Abstract
Background
The ADH1B gene has consistently been implicated in problem drinking, but rarely incorporated into gene by environment investigations of alcohol phenotypes. This study examined the joint effects of variation in ADH1B and childhood adversity – a well-documented risk factor for alcohol problems and moderator of genetic liability to psychiatric outcomes – on maximum drinks consumed in a 24-hour period (maxdrinks) and alcohol use disorder (AUD) symptoms.
Methods
Data were drawn from 2,617 African-American (AA) and 1,436 European-American (EA) participants (42% female) in a multisite genetic study of substance dependence. We tested the most significant ADH1B SNPs for alcohol dependence from a genomewide association study with this sample, ADH1B-rs1229984 (Arg48His) and ADH1B-rs2066702 (Arg370Cys), in EA and AA subsamples, respectively.
Results
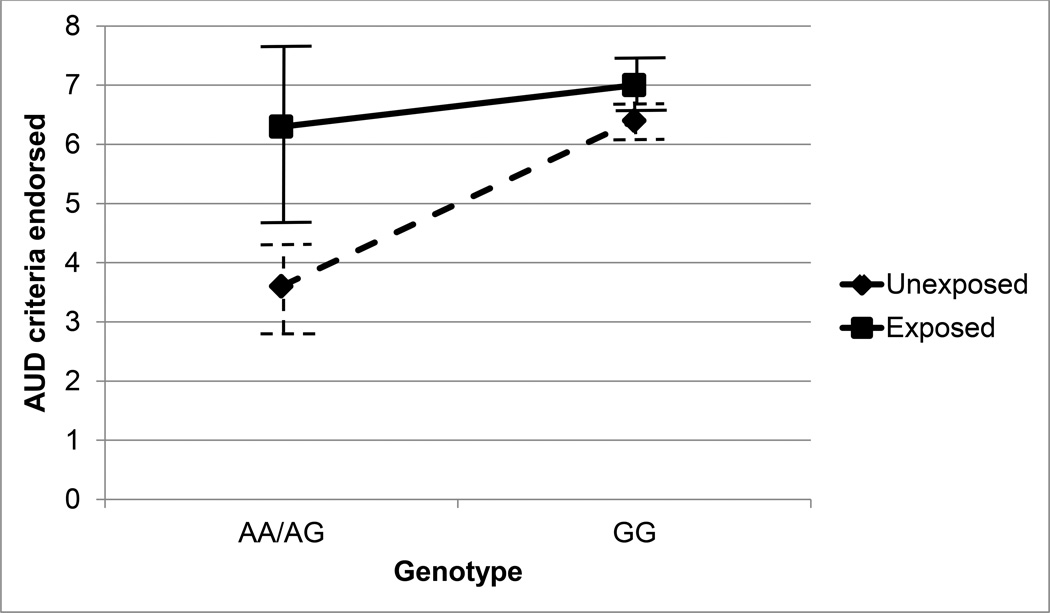
Ordinal regression analyses conducted separately by sex and population revealed significant main effects for childhood adversity both for alcohol phenotypes in AA women and men and for maxdrinks in EA women. A significant rs1229984 by childhood adversity interaction was observed for AUD symptoms in EA men. Unexposed His-allele carriers reported a mean of 3.6 AUD criteria, but adversity-exposed His-allele carriers endorsed approximately the same number (6.3) as those without the protective allele (6.3 and 7.0 for adversity-exposed and adversity-unexposed groups, respectively).
Conclusions
Results suggest that under conditions of childhood adversity, the His allele does not exert its protective effects in EA men (OR=0.57, CI:0.32–1.01; p=0.056). Findings highlight the robust risk effect conferred by childhood adversity and the importance of considering population and sex in genetically informative investigations of its association with alcohol outcomes.
Keywords: childhood adversity, ADH1B, alcohol, African-Americans
Introduction
The contribution of heritable factors to alcoholism has been documented in twin studies for over two decades (e.g., Jardine and Martin, 1984; Kaprio et al., 1987), but identification of the specific genetic variants that influence alcohol use disorder (AUD) has proven challenging. AUD is a complex and heterogeneous disorder that involves multiple biological pathways, including alcohol metabolism, GABA receptor activity, and serotonin and dopamine signaling, and as such, involves a large number of genes with diverse functions. The individual contribution of a given genetic variant is modest, typically accounting for less than 1% of genetic variance in alcohol phenotypes, and the sum of the individual contributions of known variants does not approach the 50–60% heritability estimate from twin studies (Reed et al., 1996, True et al., 1996, van den Bree et al., 1998). The so-called missing heritability problem (Maher, 2008) may be due in part to inflation in heritability estimates from twin studies, in which gene–environment correlation and interaction effects are subsumed under additive genetic effects. Thus, investigating gene–environment interplay in alcohol-related outcomes is critical to the development of valid etiological models of alcohol use and misuse.
Among the few consistent findings from gene discovery studies of alcohol phenotypes is the association of ADH1B, which encodes alcohol dehydrogenase 1B, a key enzyme in alcohol metabolism, with liability to heavy alcohol consumption and alcohol dependence. The single nucleotide polymorphism (SNP) rs1229984*G to A encodes an arginine to histidine substitution, (Hurley and Edenberg, 2012), increasing the clearance of alcohol. This effect, presumably through increased acetaldehyde concentrations, protects against alcohol misuse. The protective effect of the His allele has been well documented in Asian populations (Kim et al., 2008, Li et al., 2011, Park et al., 2013), in which the prevalence is approximately 70% (Li et al., 2011). It has also been found in Israeli Jews, 20–40% of whom carry the A allele (Carr et al., 2002, Meyers et al., 2013a, Neumark et al., 2004). Although the His allele is far less prevalent in European populations (under 4%, Hurley and Edenberg, 2012), large-scale studies have demonstrated that A-allele carriers of European descent also exhibit lower levels of heavy and problem drinking (Bierut et al., 2012, Gelernter et al., 2014, Tolstrup et al., 2008). The rs1229984 has not been implicated as a source of genetic variance in AUD in individuals of African descent, but an association between alcohol dependence and another SNP on ADH1B, rs2066702, was observed in the AA subsample in our recent genomewide association study (GWAS) (Gelernter et al., 2014).
