Abstract
Background and Objective:
The addition of fine-needle aspiration (FNA) to different imaging modalities has raised the accuracy for diagnosis of cystic pancreatic lesions. We aim to differentiate benign from neoplastic pancreatic cysts by evaluating cyst fluid carcinoembryonic antigen (CEA), carbohydrate antigen (CA19-9), and amylase levels and cytopathological examination, including mucin stain.
Patients and Methods:
This prospective study included 77 patients with pancreatic cystic lesions. Ultrasound-FNA (US-FNA) or endoscopic ultrasound-FNA (EUS-FNA) was done according to the accessibility of the lesion. The aspirated specimens were subjected to cytopathological examination (including mucin staining), tumor markers (CEA, CA19-9), and amylase level.
Results:
Cyst CEA value of 279 or more showed high statistical significance in differentiating mucinous from nonmucinous lesions with sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of 73%, 60%, 50%, 80%, and 65%, respectively. Cyst amylase could differentiate between neoplastic and nonneoplastic cysts at a level of 1043 with sensitivity of 58%, specificity of 75%, PPV of 73%, NPV of 60%, and accuracy of 66%. CA19-9 could not differentiate between neoplastic and nonneoplastic cysts. Mucin examination showed a sensitivity of 85%, specificity of 95%, PPV of 92%, NPV of 91%, and accuracy of 91% in differentiating mucinous from non-mucinous lesions. Cytopathological examination showed a sensitivity of 81%, specificity of 94%, PPV of 94%, NPV of 83%, and accuracy of 88%.
Conclusion:
US or EUS-FNA with analysis of cyst CEA level, CA19-9, amylase, mucin stain, and cytopathological examination increases the diagnostic accuracy of cystic pancreatic lesions.
Keywords: Cystic lesion, diagnosis, endoscopic ultrasound, fine needle aspiration, pancreatic lesion, ultrasound
INTRODUCTION
Pancreatic cystic lesions range from benign abnormalities needing minimal followup to premalignant or malignant lesions requiring careful monitoring or resection. These lesions present a diagnostic challenge.[1] Endoscopic ultrasound (EUS) is now used to investigate cystic pancreatic lesions, particularly as a mean of EUS-guided cyst aspiration.[2,3] Several EUS features of pancreatic cysts have been associated with an increased risk of malignancy, including thick wall, septations, presence of intramural nodules, and masses.-[4] However, recent studies indicated that pancreatic cyst appearance during EUS is not enough as an independent predictor of malignancy.[5,6] Cyst fluid amylase, tumor markers such as carcinoembryonic antigen (CEA) and carbohydrate antigen (CA19-9) and cytopathological examination, including mucin stain have the potential to improve significantly the diagnosis of pancreatic lesions.[1] The objective of this study was to evaluate and validate cyst fluid CEA, CA19-9, mucin, amylase, and cytological examination in the diagnosis of pancreatic cystic lesions.
PATIENTS AND METHODS
This prospective study included 77 patients presented with pancreatic cystic lesions based on abdominal computed tomography (CT), EUS, or abdominal ultrasound. According to accessibility and feasibility, fine-needle aspiration (FNA) was done using abdominal ultrasound or EUS. It was carried over 2 years after approval of the ethical committee. Written consents were obtained from the patients.
Inclusion criteria
Patients older than 18 years with radiological evidence (CT, magnetic resonance imaging (MRI) of pancreatic cyst and needed FNA for final diagnosis
Patients with obstructive jaundice due to a large pancreatic cyst
Patients with severe resistant abdominal pain proved to have a pancreatic cyst suggestive of intraductal papillary mucinous neoplasm (IPMN)
Patients with unexplained common bile duct strictures or pancreatic duct dilatation by endoscopic retrograde cholangiopancreatography or magnetic resonance cholangiopancreatography and sent for further delineation by EUS.
