Abstract
Increasing evidence sustains that the establishment and maintenance of many, if not all, human cancers are due to cancer stem cells (CSCs), tumor cells with stem cell properties, such as the capacity to self-renew or generate progenitor and differentiated cells. CSCs seem to play a major role in tumor metastasis and drug resistance but, albeit the potential clinical importance, their regulation at the molecular level is not clear. Recent studies have highlighted several miRNAs to be differentially expressed in normal and cancer stem cells and established their role in targeting genes and pathways supporting cancer stemness properties. This review focuses on the last advances on the role of microRNAs in the regulation of stem cell properties and cancer stem cells in different tumors.
Graphical abstract
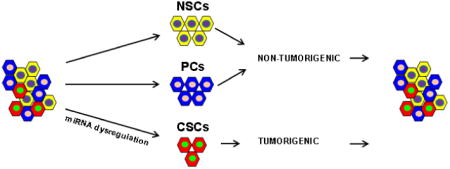
Introduction
The cancer stem cells hypothesis proposes that tumors are formed by heterogeneous cells derived from cancer stem cells, which have self-renewal, differentiation and homeostatic control capabilities. Normal stem cells are tissue specific cells with unlimited ability to self-renew or engender progenitor and differentiated cells [1]. Proper regulation of these properties is crucial in animal development, growth and reproduction. Therefore, cancer might derive from cells with stem cell properties or from the progenitors of stem cells that normally endure limited cycles of cell divisions after acquiring genetic modifications and epigenetic alterations [2] (Figure 1). The cancer stem cell hypothesis was launched more than one century ago by Cohnheim and Durante, based on the observation that embryonic tissue and cancer share common characteristics such as the formidable ability to proliferate and differentiate [3,4,5,6]. Today what it is known about the biology of CSCs is the result of experiments in normal and malignant hematopoiesis which led to the identification of hematopoietic stem cell (HSC) as well the malignant leukemia stem cell (LSC). LSCs preserve many aspects of normal HSCs [7], suggesting that the malignant stem cell population can originate from normal HSCs or from differentiated cells after the onset of mutations (Figure 1). In the late 1980s cell surface markers were identified allowing the isolation of normal HSCs cells by FACS (fluorescence-activated cell sorting) [8]. Subsequent methodologies developed in the study of hematopoietic stem cells, have provided striking evidence that the stem cell theory is true also for some solid tumors. Al-Hajj et al., identified breast tumor-initiating cells (TICs) capable to form tumors in vivo [9]. In fact, as few as 1000 purified tumor cells expressing a CD44+/CD24low Lineage- (CD is short for cluster of differentiation) cell surface phenotype were shown to initiate tumors after transplantation in NOD/SCID mice, whereas the injection of as many as 10000 CD44+/CD24+ Lineage – cells failed to initiate growth. Flow cytometry analysis of the tumors showed a population of cells identical in phenotype to those of the tumor of origin. [9]. Further evidence in support of the role for stem cells in solid cancers came from the study of brain tumors [10]. Singh et al., reported that the neural stem cell antigen CD133 expressed on brain-derived TICs cells gave rise in vitro to neurospheres capable of self-renewal, differentiation and proliferation analogous to normal brain stem cells [11]. These findings, implicate TICs as the responsible for the development of brain cancer. The fact that CSC properties were only investigated by transplantation assays in immunocompromised mice and the variable specificity of the cell-surface markers used to discriminate a CSC from a non-CSC, did not convince everyone on the existence of CSCs. Recently, Driessens et al. used a genetic labeling strategy of skin tumors that allows individual tumour cells to be marked and traced over time at different stages of tumour progression. They found that the majority of labeled tumour cells in benign papilloma have only limited proliferative potential, whereas a fraction has the capacity to persist long term, giving rise to progeny that occupy a significant part of the tumour [12]. Shepers et al. using mouse models and “lineage retracing” using the multicolor Cre-reporter R26R-Confetti, demonstrated that the stem cell marker Lgr5 (leucine-rich repeat–containing heterotrimeric guanine nucleotide–binding protein–coupled receptor 5) encoded by a Wnt target gene and itself a Wnt receptor component, marks a subpopulation of adenoma cells that fuel the growth of established intestinal adenomas [13]. Finally, Chen et al. showed that nestin-ΔTK-IRES-GFP (Nes-ΔTK-GFP) transgene that labels quiescent adult neural stem cells also labels a subset of endogenous glioma tumour cells in a glioma mouse model [14]. Using genetic labeling techniques to trace cells in solid cancers, these three new studies provide a strong evidence of the existence of cancer stem cells in different tumors. Importantly, CSCs are resistant to conventional treatments and are thus not only of academic interest, but might also be potentially useful pharmacologic targets. Therefore, therapies targeted to eliminate CSCs offer the potential for a cure. MiRNAs are small non-coding RNAs, crucial post-transcriptional regulators of gene expression. They are key players in various critical cellular processes including self-renewal and differentiation. Recently, abnormalities in non-coding RNAs have been reported to be fundamental in the regulation of CSC properties such as asymmetric cell division, tumorigenicity and drug resistance. In this review we will discuss recent findings on the role of microRNAs in cell differentiation, self-renewal and/or maintaining of cancer-stem cell properties.
Figure 1. Cancer stem cell theory.
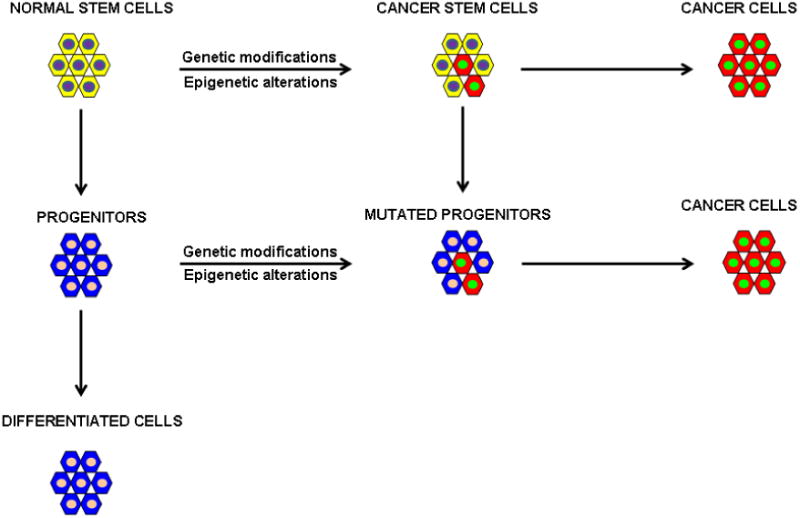
Cancer might arise from cells with stem cell properties or from the progenitors of stem cells that normally have limited numbers of cell divisions after the acquisition of genetic modifications and/or epigenetic alterations.
1. MicroRNA biogenesis
MicroRNAs or miRNAs are short (20–24-nucleotides) non-coding RNAs, that regulate gene expression at the post-transcriptional level by binding to the 3′-untraslated regions (3′UTRs) or the open reading frames of target genes, leading to the degradation of target mRNA or repression of mRNA translation. MiRNAs are transcribed as long primary transcripts characterized by hairpin structures (pri-miRNAs) whose maturation occurs through sequential processing events. First, the pri-miRNA is cleaved in the nucleus by the RNase III Drosha into roughly 70-100 nucleotide-long precursor miRNAs (pre-miRNAs) in combination with cofactors such as DGCR8 [15]. The product of pri-miRNA cleavage, the pre-miRNA is exported to the cytoplasm by Exportin 5 (Exp5) and its Ran–guanosine triphosphate (Ran-GTP) cofactor [16] and further processed by another RNase III–type class III enzyme, Dicer in a double strand RNA of about 19-24 nucleotides. While one of the two strands is selected as a guide strand, the complementary strand is usually degradated [17]. The mature miRNAs are incorporated into a complex named RISC (RNA-induced silencing complex), which contains Argonaute proteins. The function of the miR is to guide the RISC to complementary or partially complementary target sites located in the 3′ UTRs of mRNAs target inducing mRNA degradation or block of translation, respectively. Above all, miRNAs have been shown to regulate the CSC phenotype and function through multiple signalling pathways, playing important roles in tumor development and progression.