Exposure to adverse events in childhood, such as physical abuse, sexual abuse, neglect, and witnessing violence, has consistently been linked to an elevated risk of developing an AUD in adulthood (Afifi et al., 2012, Fergusson et al., 1996a, Kendler et al., 2000, Kessler et al., 1997a, Nelson et al., 2006, Schilling et al., 2007). Childhood adversity is among the most commonly studied environmental risk factors in gene by environment (GxE) studies of alcohol phenotypes. Stressful life events, defined more broadly and including primarily events in adulthood, have been studied as environmental modifiers of genetic liability to AUD as well (Bau et al., 2000, Dick et al., 2007, Madrid et al., 2001). However, we consider exposure to adverse childhood events to be a more informative environmental risk factor both because of the consistency of the literature on its association with alcohol outcomes and because, by definition, it precedes the period of peak risk for the initiation of alcohol use, that is adolescence. This allows for the interpretation that adverse events are contributors to – rather than consequences of – heavy or problem alcohol use.
The majority of studies on gene by childhood adversity effects on alcohol outcomes have investigated interactions with genes associated with stress response and serotonin regulation: The serotonin transporter linked polymorphic region (5-HTTLPR) (Kaufman et al., 2007, Laucht et al., 2009), the monoamine oxidase A (MAOA) gene (Ducci et al., 2008, Nilsson et al., 2007, Nilsson et al., 2008), and the corticotropin-releasing hormone (CRH) gene (Nelson et al., 2010). Far fewer studies have examined potential interactions with genes associated with vulnerability to addiction. Among them is a case–control study examining the influence of childhood trauma and variation in GABRA2 on alcohol, heroin, and cocaine dependence in African-American (AA) men, which found no evidence of a GxE effect on alcohol dependence (Enoch et al., 2010). The only known study to investigate child adversity as a modifier of the association of ADH1B variation with alcohol phenotypes was conducted by Meyers and colleagues (Meyers et al., 2013b) using a sample of primarily male (78.3%) Israeli Jews. They found GxE interactions on indicators of heavy consumption (maximum drinks consumed in a 24-hour period) and alcohol use disorder severity (AUD criterion count), such that the effects of rs1229984 (i.e., presence vs. absence of the His allele) on alcohol outcomes was greater in individuals who had experienced adverse events during childhood than in those who had not.
The aim of the current investigation was to examine the joint effects of ADH1B variants and childhood adversity on two alcohol phenotypes that represent the underlying continuum of drinking behaviors, maximum drinks consumed in a 24-hour period (maxdrinks), and DSM-IV alcohol abuse and dependence criterion count (AUD symptoms), in a large sample of AA and European-American (EA) women and men. The study extends the existing literature on the moderating effects of childhood adversity on genetic liability to alcohol consumption and problem drinking in several ways. First, we attempted to replicate findings from the only prior study of GxE effects of ADH1B and childhood adversity on alcohol phenotypes. Second, we assessed the generalizability of findings from that study to individuals of African descent. Drawing on results of a GWAS conducted with the current sample, which revealed that rs1229984 was the most significant ADH1B SNP in the EA subsample (p=7.77 × 10−14) and rs2066702 was the most significant SNP in the AA subsample (5.73 × 10−17) (Gelernter et al., 2014), we examined ADH1B variants of greatest effect in each population. Third, we examined potential distinctions by sex in the contributions of ADH1B variants and childhood adversity to alcohol phenotypes, a key issue to address given the lower rates of alcohol consumption and AUD (Chan et al., 2007, Keyes et al., 2008), higher prevalence of traumatic events associated with alcohol-related problems (e.g., sexual abuse) (Fergusson et al., 1996b, Pereda et al., 2009), and evidence of greater resilience to the effects of trauma on psychiatric and substance use problems (DuMont et al, 2007; Schilling et al., 2007) in women than men.
Materials and Methods
Sample and Procedures
Data for the current study were derived from a multisite study of the genetics of alcohol dependence, cocaine dependence, and opioid dependence conducted through Yale University School of Medicine, the University of Connecticut Health Center, the University of Pennsylvania Perelman School of Medicine, the Medical University of South Carolina, and McLean Hospital. The sample was composed of alcohol, cocaine, or opioid-dependent individuals and unaffected controls recruited for case–control genetic studies of substance use disorders and cocaine or opioid-dependent probands and their relatives from family-based genetic studies. Subjects from the multisite study with genotypic data for rs2066702 (AA subsample) or rs1229984 (EA subsample) who reported on their exposure to adverse childhood events and had consumed at least one alcoholic drink over the lifetime were included in the present study: 2,617 African-Americans and 1,436 European-Americans. Demographic and psychiatric characteristics of the sample are shown by population in Table 1. In both AA and EA subsamples, women comprised just under half of the subjects, mean age was about 40, approximately one-third of subjects completed fewer than 12 years of education, half reported their annual household income as below $10,000, and just over 50% were never married. Rates of psychiatric disorders, substance use disorders in particular, were high in both subsamples (as expected given the study’s ascertainment strategy). Overall severity of psychopathology was somewhat greater in the EA subsample than the AA subsample.
Table 1.
