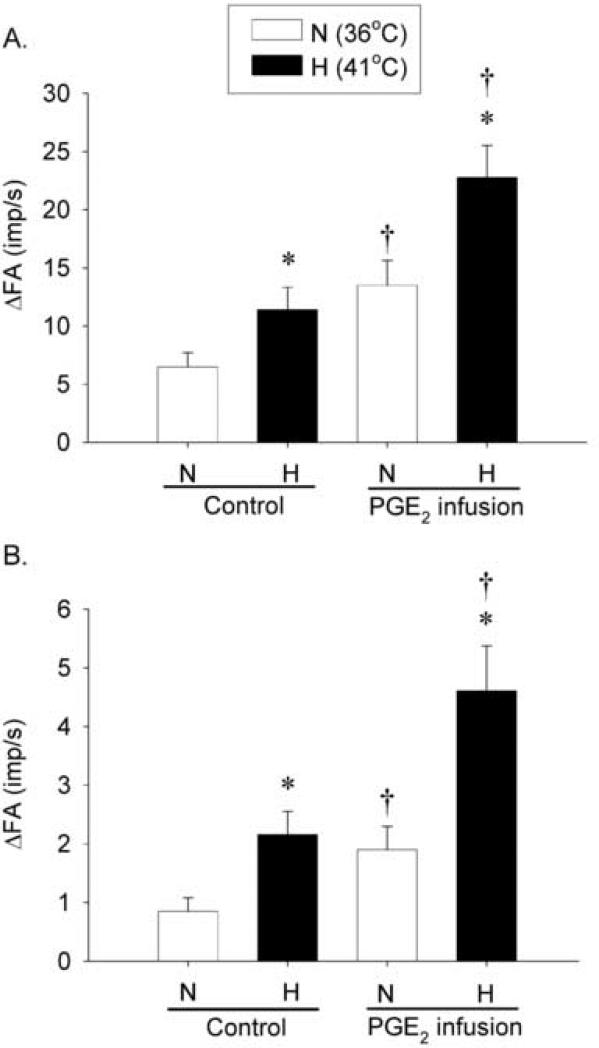
The fiber responses to right atrial injections of capsaicin (0.5-1.0 μg/kg) and adenosine (170 μg/kg) were increased when Tit was raised to H level, and these hyperthermia-induced increases in FA responses to capsaicin and adenosine were further elevated by PGE2 (Fig. 3 and 4). At control (without PGE2), capsaicin injection evoked an immediate and intense burst of activity, and the response was clearly enhanced during hyperthermia: peak FA after capsaicin injection were 6.50 ± 1.21 imp/s and 11.4 ± 1.89 imp/s at N and H levels of Tit, respectively. In comparison, during PGE2 infusion, peak FA increased to 13.5 ± 2.12 imp/s (n = 18, P < 0.05) and 22.8 ± 2.77 imp/s (n = 18, P < 0.05) at N and H, respectively (Fig. 3 and 4A). Thus, the hyperthermia-induced increase in FA response to capsaicin was significantly elevated by PGE2 (Δ = 4.93 ± 1.01 imp/s at control; Δ = 9.26 ± 1.36 imp/s during PGE2 infusion; n = 18, P < 0.05). The increased response to capsaicin during hyperthermia returned to control in 4 fibers tested 15-20 min after termination of the PGE2 infusion (e.g., Fig. 3). A similar potentiating effect of PGE2 was also found in the fiber response to adenosine injection (n = 12, P < 0.05; Fig. 4B).
Fig. 3. Experimental records illustrating the effects of PGE2 on the pulmonary C fiber responses to capsaicin injection in an anaesthetized, vagotomized and open-chest rat.
Left panel, responses to capsaicin (1 μg/kg in 0.15 ml volume) that was slowly injected into the catheter (dead space: 0.2 ml) and then flushed (at arrow) into the right atrium as a bolus with saline (0.4 ml) at Tit of 36°C; right panel, responses to the same dose of capsaicin at Tit of 41°C. The responses to capsaicin injection were tested at the last minute during the 3-min constant perfusion of thoracic chamber with isotonic saline held at a constant temperature. A, B and C: before (control), during and 20 min after the intravenous PGE2 infusion (3 μg/kg/min for 3 min), respectively. At least 20 min were allowed to elapse between tests for a complete recovery. AP, action potential; Pt, tracheal pressure; ABP, arterial blood pressure. Receptor location, right lower lobe; rat body weight, 380 g.
