Abstract
Background and Objectives:
Data on the risk of acute pancreatitis following endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) of pancreatic cystic lesions are limited. The aim of our study was to evaluate the frequency of acute pancreatitis after EUS-FNA of pancreatic cysts and solid lesions, and determine whether there was a difference in pancreatitis risk in patients with side branch intraductal papillary mucinous neoplasms (SB-IPMN).
Patients and Methods:
A retrospective review of patients who underwent EUS-FNA of pancreatic cysts and solid lesions was performed. The primary outcome measure was development of acute pancreatitis after EUS-FNA. Factors associated with acute pancreatitis were examined by statistical analysis to determine independent predictors of acute pancreatitis. Statistical significance was determined at a P ≤ 0.05.
Results:
We identified 186 patients with pancreatic cystic lesions and 557 with solid lesions in which EUS-FNA was performed. The median size of the cysts was 19 mm (range: 10-66 mm). There were 37 IPMNs, 33 mucinous cystic neoplasms, 58 serous cysts and 46 pseudocysts and 12 solid-cystic ductal carcinomas. The majority of patients (75%) with solid lesions were diagnosed with adenocarcinoma. Patients with pancreatic cysts had a statistically greater frequency of developing pancreatitis after EUS-FNA when compared to those with solid lesions (2.6% vs. 0.36% respectively; P = 0.13). In patients with cysts, there were no statistically significant differences between the two groups (with and without pancreatitis) with regard to a cyst location, size of the cyst, and number of needle passes or trainee involvement. Patients with SB-IPMN had a statistically higher frequency of pancreatitis after EUS-FNA compared to those with other cyst types (8% vs. 1.3% respectively; odds ratio = 6.4, 95% confidence intervals = 1.0-40.3, P = 0.05).
Discussion:
Patients with SB-IPMN are at a higher risk of developing acute pancreatitis after a EUS-FNA. Alternative means of diagnosis such as magnetic resonance cholangiopancreatogram might be necessary to avoid risk of EUS-FNA.
Keywords: Acute pancreatitis, endosonography, fine-needle aspiration, intraductal papillary, mucinous neoplasm
INTRODUCTION
Due to increasing utilization of cross sectional imaging, pancreatic cysts are now being increasing diagnosed. It has been predicted that up to 10% of the population over 70 years will have a pancreatic cyst.[1] There are four primary types of pancreatic cysts: serous cystadenomas and pseudocysts are typically benign, while mucinous cystic neoplasms (MCN) and intraductal papillary mucinous neoplasms (IPMN) have malignant potential.
Noninvasive imaging using computed tomography (CT) scan or magnetic resonance imaging (MRI) has been used in an attempt to characterize these cysts. However, these imaging techniques may not be as accurate as endoscopic ultrasound (EUS) in helping to determine the cyst type.[2,3] The characteristics of pancreatic cysts as visualized by EUS may help determine whether a patient can be safely observed or should undergo a morbid but potentially life-saving surgical resection.
Endoscopic ultrasound with fine-needle aspiration (FNA) of the cyst fluid can reliably characterize a mucinous from nonmucinous pancreatic cyst by measuring carcinoembryonic antigen (CEA) and amylase levels from the aspirate.[4] Targeted FNA of the cyst wall following aspiration of fluid has also been demonstrated to improve overall sensitivity in diagnosing mucinous cysts which have malignant potential or have frank malignancy which may have otherwise been misdiagnosed as benign.[5] Recent advances using cyst fluid DNA analysis have shown that KRAS mutation, amount and quality of DNA and allelic imbalance increase the sensitivity for diagnosing mucinous cysts.[6] The ongoing discussion of how to approach pancreatic cysts has led to the creation of the Fukuoka Guidelines for the management of patients presumed to have side branch (SB)-IPMN and MCNs.[7]
Due to its invasive nature, EUS-FNA does come with risks. Retrospective studies have shown that EUS-FNA of solid pancreatic masses is infrequently associated with pancreatitis.[8,9,10] Most of these studies have excluded EUS-FNA of cystic lesions. There is a theoretical risk of inducing pancreatitis with FNA of SB-IPMNs as there may be a direct communication between the aspirated cyst and the main pancreatic duct. Currently, it is unclear what the risk of post-EUS-FNA pancreatitis is for pancreatic cysts. The aim of the present study was to determine whether there was an increased risk of pancreatitis after EUS-FNA of SB-IPMNs when compared to other pancreatic cysts. Our secondary aim was to compare the risk of acute pancreatitis after EUS-FNA of SB-IPMNs to solid pancreatic lesions.