Demographic and psychiatric characteristics of African-American (AA) and European-American (EA) samples
| AA (n=2,617) |
EA (n=1,436) |
|
|---|---|---|
| Demographics | ||
| Female | 43.2% | 45.3% |
| Age: mean (SD) | 41.5 (9.2) | 38.4 (11.1) |
| Education level | ||
| Below high school | 39.1% | 33.5% |
| High school degree | 28.1% | 31.5% |
| Greater than high school | 32.8% | 38.2% |
| Household income ≤ $10,000 | 53.0% | 45.3% |
| Marital status | ||
| Currently married | 13.1% | 13.3% |
| Never married | 25.9% | 31.5% |
| Separated/divorced/widowed | 61.0% | 55.2% |
| Lifetime DSM-IV Psychiatric Disorders | ||
| Attention deficit hyperactivity disorder | 5.0% | 11.3% |
| Bipolar I disorder | 3.8% | 6.3% |
| Conduct disorder | 14.8% | 18.9% |
| Major depressive disorder | 13.4% | 18.5% |
| Panic disorder | 3.3% | 12.2% |
| Post-traumatic stress disorder | 15.4% | 18.4% |
| Social phobia | 3.5% | 8.3% |
| Substance Use Disorders | ||
| Alcohol dependence | 62.5% | 78.7% |
| Cocaine dependence | 73.8% | 75.2% |
| Cannabis dependence | 32.2% | 41.5% |
| Nicotine dependence | 58.3% | 77.4% |
| Opioid dependence | 24.5% | 60.4% |
Procedures
Subjects gave written informed consent, as approved by the institutional review board at each participating institution, and certificates of confidentiality were obtained from NIDA and NIAAA. (See Sun et al., 2012 for details of sample ascertainment and procedures.)
Assessment
Data were collected by trained interviewers who conducted in-person interviews using an electronic version of the Semi-Structured Assessment for Drug Dependence and Alcoholism (SSADDA). The SSADDA queries demographic information, lifetime diagnostic criteria for DSM-IV psychiatric disorders, and psychosocial history, including history of exposure to adverse events during childhood. A detailed history of substance use, including maximum number of drinks consumed in a 24-hour period, is also queried in the SSADDA. (Descriptions of SSADDA administration methods and reliability can be found in Feinn et al., 2009, Pierucci-Lagha et al., 2007, and Pierucci-Lagha et al., 2005).
Genotyping
Samples were genotyped on the Illumina HumanOmni1-Quad v1.0 microarray, containing 988,306 autosomal SNPs, at the Center for Inherited Disease Research and the Yale Center for Genome Analysis. Genotypes were called using GenomeStudio software V2011.1 and genotyping module V1.8.4 (Illumina, San Diego, CA, USA). (See Gelernter et al., 2014 for a detailed description of genotyping and quality control measures.)
Operationalization of Variables
Alcohol Outcomes
Maximum drinks consumed in a 24-hour period (maxdrinks) were assessed with the question, “In your lifetime, what is the largest number of drinks you have ever had in a 24-hour period (including all types of alcohol)?” Values over 50 were recoded to 50 (based on the assumption that the number was an overestimate). The distribution of maxdrinks showed peaks at multiples of 5 and 6, suggesting that participants were estimating either by rounding to the nearest multiple of 5 or by counting 6 packs of beer (i.e., not a true continuous distribution), so we chose to treat maxdrinks as an ordinal variable. The distribution was split into quintiles: 1–6, 7–14, 15–23, 24–30, and >30 drinks to create a 5-level categorical variable.
The AUD symptom count variable was derived by summing DSM-IV alcohol abuse and alcohol dependence criteria.
Childhood Adversity
Subjects were coded positive for exposure to childhood adversity if they responded “yes” to any of the following questions and negative if they did not: (1) “Did either of your parents die before you were age 6?” (2) “Did you ever witness or experience a violent crime, like a shooting or a rape, by age 13?” (3) “By the time you were age 13, were you ever sexually abused?”, or (4) “By the time you were age 13, were you ever beaten by an adult so badly that you needed medical care or had marks on your body that lasted for more than 30 days?”ADH1B Variant Groups For each SNP, we combined individuals with AA or AG genotype into a single group, that is A-allele carriers, and compared them to individuals with a GG genotype, that is noncarriers of the A allele.
Data Analysis
Analyses proceeded in four steps. Preliminary analyses (steps 1 to 3) were conducted in SAS (SAS Institute, 2008), and final models (step 4) were conducted in Stata (StataCorp, 2007). First, ordinal logistic regression analyses testing the association of rs2066702 and rs1229984 with maxdrinks and AUD symptoms were conducted separately in the AA and EA subsamples to estimate main effects and determine the specificity of the effects by population (i.e., rs2066702 in African-Americans and rs1229984 in European-Americans). Second, to rule out possible confounding effects of gene–environment correlations, we conducted logistic regression analyses with each SNP to determine whether exposure to childhood adversity varies by genotype. Third, we examined sex differences in maxdrinks, AUD symptoms, and childhood adversity in the full sample to determine the appropriateness of analyzing data from female and male subjects together. Independent t-tests were conducted to assess sex differences in mean maxdrinks and AUD criteria, and a logistic regression analysis was conducted to assess distinctions by sex in risk for exposure to childhood adversity. Fourth, based on findings from preliminary analyses (reported in the Results section), ordinal regression analyses were conducted separately by sex and population, using rs2066702 in the AA female and AA male models and rs1229984 in the EA female and EA male models. Age was included as a covariate, and models were adjusted for nonindependence of observations in family members using the clustered sandwich estimator. We applied a Bonferroni correction to account for multiple testing within each group (i.e., AA women, AA men, EA women, and EA men) such that the threshold for significance was set to 0.025.
Results
ADH1B Variants, Alcohol Outcomes, and Childhood Adversity by Population
The prevalence of rs1229984 A-allele carriers was 3.1% in the AA subsample (minor allele frequency (MAF) =1.6%) and 11.5% in the EA subsample (MAF=6.5%). The prevalence of rs2066702 A-allele carriers was 30.6% in the AA subsample (MAF=19.7%) and 1.2% in the EA subsample (MAF=0.07%). Ordinal logistic regression analyses revealed that EA carriers of the rs1229984*A allele reported fewer maxdrinks (OR=0.42, CI:0.30–0.58) and AUD symptoms (OR=0.34, CI:0.25–0.45) than noncarriers. In the AA subsample, no significant association between rs1229984 and either alcohol outcome was observed, but there was evidence of a protective effect for the rs2066702*A allele. Fewer maxdrinks (OR=0.69, CI: 0.59–0.80) and AUD symptoms (OR=0.67, CI:0.58–0.78) were reported by A-allele carriers than noncarriers. No significant association between rs2066702 and either alcohol outcome was observed in the EA subsample. Ordinal logistic regression analyses testing for GxE effects were therefore conducted with the rs2066702 SNP in the AA subsample and the rs1229984 SNP in the EA subsample. Logistic regression analyses conducted to test for gene–environment correlations (i.e., rs1229984 or rs2066702 with childhood adversity) revealed no association of either SNP with exposure to adverse childhood events.