Fig. 4. Effects of PGE2 on the baseline activity and responses of pulmonary C fiber to right-atrial bolus injections of capsaicin and adenosine.
A, average responses to capsaicin injection (0.5-1.0 μg/kg, n = 18); B, average responses to adenosine injection (170 μg/kg, n = 12). Average peak responses of pulmonary C fibers to right-atrial injections of capsaicin and adenosine were measured with or without PGE2 infusion (3 μg/kg/min, 3 min) at 2 different levels of Tit (N: 36°C, open bars; H: 41°C, closed bars). Each of the chemicals (0.15 ml) was slowly injected into the right atrium as a bolus. ΔFA represents the difference between the peak FA (average over 2-sec interval for capsaicin and 10-sec interval for adenosine injection) and the baseline FA (average over 60-sec interval) in each fiber. *, significantly different (P < 0.05) from the corresponding data at normal Tit (36°C); †, significantly different (P < 0.05) from the corresponding data at control (before PGE2 infusion). Data are mean ± S.E.M.
Study Series 2
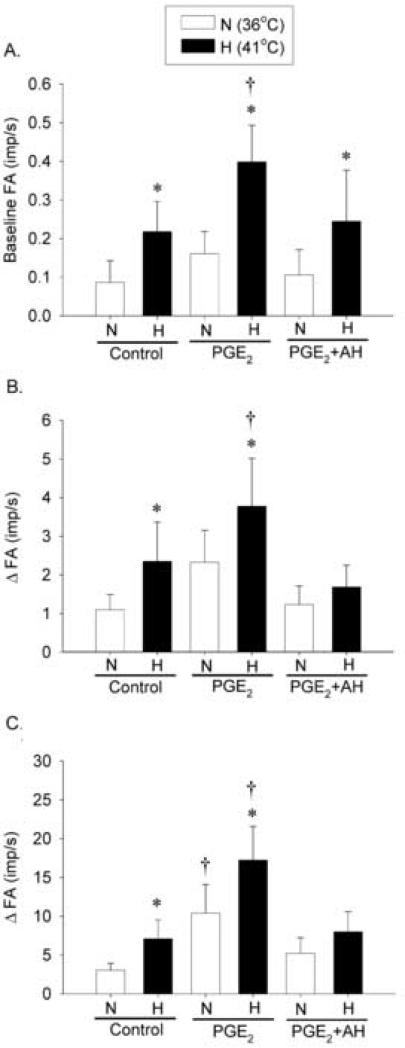
To determine the involvement of EP2 and EP4 prostanoid receptors in the potentiating effect of PGE2 found in Study Series 1, we repeated the same experimental protocols studying the responses of pulmonary C fibers to lung inflation and capsaicin injection during hyperthermia after the animals were pretreated with selective antagonists of these receptors. During PGE2 infusion (before administration of these antagonists), the hyperthermia-induced increases in baseline FA and fiber responses to lung inflation and capsaicin were significantly elevated from control (without PGE2). All these increases induced by PGE2 were completely prevented by the pretreatment with AH6809 (50 μg/kg) and AH23848 (50 μg/kg) (Fig. 5). For example, when Tit increased from N to H, the baseline FA increased by 0.13 ± 0.05 imp/s at control and the increase was almost doubled during PGE2 infusion (ΔFA = 0.24 ± 0.07 imp/s, n = 9, P < 0.05; Fig. 5A). However, this potentiating effect of PGE2 was almost completely abolished after pretreatment with AH6809 and AH23848 (ΔFA = 0.14 ± 0.07 imp/s, n = 9, P > 0.05; Fig. 5A).
Fig. 5. Effects of selective antagonists of EP2 and EP4 receptors on the baseline activity and responses of pulmonary C fiber to lung inflation and right-atrial injection of capsaicin during hyperthermia.
