Abstract
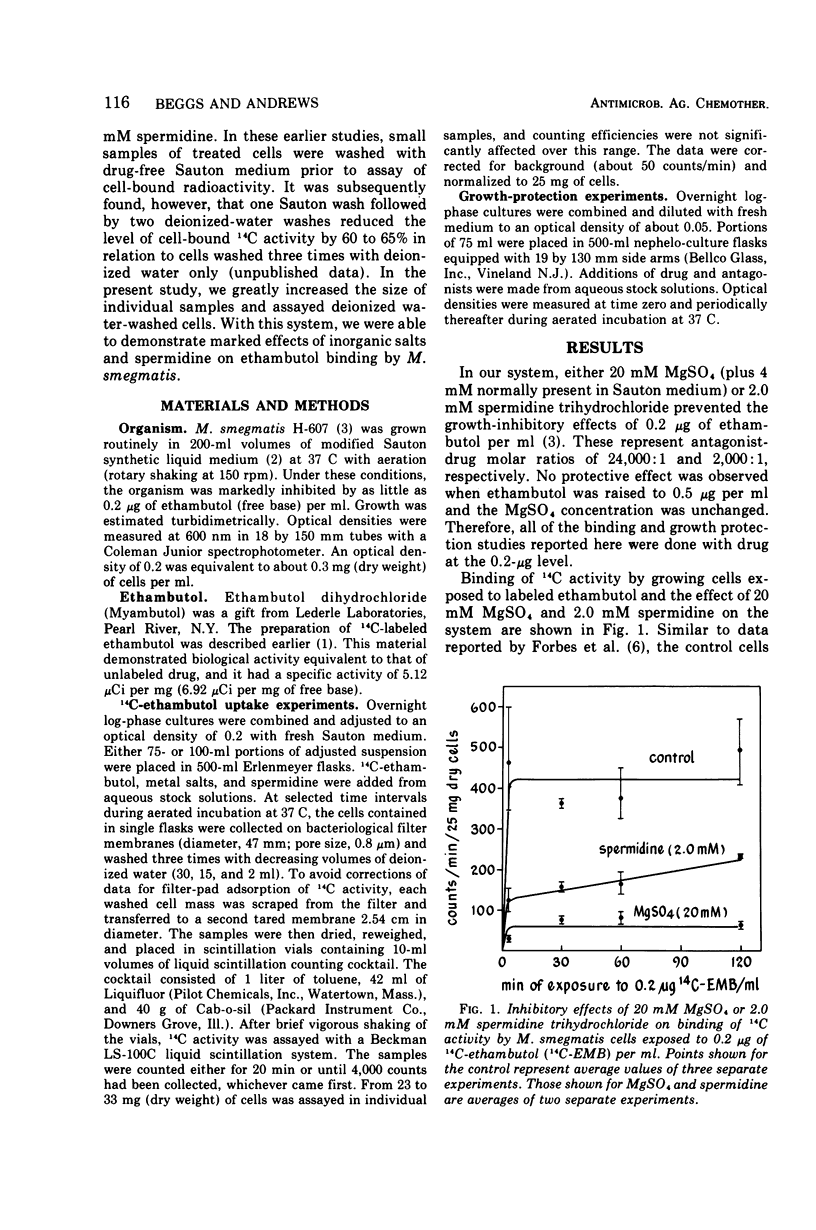
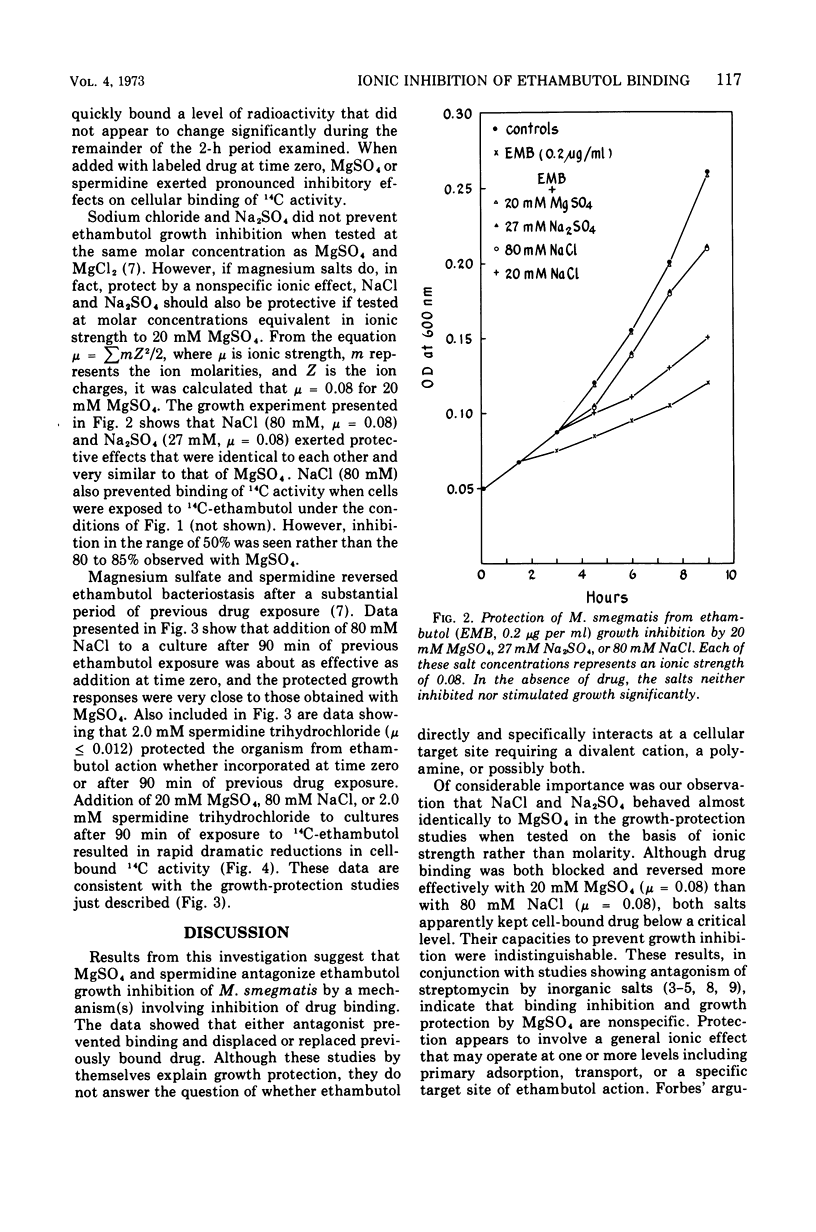
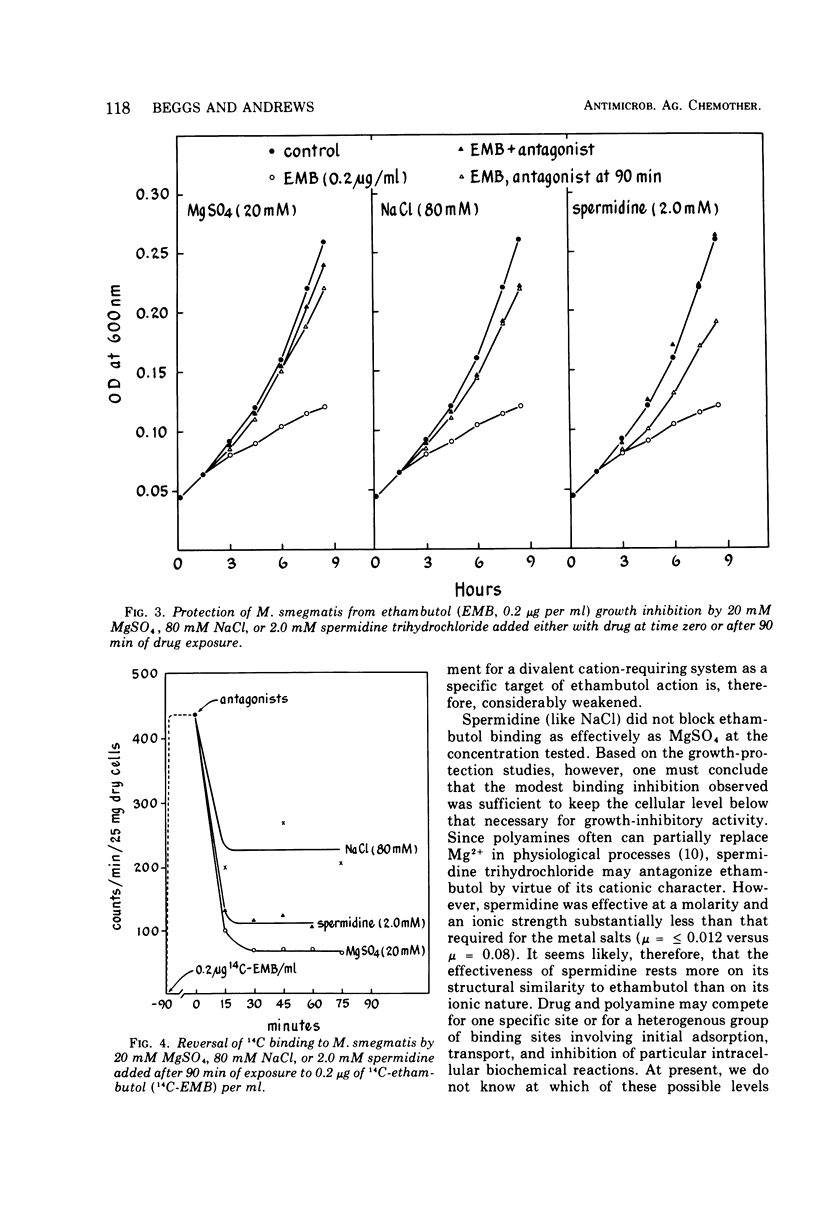
Magnesium sulfate and spermidine were tested for their effects on binding of 14C-ethambutol by Mycobacterium smegmatis. Concentrations were used that protected the organism from ethambutol inhibition. Sodium salts were examined as possible ethambutol antagonists to test the previously reported specificity of the divalent cation salt effect. Consistent with growth-protection experiments, 20 mM MgSO4 or 2.0 mM spermidine prevented and reversed 14C binding by cells shaken with 0.2 μg of 14C-ethambutol per ml of Sauton medium at 37 C. Sodium salts were not effective ethambutol antagonists when tested at 20 mM, but at concentrations equivalent in ionic strength (μ) to that provided by 20 mM MgSO4 they were effective. Thus, 20 mM MgSO4, 80 mM NaCl, or 27 mM Na2SO4 (μ = 0.08) all gave similar results in growth protection and binding experiments, suggesting that MgSO4 antagonism is a nonspecific ionic effect. Because spermidine (μ ≤ 0.012) antagonized ethambutol at an ionic strength substantially less than that required for the metal salts, its effect may hinge on structural similarity to ethambutol rather than its cationic character. Drug and polyamine may compete for one site or a