2. microRNAs and embryonic stem cells (ESCs)
The most primitive stem cell is the ESC, which derives from the internal cell mass of the blastocyst. The ESC is pluripotent and can therefore generate all the tissues of the body [18]. The role for miRNAs in regulating stem cells was first identified by Lee and colleagues who reported that two microRNAs, lin-4 and let-7, regulated the timing of larval to adult cell fates in C. elegans [19]. Lin-4 and let-7 expression was null in the embryo and increased during the larval stage and in the adult, suggesting that they might play a key role in differentiation [20,21]. Mouse and human ESCs lacking Dicer1 and DGCR8, both critical for miRNA biogenesis, have been utilized to study the involvement of miRNAs in these cells. Deletion of Dicer1 led to embryonic lethality in mice [22]. DGCR8-knockout mouse ESCs showed alterations in the regulation of the cell cycle and differentiation that are associated with failure to silence stemness transcription factors, such as Oct4, Rex1, Sox2 and Nanog, which control ESC renewal and pluripotency [23,24] and delayed expression of differentiation markers [25]. In a comparative transcriptome analysis, Sinkkonen et al. showed that members of the miR-290 family were able to rescue the differentiation defects of Dicer–/– mouse ESCs by downregulating a transcriptional repressor of de novo methyltransferases. This regulation was necessary for Oct4 stable repression [26]. Card et al. demonstrated that Oct4 and Sox2 bind to the promoter region of miR-302 cluster, specifically expressed in ESCs and pluripotent cells. Expression of miR-302a in primary and transformed cell lines induced the transition from the phase G1 to the phase S. Conversely, the inhibition of miR-302 caused hESCs to accumulate in G(1) phase by targeting an important G(1) regulator, cyclin D1 [27].
Therefore, miRNAs such as the miR-290 cluster in mouse and miR-302 family in human are specifically expressed in stem cells and control self-renewal and differentiation by negatively regulating the expression of key genes in stem cells.
Melton et al. showed that let-7 miRNA family repress self-renewal in Dgcr8(-/-) but not wild-type ESCs by downregulating Oct4, Sox2 and Nanog. [28]. MiR-34 has been involved in the differentiation of human erythroleukemia cells, monocyte-derived dendritic cells and mouse embryonic stem cells. Members of the miR-34 family of miRNAs exhibit p53-dependent induction during reprogramming. P53 deficiency enhances reprogramming by suppressing miR-34 family and consequent upregulation of pluripotency genes, including Nanog, Sox2 and N-Myc [29]. Arahna and colleagues provided new insights in mouse neuronal stem cells differentiation, showing that miR-34a regulates neuronal differentiation by targeting SIRT1 [30]. Altogether, these findings suggest that stem cells-specific miRNAs may be involved in the regulation and control of stem cell properties [80].
2.1 Liver cancer
In addition to regulating stem cells, miRNAs seem to be involved in CSC self-renewal, differentiation, drug resistance and metastasis. An increasing number of studies have pointed out altered miRNA expression in liver CSC subsets compared with non-CSC subsets or normal liver tissue. Other studies have identified various miRNAs that control the expression of liver CSC markers [31]. Recent findings indicate that dysregulation of the pathways involved in normal stem cell self-renewal such as the Hedgehog, Wnt/b-catenin, Notch, and polycomb genes affect proliferation of CSCs. The Hedgehog pathaway activates Nanog and Oct4 through Gli1 and Gli2 transcription factors [32,33]. WNT signalling regulates Nanog-, Oct4-, Sox2-, and Klf4 pluripotency mantaining factors [34]. Recently, van den Berg and colleagues found that Oct4 associated with Rbpi, the nuclear effector of the Notch signalling pathway, implying a connection between Oct4 and the Notch-regulated gene expression [35] Ji et al. described that multiple members of the miR-181 family, including miR-181a, miR181b, miR181c, and miR181d, are consistently up-regulated in the liver CSC subset marked with EpCAM+AFP+ surface markers. Further, miR-181s maintained stemness by directly targeting GATA6 (GATA-binding protein 6) and CDX2 (caudal type homeobox 2) to block cell differentiation and NLK (nemo-like kinase) to activate the Wnt/β-catenin pathway [36] Table 1(Figure 2). Interestingly, the expression of miR-181 transcripts was directly induced upon activation of the Wnt/β-catenin pathway and was inhibited upon its inactivation. CD133 has been proved to be a marker to isolate liver CSCs; CD133+ HCC (hepatocellular carcinoma) subpopulations presented stem cell properties whereas the CD133- subpopulations included differentiated tumor cells [37,38]. Zhang et al. compared the miRNA profiles of CSCs with that of the non-stem cell population in HCC. They found upregulation of miR-150 in CD133- cells. Overexpression of miR-150 led to a significant reduction of CD133+ cells and to an important inhibition of cell growth and tumorsphere formation and induced cell cycle arrest and apoptosis in CD133+ cells by targeting c-myb and cyclin D1 [39]. Ma et al., reported the overexpression of miR-130b in CD133+ liver CSCs isolated from both HCC cell lines and freshly resected clinical samples. The ectopic expression of miR-130b was found to enhance chemoresistance, self-renewal ability in vitro, and tumorigenicity in vivo in CD133- cells by targeting the tumor suppressor gene TP53INP1, a pro-apoptotic stress-induced p53 target gene with both anti-proliferative and pro-apoptotic activities [40]. More recently, Liu et al. found that miR-130b (as well as miR-15b) was consistently and significantly up-regulated in HCC tissues, cell lines, and patient serum samples. Remarkably, the serum levels of these miRNAs were found to be significantly reduced after surgery, indicating that these circulating miRNAs originated from the tumor [41]. Jia et al. showed that miR-145 expression is lower in HCC cancer stem cells derived from hepatocarcinoma cell line T3A-A3 than in the HCC cell line BEL-7402 or a normal liver sinusoidal endothelial cell line. Overexpression of miR-145 in T3A-A3 cells resulted in cell cycle arrest, inhibition of colony and spheroid formation, and the inhibition of tumor formation in nude mice by targeting Oct4. The results suggest that miR-145 exerts its tumor-suppressive effect in HCC via modulation of this stem cell marker [42]. Table 1.
Table 1. MicroRNAs dysregulated in CSCs.
| Cancer | microRNA | Expression | Process | Target | CSCs marker | Reference |
|---|---|---|---|---|---|---|
| Liver cancer | miR-181s | Upregulated | Differentiation | GATA6- | EpCAM+AFP+ | 36 |
| miR-150 | Downregulated | Self-renewal | CDX2-NLK | CD133 | 39 | |
| miR-130b | Upregulated | Self-renewal, chemoresistance | c-myb-cyclin D1 | CD133 | 40,41 | |
| miR-145 | Downregulated | Self-renewal | TP53INP1 Oct4 | CD133 | 42 | |
| Leukemia | miR-126 | Upregulated | Self-renewal | N/A | CD34+CD38- | 45 |
| miR-29 | Upregulated | Proliferation | HBP1 | CD34+CD38- | 46 | |
| miR-22 | Upregulated | Self-renewal | TET2 | CD34+ | 47 | |
| miR-150 | Downregulated | Differentiation | MYB | CD34+ | 48 | |
| miR-326 | Downregulated | Proliferation | Smo | CD34+ | 49 | |
| Breast cancer | Let-7 | Downregulated | Self-renewal | IL-6, ER-α | CD44+CD24- | 52,54 |
| miR-200-141 | Downregulated | Self-renewal, EMT | Zeb1, Zeb2 | CD44+CD24- | 95 | |
| miR-7 | Downregulated | Differentiation | KLF4 | CD44+CD24- | 55 | |
| miR-30s | Downregulated | Self-renewal | Ubc9, ITGB3 | CD44+CD24- | 56 | |
| miR-128 | Downregulated | Self-renewal, EMT | BMI1, ABCC5, | CD44+ | 57,97 | |
| miR-140 | Downregulated | Self-renewal | Lin28, Nanog, Snail | CD44+ | 59 | |
| miR-181 | Upregulated | Self-renewal | Sox9, ALDH1 | CD44+ | 58 | |
| miR-125 | Upregulated | Chemoresistance, EMT | BRCA1 | CD44+ | 99 | |
| miR-15/16b | Downregulated | Self-renewal, EMT | Bak1 | CD44+ | 103 | |
| miR-103/107 | Downregulated | Self-renewal, EMT | Suz12, Suz12 | 103 | ||
| Colon cancer | miR-451 | Downregulated | Self-renewal, | ABCB1 | CD133+/CD44+ | 61 |
| miR-21 | Upregulated | chemoresistance | TGFbR2 | CD44+ | 64 | |
| miR-34a | Downregulated | Differentiation Differentiation, EMT | Notch1, Zeb1, Snail, Slug | CD133+/CD44+ | 66,98 | |
| Lung cancer | miR-34 | Downregulated | Self-renewal | N/A | CD44+ | 75 |
| miR-27 | Downregulated | Self-renewal | N/A | Upar/CD133+ | 77 | |
| miR-191 | Upregulated | Self-renewal | BASP1 | CD133+/CD44+ | 79 | |
| Braintumors | miR-218 | Downregulated | Self-renewal | BMI | CD133+, nestin | 81 |
| miR-107 | Downregulated | Proliferation | Notch2 | CD133+, nestin | 82 | |
| miR-128 | Downregulated | Self-renewal | Suz12 | CD133+ | 83 | |
| miR-134 | Downregulated | Proliferation | Nanog | CD133+ | 84 | |
| miR-153 | Differentiation | Self-renewal | BCL2, MCL1 | 85 | ||
| miR-204 | Downregulated | Self-renewal | Sox4, EphB2 | 86 | ||
| Prostate cancer | miR-34a/let-7b | Downregulated | Self-renewal | CD44 | CD44+ | 80 |
| miR-145 | Downregulated | Self-renewal | CD44, Oct4, c-Myc, KLF4 | CD44+ | 81 | |
| Ovarian Cancer | miR-134 | Downregulated | Differentiation | Nanog, LRH1 | CD44+ | 89 |
| miR-708 | Downregulated | Proliferation | AKT2, LRH1 | CD44+ | 92 | |
| miR-199a/214 | Upregulated | Differentiation, EMT | TWIST | CD44+ | 100 |
Figure 2. Involvement of Wnt, Sonic Hedgehog (SHH) and Notch in CSCs.