PATIENTS AND METHODS
Patients
We conducted a retrospective analysis of consecutive patients with solid and cystic pancreatic lesions found on CT or MRI imaging ≥10 mm in dimension who underwent an initial EUS-FNA at Thomas Jefferson University Hospital from June 2004 to August 2010. Patient medical records, endoscopy reports, operative reports and review of the solid pancreatic mass and cyst pathology were evaluated. Data was also gathered on patient demographics, symptomatology, a history of pancreatitis, and imaging studies. EUS characteristics of the pancreatic cyst and solid lesions were recorded. Pancreatic cyst fluid analysis from EUS-FNA including CEA, amylase, cytology, and DNA molecular analysis were recorded to determine etiology of the pancreatic cyst. All patients with pancreatic cysts enrolled in the study had a minimum of 18 months follow-up with semi-annual or annual abdominal imaging by CT or MRI scan to follow progression of their pancreatic cyst. This study was approved by the Institutional Review Board at Thomas Jefferson University Hospital.
Endoscopic procedures
All EUS-FNA procedures were performed by three experienced faculty endoscopists with >5 years of experience.
The following cyst features on EUS were noted: Size, location, dilation of the main pancreatic duct or side branches, any direct communication between cyst and main pancreatic duct, and the presence of papillary projections, debris, septations or solid mass lesions in the cyst. Once the pancreatic cyst was visualized, a curvilinear echoendoscope (Olympus GF UCT 140 or UCT 160, Melville, NY, US) was used to perform the EUS-FNA. Lesions within the pancreatic head and uncinate process were aspirated via a trans-duodenal approach while lesions in the neck, body, or tail of the pancreas were accessed via the trans-gastric approach. A 22-gauge FNA needle (Echotip, Cook Medical, Winston-Salem, NC, US) was utilized for cyst fluid aspiration, followed by targeted cyst wall puncture in order to obtain wall cytology. Intravenous antibiotics were administered to all patients during the procedure.
For solid pancreatic lesions, a 22-gauge FNA needle was utilized to obtain cytological samples and the smears were immediately reviewed by an on-site cytology technician. At least six passes were obtained from the pancreatic lesion unless the technician established the presence of malignant appearing cells.
Assessment of acute pancreatitis after endoscopic ultrasound-fine needle aspiration
All patients were observed in the recovery room for 1 h after the procedure with recordings of vital signs every 10 min. Any abdominal pain during the recovery time was assessed by the endoscopist. Serum amylase and lipase were measured if pancreatitis was suspected. Radiologic imaging was also performed if abdominal pain persisted.
Acute pancreatitis was defined as upper abdominal pain (with or without nausea and/or vomiting) accompanied by at least three-fold elevation of serum amylase or lipase with 24 h of the procedure. Severity of pancreatitis was classified according to the Atlanta classification.[11] An experienced gastrointestinal nurse called all patients 24-72 h after the procedure to follow-up on any potential complications. For the patients who could not be successfully contacted, information was collected from the medical records and from clinic follow-up.
Cyst classification
Cyst classification was performed using cyst fluid analysis, surgical histology (when available), and imaging studies. A staff cytopathologist also interpreted the cyst wall cytology obtained via EUS-FNA. Cytology features consistent with IPMN featured highly cellular cells arranged predominantly in tall papillary groups distributed in a background of abundant mucin. MCN featured variable columnar to cuboidal cells with cytoplasmic mucin which were typical for its cellular cytomorphology, along with positive staining with mucicarmine stain. Cytology in patients with serous cystadenomas showed small clusters of uniform cuboidal epithelial cells with finely granular cytoplasm and round nuclei. Patients with pseudocysts revealed neutrophils and/or histiocytes with some epithelial clusters.