A, average baseline FA (n = 9); B, average responses to lung inflation at Pt = 30 cmH2O (n = 9); C, average responses to capsaicin injection (0.5 μg/kg) (n = 9). The baseline FA was averaged over 60 sec in each fiber. ΔFA represents the difference between the peak FA (average over 10-sec interval for lung inflation; average over 2-sec interval for capsaicin injection) and the average baseline FA in each fiber. AH6809 (50 μg/kg) and AH23848 (50 μg/kg) were administrated ~5 min before the PGE2 infusion. *, significantly different (P < 0.05) from the corresponding data at normal Tit (36°C); †, significantly different (P < 0.05) from the corresponding data at control (before PGE2 infusion). Data are mean ± S.E.M.
Similarly, pretreatment with these antagonists also very effectively blocked the PGE2 - induced increase in fiber responses to lung inflation (Fig. 5B) and capsaicin injection (Fig. 5C) during hyperthermia. The attenuated responses were not caused by a progressive attenuation of the responses to lung inflation and capsaicin over time because the sequence of the control and after-treatment tests was reversed in 4 of these 9 fibers studied. To further test this possibility, in a separate group of pulmonary C fibers the same experimental protocol testing the PGE2 effect was repeated in the same fibers 25-30 minutes later, after the vehicles of these EP2 and EP4 antagonists were administered. We found no difference in the augmenting effect of PGE2 on either the baseline FA or the fiber response to capsaicin between the two consecutive PGE2 tests; the hyperthermia-induced increase in the FA response to capsaicin was 11.9 ± 2.49 imp/s during the first PGE2 infusion, and 13.3 ± 2.97 imp/s (n = 5, P > 0.05) during the second PGE2 infusion after pretreatment with the vehicles of AH6809 and AH23848 (DMSO; ~2.7% V/V, 0.3 ml).
4. Discussion
Results of this study show that intrathoracic hyperthermia increased the baseline activity of vagal pulmonary C-fibers, and the increased fiber activity was further augmented by PGE2. In addition, PGE2 also potentiated the hyperthermia-induced hypersensitivity of pulmonary C-fibers to lung inflation and chemical stimulations. This potentiating effect of PGE2 on C-fibers appeared to be mediated primarily through the activation of EP2 and EP4 prostanoid receptors because the effect was completely prevented by pretreatment with the selective antagonists of these receptors.
PGE2, a potent autacoid derived from arachidonic acid metabolism through the enzymatic action of cyclooxygenase and PGE synthase, is released from a number of cells in the lungs during various airway inflammatory reactions (Holtzman, 1991). PGE2 causes airway and vascular smooth muscle relaxation, and can modulate the functions of other inflammatory cells (e.g., neutrophils) (Eglin and Whiting, 1988; Narumiya et al., 1999; Talpain et al., 1995). Inhalation of PGE2 aerosol enhances the sensitivity of the cough reflex elicited by capsaicin in healthy human subjects (Choudry et al., 1989), and also induces reflex bronchoconstriction in asthmatic patients (Holtzman, 1991), suggesting a PGE2-induced sensitization of pulmonary afferents. Indeed, Ho and co-workers reported that exogenous PGE2 markedly enhances the excitabilities of pulmonary C fibers to chemical stimulants and to lung inflation (2000). Results of the present study have further shown that PGE2 augments the potentiating effects of hyperthermia on pulmonary C-fiber afferents. However, physiological implications of this synergistic effect of PGE2 and airway hyperthermia in the regulation of airway function remain to be further explored.
Hyperthermia can occur under both normal and pathophysiological conditions. The most common cause of hyperthermia is an increase in metabolic rate such as during vigorous exercise. Hyperthermia (>41°C) also occurs frequently under pathophysiological conditions caused by endogenous pyrogens or infection, such as in patients suffering from acute heatstroke or severe fever (Bouchama et al., 1991). Moreover, tissue inflammation is known to lead to local hyperemia and an increase in temperature in the inflamed area (Gourine et al., 2001, Planas et al., 1995); for example, when an inflammatory reaction was induced by injection of carrageenan in the rat paw, the local tissue temperature was elevated by 3-4°C (Planas et al.,1995). A recent report has further revealed a higher tissue temperature in the airways of asthmatic patients (Paredi et al., 2002). Therefore, it is quite possible that tissue hyperthermia and increasing release of PGE2 in the airways may occur concurrently during various airway inflammatory reactions.