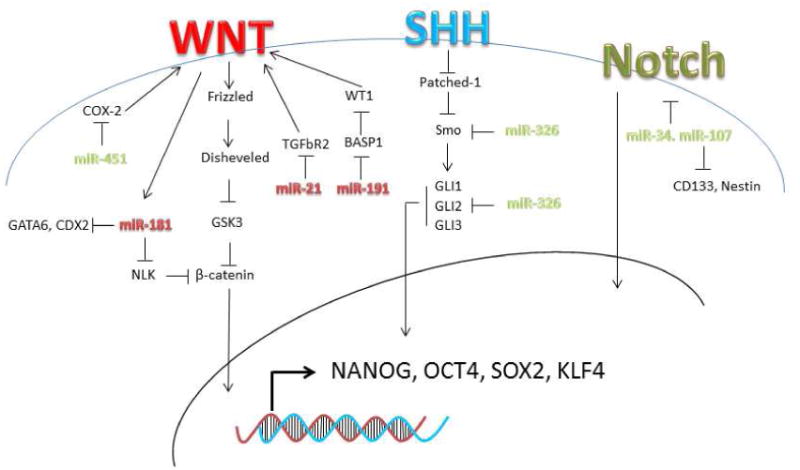
In red are the upregulated and in green the downregulated microRNAs involved the activation/inactivation of Wnt, SHH and Notch in CSCs.
2.2 Leukemia
Recently, dysregulation of miRNAs was shown to contribute to hematological malignancies, including AML and myelodysplastic syndrome [43]. In spite of very high remission rates after therapy, 60-70% of acute myeloid leukemia (AML) patients dye commonly five years after their initial diagnosis. One of the reasons of treatment failures is the incomplete elimination of leukemic stem-like cells (LSC), which give rise to more differentiated leukemic progenitors and relapse of the disease. In 1994 John Dick's laboratory isolated a sub-population (CD34+CD38-) from patients with acute myeloid leukemia (AML), demonstrating the existence of LSCs [44]. de Leeuw et al. analyzed microRNA expression profile in healthy CD34+CD38- hematopoietic stem cells (HSC), CD34+CD38- LSC and CD34+CD38+ LP (leukemic progenitors), derived from the same patients' bone marrow specimens. They found that miR-126 was mighty expressed in HSC and its levels increased in LSC compared to LP. High miR-126 expression in AML was associated with poor survival and higher chance of relapse. MiR-126 downregulation in LSC and LP cells reduced their clonogenic capacity and eliminated leukemic cells, suggesting that this microRNA is important in cancer stem cell phenotype maintainance [45]. Han et al. showed that miR-29a is highly expressed in HSC and down-regulated in hematopoietic progenitors. Mouse HSC/progenitors cells overexpressing miR-29a transplanted in mice gave rise to a myeloproliferative disorder that progressed to acute myeloid leukemia (AML). MiR-29a promoted proliferation, accelerating the transition G1 to S/G2, by targeting HBP1, a negative regulator of cell cycle progression, at the G1 to S/G2 phase transition [46]. Arnold et al., reported miRNA expression profile in different adult tissue-specific stem cells and their differentiated counterparts. They identified a stem/progenitor transition miRNA (SPT-miRNA) signature and demonstrated that SPT-miRNAs coordinately regulate genes, such as Hoxb6 and Hoxa4, with a known role in controlling HSC self-renewal [47]. MiR-22 overexpression in myelodysplastic syndrome (MDS) and leukemia correlates with poor survival. Mice conditionally expressing miR-22 in the hematopoietic compartment showed decreased levels of 5-hydroxymethylcytosine (5-hmC) and high hematopoietic stem cell self-renewal and developed MDS and hematological malignancies. Moreover, they identified TET2, (ten-eleven-translocation gene 2), located in 4q24 and whose mutation or deletion is extremely frequent in hematological malignancies, as a target of miR-22. Interestingly, TET2 enforced expression rescued the miR-22-induced phenotypes [47]. In acute myeloid leukemia (AML) and blast crisis (BC) chronic myeloid leukemia (CML) normal differentiation, critical for normal blood cell function, is impaired. Morris et al., analyzed the miRNA expression profile in AML and BC CML. They observed that miR-150 is low or absent in BC CML and AML patient samples and cell lines. Enforced expression of miR-150 promoted myeloid differentiation by targeting MYB. The Hedgehog (Hh) signalling has a major role in development and has been proven as a functional pathway for LSCs; indeed, its loss impairs the development of CML and depletes CML stem cells [48]. Babashab et al., reported that upregulation of the Hh smoothened (Smo) signal transducer was inversely related to miR-326 in the CD34(+) cells from a group of patients with CML. Enforced expression of miR-326 induced downregulation of Smo, reduced cell proliferation and increased the rate of apoptosis in CML CD34(+) cells. Importantly, the restoration of miR-326 expression could eradicate CD34(+) CML stem/progenitor cells, a potential source of relapse in patients suffering CML [49]. (Table 1 and Figure 2)
2.3 Breast cancer
Al-Hajj et al. isolated breast cancer stem cells (BCSCs) in 2003 based on the expression of the surface markers CD44+, CD24-/low and ESA+ (ESA is short for epithelial specific antigen)[9,50]. Few years later, Ginestier et al. identified high aldehyde dehydrogenase 1 (ALDH1) expression in BCSCs extending the BCSC phenotype on CD44+, CD24-/low, ESA+ and alternatively ALDH+ [50,51]. Iliopulos et al. reported that transient activation of Src oncoprotein induced an epigenetic switch from immortalized breast cells to mammospheres that contain cancer stem cells through Lin28-mediated repression of let-7. Let-7 directly targets IL-6 which is foundamental for STAT3 activation, necessary for transformation [52]. Recent findings reported that miR-200 family and its target mRNAs are involved in the maintenance and regulation of the BCSC phenotype. Lim and colleagues investigated the role of the miR-200 family during their conversion to a stem-like phenotype utilizing immortalized human mammary epithelial (HMLE) cells. Remarkably, loss of miR-200 expression converted HMLE cells from a non-stem to a stem-like phenotype. Modifications mediated by a polycomb group were responsible for the silencing of miR-200b-200a-429 cluster in the stem-like phenotype whilst the miR-200c-141 cluster was repressed by DNA methylation. The results pointed out that different epigenetic-based mechanisms regulate each miR-200 gene in the transition between stem-like and non-stem phenotypes [53]. Sun et al., defined that let-7 acts as a tumor suppressor by inhibiting ERα-mediated cellular malignant growth in ER-positive breast cancer stem cells, suggesting that let-7 overexpression may be a promising strategy for the elimination of cancer stem cells [54]. Okuda et al., analyzed the microRNA expression profile in breast CSCs highly metastatic to bone and brain compared to non CSCs populations. MiR-7 was significantly downmodulated in CSCs and was able to modulate Kruppel-like factor 4 (KLF4) a gene with a fundamental role in maintaining embryonic stem cells and in preventing their differentiation. Interestingly, miR-7 enforced expression drastically suppressed the capacity of CSCs to metastasize to brain but not to bone in mice [55]. Also members of miR-30 family, including miR-30d, miR-30a-5p, miR-30e-5p, miR-30b and miR-30c, are reduced in mammospheric SK-3rd cells. Enforced expression of miR-30e in BT-ICs (breast tumor initiating cells) inhibits their self-renewal capacity by reducing Ubc9 (ubiquitin-conjugating enzyme 9), and induces apoptosis through silencing ITGB3 (integrin beta3). Ectopic expression of miR-30e in BT-IC xenografts reduced tumorigenesis and lung metastasis in immunodeficient mice [56,50]. Zhu and colleagues reported reduced miR-128 expression levels in mammospheric BCSCs in two breast cancer cell lines (SK-3rd and MCF-7) and in BCSCs isolated from primary breast cancer patients, whereas protein levels of the polycomb oncogene BMI1 and ATP-binding cassette sub-family C member 5 (ABCC5), targets of miR-128, were increased [57]. A loss of function (LOH) at chromosome 3p has been reported in 87% of primary breast cancers. Levels of miR-181 family members are upregulated in tumor initiating mammospheres compared to non-tumorigenic parental cells. Liu and colleagues identified breast cancer 1 (BRCA1) as a target downregulated by TGFbeta through the miR-181 family. They also found an inverse correlation between TGFbeta and miR-181 with BRCA1 expression in vivo in breast tumor samples [58].The microRNA expression profile of normal mammary stem cells and cancer stem-like cells from ductal carcinoma in situ (DCIS) was analyzed by Li and colleagues. MiR-140 was significantly downregulated in cancer stem-like cells compared to the normal stem cells and was critical in self-renewal through Sox9 and ALDH1 downregulation [59]. Table 1.