Alcohol Outcomes and Childhood Adversity by Sex
Independent t-tests conducted on the full sample (i.e, AA and EA subjects) revealed significant differences by sex for maxdrinks (t(3786.3)=18.52, p<0.0001) and AUD symptoms (t(3581.5)=10.53, p<0.0001). Women reported a mean of 16.3 (SD=13.2) drinks, and men reported a mean of 24.3 (SD=14.1) drinks. Means for AUD symptoms were 4.3 (SD=3.8) and 5.6 (SD=3.6) for women and men, respectively. Sex differences were also observed for exposure to childhood adversity in the full sample; the prevalence was significantly higher in women than men (OR=1.25, CI: 1.10–1.41): 39.1% vs. 34.0%. The final models, which included ADH1B by childhood adversity interaction effects, were therefore conducted separately for AA women (n=1,131), AA men (n=1,486), EA women (n=586), and EA men (n=850). The prevalence of A-allele carriers and exposure to adverse events in childhood are shown by race/ethnicity and sex in Table 2.
Table 2.
Prevalence of childhood adversity and AA or AG genotype by race/ethnicity and sex
| AA | EA | |||
|---|---|---|---|---|
| Women (n=1,131) |
Men (n=1,486) |
Women (n=586) |
Men (n=850) |
|
| Experienced adverse events in childhood | 37.9% | 38.3% | 41.5% | 26.5% |
| AA or AG genotype | 33.2% | 28.6% | 11.7% | 11.3% |
AA= African-American; EA=European-American
Moderation by Childhood Adversity of ADH1B Variant Effects on Alcohol Outcomes
Results of ordinal logistic regression analyses of moderation by childhood adversity of the effects of rs2066702 on maxdrinks and AUD symptoms in African-Americans are shown separately by sex in Tables 3 and 4. Results of ordinal logistic regression analyses testing for moderation by childhood adversity of the effects of rs1229984 on maxdrinks and AUD symptoms in European-Americans are shown by sex in Tables 5 and 6.
Table 3.
Results of ordinal logistic regression analyses using ADH1B-rs2066702 and childhood adversity to predict maximum drinks consumed in 24 hours and alcohol use disorder symptoms in African-American womena
| Max drinks | AUD symptoms | |
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| Childhood adversity | 1.92 (1.45–2.53)* | 1.71 (1.31–2.22)* |
| ADH1B-rs2066702b | 0.72 (0.54–0.97) | 0.70 (0.53–0.93)* |
| ADH1B-rs2066702*childhood adversity | 1.09 (0.68–1.75) | 1.22 (0.77–1.93) |
p <0.025;
adjusted for age;
0=GG genotype, 1=AA or AG genotype; OR= odds ratio; 95% CI = 95% confidence intervals; max drinks = maximum drinks consumed in a 24-hour period; AUD=alcohol use disorder
Table 4.
Results of ordinal logistic regression analyses using ADH1B-rs2066702 and childhood adversity to predict maximum drinks consumed in 24 hours and alcohol use disorder symptoms in African-American mena
| Max drinks | AUD symptoms | |
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| Childhood adversity | 1.67 (1.32–2.12)* | 1.78 (1.41–2.25)* |
| ADH1B-rs2066702b | 0.74 (0.57–0.97) | 0.72 (0.56–0.93)* |
| ADH1B-rs2066702*childhood adversity | 0.78 (0.51–1.20) | 0.79 (0.52–1.20) |
p <0.025;
adjusted for age;
0=GG genotype, 1=AA or AG genotype; OR= odds ratio; 95% CI = 95% confidence intervals; max drinks = maximum drinks consumed in a 24-hour period; AUD=alcohol use disorder
Table 5.
Results of ordinal logistic regression analyses using ADH1B-rs1229984 and childhood adversity to predict maximum drinks consumed in 24 hours and alcohol use disorder symptoms in European-American womena
| Max drinks | AUD symptoms | |
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| Childhood adversity | 1.77 (1.28–2.44)* | 1.33 (0.97–1.81) |
| ADH1B-rs1229984b | 0.29 (0.15–0.55)* | 0.30 (0.14–0.62)* |
| ADH1B-rs1229984 *childhood adversity | 1.40 (0.58–3.40) | 1.67 (0.68–4.07) |
p <0.025;
adjusted for age;
0=GG genotype, 1=AA or AG genotype; OR= odds ratio; 95% CI = 95% confidence intervals; max drinks = maximum drinks consumed in a 24-hour period; AUD=alcohol use disorder
Table 6.
Results of ordinal logistic regression analyses using ADH1B-1229984 and childhood adversity to predict maximum drinks consumed in 24 hours and alcohol use disorder symptoms in European-American mena
| Max drinks | AUD symptoms | |
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| Childhood adversity | 1.40 (1.04–1.88) | 1.32 (1.00–1.76) |
| ADH1B-rs1229984 b | 0.46 (0.29–0.73)* | 0.24 (0.15–0.36)* |
| ADH1B-rs1229984 *childhood adversity | 0.97 (0.30–3.10) | 2.39 (1.17–4.88)* |
p <0.025;
adjusted for age;
0=GG genotype, 1=AA or AG genotype; OR= odds ratio; 95% CI = 95% confidence intervals; max drinks = maximum drinks consumed in a 24-hour period; AUD=alcohol use disorder
Childhood adversity was associated with a higher number of maxdrinks in AA women, AA men, and EA women, with odds ratios ranging from 1.67 (1.32–2.12) to 1.92 (CI: 1.45–2.53). For AUD symptoms, main effects of childhood adversity were evident only in African-Americans (OR=1.71, CI: 1.31–2.22 for women and OR=1.78, CI: 1.41–2.25 for men). A moderating effect of childhood adversity on the association between ADH1B variants and alcohol outcomes was observed exclusively in EA men for AUD symptoms. Exposure to childhood adversity was associated with a significant increase in risk for AUD symptoms in carriers of the A (His) allele in rs1229984 (OR=3.16 (1.32*2.39), CI:1.64–6.09; p=0.017); that is, the protective effect of the A allele was significantly reduced in EA men who had experienced childhood adversity. Mean AUD symptoms by genotype and childhood adversity status in EA men are shown with 95% confidence intervals in Figure 1. Carriers of the A allele who did not endorse adverse childhood experiences reported a mean of 3.6 (SD=3.3) AUD symptoms, whereas those who endorsed adverse childhood events reported a mean of 6.3 (SD=2.9) symptoms, much closer to the mean symptom counts of noncarriers (6.3 (SD=3.5) and 7.0 (SD=3.3) for unexposed and exposed groups, respectively. An independent t-test comparing childhood adversity-exposed versus unexposed carriers of the protective allele revealed a statistically significant group difference (t(90)=3.16, p=0.002).