Exclusion criteria
Patients having pancreatic cyst smaller than 1 cm
Patient with gall bladder stones and severe colicky pains in whom their pancreatic cysts are suggested to be inflammatory pseudocysts
Patients having platelet count <50,000/cmm or PC <60%
Poor risk patients for deep sedation by Propofol
Patients refused to sign the consent
Patients missed for followup or patients whose lab and histological examination were not available, so the final diagnosis was not settled.
Ultrasound examination
Ultrasound was done using Hitachi machine EUB-7000. FNA was done using Chiba needles (16-22G) under complete guidance technique with biopsy attachment.
Endoscopic ultrasound examination
Endoscopic ultrasound examination was done using a Pentax machine, EG3830UT (HOYA Corporation, PENTAX Lifecare Division, Showanomori Technology Center, Tokyo, Japan) attached to Hitachi machine EUB-7000. FNA was done using 22 or 19G Echotip needless (Cook, Endoscopy, Winston-Salem, NC), no on-site cytopathological examination was available.
Deep sedation with intravenous Propofol was used in all patients even in cases of US-FNA. All patients were given 1 g Ceftriaxone within 6 h before the FNA.
The aim was to evacuate the cysts completely with a single-needle pass. Samples were spread over dry slides and also preserved in formalin and sent for cytopathological examination and mucin stain (by PAS diastase). It was also examined for CEA, CA19-9, and amylase levels.
All EUS and US were done by a single gastroenterologist. The cytopathologist was blinded to the US and EUS findings.
Eight patients suffered from tolerable epigastric pains for <1 day, and one patient suffered from acute pancreatitis required ICU admission for 2 days with complete relief thereafter. No serious complications as severe pancreatitis or bleeding, and no mortality were recorded.
Final diagnosis was based on the presence of one of the following
Malignant cells in the FNA
Presence of distant metastasis