In a recent study in our laboratory, we found that hyperthermia elevated the baseline activity of vagal pulmonary C-fiber endings in anesthetized rats (Ruan et al., 2005). Furthermore, hyperthermia produced a distinct increase in the sensitivities of these afferents to chemical stimulants and to lung inflation, and the temperature threshold for activating these afferents is about 39.2°C. However, the mechanism underlying the hyperthermia-induced C-fiber hypersensitivity was not fully understood. One possibility is that hyperthermia may increase the release of certain proinflammatory cytokines (e.g. tumor necrosis factor α, interleukin 6, etc.) in the circulating blood (Bouchama et al., 1991). Since C-fiber nociceptors are known to be activated by some of these cytokines (Sommer and Kress, 2004), it seems possible that the enhanced C-fiber sensitivity is generated indirectly, in part, from the chemical substances released locally in the lung tissue during hyperthermia. On the other hand, hyperthermia may also activate certain temperature sensitive ion channels, particularly the transient receptor potential vanilloid type (TRPV) channels. In fact, expression of the TRPV1 receptors on the sensory terminals has been considered one of the most distinct characteristic features of the pulmonary C-fiber afferents (Ho et al., 2001), although the temperature threshold for activating the TRPV1 (> 43°C) exceeds the physiological range of body temperature that was applied in our studies. To test this possibility, a follow-up study was recently carried out in isolated rat vagal pulmonary sensory neurons (Ni et al., 2006). Results of that study clearly showed that cultured jugular and nodose pulmonary sensory neurons can be directly activated by an increase in temperature within the normal physiological range. The distinctly high temperature coefficient (Q10 = 29.5) of these neurons over the range of 35–41°C further suggests that the increase in temperature probably leads to the opening of temperature-sensitive ion channels (Hille, 2001). Approximately half of the inward current induced by hyperthermia was blocked by capsazepine, a selective TRPV1 antagonist, and the response was almost completely abolished by ruthenium red, an effective but non-selective blocker of TRPV1–4 channels. These data strongly suggest the involvement of TRPV1 as well as other subtypes of temperature-sensitive TRPV channels in the responses of these neurons to hyperthermia. In addition, the expressions of TRPV1–4 channel transcripts and proteins, respectively, in vagal pulmonary sensory neurons were further demonstrated in the reverse transcriptase-polymerase chain reaction and immunohistochemistry studies (Ni et al., 2006).
TRPVs are a subfamily of the TRP superfamily of ion channel proteins containing six trans-membrane domains that form non-selective, non-voltage-gated cationic channels (Clapham et al., 2001; Clapham, 2003). The subtypes of TRPV channels, TRPV1-4, are generally considered as the primary thermal sensors in mammalian species, and each type of TRPVs is activated in a different temperature range (Benham et al., 2003; Patapoutian et al., 2003). In addition, the TRPV channels can also be activated by a variety of physiological and pharmacological stimuli. More importantly, a number of endogenous inflammatory mediators, including PGE2, can sensitize TRPV1 during tissue inflammation, which leads to nociceptor hypersensitivity and hyperalgesia. Increasing evidence from recent studies has further suggested that TRPV1 may also play an important role in the manifestation of various symptoms of airway hypersensitivity associated with airway inflammatory reactions (Jia et al., 2005; Rami et al., 2004; Mitchell et al., 2005). The mechanisms underlying the sensitization of TRPV1 by PGE2 are not yet fully understood, but the involvement of certain signal transduction pathways has been suggested (Kwong and Lee, 2002; Gu et al., 2003). Among the several types of prostanoid receptors, the EP receptor has the highest affinity for PGE2 based upon the ligand-binding studies, and some of the subtypes of the EP receptor, such as EP2, EP3 and EP4 receptors, are known to be present on the sensory nerves and may therefore be involved in mediating the sensitizing effects of PGE2 on these endings (Coleman et al., 1994; Naruyima et al., 1999). Several species of G protein are known to participate in signal transduction via the EP receptors (Naruyima et al., 1999). Both EP2 and EP4 receptors are coupled to Gs proteins that upon activation increase the enzymatic activity of adenylyl cyclase. The resulting elevation of cAMP may then stimulate protein kinase A, which can in turn enhance the neuronal excitability by increasing the phosphorylation of both ligand-gated channels and voltage-sensitive channels, as shown in recent studies in isolated pulmonary sensory neurons (Kwong and Lee, 2002; Gu et al., 2003; Kwong and Lee, 2005). These findings of a sensitizing effect of PGE2 on the TRPV1 channel via an activation of the cAMP/PKA pathway (Kwong and Lee, 2002; Gu et al., 2003) provide further support to the possible involvement of EP2 and EP4 receptors in this study. However, we can not evaluate the relative contributes of EP2 and EP4 receptors in the observed responses since these antagonists were administered together in this study.