We used the cystic fluid CEA and amylase levels to assist in classification as mucinous and nonmucinous. CEA and amylase values were determined using a Roche Elecysy 2010 and a Roche COBAS integral 400 Plus, respectively. Molecular analysis was performed in a Clinical Laboratory Improvement Amendments-certified, College of American Pathologists-accredited laboratory (PathFinderTG®-Pancreas; RedPath Integrated Pathology, Inc., Pittsburgh, PA, US). This included checking for quantification of molecular parameters consisting of DNA quantity/quality, oncogene (k-ras, GNAS) point mutation and extent of clonal expansion, and tumor suppressor gene mutation and extent of clonal expansion, as determined by allelic imbalance (loss of heterozygosity determination). The presence of a KRAS mutation was indicative of a mucinous cyst.[4] A review of the CT, MRI and EUS morphologies supported cyst diagnosis when using cytology, CEA levels, amylase levels, and molecular analysis.
Solid pancreatic lesions
Endoscopic ultrasound-FNA cytology samples considered to be adequate were interpreted as malignant, suspicious for malignancy, atypical cells, benign and nondiagnostic. Patients in the current study were classified as having a benign versus malignant lesion. A final diagnosis of a pancreatic malignancy was based upon (1) cytologic or histologic evidence of malignancy based upon material obtained by EUS-FNA, endoscopic retrograde cholangiopancreatography (ERCP), or surgical resection (2) clinical course based upon follow-up of that patient over a period of at least 6 months.
Statistical analysis
Each procedure was classified as a separate data point. Each EUS-FNA procedure was classified according the absence or presence of acute pancreatitis after EUS-FNA of the cyst and solid mass. Categorical variables were reported as frequency with percentages while continuous variables were reported as medians.
We compared the groups with and without acute pancreatitis with regards to patient demographics, clinical history and cyst characteristics, and cyst etiology. We also compared the risk of acute pancreatitis after EUS-FNA in patients with pancreatic cysts and solid pancreatic lesions. Risk factors for acute pancreatitis after EUS-FNA were assessed using Fisher exact two-tail test for dichotomous variable and simple logistic regression for continuous variables. We also calculated the odds ration with exact 95% confidence intervals (CI) for proportions. Statistical significance was determined a priori at P ≤ 0.05. Analyses were performed using SAS V9.1 (SAS Institute, Cary, NC, US).
RESULTS
Pancreatic cyst subjects
We identified 186 consecutive patients (65% females, mean age 64.2 ± 1.4 standard error [SE] years) with pancreatic cystic lesions found on imaging in which EUS-FNA was performed. Most of the patients were white (86.1%). The most common symptoms on presentation were abdominal pain (20%), abdominal fullness (5%), jaundice (5%) and fatigue and/or malaise (6%). Twenty-nine (15%) patients had a previous history of acute pancreatitis. Overall, 54% of patients were asymptomatic and the cysts were identified incidentally on imaging as part of the workup of a different problem.
Pancreatic solid mass subjects
We identified 557 consecutive patients (48% females, mean age 65.8 ± 2.3 [SE] years) with solid pancreatic lesions found on imaging in which EUS-FNA was performed. The patients were predominantly white (71.6%). The most common symptoms on presentation were abdominal pain (23%), jaundice (59%), weight loss (49%) and fatigue and/or malaise (26%).
Pancreatic cyst characteristics
The median dimensions of the pancreatic cysts were 25.1 mm (range: 10-66 mm) (long axis). Locations of the cysts within the pancreas were as follows: Pancreatic head, 71 (38%); body, 82 (44%); and tail, 33 (18%). One hundred and forty-two (76%) had only one needle pass. The median number of needle passes per lesion was 1 (range: 1-2). Prophylactic antibiotics were administered in all cases. The median cyst aspirate amount was 2 mL (range: 0.5-17 mL). A fellow-in-training was involved in 76 (41%) of cases. Final pathology diagnosis by surgical resection was available in 24 (13%) patients.
Based upon a combination of abdominal imaging, cyst fluid CEA and amylase levels, cyst fluid DNA analysis, cyst fluid cytology and cyst wall cytology, pancreatic cysts were classified as following: Thirty seven SB-IPMNs, 33 MCN, 58 serous cysts, 46 pseudocysts and 12 solid-cystic ductal carcinomas. Median follow-up for all the cystic lesions was 24 months (range: 18-47 months).