Followup of benign lesions for at least 18 months with no change in size
Surgical resection and biopsy results.
T-test was used, significance was taken at the level of 5%. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy were all calculated manually based on published methodology.
RESULTS
Patients’ demographics
Seventy-seven patients satisfied the inclusion criteria of the study (29 females, 48 males, mean age for females was 48 ± 8 years and the mean age for males was 52 ± 4 years). After the initial EUS-FNA or US-FNA, surgical removal of the pancreatic cysts was the way of diagnosis in 11 patients, 14 cases were diagnosed by followup and the remaining 52 patients were diagnosed by cytopathology. US-FNA was done for 53 patients while EUS-FNA was done to the remaining 24 patients.
Based on the final diagnosis, cysts were classified as nonneoplastic and neoplastic [Table 1]
Table 1.
Final diagnosis of pancreatic cysts
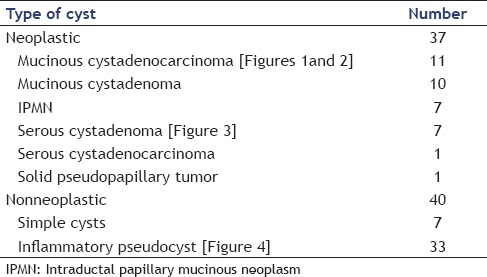
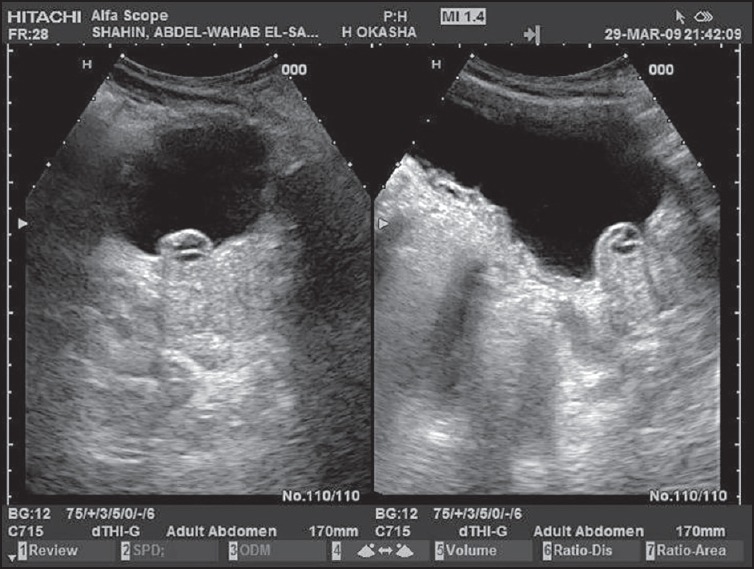
Eleven out of 28 (39.3%) mucinous cystic neoplasms had mural nodules on ultrasonographic or EUS examination [Figures 1 and 2], 9 of them proved to be mucinous cystadenocarcinoma representing 81.8% of all cases of mucinous adenocarcinoma (9 out of 11) and 2 of them proved to be IPMN representing 28.6% of all cases of IPMN (3 out of 7). All cases were confirmed by triphasic spiral or multislice CT scan. Soft tissue debris, turbid fluid, and/or irregular wall were found in inflammatory cysts, but no mural nodules were found in such lesions [Figure 4].
Figure 1.
A pancreatic mucinous cystadenocarcinoma with a prominent mural nodule as seen by ultrasound
Figure 2.
A pancreatic mucinous cystadenocarcinoma with turbid fluid and small mural nodules at its posterior wall as seen by endoscopic ultrasound
Figure 4.
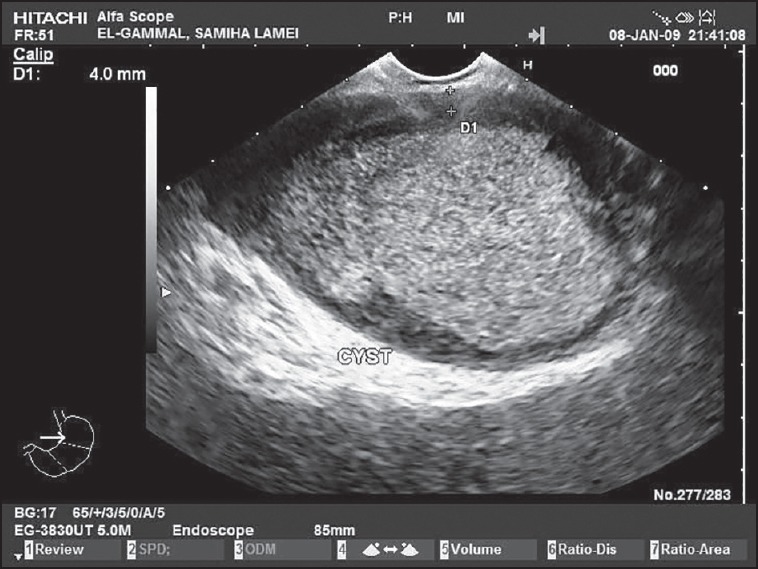
An inflammatory pancreatic pseudocyst with thick wall and dense turbid fluid inside as seen by endoscopic ultrasound
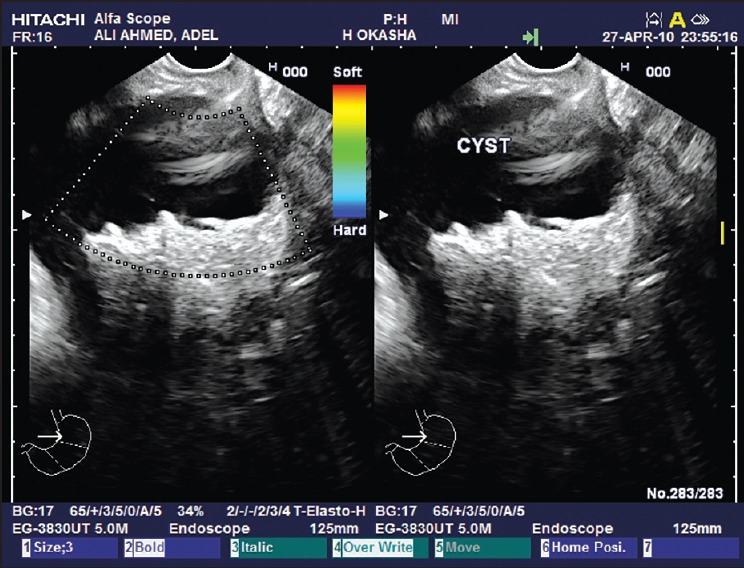
Figure 3.