The potentiating effect of PGE2 was also found in the responses of pulmonary C-fiber afferents to adenosine and lung inflation in this study; both of which involve transduction mechanisms different from that of capsaicin (Lee and Undem, 2005). Therefore, these results suggest that the positive interaction between PGE2 and hyperthermia in the C-fiber activation is not limited solely to the responses mediating through the TRPV channels.
PGE2 is also known for its pyrogenic effect via an action on the hypothalamus. Indeed, our data indicate a very small but consistent increase in rectal temperature during infusion of PGE2 (~0.2°C; Table 1). However, we do not believe this small additional increase in rectal temperature had any influence on the PGE2-induced potentiation of C-fiber responses observed in this study for the following reasons: 1) Pulmonary C-fiber endings reside in the lung tissue, the temperature of which is almost identical to that of the perfusate (isotonic saline) in the thoracic chamber (intrathoracic temperature), and independent of the body temperature (Ruan et al., 2005); 2) Because the intrathoracic temperature was maintained by an external heating device, there was no difference in Tit between control and during the PGE2 infusion (Table 1).
In summary, this study has clearly demonstrated that PGE2 enhances the hyperthermia-induced hypersensitivity of pulmonary C-fibers, and this potentiating effect is mediated through activation of EP2 and EP4 prostanoid receptors that are, presumably, expressed on the sensory terminals of these afferents. The fact that both an elevation of airway temperature and increased local release of PGE2 can occur concurrently during various airway inflammatory reactions suggests a possibility that such an interaction may take place under those conditions. Furthermore, activation of the pulmonary C-fiber afferents is known to elicit powerful and extensive systemic reflex responses such as rapid shallow breathing, bronchoconstriction, hypersecretion of mucus, cough, arterial hypotension, etc. (Coleridge and Coleridge, 1984; Lee and Pisarri, 2002). This synergistic effect may, therefore, play a part in the manifestation of airway hypersensitivity associated with airway inflammatory reactions.