Figure 1.
Mean alcohol use disorder (AUD) symptoms in European-American men by ADH1B-rs1229984 genotype and childhood adversity status
Discussion
Our investigation extends the literature on the contribution of adverse childhood experiences to the manifestation of genetic liability to alcohol problems by examining risk variants associated with alcohol dependence – rarely the subject of GxE investigations in this area – in a large sample with greater representation of African-Americans and women than prior GxE studies of alcohol phenotypes. The ADH1B SNPs that we investigated, the most significant ADH1B SNPs identified in a GWAS of alcohol dependence conducted with this sample (rs2066702 in the AA subsample, rs1229984 in the EA subsample), predicted both AUD symptoms and maxdrinks (when analyzed across sex), indicating that the effects are not specific to an extreme alcohol phenotype. Childhood adversity also predicted maxdrinks in AA women, AA men, and EA women as well as AUD symptoms in African-Americans, a noteworthy finding given the high rate of exposure to adverse childhood events and the heaviness of alcohol consumption in the sample. Finally, we found support for an interaction effect of rs1229984 with childhood adversity on AUD symptoms exclusively in EA men. Specifically, the A allele’s protective effects against the development of AUD symptoms were substantially reduced in EA men exposed to adverse childhood events. Our findings are partially consistent with the only other known GxE study of rs1229984 and childhood adversity, which was conducted in a largely male sample of Israeli Jews.
Specificity of the Observed GxE Effects
The specificity of the GxE effects to AUD symptoms in EA men raises questions regarding possible sources of distinction by race/ethnicity, sex, and alcohol phenotype. Findings indicate that African-Americans differ from European-Americans in the impact of childhood adversity on genetic liability to alcohol problems. There is some evidence that African-Americans are more resilient than European-Americans to the potential psychiatric sequelae of trauma (Schilling et al., 2007). Although the statistically significant main effects for childhood adversity in AA women and men appear to contradict this contention, the absence of an interaction effect indicates that experiencing adverse events in childhood does not attenuate the protective effects of the minor allele on the ADHB1 variant, as it does in EA males. This distinction may reflect differences by population in the mechanisms underlying the link between childhood adversity and alcohol outcomes.
Greater resilience to psychiatric and substance use problems following trauma exposure has also been observed in women compared with men (DuMont et al., 2007, Schilling et al., 2007), so the same argument for differences in the underlying mechanisms can be made for sex as for population differences. The odds ratio for the interaction term in the AUD symptom model for EA women was in the same direction as in the model for EA men, so another possible explanation for the lack of interaction effects in EA women is reduced power (estimated at below 0.2) associated with a smaller number of EA women than men (n= 586 vs. 850). The one known GxE study of adverse events and alcohol phenotypes to test for sex differences was conducted with a small sample of adolescents and young adults in Germany (167 females and 142 males) (Laucht et al., 2009). A significant interaction was observed between early adversity and 5-HTTLPR on alcohol consumption in both sexes, but interaction effects with current stressful life events were observed for consumption and binge drinking only in males, suggestive of greater genotypic sensitivity in males to certain environmental stressors.
The specificity of the interaction effects for the AUD symptom phenotype is surprising given the evidence of overlap in genetic effects on alcohol dependence and maxdrinks (Grant et al., 2009, Kendler et al., 2010) and Meyers et al.’s (2013b), finding that childhood adversity moderated the influence of rs1229984 on both alcohol phenotypes. The absence of an effect on maxdrinks may be due in part to the distinction between the two alcohol-related phenotypes (although it is unclear why this distinction has not impacted results in other studies). Whereas AUD symptom count is a direct measure of problems resulting from alcohol use, that is pathological use, maxdrinks captures a snapshot of peak alcohol use that may or may not be representative of long-term patterns of alcohol consumption.
Contrast with Interpretation of Results from the only Prior Study of ADH1B and Childhood Adversity Effects on Alcohol-Related Phenotypes
Although ADH1B variants are among those most consistently identified in genetic studies of alcohol phenotypes (e.g., Bierut et al., 2012, Carr et al., 2002, Gelernter et al., 2014, Kim et al., 2008, Li et al., 2011, Meyers et al., 2013a, Neumark et al., 2004, Park et al., 2013, Tolstrup et al., 2008), we know of only one previous GxE investigation of childhood adversity that examined ADH1B, a study based on a sample of 1,143 Israeli Jews (78.3% male) (Meyers et al., 2013b). The inclusion of a substantial number of African-Americans and women in this study allowed us to examine sex differences and determine the generalizability of their findings in another population. Meyers and colleagues found that the relative influence of rs1229984 on maxdrinks and AUD symptoms was greater among individuals exposed to adverse events in childhood than among those who were not exposed. Our results are only partially consistent with theirs. In addition to the fact that we observed an interaction only for AUD symptoms and Meyers et al. (2013b) found an interaction for both maxdrinks and AUD symptom phenotypes, the studies differ in the nature of the GxE interaction. Whereas we observed a protective effect of the rs1229984 A allele in EA men who had not been exposed to adverse events in childhood (OR=0.24, CI:0.15–0.36, p<0.001), but no protective effect in A-allele carriers who had been exposed (OR=0.57, CI:0.32–1.01, p=0.056), Meyers and colleagues found that the contribution of the rs1229984 genotype was greater in the childhood adversity-exposed than the unexposed group. The outliers in our study were the A-allele carriers who had not been exposed to childhood adversity. They endorsed an average of 3.6 AUD criteria, approximately 3 fewer than the exposed carriers (6.3), unexposed noncarriers (6.3), and exposed noncarriers (7.0). By contrast, the outliers in Meyers et al.’s study were the noncarriers exposed to adversity in childhood, who endorsed the largest number of AUD criteria. In other words, we found that childhood adversity attenuated the protective effects of the A allele, whereas Meyers et al. found that the A allele maintained its protective effect under conditions of childhood adversity, but in the absence of the A allele, childhood adversity was associated with heavy and problem drinking. The differences between the two studies in the observed joint effects of rs1229984 and childhood adversity on AUD symptoms are likely related to sample differences: one drawn from a population with relatively low rates of alcohol consumption and problem drinking versus one ascertained on substance use disorder status (in the individual or a family member) reporting high rates of alcohol use and drinking problems. A reduction in the protective effect of the A allele under conditions of childhood adversity may only be observable in the context of heavy alcohol consumption.