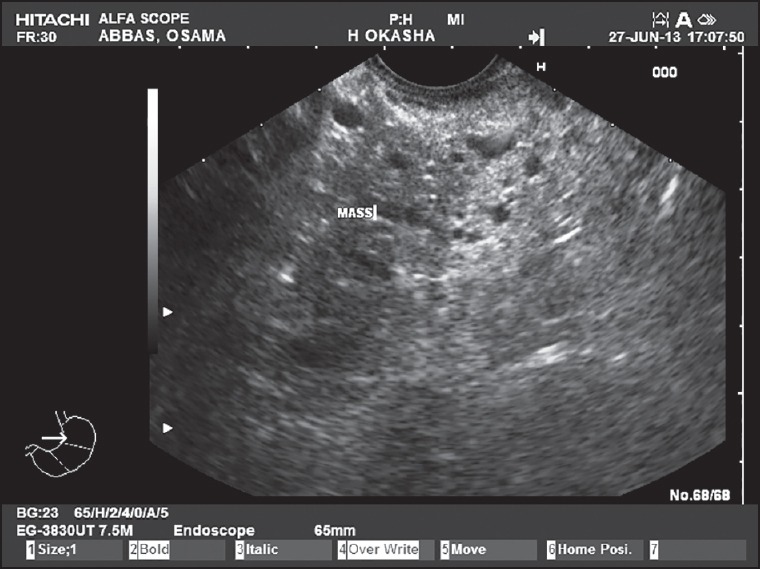
A pancreatic serous cystadenoma giving a honey-comb appearance as seen by endoscopic ultrasound
CEA was done in 62 out of 77 patients (80.5%), the remaining 15 did not have enough aspirate fluid for analysis. With a cutoff value of 105 ng/mL, there is a high statistical significant difference of CEA between mucinous (23 cases) and non-mucinous (32 cases) cysts, and it had a sensitivity of 80%, specificity of 77%, PPV of 67%, NPV of 87%, and accuracy of 78% for mucinous lesions.
The test was true positive in 16 patients and true negative in 24 patients, 16 cases were false positive and 6 cases were false negative with sensitivity, specificity, PPV, NPV, and accuracy of 73%, 60%, 50%, 80%, and 65%, respectively as shown in Table 2
Table 2.
Cyst CEA in differentiating mucinous from nonmucinous lesions

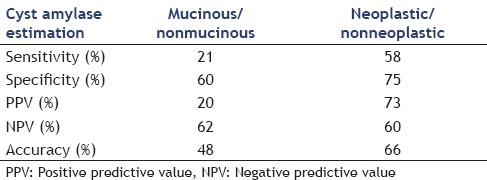
Cyst amylase was done in 44 patients and could differentiate between mucinous and non-mucinous lesions at the level of 24150 or above and between neoplastic and nonneoplastic cysts at a level of 1043 or above with the following sensitivity, specificity, PPV, NPV, and accuracy in Table 3. The amylase level showed statistical significant difference between neoplastic and benign lesions being higher in benign cysts with P < 0.001. All cases with inflammatory pseudocysts had cyst fluid amylase level above 250 ng/mL, so, levels below that cut-off value virtually exclude the diagnosis of inflammatory pseudocysts
Table 3.