Acknowledgements
This study was supported in part by grants from NIH HL-67379 and Kentucky Lung Cancer Research Program. The authors are thankful to Michelle Wiggers for her technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramovitz M, Adam M, Boie Y, Carriere M, Denis D, Godbout C, Lamontagne S, Rochette C, Sawyer N, Tremblay NM, Belley M, Gallant M, Dufresne C, Gareau Y, Ruel R, Juteau H, Labelle M, Ouimet N, Metters KM. The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim Biophys Acta. 2000;1483:285–293. doi: 10.1016/s1388-1981(99)00164-x. [DOI] [PubMed] [Google Scholar]
- Benham CD, Gunthorpe MJ, Davis JB. TRPV channels as temperature sensors. Cell Calcium. 2003;33:479–487. doi: 10.1016/s0143-4160(03)00063-0. [DOI] [PubMed] [Google Scholar]
- Bouchama A, Parhar RS, el-Yazigi A, Sheth K, al-Sedairy S. Endotoxemia and release of tumor necrosis factor and interleukin 1 alpha in acute heatstroke. J Appl Physiol. 1991;70:2640–2644. doi: 10.1152/jappl.1991.70.6.2640. [DOI] [PubMed] [Google Scholar]
- Briese E. Normal body temperature of rats: the setpoint controversy. Neurosci Biobehav Rev. 1998;22:427–436. doi: 10.1016/s0149-7634(97)00051-1. [DOI] [PubMed] [Google Scholar]
- Choudry NB, Fuller RW, Pride NB. Sensitivity of the human cough reflex: effect of inflammatory mediators prostaglandin E2, bradykinin, and histamine. Am Rev Respir Dis. 1989;140:137–141. doi: 10.1164/ajrccm/140.1.137. [DOI] [PubMed] [Google Scholar]
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Clapham DE, Runnels LW, Strubing C. The TRP ion channel family. Nat Rev Neurosci. 2001;2:387–396. doi: 10.1038/35077544. [DOI] [PubMed] [Google Scholar]
- Clarke DL, Belvisi MG, Catley MC, Yacoub MH, Newton R, Giembycz MA. Identification in human airways smooth muscle cells of the prostanoid receptor and signalling pathway through which PGE2 inhibits the release of GM-CSF. Br J Pharmacol. 2004;141:1141–1150. doi: 10.1038/sj.bjp.0705716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman RA, Smith WL, Narumiya S. International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev. 1994;46:205–229. [PubMed] [Google Scholar]
- Coleridge JC, Coleridge HM. Afferent vagal C fibre innervation of the lungs and airways and its functional significance. Rev Physiol Biochem Pharmacol. 1984;99:1–110. doi: 10.1007/BFb0027715. [DOI] [PubMed] [Google Scholar]
- Costello JF, Dunlop LS, Gardiner PJ. Characteristics of prostaglandin induced cough in man. Br J Clin Pharmacol. 1985;20:355–359. doi: 10.1111/j.1365-2125.1985.tb05077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eglen RM, Whiting RL. The action of prostanoid receptor agonists and antagonists on smooth muscle and platelets. Br J Pharmacol. 1988;94:591–601. doi: 10.1111/j.1476-5381.1988.tb11565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Rudolph K, Korsak AS, Kubatko J, Tesfaigzi J, Kozak W, Kluger MJ. Role of capsaicin-sensitive afferents in fever and cytokine responses during systemic and local inflammation in rats. Neuroimmunomodulation. 2001;9:13–22. doi: 10.1159/000049003. [DOI] [PubMed] [Google Scholar]
- Gu Q, Kwong K, Lee LY. Ca2+ transient evoked by chemical stimulation is enhanced by PGE2 in vagal sensory neurons: role of cAMP/PKA signaling pathway. J Neurophysiol. 2003;89:1985–1993. doi: 10.1152/jn.00748.2002. [DOI] [PubMed] [Google Scholar]
- Hille B. Ion channels of excitable membranes. Sinauer Associates; Sunderland, Massachusettes: 2001. [Google Scholar]
- Ho CY, Gu Q, Hong JL, Lee LY. Prostaglandin E(2) enhances chemical and mechanical sensitivities of pulmonary C fibers in the rat. Am J Respir Crit Care Med. 2000;162:528–533. doi: 10.1164/ajrccm.162.2.9910059. [DOI] [PubMed] [Google Scholar]
- Ho CY, Gu Q, Lin YS, Lee LY. Sensitivity of vagal afferent endings to chemical irritants in the rat lung. Respir Physiol. 2001;127:113–124. doi: 10.1016/s0034-5687(01)00241-9. [DOI] [PubMed] [Google Scholar]