3.4 Colon cancer
Fang and colleagues were the first to isolate populations of colon CSCs with the CD133+/CD44+ and CD133-/CD44- surface phenotype from a human SW1116 colon adenocarcinoma cell line, evaluating the miRNA expression differences between colon CSCs and non-stem cells. They found 62 miRNAs and 2049 mRNAs differentially expressed in colon stem cells compared to non-stem cells. Among these differentially expressed miRNAs, 31 miRNAs were overexpressed in colon stem cells, whereas the remaining 31 miRNAs were underexpressed. Overexpression of miR-29a, miR-29b and underexpression of miR-449b, miR-4524 were confirmed by quantitative RT-PCR assay [60]. Bitarte et al. analyzed the microRNA expression profile in different colon carcinoma cells. The results showed that miR-451 was downregulated in colonspheres versus parental cells. Enforced expression of miR-451 decreased self-renewal and chemoresistance of colonspheres to irinotecan by indirectly targeting cyclooxygenase-2 (COX-2), which activates Wnt, essential for CSC growth. (Figure 2). MiR-451 restoration also reduced the expression of the ATP-binding cassette drug transporter ABCB1, improving the response of colon cancer cells irinotecan [61].The Wnt pathway is an important regulator of normal intestinal stem cells [62], and more recently has been recognized a regulator of colon CSCs [63]. When the Wnt signalling is not activated, β-catenin is degradated in the cytosol by the proteasome following glycogen synthase 3 (GSK3) mediated phosphorylation. Inhibition of Wnt signalling blocks epithelial renewal. Yu et al. showed that miR-21 over-expression increased Wnt activity and tumour initiating ability causing a downregulation of the tumor suppressor gene TGFbR2 (Transforming growth factor, beta receptor II), involved in differentiation [64] (Figure 2). Notch is a fundamental pathway regulating intestinal stem cells. In mammals have been reported four Notch genes, which act as receptors for Jagged 1 and 2, and Delta Like (Dll) 1,3 and 4. Activation of Notch-1 signalling reduces differentiation and increases progenitor proliferation [65]. Interestingly, Bu et al. showed that miR-34a play an important role in CCSCs from early-stage colorectal cancer (CRC). The decision of a CCSC to produce two CCS daughter cells or a CCS daughter cell and a differentiated non-CCS daughter cell is closely regulated by miR-34a. High miR-34a levels silence Notch inhibiting its signalling and promoting differentiation, whereas low miR-34a levels induce activation of the Notch signalling, favoring CCSCs generation [66] (Table 1 and Figure 2).
3.5 Lung cancer
Potential lung CSCs have been purified using functional assays. Many attempts to isolate CSCs from both cell lines and primary tumors have been performed during the last years. The first attempt was based on the side population (SP) phenotype (low Hoechst 33342 staining pattern) of stem cells. SP lung cancer cells isolated from different cell lines, showed enhanced invasiveness and higher resistance to chemotherapeutic drugs [67]. A second attempt was based on their resistance to different drugs, such as cisplatin, doxorubicin or etoposide [68]. The third attempt was based on increased ALDH activity. Jiang et al. demonstrated that ALDH-positive cells isolated from lung cancer cell lines showed characteristics of CSCs both in vitro and in vivo [69]. Subsequently, several membrane-bound surface markers to identify CSC in lung cancer were investigated. Of all, CD44 and CD133 seemed to be the most promising. [70,71,72]. CD133 is a member of prominin family, and was first discovered from hematopoietic stem cells as their marker and found later in certain types of leukemic cells. It is an antigen of a 120 kDa five-transmembrane glycoprotein whose expression of CD133 has been reported in CSCs from a variety of solid tumors including brain, prostate, pancreas, colorectum, melanoma, liver and bile duct, lung, ovary, etc. CD44 is a transmembrane glycoprotein activated in a wide range of tumours where it plays a crucial role in migration, invasion and survival. [73] Lungs are constituted by the mosaic of specialized cells that form millions of tiny, exceptionally thin-walled air sacs called alveoli. Alveoli are gas-exchange sacs composed by squamous alveolar type (AT) 1 cells and surfactant secreting AT2 cells. Very recently Desai et al., reported that during development AT1 and AT2 cells arise directly from a bipotent progenitor, whilst after birth new AT1 cells derive from rare, self-renewing AT2 cells. The stem-cell function is activated by AT1 injury and AT2 self-renewal is induced in vivo by KRAS, resulting in cancer. This very interesting study is a further confirmation not only of the existence of CSCs but also of the importance of CSCs in lung cancer formation [74]. Shi et al. showed that overexpression of miR-34a in purified CD44 high H460 cells inhibited tumor outgrowth. In contrast, knockdown of miR-34a in the CD44low H460 cells promoted tumor development, suggesting that miR-34a is a negative regulator of lung CSCs [75]. Gutova et al. identified subpopulations of urokinase plasminogen activator receptor uPAR- (CD87) positive cells in six SCLC cell lines, with multidrug resistance and clonogenic activity in vitro [76]. Miao et al., compared miRNA expression in stem-like cells, uPAR(+) and CD133+, and differentiated cells from small cell lung cancer (SCLC). They found 86 miRNAs that were differentially expressed, including 48 upregulated miRNAs and 38 downregulated miRNAs between sphere-forming cells and parental cells. Among the downregulated miRs, miR-27a had very low expression in sphere-forming cells of different cell lines. Interestingly, inhibition of miR-27a in parental cells enhanced proliferation, self-renewal, and the proportion of undifferentiated cells in vitro [77]. Because of the high rate of recurrence following therapy in all forms of lung cancer, the possibility to block their CSC-like activity is a very attractive treatment option. Polemics widely arise from the lack of specificity of the markers so far identified. Identification of new markers and pathways for lung CSCs isolation is necessary. Moreover, the involvement of microRNAs in lung cancer stem cells maintaining is still in its infancy and other studies should be done to obtain progresses in lung cancer diagnosis and prognosis and to improve tumor eradicating therapies. Dysregulation of embryonic signalling pathways such as Hedgehog (Hh), Notch, Wnt is believed to be involved in driving CSC activity also in lung cancer [66, 78]. Xu et al., demonstrated that miR-191 was up- regulated in human bronchial epithelial (HBE) cells malignantly transformed by arsenite compared to normal HBE cells. MiR-191 directly targets BASP1, increasing the expression of WT1 and promoting the activation of Wnt/β-catenin pathway (Figure 2)[79]. Very recently, Jiang et al., showed that miR-326 acts as a negative regulator of Shh signalling by directly targeting Smo and Gli2 [80]. (Table 1 and Figure 2).
3.6 Brain tumors
As with other cancers it has been suggested that also glioblastoma (GBM) contains functionally subsets of cells with stem-like properties, characterized by resistance to chemotherapics and considered responsible for tumor relapse. Several studies reported that a hypoxic microenvironment play a crucial role in controlling GSC. Malignant glioma is the prevalent central nervous system tumor and the molecular mechanism driving its development and recurrence is barely known. Tu et al., showed that miR-218, a commonly downregulated microRNA in glioblastoma, inhibited the self-renewal of glioma stem-like cells, by targeting stem cell-promoting oncogene BMI1, a component of PCR1 (The Polycomb Repressor Complex) an epigenetic regulator of transcription involved in cancer stem cell maintenance and radioresistance [81].Chen et al., found that miR-107 was down-regulated in GSCs. Overexpression of miR-107 in U87 GSCs suppressed proliferation and down-regulated Notch2 protein and stem cell marker such as CD133 and Nestin (Figure 2) [82]. In another study Peruzzi et al. reported that miR-128 directly targeted the mRNA of Suz12, a key component of PRC2 [83]. Niu et al. found that miR-134 was downregulated in GBM. MiR-134 overexpression decreased proliferation, invasiveness and migration capability of U87 cells promoting apoptosis in vitro and suppressing the growth of tumors in vivo by targeting Nanog [84]. Zhao et al., reported that miR-153 expression was down-regulated in CD133 positive cells compared to CD133 negative cells and enforced expression of miR-153 into GBM-SCs impairs self-renewal ability inducing differentiation [85]. Ying et al., found that miR-204 inhibited stem cell properties and migration of glioma cells by targeting the transcription factor SOX4 and the migration-promoting receptor EphB2 [86]. Table 1.