Pancreatic mass lesion characteristics
The median dimensions of the pancreatic mass were 29 mm (range: 10-83 mm) (long axis). Locations of the mass within the pancreas were as follows: Pancreatic head, 384 (69%); body, 56 (10%); and tail, 117 (21%). The median number of needle passes per lesion was 3.9 (range: 1-7). Based upon cytology and/or surgical biopsy results, the pancreatic mass lesions were classified as following: Four hundred and twenty adenocarcinomas, 71 chronic focal pancreatitis, 38 neuroendocrine tumors, 4 lymphomas, and 16 metastatic lesions.
Acute pancreatitis after endoscopic ultrasound-fine-needle aspiration
Acute pancreatitis developed in 5/186 (2.6%) patients after EUS-FNA of a pancreatic cyst and in 2/557 (0.36%) of patients with solid pancreatic lesions. Patients with pancreatic cysts had a statistically greater frequency of developing pancreatitis after EUS-FNA when compared to those with solid lesions (P = 0.13). Of the pancreatic cyst patients who developed acute pancreatitis, 3 had SB-IPMN and 2 had serous cyst adenomas. Both patients with solid pancreatic lesions who developed pancreatitis after EUS-FNA had adenocarcinoma. All patients developed pancreatitis within 24 h of the procedure.
To determine factors associated with the development of post-EUS-FNA acute pancreatitis in patients with cysts, we compared the groups with and without pancreatitis with regard to clinical presentation, mass characteristics, and technical details of the procedure. Patients with SB-IPMN had a statistically higher frequency of pancreatitis after EUS-FNA compared to those with other cyst types (8% vs. 1.3% respectively; odds ratio [OR] = 6.4, 95% CI = 1.0-40.3, P = 0.05). There were no statistically significant differences between the two groups (with and without pancreatitis) with regard to a previous history of pancreatitis, cyst location, size of the cyst, number of needle passes, or trainee involvement. These findings are summarized in Table 1 multiple regression analysis revealed that a previous history of acute pancreatitis and patients with SB-IPMN had a statistically higher frequency of pancreatitis after EUS-FNA (0.02 and 0.03 respectively). These findings are summarized in Table 2.
Table 1.
Predictors of EUS-FNA – associated pancreatitis in patients with pancreatic cysts
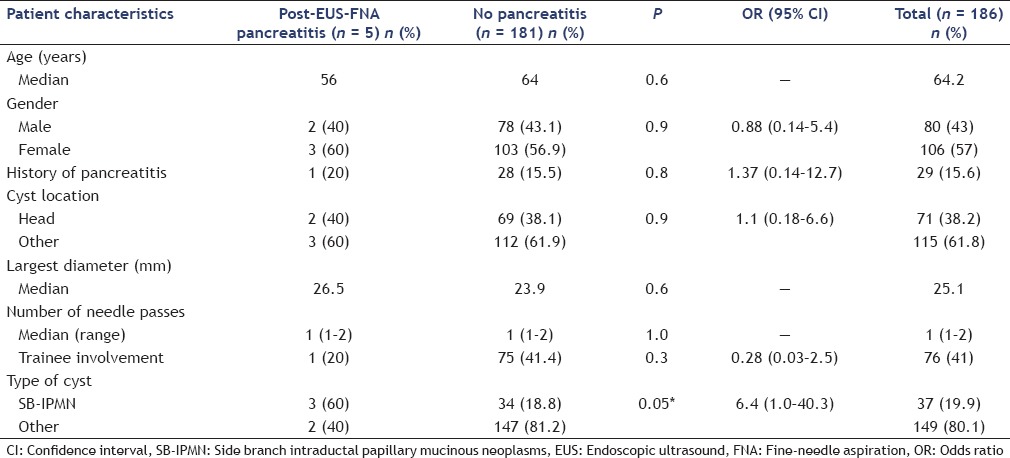
Table 2.
Multiple regression analysis of EUS-FNA – associated pancreatitis in patients with pancreatic cysts
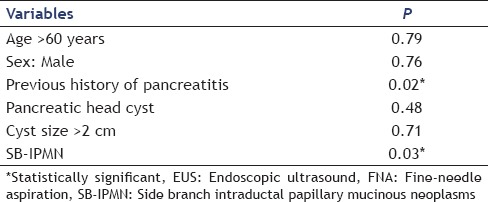
Patients with SB-IPMN had a 24 times greater frequency of developing post-EUS-FNA pancreatitis compared with those with solid pancreatic lesions (OR = 24.4, 95% CI = 3.9-151.4, P = 0.02).