Limitations
Certain limitations should be considered in interpreting findings from the current study. First, the sample was composed of participants from several different studies that employed different study designs (e.g., case–control, high-risk family), resulting in an overrepresentation of heavy substance users in a heterogeneous sample. The prevalence of exposure to childhood adversity and endorsement of AUD symptoms and maxdrinks are therefore neither representative of clinical populations nor of the general population. However, as the association of genetic background and childhood adversity with alcohol outcomes would not be impacted by the heterogeneity of the sample or high concentration of childhood adversity and problem alcohol use, variability in the sample ascertainment does not limit interpretation of the key findings. Second, although preliminary phenotypic analyses supported conducting GxE analyses separately by sex as well as population, the resulting increase in tests raises the possibility of Type I error inflation. However, even after applying a Bonferroni correction for multiple testing within groups – a conservative adjustment given the nonindependence of the two alcohol outcomes – the GxE interaction in the EA male model remained statistically significant…
Conclusions and Future Directions
The protective effects of variants in ADH1B that were observed in prior alcohol studies were evident for the maximum number of drinks consumed in a 24-hour period and AUD symptoms in both populations (when analyzed across sex). However, among EA males, the protective effect against development of AUD symptoms conferred by the A allele on ADH1B-rs1229984 was not present in individuals who had experienced adverse events in childhood. Consistent with the extensive literature on early adversity and alcohol-related problems (Afifi et al., 2012, Fergusson et al., 1996a, Kendler et al., 2000, Kessler et al., 1997b, Nelson et al., 2006, Schilling et al., 2007), childhood adversity predicted both alcohol outcomes in African-Americans as well as AUD symptoms in EA women, but there was evidence of moderation of the genetic effect only in EA men. There is a paucity of research on sex and population differences in the joint contributions of environmental factors and genetic variants associated with AUD to alcohol phenotypes, yet this line of research is highly informative in identifying resilient and high-risk populations. Exploration of moderation by other environmental influences at the familial (e.g., parental monitoring) and community (e.g., neighborhood poverty) levels is therefore merited. Furthermore, examination of sex and racial/ethnic differences in GxE effects using a broader range of genetic variants associated with the many biological pathways involved in alcohol consumption and addiction vulnerability will lead to a clearer understanding of the mechanisms underlying gene–environment interplay as well as sources of sex and racial/ethnic differences in moderation effects.
Acknowledgments
The following investigators oversaw subject recruitment and assessment at their respective sites: Roger Weiss, M.D. (McLean Hospital), Kathleen Brady, M.D., Ph.D. and Raymond Anton, M.D. (Medical University of South Carolina), and David Oslin, M.D. (University of Pennsylvania).
This work was supported by NIH grants DA12849, DA00167, DA12690, AA017921, AA11330, and AA13736, CTSA KL2 RR024138, the Robert E. Leet and Clara Guthrie Patterson Trust, the APA/Merck Early Academic Career Award Program, and the VA CT and Philadelphia VA Mental Illness Research, Education, and Clinical Centers
References
- Afifi TO, Henriksen CA, Asmundson GJ, Sareen J. Childhood maltreatment and substance use disorders among men and women in a nationally representative sample. Can J Psychiatry. 2012;57:677–686. doi: 10.1177/070674371205701105. [DOI] [PubMed] [Google Scholar]
- Bau CH, Almeida S, Hutz MH. The TaqI A1 allele of the dopamine D2 receptor gene and alcoholism in Brazil: association and interaction with stress and harm avoidance on severity prediction. Am J Med Genet. 2000;96:302–306. doi: 10.1002/1096-8628(20000612)96:3<302::aid-ajmg13>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Goate AM, Breslau N, Johnson EO, Bertelsen S, Fox L, Agrawal A, Bucholz KK, Grucza R, Hesselbrock V, Kramer J, Kuperman S, Nurnberger J, Porjesz B, Saccone NL, Schuckit M, Tischfield J, Wang JC, Foroud T, Rice JP, Edenberg HJ. ADH1B is associated with alcohol dependence and alcohol consumption in populations of European and African ancestry. Mol Psychiatry. 2012;17:445–450. doi: 10.1038/mp.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr LG, Foroud T, Stewart T, Castelluccio P, Edenberg HJ, Li TK. Influence of ADH1B polymorphism on alcohol use and its subjective effects in a Jewish population. Am J Med Genet. 2002;112:138–143. doi: 10.1002/ajmg.10674. [DOI] [PubMed] [Google Scholar]
- Chan KK, Neighbors C, Gilson M, Larimer ME, Marlatt GA. Epidemiological trends in drinking by age and gender: providing normative feedback to adults. Addict Behav. 2007;32:967–976. doi: 10.1016/j.addbeh.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Dick DM, Plunkett J, Hamlin D, Nurnberger J, Jr, Kuperman S, Schuckit M, Hesselbrock V, Edenberg H, Bierut L. Association analyses of the serotonin transporter gene with lifetime depression and alcohol dependence in the Collaborative Study on the Genetics of Alcoholism (COGA) sample. Psychiatr Genet. 2007;17:35–38. doi: 10.1097/YPG.0b013e328011188b. [DOI] [PubMed] [Google Scholar]