3.7 Prostate cancer
Liu et al. profiled, for the first time, miRNA expression in prostate CSC and/or progenitor cells. They identified miR-34a and let-7b, to be commonly under-expressed in all marker-positive cell populations. Overexpression of miR-34a, by using miRNA mimics or lentiviral vectors, in purified CD44+ cells inhibited tumor growth and metastasis in vivo. Interestingly, CD44 was a direct target of miR-34 [87]. Ren et al., showed that miR-145, a p53-regulated microRNA, suppressed colony formation, tumor sphere formation and expression of CSC markers and stemness factors including CD44, Oct4, c-Myc and KLF4 in PC-3 cells [88]. Similarly, miR-134 promotes stem cells differentiation by repressing Nanog and LRH1, positive regulators of Oct4/POU5F1 and mouse embryonic stem cells (mESC) growth [89]; thus, decreased miR-134 expression helps maintain SC properties. Inorganic arsenic (iAs) is a human carcinogen that malignantly transforms human prostate epithelial line in cancer cell lines. To investigate differences in miRNA expression profile between the arsenic-transformed epithelial cell population and the transformed SC population, Ngalame et al., analyzed the microRNA expression profile of human prostate epithelial cells (CAsE-PE) and stem cells (As-CSCs) that had been malignantly transformed by chronic iAs exposure. In both transformants, there was a downregulation of miRNAs targeting KRAS and RAS superfamily member. Therefore, dysregulated miRNA expression appears to impact RAS activation, important to arsenic transformation in these cells [90]. Iliopoulos et al., reported that NSCCs (non-stem cancer cells) and CSCs presented different microRNA expression profiles and medium from the transformed population stimulated NSCCs to become CSCs in vitro and in vivo. Intriguingly, IL6 was sufficient to convert NSCCs to CSCs in different breast cell lines, human breast tumors, and a prostate cell line. In this study, they showed that tumor heterogeneity derives from a dynamic equilibrium between CSCs and NSCCs mediated by IL6 [91].
Saini et al. showed that miR-708 was downregulated in CD44(+) cells from prostate cancer xenografts. Forced expression of miR-708 in prostate cancer cell lines or CD44(+) prostate cancer cells induced the downregulation of AKT2 and CD44 and led to decreased tumorigenicity in vitro [92]. Overall these examples indicate that several specific miRNAs have a prime role in the regulation of CSCs by regulating self-renewal, proliferation and differentiation through the downregulation of CSCs specific genes. (Table 1).
CSCs and EMT
It has been proposed that non-cancer stem cells acquire CSC-like properties through the EMT process [93], by which cells lose their epithelial properties and gain migratory and invasive properties to become mesenchymal stem cells. The switch in gene expression and synthesis of mesenchymal proteins is mediated mainly by three families of transcription factors, including SNAI1/2, ZEB1/2, and TWIST1/2. Chaffer and colleagues, demonstrated that non-CSCs of human breast cancers can switch from a non-CSC to a CSC state. This switch is dependent on ZEB1, a key regulator of the epithelial-mesenchymal transition [94]. The miR-200 family, often downregulated in CSCs, was found to directly target the mRNA of the E-cadherin transcriptional repressors ZEB1 and ZEB2. Enforced expression of miR-200 caused up-regulation of E-cadherin in cancer cell lines and reduced their motility in different cancer cell lines [95]. Also, ZEB1 represses miR-200 in a mutual repression loop [96]. Qian et al. showed that miR-128-2 silencing promoted EMT in breast cancer cells through the derepression of a cohort of direct targets (BMI1, CSF1, KLF4, LIN28A, NANOG, and SNAIL), which together induced activation of the PI3K/AKT and STAT3 signalling pathways [97]. Siemens et al. demonstrated that p53 induced the downregulation of Snail through miR-34 family which also down-regulated SLUG and ZEB1 in colorectal cancer. Conversely, SNAIL and ZEB1 bind to E-boxes in the miR-34a/b/c promoters, repressing miR-34a and miR-34b/c expression [98] Upregulation of miR-125b by Snail through Wnt signalling enriched cancer stem cells (CD24-CD44+) and induced chemoresistance in breast cancer cells through Bak1 silencing [99]. Yin et al. showed that epithelial ovarian cancer (EOC) stem cells presented high levels of miR-199a and miR-214. They identified Twist1 as a regulator of this microRNA cluster responsible for the regulation of the IKKbeta/NF-kappaB and PTEN/AKT pathways, suggesting that Twist may be an important regulator of ‘stemness’ in EOC cells [100]. NVP-LDE-225, a smoothened inhibitor, inhibited pluripotency-maintaining factors such as Nanog, Oct-4, c-Myc and Sox-2. TNVP-LDE-225 also suppressed EMT in prostate cancer cells by upregulating E-cadherin and inhibiting N-cadherin, Snail, Slug and Zeb1 through the miR-200 family [101]. In turn, Snail can repress miR-200 family transcriptional activation [102]. Polytarchou et al. identified microRNAs that are down-regulated in CSCs and inhibit CSC growth, including miR-16, miR-15b and miR-103/107 families. These miRNAs commonly target Suz12, a component of the polycomb repressor complexes Suz12 downregulation induces upregulation of E-cadherin and consequent downmodulation of ZEB1 and ZEB2 [103]. (Table 1 and Figure 3).
Figure 3. Link between CSCs and EMT.
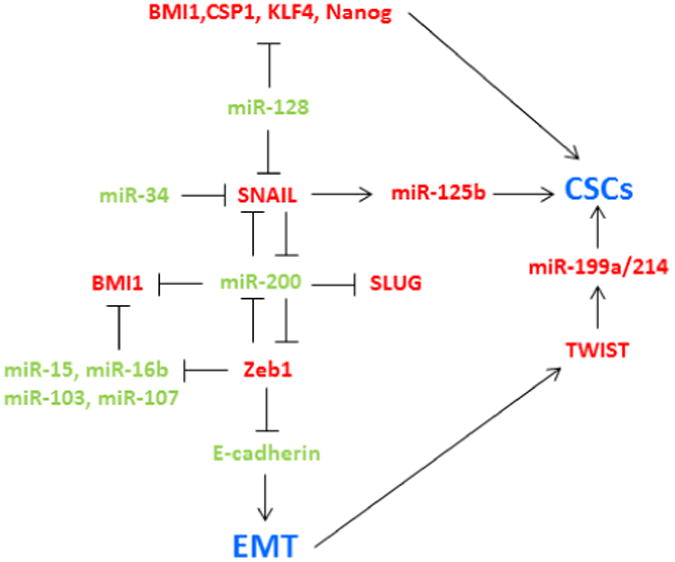
In red are reported the upregulated and in green the downregulated microRNAs and genes involved in cancer stem cell properties and EMT.
3. Concluding remarks
Doubtless CSCs do exist in most tumors. Therefore, the characterization and specific targeting of CSCs for therapeutic purposes should be addressed. Recent research has made increasingly clear that cancer cells display features of normal tissue organization, where CSCs can drive tumor growth. The fact that cancer is mainly driven by a small population of stem cells has important implications. If new anti-cancer therapies are not able to eliminate the cancer stem cells, the tumor will relapse. Therefore there is an urgent need to further characterize cancer stem cells and find new strategies to destroy them, contributing enormously to the therapeutic management of malignant cancers. MiRNAs play crucial roles in the post-transcriptional regulation of genes. Emerging evidence suggest that miRNAs have important roles in the regulation of angiogenesis, drug resistance and metastasis. Given that CSCs are believed to be responsible for cancer initiation, propagation and chemotherapy resistance, a better understanding of how microRNAs mediate gene expression in CSCs will help identify novel cancer biomarkers and therapeutic targets and will aid in the development of better strategy for cancer treatment. The development of therapies against CSCs should aim to the elimination of both bulk cancer cells and CSCs. One of the most promising approaches is the cell based delivery of miRNAs or miRNA inhibitors. Unfortunately, some CSC markers, such as CD44 and CD133, are also expressed in normal stem and progenitor cells [104] and this might have negative consequences for the development of CSC-targeted therapy. The problem could be overcome by the development of antibodies against specific glycans on CSC conjugated to liposomes or nanoparticles for the selective delivery of miRNAs. In conclusion, the use of miRNAs as biomarkers in clinical practice is a potentially powerful tool for non-invasive analysis. A more detailed understanding of the role of miRNAs in CSC biology may improve cancer treatments and possibly lead to the clinical application of miRNAs in cancer diagnosis, prognosis and treatment.