heterogeneous group of binding sites involving adsorption, transport, and intracellular target reactions. Until we know at which of these levels spermidine antagonizes ethambutol binding, the relationship between polyamines and ethambutol action will remain obscure. However, these studies have weakened the earlier argument for a divalent cation-requiring system as a specific ethambutol target site.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAGG P. D., POLGLASE W. J. ACTION OF DIHYDROSTREPTOMYCIN AND ANTAGONISM BY CATIONS. J Bacteriol. 1963 Mar;85:590–594. doi: 10.1128/jb.85.3.590-594.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs W. H., Auran N. E. Uptake and binding of 14C-ethambutol by tubercle bacilli and the relation of binding to growth inhibition. Antimicrob Agents Chemother. 1972 Nov;2(5):390–394. doi: 10.1128/aac.2.5.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs W. H., Jenne J. W. Mechanism for the pyridoxal neutralization of isoniazid action of Mycobacterium tuberculosis. J Bacteriol. 1967 Oct;94(4):793–797. doi: 10.1128/jb.94.4.793-797.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs W. H., Williams N. E. Protection of Mycobacterium smegmatis from Ethambutol and Streptomycin Inhibition by MgSO(4) and Polyamines. Infect Immun. 1971 Mar;3(3):496–497. doi: 10.1128/iai.3.3.496-497.