Management of acute pancreatitis
All patients with acute pancreatitis after EUS-FNA required hospitalization and were treated conservatively with analgesics and intravenous fluid hydration. The median duration of hospitalization for treatment of pancreatitis was 4 days (range: 1-11 days). The pancreatitis was classified as mild in three cases and moderate in one case according to the Atlanta severity classification of acute pancreatitis. None of the patients had any long-term sequel of their pancreatitis.
DISCUSSION
Endoscopic ultrasound-guided puncture of pancreatic cystic lesions is increasingly being used to differentiate benign cysts (pseudocyst, serous cystadenoma) from cysts with intermediate malignant potential (SB-IPMN) or those with high malignant potential (mucinous cystadenoma, main duct IPMN).[5,6,7] Although there are several trials evaluating the risk of pancreatitis associated with EUS-FNA of solid pancreatic lesions,[8,9] there is very limited data on the frequency and risk factors of acute pancreatitis after the EUS-FNA in pancreatic cystic lesions.
The present study demonstrates a small albeit important risk of pancreatitis is association with EUS-FNA of SB-IPMNs. Our study demonstrates that the risk of pancreatitis after EUS-FNA of SB-IPMNs is 6 times greater compared to those with other cyst types. Because patients with SB-IPMN have cysts that directly communicate with the main pancreatic duct, we postulated that FNA causes trauma to the pancreatic duct and therefore place these patients at a higher risk for this complication. In addition, our findings show that patients with SB-IPMN had a significantly greater frequency of developing post-EUS-FNA pancreatitis compared with those with solid pancreatic lesions.
The risk of iatrogenic pancreatitis as a result of EUS-FNA of solid and cystic pancreatic lesions is reported to range from 0% to 2%.[5,6,8,9,12,13] EUS-FNA of cystic lesions has been demonstrated to have a high complication rate when compared to that of solid lesions.[8] This is in concordance with findings of the current study. O’Toole et al. performed a retrospective that noted a complication rate of 3.5% for cystic lesions after EUS-FNA where there were three cases of pancreatitis.[13] Lee et al. specially evaluated the complications of EUS-guided pancreatic cyst aspiration and found the rate of pancreatitis was 1.0%.[12] However, unlike the present study, they did not specially evaluate whether the type of cyst affected the risk of postprocedure pancreatitis.
Studies have also suggested that a previous history of pancreatitis may increase the risk for pancreatitis after EUS-FNA.[7] In our study, there was no statistically significant difference in the frequency of acute pancreatitis after EUS-FNA between patients with and without a previous history of pancreatitis (P = 0.8). Another study suggested the FNA of pancreatic head and uncinate cysts may promote pancreatitis due to the longer distance the needle passed through normal pancreas tissue during aspiration. In our study, 2/5 (40%) of patients who developed pancreatitis had cysts in the head with 3 (60%) cysts in the body and tail. We did not find any correlation between cyst location and the risk of developing pancreatitis after FNA. Similarly, cyst diameter, number of needle passes and trainee involvement were not predictors of pancreatitis. Again, these findings are consistent with other studies which evaluated EUS-FNA complications.
Unlike trials evaluating risk factors for post-ERCP pancreatitis, trainee involvement did not increase the risk of EUS-FNA induced pancreatitis.[14] Only 1/76 (1.3%) patients in which the trainee was involved developed pancreatitis. These finding are similar to Coté et al. who found that attending-supervised, fellow-directed EUS-FNA is safe and accurate.[15]
The present study has certain limitations. Firstly, it is retrospective in nature. However, our overall rate of pancreatitis after EUS-FNA closely matches recent studies evaluating complications of EUS-FNA after cyst aspiration.[12,13] Secondly, the small number of patients who developed acute pancreatitis after EUS-FNA may have the potential to introduce bias when identifying significant predictors of this complication. Finally, the etiology of the cyst was based predominantly on cyst fluid analysis, cytology and imaging characteristics since surgical pathology was available in only 24 patients. However, recent recommendations demonstrate that a conglomeration of all these tests has a high sensitivity, specificity and diagnostic accuracy to predict the etiology of the pancreatic cyst.