- Holtzman MJ. Sources of inflammatory mediators in the lung: the role of epithelial and leukocyte pathways for arachidonic acid oxygenation. Chapter 6 in: Mediators of Pulmonary Inflation. In: Bray MA, Anderson WH, editors. Lung Biology in Health and Disease Series. Vol. 54. Dekker; New York: 1991. [Google Scholar]
- Jia Y, McLeod RL, Hey JA. TRPV1 receptor: a target for the treatment of pain, cough, airway disease and urinary incontinence. Drug News Perspect. 2005;18:165–171. doi: 10.1358/dnp.2005.18.3.892761. [DOI] [PubMed] [Google Scholar]
- Kwong K, Lee LY. PGE(2) sensitizes cultured pulmonary vagal sensory neurons to chemical and electrical stimuli. J Appl Physiol. 2002;93:1419–1428. doi: 10.1152/japplphysiol.00382.2002. [DOI] [PubMed] [Google Scholar]
- Kwong K, Lee LY. Prostaglandin E2 potentiates a TTX-resistant sodium current in rat capsaicin-sensitive vagal pulmonary sensory neurones. J Physiol. 2005;564:437–450. doi: 10.1113/jphysiol.2004.078725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LY, Pisarri TE. Afferent properties and reflex functions of bronchopulmonary C-fibers. Respir Physiol. 2001;125:47–65. doi: 10.1016/s0034-5687(00)00204-8. [DOI] [PubMed] [Google Scholar]
- Lee LY, Undem BJ. In: Bronchopulmonary vagal sensory nerves. Chapter 11. In: Advances in Vagal Afferent Neurobiology: Frontiers in Neurosciences Series. Undem BJ, Weinreich D, editors. CRC Press; 2005. [Google Scholar]
- Mitchell JE, Campbell AP, New NE, Sadofsky LR, Kastelik JA, Mulrennan SA, Compton SJ, Morice AH. Expression and characterization of the intracellular vanilloid receptor (TRPV1) in bronchi from patients with chronic cough. Exp Lung Res. 2005;31:295–306. doi: 10.1080/01902140590918803. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- Ni D, Gu Q, Hu HZ, Gao N, Zhu MX, Lee LY. Thermal sensitivity of isolated vagal pulmonary sensory neurons: role of transient receptor potential vanilloid receptors. Am J Physiol Regul Integr Comp Physiol. 2006;291:R541–550. doi: 10.1152/ajpregu.00016.2006. [DOI] [PubMed] [Google Scholar]
- Norel X, Walch L, Labat C, Gascard JP, Dulmet E, Brink C. Prostanoid receptors involved in the relaxation of human bronchial preparations. Br J Pharmacol. 1999;126:867–872. doi: 10.1038/sj.bjp.0702392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredi P, Kharitonov SA, Barnes PJ. Faster rise of exhaled breath temperature in asthma: a novel marker of airway inflammation? Am J Respir Crit Care Med. 2002;165:181–184. doi: 10.1164/ajrccm.165.2.2103053. [DOI] [PubMed] [Google Scholar]
- Patapoutian A, Peier AM, Story GM, Viswanath V. ThermoTRP channels and beyond: mechanisms of temperature sensation. Nat Rev Neurosci. 2003;4:529–539. doi: 10.1038/nrn1141. [DOI] [PubMed] [Google Scholar]
- Planas ME, Rodriguez L, Sanchez S, Pol O, Puig MM. Pharmacological evidence for the involvement of the endogenous opioid system in the response to local inflammation in the rat paw. Pain. 1995;60:67–71. doi: 10.1016/0304-3959(94)00090-2. [DOI] [PubMed] [Google Scholar]
- Rami HK, Gunthorpe MJ. The therapeutic potential of TRPV1 antagonists: clinical answers await. Drug Discovery Today: Therapeutic Strategies. 2004;1:97–104. [Google Scholar]
- Ruan T, Gu Q, Kou YR, Lee LY. Hyperthermia increases sensitivity of pulmonary C-fibre afferents in rats. J Physiol. 2005;565:295–308. doi: 10.1113/jphysiol.2005.084319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett. 2004;361:184–187. doi: 10.1016/j.neulet.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Talpain E, Armstrong RA, Coleman RA, Vardey CJ. Characterization of the PGE receptor subtype mediating inhibition of superoxide production in human neutrophils. Br J Pharmacol. 1995;114:1459–1465. doi: 10.1111/j.1476-5381.1995.tb13370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]