Acknowledgments
M.G. is recipient of the Kimmel Award (2011).
List of abbreviations
- CSCs
cancer stem cells
- NSCs
normal stem cells
- PCs
progenitor cells
- TICs
tumor-initiating cells
- CD
cluster of differentiation
- ESC
embryonic stem cells
- HSC
hematopoietic stem cells
- AML
acute myeloid leukemia
- LSC
leukemic stem-like cells
- LP
leukemic progenitors
- MDS
myelodysplastic syndrome
- STP miRNA
stem/progenitor transition miRNA
- CML
chronic myeloid leukemia
- BCSC
breast cancer stem cells
- BT-ICs
breast tumor initiating cells
- ESA
epithelial specific antigen
- HMLE
mammary epithelial cells
- DCIS
ductal carcinoma in situ
- PCR
Polycomb Repressor Complex
- NSCCs
non-stem cancer cells
- EMT
epithelial-mesenchymal transition
- HBE
human bronchial epithelial
- EOC
epithelial ovarian cancer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;311:525–32. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 2.Passegué E, Jamieson CH, Ailles LE, Weissman IL. Normal and leukemic hematopoiesis: are leukemias a stem cell disorder or a reacquisition of stem cell characteristics? Proc Natl Acad Sci U S A. 2003 doi: 10.1073/pnas.2034201100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sell S. Stem cell origin of cancer and differentiation therapy. Crit Rev Oncol Hematol. 2004;51:1–28. doi: 10.1016/j.critrevonc.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Durante F. Nesso fisio-pathologico tra la struttura dei nei materni e la genesi di alcuni tumori maligni. Arch Memor Observ Chir Pract. 1874;11:217–26. [Google Scholar]
- 5.Cohnheim J. Ueber entzundung und eiterung. Path Anat Physiol Klin Med. 1867;40:1–79. [Google Scholar]
- 6.Cohnheim J. Congenitales, quergestreiftes Muskelsarkonder Nireren. Virchows Arch. 1875;65:64. [Google Scholar]
- 7.Jordan CT. Unique molecular and cellular features of acute myelogenous leukemia. stem cells Leukemia. 2002;16:559–62. doi: 10.1038/sj.leu.2402446. [DOI] [PubMed] [Google Scholar]
- 8.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic. stem cells Science. 1988;244:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 9.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ignatova TN, Kukekov VG, Laywell ED, Suslov ON, Vrionis FD, Steindler DA. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;199:193–206. doi: 10.1002/glia.10094. [DOI] [PubMed] [Google Scholar]
- 11.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–8. [PubMed] [Google Scholar]
- 12.Driessens G, Beck B, Caauwe A, Simons BD, Blanpain C. Defining the mode of tumour growth by clonal analysis. Nature. 2012;488:527–30. doi: 10.1038/nature11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schepers AG, Snippert HJ, Stange DE, van den Born M, van Es JH, van de Wetering M, et al. Lineage tracing reveals Lgr5+stem cell activity in mouse intestinal adenomas. Science. 2012;333:730–5. doi: 10.1126/science.1224676. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Li Y, Yu TS, McKay RM, Burns DK, Kernie SG, et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522–6. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gregory RI, Yan KP, Amuthan G, Chendrimada T, Doratotaj B, Cooch N, Shiekhattar R. The Microprocessor complex mediates the genesis of microRNAs. Nature. 2004;433:235–40. doi: 10.1038/nature03120. [DOI] [PubMed] [Google Scholar]
- 16.Bohnsack MT, Czaplinski K, Gorlich D. Exportin 5 is a RanGTP-dependent dsRNA-binding protein that mediates nuclear export of pre-miRNAs. RNA. 2004;10:185–91. doi: 10.1261/rna.5167604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol. 2009;10:126–39. doi: 10.1038/nrm2632. [DOI] [PubMed] [Google Scholar]
- 18.Smith AG. Embryo-derived stem cells: of mice and men. Annu Rev Cell Dev Biol. 2001;17:435–462. doi: 10.1146/annurev.cellbio.17.1.435. [DOI] [PubMed] [Google Scholar]
- 19.Lee RC, Feinbaum RL, Ambros V. The C legans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–54. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 20.Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, et al. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;400:901–6. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- 21.Pasquinelli AE, Reinhart BJ, Slack F, Martindale MQ, Kuroda MI, Maller B, D C, et al. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature. 2000;408:86–9. doi: 10.1038/35040556. [DOI] [PubMed] [Google Scholar]
- 22.Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, et al. Dicer is essential for mouse development. Nat Genet. 2003;35:215–7. doi: 10.1038/ng1253. [DOI] [PubMed] [Google Scholar]
- 23.Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–56. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loh HY, Wu Q, Chew JL, Vega VB, Zhang W, Chen X, G, et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38:431–40. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- 25.Wang S, Tang X, Niu Y, Chen H, Li B, Li T, et al. Generation and characterization of rabbit embryonic stem cells. Stem Cells. 2007;25:481–9. doi: 10.1634/stemcells.2006-0226. [DOI] [PubMed] [Google Scholar]
- 26.Sinkkonen L, Hugenschmidt T, Berninger P, Gaidatzis D, Mohn F, Artus-Revel CG, et al. MicroRNAs control de novo DNA methylation through regulation of transcriptional repressors in mouse embryonic stem cells. Nat Struct Mol Biol. 2008;15:259–67. doi: 10.1038/nsmb.1391. [DOI] [PubMed] [Google Scholar]
- 27.Card DA, Hebbar PB, Li L, Trotter KW, Komatsu Y, Mishina Y, et al. Oct4/Sox2-regulated miR-302 targets cyclin D1 in human embryonic stem cells. Mol Cell Biol. 2008;28:6426–38. doi: 10.1128/MCB.00359-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melton C, Judson RL, Blelloch R. Opposing microRNA families regulate self-renewal in mouse embryonic stem cells. Nature. 2010;466:621–6. doi: 10.1038/nature08725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi YJ, Lin CP, Ho JJ, He X, Okada N, et al. miR-34 miRNAs provide a barrier for somatic cell reprogramming. Nat Cell Biol. 2011;13:1353–60. doi: 10.1038/ncb2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aranha MM, Santos DM, Solá S, Steer CJ, Rodrigues CM. miR-34a regulates mouse neural stem cell differentiation. PLoS One. 2011;6:e21396. doi: 10.1371/journal.pone.0021396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao X, Yang Z, Li G, Li D, Zhao Y, Wu Y, et al. The role and clinical implications of microRNAs in hepatocellular carcinoma. Sci China Life Sci. 2012;55:906–919. doi: 10.1007/s11427-012-4384-x. [DOI] [PubMed] [Google Scholar]
- 32.Po A, Ferretti E, Miele E, De Smaele E, Paganelli A, Canettieri G. Hedgehog controls neural stem cells through p53-independent regulation of Nanog. EMBO J. 2010;29:2646–58. doi: 10.1038/emboj.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodova M, Fu J, Watkins DN, Srivastava RK, Shankar S. Sonic hedgehog signaling inhibition provides opportunities for targeted therapy by sulforaphane in regulating pancreatic cancer stem cell self-renewal. PLoS One. 2012;7:e46083. doi: 10.1371/journal.pone.0046083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng Y, Cheung AK, Ko JM, Phoon YP, Chiu PM, Lo PH. Physiological β-catenin signaling controls self-renewal networks and generation of stem-like cells from nasopharyngeal carcinoma. BMC Cell Biol. 2013;14:44. doi: 10.1186/1471-2121-14-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van den Berg DL, Snoek T, Mullin NP, Yates A, Bezstarosti K, Demmers J. An Oct4-centered protein interaction network in embryonic stem cells. Cell Stem Cell. 2010;6:369–81. doi: 10.1016/j.stem.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ji J, Yamashita T, Budhu A, Forgues M, Jia HL, Li C, et al. Identification of microRNA-181 by genome-wide screening as a critical player in EpCAM-positive hepatic cancer stem cells. Hepatology. 2009;50:472–480. doi: 10.1002/hep.22989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suetsugu A, Nagaki M, Aoki H, Motohashi T, Kunisada T, Moriwaki H. Characterization of CD133+ hepatocellular carcinoma cells as cancer stem/progenitor cells. Biochem Biophys Res Commun. 2006;351:820–824. doi: 10.1016/j.bbrc.2006.10.128. [DOI] [PubMed] [Google Scholar]