In summary, patients with SB-IPMN are at a 6 times higher risk of developing acute pancreatitis after a EUS-FNA compared to other types of pancreatic cysts. Because patients with SB-IPMN have cysts that directly communicate with the main pancreatic duct, FNA may place these patients at a higher risk for this complication. Alternative means of diagnosis such as magnetic resonance cholangiopancreatogram might be necessary to avoid risk of EUS-FNA. A prospective, multicenter study of the risk of acute pancreatitis after EUS-FNA of SB-IPMN is warranted.
Footnotes
Source of Support: This study was funded entirely through existing intramural funds and salary support.
Conflict of Interest: None declared.
REFERENCES
- 1.de Jong K, Nio CY, Mearadji B, et al. Disappointing interobserver agreement among radiologists for a classifying diagnosis of pancreatic cysts using magnetic resonance imaging. Pancreas. 2012;41:278–82. doi: 10.1097/MPA.0b013e31822899b6. [DOI] [PubMed] [Google Scholar]
- 2.Farrell JJ, Fernández-del Castillo C. Pancreatic cystic neoplasms: Management and unanswered questions. Gastroenterology. 2013;144:1303–15. doi: 10.1053/j.gastro.2013.01.073. [DOI] [PubMed] [Google Scholar]
- 3.Kalra MK, Maher MM, Mueller PR, et al. State-of-the-art imaging of pancreatic neoplasms. Br J Radiol. 2003;76:857–65. doi: 10.1259/bjr/16642775. [DOI] [PubMed] [Google Scholar]
- 4.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: A report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–6. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Hong SK, Loren DE, Rogart JN, et al. Targeted cyst wall puncture and aspiration during EUS-FNA increases the diagnostic yield of premalignant and malignant pancreatic cysts. Gastrointest Endosc. 2012;75:775–82. doi: 10.1016/j.gie.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 6.Khalid A, Zahid M, Finkelstein SD, et al. Pancreatic cyst fluid DNA analysis in evaluating pancreatic cysts: A report of the PANDA study. Gastrointest Endosc. 2009;69:1095–102. doi: 10.1016/j.gie.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka M, Fernández-del Castillo C, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–97. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Eloubeidi MA, Gress FG, Savides TJ, et al. Acute pancreatitis after EUS-guided FNA of solid pancreatic masses: A pooled analysis from EUS centers in the United States. Gastrointest Endosc. 2004;60:385–9. doi: 10.1016/s0016-5107(04)01714-6. [DOI] [PubMed] [Google Scholar]
- 9.Gress F, Michael H, Gelrud D, et al. EUS-guided fine-needle aspiration of the pancreas: Evaluation of pancreatitis as a complication. Gastrointest Endosc. 2002;56:864–7. doi: 10.1067/mge.2002.129602. [DOI] [PubMed] [Google Scholar]
- 10.Katanuma A, Maguchi H, Yane K, et al. Factors predictive of adverse events associated with endoscopic ultrasound-guided fine needle aspiration of pancreatic solid lesions. Dig Dis Sci. 2013;58:2093–9. doi: 10.1007/s10620-013-2590-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis – 2012: Revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–11. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 12.Lee LS, Saltzman JR, Bounds BC, et al. EUS-guided fine needle aspiration of pancreatic cysts: A retrospective analysis of complications and their predictors. Clin Gastroenterol Hepatol. 2005 doi: 10.1016/s1542-3565(04)00618-4. [DOI] [PubMed] [Google Scholar]
- 13.O’Toole D, Palazzo L, Arotçarena R, et al. Assessment of complications of EUS-guided fine-needle aspiration. Gastrointest Endosc. 2001;53:470–4. doi: 10.1067/mge.2001.112839. [DOI] [PubMed] [Google Scholar]
- 14.Cheng CL, Sherman S, Watkins JL, et al. Risk factors for post-ERCP pancreatitis: A prospective multicenter study. Am J Gastroenterol. 2006;101:139–47. doi: 10.1111/j.1572-0241.2006.00380.x. [DOI] [PubMed] [Google Scholar]
- 15.Coté GA, Hovis CE, Kohlmeier C, et al. Training in EUS-guided fine needle aspiration: Safety and diagnostic yield of attending supervised, trainee-directed FNA from the onset of training. Diagn Ther Endosc 2011. 2011 doi: 10.1155/2011/378540. 378540. [DOI] [PMC free article] [PubMed] [Google Scholar]