- Ducci F, Enoch MA, Hodgkinson C, Xu K, Catena M, Robin RW, Goldman D. Interaction between a functional MAOA locus and childhood sexual abuse predicts alcoholism and antisocial personality disorder in adult women. Mol Psychiatry. 2008;13:334–347. doi: 10.1038/sj.mp.4002034. [DOI] [PubMed] [Google Scholar]
- DuMont KA, Widom CS, Czaja SJ. Predictors of resilience in abused and neglected children grown-up: the role of individual and neighborhood characteristics. Child Abuse Negl. 2007;31:255–274. doi: 10.1016/j.chiabu.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Enoch MA, Hodgkinson CA, Yuan Q, Shen PH, Goldman D, Roy A. The influence of GABRA2, childhood trauma, and their interaction on alcohol, heroin, and cocaine dependence. Biol Psychiatry. 2010;67:20–27. doi: 10.1016/j.biopsych.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinn R, Gelernter J, Cubells JF, Farrer L, Kranzler HR. Sources of unreliability in the diagnosis of substance dependence. J Stud Alcohol Drugs. 2009;70:475–481. doi: 10.15288/jsad.2009.70.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Lynskey MT. Childhood sexual abuse and psychiatric disorder in young adulthood: II. Psychiatric outcomes of childhood sexual abuse. J Am Acad Child Adolesc Psychiatry. 1996a;35:1365–1374. doi: 10.1097/00004583-199610000-00024. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Lynskey MT, Horwood LJ. Childhood sexual abuse and psychiatric disorder in young adulthood: I. Prevalence of sexual abuse and factors associated with sexual abuse. J Am Acad Child Adolesc Psychiatry. 1996b;35:1355–1364. doi: 10.1097/00004583-199610000-00023. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Kranzler HR, Sherva R, Almasy L, Koesterer R, Smith AH, Anton R, Preuss UW, Ridinger M, Rujescu D, Wodarz N, Zill P, Zhao H, Farrer LA. Genome-wide association study of alcohol dependence:significant findings in African- and European-Americans including novel risk loci. Mol Psychiatry. 2014;19:41–49. doi: 10.1038/mp.2013.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JD, Agrawal A, Bucholz KK, Madden PAF, Pergadia ML, Nelson EC, Lynskey MT, Todd RD, Todorov A, Hansell NK, Whitfield JB, Martin NG, Heath AC. Alcohol consumption indices of genetic risk for alcohol dependence. Biol Psychiatry. 2009;66:795–800. doi: 10.1016/j.biopsych.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley TD, Edenberg HJ. Genes encoding enzymes involved in ethanol metabolism. Alcohol Res. 2012;34:339–344. doi: 10.35946/arcr.v34.3.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Yang B-Z, Douglas-Palumberi H, Crouse-Artus M, Lipschitz D, Krystal JH, Gelernter J. Genetic and environmental predictors of early alcohol use. Biol Psychiatry. 2007;61:1228–1234. doi: 10.1016/j.biopsych.2006.06.039. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Bulik CM, Silberg J, Hettema JM, Myers J, Prescott CA. Childhood sexual abuse and adult psychiatric and substance use disorders in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57:953–959. doi: 10.1001/archpsyc.57.10.953. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Myers J, Dick D, Prescott CA. The relationship between genetic influences on alcohol dependence and on patterns of alcohol consumption. Alcohol Clin Exp Res. 2010;34:1058–1065. doi: 10.1111/j.1530-0277.2010.01181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R, Crum RM, Warner L, Nelson CB, Schulenberg JE, Anthony JC. Lifetime co-occurence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Arch Gen Psychiatry. 1997a;54:313–321. doi: 10.1001/archpsyc.1997.01830160031005. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Davis CG, Kendler KS. Childhood adversity and adult psychiatric disorder in the US National Comorbidity Survey. Psychol Med. 1997b;27:1101–1119. doi: 10.1017/s0033291797005588. [DOI] [PubMed] [Google Scholar]
- Keyes KM, Grant BF, Hasin DS. Evidence for a closing gender gap in alcohol use, abuse, and dependence in the United States population. Drug Alcohol Depend. 2008;93:21–29. doi: 10.1016/j.drugalcdep.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DJ, Choi IG, Park BL, Lee BC, Ham BJ, Yoon S, Bae JS, Cheong HS, Shin HD. Major genetic components underlying alcoholism in Korean population. Hum Mol Genet. 2008;17:854–858. doi: 10.1093/hmg/ddm357. [DOI] [PubMed] [Google Scholar]
- Laucht M, Treutlein J, Schmid B, Blomeyer D, Becker K, Buchmann AF, Schmidt MH, Esser G, Jennen-Steinmetz C, Rietschel M, Zimmermann US, Banaschewski T. Impact of psychosocial adversity on alcohol intake in young adults: moderation by the LL genotype of the serotonin transporter polymorphism. Biol Psychiatry. 2009;66:102–109. doi: 10.1016/j.biopsych.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Li D, Zhao H, Gelernter J. Strong association of the alcohol dehydrogenase 1B gene (ADH1B) with alcohol dependence and alcohol-induced medical diseases. Biol Psychiatry. 2011;70:504–512. doi: 10.1016/j.biopsych.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid GA, MacMurray J, Lee JW, Anderson BA, Comings DE. Stress as a mediating factor in the association between the DRD2 TaqI polymorphism and alcoholism. Alcohol. 2001;23:117–122. doi: 10.1016/s0741-8329(00)00138-5. [DOI] [PubMed] [Google Scholar]