Cyst amylase in differentiating mucinous from nonmucinous lesions and neoplastic from nonneoplastic ones
Cyst CA19-9 was not valuable in differentiating neoplastic from nonneoplastic lesions but at a level of 447 or above showed sensitivity of 77%, specificity of 61%, PPV of 43%, NPV of 87%, and accuracy of 65%. Table 4 shows the results of the analysis
Table 4.
Cyst CA 19-9 in differentiating mucinous from nonmucinous cysts

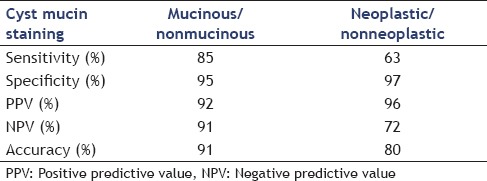
The sensitivity, specificity, PPV, NPV, and accuracy of positive mucin stain in differentiating mucinous from nonmucinous lesions and neoplastic from nonneoplastic ones are shown in Table 5
Table 5.
Mucin stain in differentiating mucinous from nonmucinous lesions and neoplastic from nonneoplastic ones
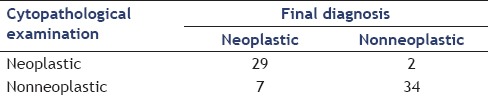
Cytopathological examination showed sensitivity of 81%, specificity of 94%, PPV of 94%, NPV of 83%, and accuracy of 88% as shown in Table 6
Table 6.
Diagnosis examination
DISCUSSION
The recent advancement of the imaging modalities such as multi-detector CT scan and high speed MRI leads to many challenging questions; for example, is EUS still necessary if multi-detectors CT can provide sufficient diagnostic information for a large cystic tumor with septations? Should surgery always be guided by EUS with cystic fluid analysis?[7]
For this, a variety of studies has been done to assess the role of EUS in discriminating benign pancreatic cysts from malignant or potentially malignant cystic lesions. Cysts in the ‘suspicious of malignancy’ category demonstrate features such as mural nodules, solid components, lymph node enlargement, dilated pancreatic duct, and elevated serum tumor markers and should in most instances undergo resection. Cysts with these suspicious features of malignancy had a more than 80% chance of harboring a malignant or potentially malignant lesion.[8] In our study, 11 out of 28 (39.3%) of all mucinous cystic neoplasms, 81.8% of all cases of mucinous adenocarcinoma, and 28.6% of all cases of IPMN had mural nodules on US or EUS examination. None of the inflammatory or simple cysts had mural nodules.
However, a study done by Brugge said that unless a solid mass or invasive tumor was seen, morphological features did not appear to be specific enough to differentiate between malignant and potentially malignant cysts.[9]
The clinical challenge in the management of pancreatic cysts is to identify those patients who should undergo pancreatic resection for early non-metastatic cancer and high-risk precancerous cystic lesions while appropriately observing those patients with limited or no potential for malignant transformation.
Cyst fluid analysis has been used for this purpose including CEA, CA19-9, amylase, mucin, and cytopathological examination to diagnose type of the pancreatic cyst and the consequent management. Previous work by Brugge et al.[2] evaluated various cyst fluid markers in 112 patients and identified CEA as having the highest clinical utility in discriminating mucinous from non-mucinous cystic lesions. They reported that a level >192 ng/mL had a sensitivity, specificity, and diagnostic accuracy of 73%, 84%, and 79%, respectively, for mucinous cystic lesions. When we investigated CEA level at 279 ng/mL or above it showed sensitivity, specificity, PPV, NPV, and accuracy of 73%, 60%, 50%, 80%, and 65%, respectively. Similarly, a cut off value of 24150 of cyst amylase could differentiate between mucinous and non-mucinous lesions with sensitivity, specificity, PPV, NPV, and accuracy of 21%, 60%, 20%, 62%, and 48%, respectively, and between neoplastic and nonneoplastic cysts at a level of 1043 with sensitivity of 58%, specificity of 75%, PPV of 73%, NPV of 60%, and accuracy of 66%.