- 38.Yin S, Li J, Hu C, Chen X, Yao M, Yan M, et al. CD133 positive hepatocellular carcinoma cells possess high capacity for tumorigenicity. Int J Cancer. 2007;120:1444–1450. doi: 10.1002/ijc.22476. [DOI] [PubMed] [Google Scholar]
- 39.Zhang J, Luo N, Luo Y, Peng Z, T, Li S. microRNA-150 inhibits human CD133-positive liver cancer stem cells through negative regulation of the transcription factor c-Myb. Int J Oncol. 2012;40:747–56. doi: 10.3892/ijo.2011.1242. [DOI] [PubMed] [Google Scholar]
- 40.Ma S, Tang KH, Chan YP, Lee TK, Kwan PS, Castilho A, et al. Cell Stem Cell. 2010;7:694–707. doi: 10.1016/j.stem.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 41.Liu AM, Yao TJ, Wang W, Wong KF, Lee NP, Fan ST, et al. Circulating miR-15b and miR-130b in serum as potential markers for detecting hepatocellular carcinoma: a retrospective cohort study. BMJ Open. 2012;2:e000825. doi: 10.1136/bmjopen-2012-000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jia Y, Liu H, Zhuang Q, Xu S, Yang Z, Li J, et al. Tumorigenicity of cancer stemlike cells derived from hepatocarcinoma is regulated by microRNA-145. Oncol Rep. 2012;27:1865–72. doi: 10.3892/or.2012.1701. [DOI] [PubMed] [Google Scholar]
- 43.Han YC, Park CY, Bhagat G, Zhang J, Wang Y, Fan JB, et al. microRNA-29a induces aberrant self-renewal capacity In hematopoietic progenitors, biased myeloid development, and acute myeloid leukemia. J Exp Med. 2010;200:475–89. doi: 10.1084/jem.20090831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 367:645–8. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 45.de Leeuw DC, Denkers F, Olthof M, Rutten A, Pouwels W, et al. Attenuation of microRNA-126 expression that drives CD34+38- stem/progenitor cells in acute myeloid leukemia leads to tumor eradication. Cancer Res. 2014 doi: 10.1158/0008-5472.CAN-13-1733. [DOI] [PubMed] [Google Scholar]
- 46.Arnold CP, Tan R, Zhou B, Yue SB, Schaffert S, Biggs JR, Doyonnas R, et al. MicroRNA programs in normal and aberrant stem and progenitor cells. Genome Res. 2011;21:798–810. doi: 10.1101/gr.111385.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song SJ, Ito K, Ala U, Kats L, Webster K, Sun SM, et al. The oncogenic microRNA miR-22 targets the TET2 tumor suppressor to promote hematopoietic stem cell self-renewal and transformation. Cell Stem Cell. 2013;13:87–101. doi: 10.1016/j.stem.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morris VA, Zhang A, Yang T, Stirewalt DL, Ramamurthy R, Meshinchi S, et al. MicroRNA-150 expression induces myeloid differentiation of human acute leukemia cells and normal hematopoietic progenitors. PLoS One. 2013;8:e75815. doi: 10.1371/journal.pone.0075815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Babashah S, Sadeghizadeh M, Hajifathali A, Tavirani MR, Zomorod MS, Ghadiani M, et al. Targeting of the signal transducer Smo links microRNA-326 to the oncogenic Hedgehog pathway in CD34+ CML stem/progenitor cells. Int J Cancer. 2013;133:579–89. doi: 10.1002/ijc.28043. [DOI] [PubMed] [Google Scholar]
- 50.Schwarzenbacher D, Balic M, Pichler M. The role of microRNAs in breast cancer stem cells. Int J Mol Sci. 2013;14:14712–23. doi: 10.3390/ijms140714712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–67. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lim YY, Wright JA, Attema JL, Gregory PA, Bert AG, Smith E, et al. Epigenetic modulation of the miR-200 family is associated with transition to a breast cancer stem-cell-like state. J Cell Sci. 2013;122:2256–66. doi: 10.1242/jcs.122275. [DOI] [PubMed] [Google Scholar]
- 54.Sun X, Qin S, Fan C, Xu C, Du N, Ren H. Let-7: a regulator of the ERα signaling pathway in human breast tumors and breast cancer stem cells. Oncol Rep. 2013;29:2079–87. doi: 10.3892/or.2013.2330. [DOI] [PubMed] [Google Scholar]
- 55.Okuda H, Xing F, Pandey PR, Sharma S, Watabe M, Pai SK, et al. miR-7 suppresses brain metastasis of breast cancer stem-like cells by modulating KLF4. Cancer Res. 2013;73:1434–44. doi: 10.1158/0008-5472.CAN-12-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu F, Deng H, Yao H, Liu Q, Su F, Song E. Mir-30 reduction maintains self-renewal and inhibits apoptosis in breast tumor-initiating cells. Oncogene. 2010;29:4194–204. doi: 10.1038/onc.2010.167. [DOI] [PubMed] [Google Scholar]
- 57.Zhu Y, Yu F, Jiao Y, Feng J, Tang W, Yao H, Gong C, et al. Reduced miR-128 in breast tumor-initiating cells induces chemotherapeutic resistance via Bmi-1 and ABCC5. Clin Cancer Res. 2011;17:7105–15. doi: 10.1158/1078-0432.CCR-11-0071. [DOI] [PubMed] [Google Scholar]
- 58.Liu L, Zhou W, Cheng CT, Ren X, Somlo G, Fong MY. TGFbeta Induces ‘BRCAness’ and Sensitivity to PARP Inhibition in Breast Cancer by Regulating DNA Repair Genes. Mol Cancer Res. 2014 doi: 10.1158/1541-7786.MCR-14-0201. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Q, Yao Y, Eades G, Liu Z, Zhang Y, Zhou Q. Downregulation of miR-140 promotes cancer stem cell formation in basal-like early stage breast cancer, Oncogene. 2014;33:2589–600. doi: 10.1038/onc.2013.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fang Y, Xiang J, Chen Z, Gu X, Li Z, Tang F, et al. miRNA expression profile of colon cancer stem cells compared to non-stem cells using the SW1116 cell line. Oncol Rep. 2012;28:2115–24. doi: 10.3892/or.2012.2054. [DOI] [PubMed] [Google Scholar]
- 61.Bitarte N, Bandres E, Boni V, Zarate R, Rodriguez J, Gonzalez-Huarriz M, et al. MicroRNA-451 is involved in the self-renewal, tumorigenicity, and chemoresistance of colorectal cancer stem cells. Stem Cells. 2011;29:1661–71. doi: 10.1002/stem.741. [DOI] [PubMed] [Google Scholar]
- 62.Pinto D, Gregorieff A, Begthel H, Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17:1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vermeulen L, De Sousa EMF, van der Heijden M, Cameron K, de Jong JH, Borovski T, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468–476. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 64.Yu Y, Kanwar SS, Patel BB, Oh PS, Nautiyal J, Sarkar FH, Majumdar AP. MicroRNA-21 induces stemness by downregulating transforming growth factor beta receptor 2 (TGFbetaR2) in colon cancer cells. Carcinogenesis. 2012;33:68–76. doi: 10.1093/carcin/bgr246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fre S, Huyghe M, Mourikis P, Robine S, Louvard D, Artavanis-Tsakonas S. Notch signals control the fate of immature progenitor cells in the intestine. Nature. 2005;435:964–968. doi: 10.1038/nature03589. [DOI] [PubMed] [Google Scholar]
- 66.Bu P, Chen KY, Chen JH, Wang L, Walters J, Shin YJ, et al. A microRNA miR-34a-regulated bimodal switch targets Notch in colon cancer stem cells. Cell Stem Cell. 2013;12:602–15. doi: 10.1016/j.stem.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ho MM, Ng AV, Lam S, Hung YJ. Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res. 2007;67:4827–33. doi: 10.1158/0008-5472.CAN-06-3557. [DOI] [PubMed] [Google Scholar]
- 68.Levina V, Marrangoni AM, DeMarco R, Gorelik E, Lokshin AE. Drug-selected human lung cancer stem cells: cytokine network, tumorigenic and metastatic properties. PLoS One. 2008;3:e3077. doi: 10.1371/journal.pone.0003077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiang F, Qiu Q, Khanna A, Todd NW, Deepak J, Xing L, et al. Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Mol Cancer Res. 2009;7:330–8. doi: 10.1158/1541-7786.MCR-08-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, et al. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15:504–14. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- 71.Leung EL, Fiscus RR, Tung JW, Tin VP, Cheng LC, Sihoe AD, et al. Non-small cell lung cancer cells expressing CD44 are enriched for stem cell-like properties. PLoS One. 2010;5:e14062. doi: 10.1371/journal.pone.0014062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qiu X, Wang Z, Li Y, Miao Y, Ren Y, Luan Y. Characterization of sphere-forming cells with stem-like properties from the small cell lung cancer cell line H446. Cancer Lett. 2012;322:161–70. doi: 10.1016/j.canlet.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 73.Marhaba R, Zoller M. CD44 in cancer progression: adhesion, migration and growth regulation. J Mol Histol. 2004;35:211–31. doi: 10.1023/b:hijo.0000032354.94213.69. [DOI] [PubMed] [Google Scholar]