- Maher B. Personal genomes: The case of the missing heritability. Nature. 2008;456:18–21. doi: 10.1038/456018a. [DOI] [PubMed] [Google Scholar]
- Meyers JL, Shmulewitz D, Aharonovich E, Waxman R, Frisch A, Weizman A, Spivak B, Edenberg HJ, Gelernter J, Hasin DS. Alcohol-metabolizing genes and alcohol phenotypes in an Israeli household sample. Alcohol Clin Exp Res. 2013a;37:1872–1881. doi: 10.1111/acer.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers JL, Shmulewitz D, Wall MM, Keyes KM, Aharonovich E, Spivak B, Weizman A, Frisch A, Edenberg HJ, Gelernter J, Grant BF, Hasin D. Childhood adversity moderates the effect of ADH1B on risk for alcohol-related phenotypes in Jewish Israeli drinkers. Addict Biol. 2013b doi: 10.1111/adb.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EC, Agrawal A, Pergadia ML, Wang JC, Whitfield JB, Saccone FS, Kern J, Grant JD, Schrage AJ, Rice JP, Montgomery GW, Heath AC, Goate AM, Martin NG, Madden PA. H2 haplotype at chromosome 17q21.31 protects against childhood sexual abuse-associated risk for alcohol consumption and dependence. Addict Biol. 2010;15:1–11. doi: 10.1111/j.1369-1600.2009.00181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EC, Heath AC, Lynskey MT, Bucholz KK, Madden PA, Statham DJ, Martin NG. Childhood sexual abuse and risks for licit and illicit drug-related outcomes: a twin study. Psychol Med. 2006;36:1473–1483. doi: 10.1017/S0033291706008397. [DOI] [PubMed] [Google Scholar]
- Neumark YD, Friedlander Y, Durst R, Leitersdorf E, Jaffe D, Ramchandani VA, O'Connor S, Carr LG, Li TK. Alcohol dehydrogenase polymorphisms influence alcohol-elimination rates in a male Jewish population. Alcohol Clin Exp Res. 2004;28:10–14. doi: 10.1097/01.ALC.0000108667.79219.4D. [DOI] [PubMed] [Google Scholar]
- Nilsson KW, Sjoberg RL, Wargelius HL, Leppert J, Lindstrom L, Oreland L. The monoamine oxidase A (MAO-A) gene, family function and maltreatment as predictors of destructive behaviour during male adolescent alcohol consumption. Addiction. 2007;102:389–398. doi: 10.1111/j.1360-0443.2006.01702.x. [DOI] [PubMed] [Google Scholar]
- Nilsson KW, Wargelius HL, Sjoberg RL, Leppert J, Oreland L. The MAO-A gene, platelet MAO-B activity and psychosocial environment in adolescent female alcohol-related problem behaviour. Drug Alcohol Depend. 2008;93:51–62. doi: 10.1016/j.drugalcdep.2007.08.022. [DOI] [PubMed] [Google Scholar]
- Park BL, Kim JW, Cheong HS, Kim LH, Lee BC, Seo CH, Kang TC, Nam YW, Kim GB, Shin HD, Choi IG. Extended genetic effects of ADH cluster genes on the risk of alcohol dependence: from GWAS to replication. Hum Genet. 2013;132:657–668. doi: 10.1007/s00439-013-1281-8. [DOI] [PubMed] [Google Scholar]
- Pereda N, Guilera G, Forns M, Gomez-Benito J. The prevalence of child sexual abuse in community and student samples: a meta-analysis. Clin Psychol Rev. 2009;29:328–338. doi: 10.1016/j.cpr.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Pierucci-Lagha A, Gelernter J, Chan G, Arias A, Cubells JF, Farrer L, Kranzler HR. Reliability of DSM-IV diagnostic criteria using the semi-structured assessment for drug dependence and alcoholism (SSADDA) Drug Alcohol Depend. 2007;91:85–90. doi: 10.1016/j.drugalcdep.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierucci-Lagha A, Gelernter J, Feinn R, Cubells JF, Pearson D, Pollastri A, Farrer L, Kranzler HR. Diagnostic reliability of the Semi-structured Assessment for Drug Dependence and Alcoholism (SSADDA) Drug Alcohol Depend. 2005;80:303–312. doi: 10.1016/j.drugalcdep.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Reed T, Page WF, Viken RJ, Christian JC. Genetic predisposition to organ-specific endpoints of alcoholism. Alcohol Clin Exp Res. 1996;23:1528–1533. doi: 10.1111/j.1530-0277.1996.tb01695.x. [DOI] [PubMed] [Google Scholar]
- Schilling EA, Aseltine RH, Jr, Gore S. Adverse childhood experiences and mental health in young adults: a longitudinal survey. BMC Public Health. 2007;7:30. doi: 10.1186/1471-2458-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp. Stata. College Station, TX: 2007. [Google Scholar]
- Sun J, Bi J, Chan G, Oslin D, Farrer L, Gelernter J, Kranzler HR. Improved methods to identify stable, highly heritable subtypes of opioid use and related behaviors. Addict Behav. 2012;37:1138–1144. doi: 10.1016/j.addbeh.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolstrup JS, Nordestgaard BG, Rasmussen S, Tybjaerg-Hansen A, Gronbaek M. Alcoholism and alcohol drinking habits predicted from alcohol dehydrogenase genes. Pharmacogenomics J. 2008;8:220–227. doi: 10.1038/sj.tpj.6500471. [DOI] [PubMed] [Google Scholar]
- True WR, Heath AC, Bucholz K, Slutske W, Romeis JC, Scherrer JF, Lin N, Eisen SA, Goldberg J, Lyons MJ, Tsuang MT. Models of treatment seeking for alcoholism: the role of genes and environment. Alcohol Clin Exp Res. 1996;20:1577–1581. doi: 10.1111/j.1530-0277.1996.tb01702.x. [DOI] [PubMed] [Google Scholar]
- van den Bree MBM, Johnson EO, Neale MC, Svikis DS, McGue M, Pickens RW. Genetic analysis of diagnostic systems of alcoholism in males. Biol Psychiatry. 1998;43:139–145. doi: 10.1016/S0006-3223(97)00225-4. [DOI] [PubMed] [Google Scholar]