A study was done to assess the role of mucin staining in relation to pancreatic cystic lesions and found that the presence of mucin had a PPV of 83%.[10] This was similar to our study that showed that mucin staining in pancreatic cysts had a PPV of 96% for differentiating neoplastic from nonneoplastic and PPV of 92% in differentiating mucinous from non-mucinous lesions.
CONCLUSION
This study finds that current cyst fluid analysis including tumor markers such as CEA, CA19-9, mucin, amylase and cytopathological examination can increase the diagnostic yield and support the management of pancreatic cystic lesions. This may be particularly helpful in cases when the nature of the cyst remains indeterminate despite clinical and imaging data. The shortcomings of these biomarkers to better predict which cysts harbor malignancy or will become malignant argue for the need for new biomarkers. Studies using DNA analysis seem to be promising but require further studies.
ACKNOWLEDGEMENT
We thank Prof. Mazen Naga, Chairman of El-Ebrashi Unit of gastroenterology, Internal Medicine Department, Cairo University, for his generous and continuous support throughout the work.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Snozek CL, Mascarenhas RC, O’Kane DJ. Use of cyst fluid CEA, CA19-9, and amylase for evaluation of pancreatic lesions. Clin Biochem. 2009;42:1585–8. doi: 10.1016/j.clinbiochem.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 2.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: A report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–6. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Bose D, Tamm E, Liu J, et al. Multidisciplinary management strategy for incidental cystic lesions of the pancreas. J Am Coll Surg. 2010;211:205–15. doi: 10.1016/j.jamcollsurg.2010.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gress F, Gottlieb K, Cummings O, et al. Endoscopic ultrasound characteristics of mucinous cystic neoplasms of the pancreas. Am J Gastroenterol. 2000;95:961–5. doi: 10.1111/j.1572-0241.2000.01976.x. [DOI] [PubMed] [Google Scholar]
- 5.Huang ES, Turner BG, Fernandez-Del-Castillo C, et al. Pancreatic cystic lesions : Clinical predictors of malignancy in patients undergoing surgery. Aliment Pharmacol Ther. 2010;15(31):285–94. doi: 10.1111/j.1365-2036.2009.04173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buscaglia JM, Giday SA, Kantsevoy SV, et al. Patient- and cyst-related factors for improved prediction of malignancy within cystic lesions of the pancreas. Pancreatology. 2009;9:631–8. doi: 10.1159/000181173. [DOI] [PubMed] [Google Scholar]
- 7.Kongkam P, Rerknimitr R, Ridtitid W, et al. EUS-FNA for pancreatic cyst lesion, today and tomorrow in the Kingdom of Thailand. Dig Endosc. 2011;23(Suppl 1):54–7. doi: 10.1111/j.1443-1661.2011.01140.x. [DOI] [PubMed] [Google Scholar]
- 8.Goh BK, Tan YM, Thng CH, et al. How useful are clinical, biochemical, and cross-sectional imaging features in predicting potentially malignant or malignant cystic lesions of the pancreas. Results from a single institution experience with 220 surgically treated patients? J Am Coll Surg. 2008;206:17–27. doi: 10.1016/j.jamcollsurg.2007.06.312. [DOI] [PubMed] [Google Scholar]
- 9.Brugge WR. Cystic pancreatic lesions: Can we diagnose them accurately what to look for? FNA marker molecular analysis resection, surveillance, or endoscopic treatment? Endoscopy. 2006;38(Suppl 1):S40–7. doi: 10.1055/s-2006-946650. [DOI] [PubMed] [Google Scholar]
- 10.Walsh RM, Henderson JM, Vogt DP, et al. Prospective preoperative determination of mucinous pancreatic cystic neoplasms. Surgery. 2002;132:628–33. doi: 10.1067/msy.2002.127543. [DOI] [PubMed] [Google Scholar]