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovick R., Bayan A. P., Canales P., Pansy F. The Influence of Certain Substances on the Activity of Streptomycin: III. Differential Effects of Various Electrolytes on the Action of Streptomycin. J Bacteriol. 1948 Jul;56(1):125–137. [PMC free article] [PubMed] [Google Scholar]
- FORBES M., KUCK N. A., PEETS E. A. EFFECT OF ETHAMBUTOL ON NUCLEIC ACID METABOLISM IN MYCOBACTERIUM SMEGMATIS AND ITS REVERSAL BY POLYAMINES AND DIVALENT CATIONS. J Bacteriol. 1965 May;89:1299–1305. doi: 10.1128/jb.89.5.1299-1305.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORBES M., KUCK N. A., PEETS E. A. Mode of action of ethambutol. J Bacteriol. 1962 Nov;84:1099–1103. doi: 10.1128/jb.84.5.1099-1103.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGER J., BENEDICT M., ARTMAN M. A common site of action of polyamines and streptomycin. Biochim Biophys Acta. 1962 Jul 30;62:202–204. doi: 10.1016/0006-3002(62)90519-x. [DOI] [PubMed] [Google Scholar]
- PLOTZ P. H., DUBIN D. T., DAVIS B. D. Influence of salts on the uptake of streptomycin by Escherichia coli. Nature. 1961 Sep 23;191:1324–1325. doi: 10.1038/1911324a0. [DOI] [PubMed] [Google Scholar]
- TABOR H., TABOR C. W. SPERMIDINE, SPERMINE, AND RELATED AMINES. Pharmacol Rev. 1964 Sep;16:245–300. [PubMed] [Google Scholar]