- 74.Desai TJ, Brownfield DG, Krasnow MA. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature. 2014;500:190–4. doi: 10.1038/nature12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shi Y, Liu C, Liu X, Tang DG, Wang J. The microRNA miR-34a inhibits non-small cell lung cancer (NSCLC) growth and the CD44hi stem-like NSCLC cells. PLoS One. 2014;9:e90022. doi: 10.1371/journal.pone.0090022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gutova M, Najbauer J, Gevorgyan A, Metz MZ, Weng Y, Shih CC. Identification of uPAR-positive chemoresistant cells in small cell lung cancer. PLoS One. 2007;2:e243. doi: 10.1371/journal.pone.0000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Miao Y, Li J, Qiu X, Li Y, Wang Z, Luan Y. miR-27a regulates the self-renewal of the H446 small cell lung cancer cell line in vitro. Oncol Rep. 2013;29:161–8. doi: 10.3892/or.2012.2095. [DOI] [PubMed] [Google Scholar]
- 78.Vaz AP, Ponnusamy MP, Batra SK. Cancer Stem Cells and Therapeutic Targets: An Emerging Field for Cancer Treatment. Drug Deliv Transl Res. 2013;3:113–120. doi: 10.1007/s13346-012-0095-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu W, Ji J, Xu Y, Liu Y, Shi L, Liu Y. MicroRNA-191, by promoting the EMT and increasing CSC-like properties, is involved in neoplastic and metastatic properties of transformed human bronchial epithelial cells. Mol Carcinog. 2014 doi: 10.1002/mc.22221. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 80.Jiang Z, Cushing L, Ai X, Lü J. miR-326 is downstream of Sonic hedgehog signaling and regulates the expression of Gli2 and smoothened. Am J Respir Cell Mol Biol. 2014;51:273–83. doi: 10.1165/rcmb.2013-0127OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tu Y, Gao X, Li G, Fu H, Cui D, Liu H, et al. MicroRNA-218 inhibits glioma invasion, migration, proliferation, and cancer stem-like cell self-renewal by targeting the polycomb group gene Bmi1. Cancer Res. 2013;73:6046–55. doi: 10.1158/0008-5472.CAN-13-0358. [DOI] [PubMed] [Google Scholar]
- 82.Chen L, Chen XR, Chen FF, Liu Y, Li P, Zhang R, et al. MicroRNA-107 inhibits U87 glioma stem cells growth and invasion. Cell Mol Neurobiol. 2013;33:651–7. doi: 10.1007/s10571-013-9927-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peruzzi P, Bronisz A, Nowicki MO, Wang Y, Ogawa D, Price R, et al. miR-128 coordinately targets Polycomb Repressor Complexes in glioma stem cells. Neuro Oncol. 2013;15:1212–24. doi: 10.1093/neuonc/not055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Niu CS, Yang Y, Cheng CD. MiR-134 regulates the proliferation and invasion of glioblastoma cells by reducing Nanog expression. Int J Oncol. 2013;42:1533–40. doi: 10.3892/ijo.2013.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao S, Deng Y, Liu Y, Chen X, Yang G, Mu Y, et al. MicroRNA-153 is tumor suppressive in glioblastoma stem cells. Mol Biol Rep. 2013;40:2789–98. doi: 10.1007/s11033-012-2278-4. [DOI] [PubMed] [Google Scholar]
- 86.Ying Z, Li Y, Wu J, Zhu X, Yang Y, Tian H, et al. Loss of miR-204 expression enhances glioma migration and stem cell-like phenotype. Cancer Res. 2013;73:990–9. doi: 10.1158/0008-5472.CAN-12-2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H, et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med. 2011;17:211–5. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ren D, Wang M, Guo W, Zhao X, Tu X, Huang S, et al. Wild-type p53 suppresses the epithelial-mesenchymal transition and stemness in PC-3 prostate cancer cells by modulating miR-145. Int J Oncol. 2013;42:1473–81. doi: 10.3892/ijo.2013.1825. [DOI] [PubMed] [Google Scholar]
- 89.Tay YM, Tam WL, Ang YS, Gaughwin PM, Yang H, Wang W, et al. MicroRNA-134 modulates the differentiation of mouse embryonic stem cells, where it causes post-transcriptional attenuation of Nanog and LRH1. Stem Cells. 2008;26:17–29. doi: 10.1634/stemcells.2007-0295. [DOI] [PubMed] [Google Scholar]
- 90.Ngalame NN, Tokar EJ, Person RJ, Xu Y, Waalkes MP. Aberrant microRNA Expression Likely Controls RAS Oncogene Activation During Malignant Transformation of Human Prostate Epithelial and Stem Cells by Arsenic. Toxicol Sci. 2014;138:268–77. doi: 10.1093/toxsci/kfu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Iliopoulos D, Hirsch HA, Wang G, Struhl K. Inducible formation of breast cancer stem cells and their dynamic equilibrium with non-stem cancer cells via IL6 secretion. Proc Natl Acad Sci U S A. 2011;108:1397–402. doi: 10.1073/pnas.1018898108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Saini S, Majid S, Shahryari V, Arora S, Yamamura S, Chang I. miRNA-708 control of CD44(+) prostate cancer-initiating cells. Cancer Res. 2012;72:3618–30. doi: 10.1158/0008-5472.CAN-12-0540. [DOI] [PubMed] [Google Scholar]
- 93.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chaffer CL, Marjanovic ND, Lee T, Bell G, Kleer CG, Reinhardt F. Poised chromatin at the ZEB1 promoter enables breast cancer cell plasticity and enhances tumorigenicity. Cell. 2013;154:61–74. doi: 10.1016/j.cell.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Park SM, Gaur AB, Lengyel E, Peter ME. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22:894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Qian P, Banerjee A, Wu ZS, Zhang X, Wang H, Pandey V. Loss of SNAIL regulated miR-128-2 on chromosome 3p22.3 targets multiple stem cell factors to promote transformation of mammary epithelial cells. Cancer Res. 2012;72:6036–50. doi: 10.1158/0008-5472.CAN-12-1507. [DOI] [PubMed] [Google Scholar]
- 97.Liu Y, Sánchez-Tilló E, Lu X, Huang L, Clem B, Telang S. The ZEB1 transcription factor acts in a negative feedback loop with miR200 downstream of Ras and Rb1 to regulate Bmi1 expression. J Biol Chem. 2014;14:4116–25. doi: 10.1074/jbc.M113.533505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Siemens H, Jackstadt R, Hünten S, Kaller M, Menssen A, Götz U, et al. miR-34 and SNAIL form a double-negative feedback loop to regulate epithelial-mesenchymal transitions. Cell Cycle. 2011;10:4256–71. doi: 10.4161/cc.10.24.18552. [DOI] [PubMed] [Google Scholar]
- 99.Liu Z, Liu H, Desai S, Schmitt DC, Zhou M, Khong HT. miR-125b functions as a key mediator for snail-induced stem cell propagation and chemoresistance. J Biol Chem. 2013;288:4334–45. doi: 10.1074/jbc.M112.419168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yin G, Chen R, Alvero AB, Fu HH, Holmberg J, Glackin C. TWISTing stemness, inflammation and proliferation of epithelial ovarian cancer cells through MIR199A2/214. Oncogene. 2010;29:3545–53. doi: 10.1038/onc.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nanta R, Kumar D, Meeker D, Rodova M, Van Veldhuizen PJ, Shankar S. NVP-LDE-225 (Erismodegib) inhibits epithelial-mesenchymal transition and human prostate cancer stem cell growth in NOD/SCID IL2Rγ null mice by regulating Bmi-1 and microRNA-128. Oncogenesis. 2013;2:e42. doi: 10.1038/oncsis.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gill JG, Langer EM, Lindsley RC, Cai M, Murphy TL, Murphy KM. Snail promotes the cell-autonomous generation of Flk1(+) endothelial cells through the repression of the microRNA-200 family. Stem Cells Dev. 2012;21:167–76. doi: 10.1089/scd.2011.0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Polytarchou C, Iliopoulos D, Struhl K. An integrated transcriptional regulatory circuit that reinforces the breast cancer stem cell state. Proc Natl Acad Sci U S A. 2012;109:14470–5. doi: 10.1073/pnas.1212811109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Karsten U, Goletz S. What makes cancer stem cell markers different? Springerplus. 2013;2:301. doi: 10.1186/2193-1801-2-301. [DOI] [PMC free article] [PubMed] [Google Scholar]


