Abstract
Background:
The utility of endoscopic ultrasound (EUS) compared with standard white light endoscopy (WLE) following recent polypectomy of high-risk colorectal polyps is unknown.
Objective:
To assess the incremental yield of EUS after endoscopic polypectomy of a high-risk rectal lesion.
Design:
Retrospective cohort.
Setting:
Tertiary referral center.
Materials and Methods:
Patients referred for EUS following attempted endoscopic resection of a high-risk rectal neoplasm, defined as a tubulovillous adenoma, tubular adenoma with high-grade dysplasia, carcinoid, carcinoma in-situ or adenocarcinoma (CA).
Interventions:
Sigmoidoscopy ± mucosal biopsy and EUS ± fine-needle aspiration (FNA) to evaluate for: (1) Residual polyp/tumor in the rectal wall or (2) peritumoral adenopathy.
Main Outcome:
Sensitivity and specificity for detection of residual neoplasia for WLE ± biopsy (WLE/BX) and EUS ± FNA for cancer (CA group) or benign disease (non-CA group). The incremental yield of EUS defined as: (1) Residual intramural neoplasia not present on WLE ± BX and; (2) abnormal peritumoral adenopathy.
Results:
A total of 70 patients (mean age 64 ± 11 years, 61% male) with a final diagnosis of CA (n = 38) and non-CA (n = 32) were identified. There was no difference between the sensitivity and specificity of WLE alone (65% and 84%), WLE with biopsy (71% and 95%), and EUS (59% and 84%), for the detection of residual neoplasia (P > 0.05 for all). EUS identified 3 masses missed by WLE, all in the CA group. A malignant (n = 2) or benign (n = 3) node was identified in 5 (13%) CA patients; EUS-FNA in two showed residual malignancy in one and a reactive lymph node (LN) in one. No LNs were identified in the non-CA patients.
Limitations:
Retrospective design, incomplete follow-up in some patients.
Conclusion:
Following endoscopic polypectomy of high-risk rectal neoplasia, the incremental yield of EUS compared with WLE/BX for evaluation of residual disease appears limited, especially in patients with benign disease.
Keywords: Colonic polyps, endosonography, rectal neoplasms
INTRODUCTION
Colonoscopic polypectomy may occasionally identify high-risk histopathology including high-grade dysplasia (HGD), adenocarcinoma (CA) or a carcinoid tumor. Surveillance guidelines recommend a follow-up exam 2-6 months after piecemeal resection of any sessile adenoma, and a 3 years interval for a completely removed advanced adenoma (>1 cm, any villous features or HGD).[1,2,3,4,5] A repeat exam is also warranted following incomplete polypectomy, particularly if pathology shows HGD[1,2,3,4,5] with a shorter interval if any question remains about completeness of resection.
Since polyps with HGD are confined to the mucosa and possess no risk of lymph node (LN) metastases, complete polypectomy offers definitive treatment. Colon polyps containing invasive carcinoma penetrating the submucosal layer may be found in approximately 2-4% of endoscopically removed colonic polyp and carry a risk of local LN metastasis.[6,7] The management of those cases represents a challenge[8] and follow-up is often based on the presence or absence of unfavorable histologic features[6,9] (poor differentiation, lymphovascular invasion, a positive margin, and incomplete resection).[6,8,9,10,11,12]
Transrectal endoscopic ultrasound (EUS) is established as a safe and accurate initial method for the staging of newly diagnosed rectal cancers or masses.[6,7,13,14,15,16,17,18] But its role following polypectomy of malignant polyps or advanced adenomas[19] is uncertain. There are also no data or guidelines regarding the use of transrectal EUS. The purpose of this study is to assess the incremental yield of EUS over standard white light endoscopy (WLE) and endoscopic forceps biopsy (WLE/BX) to identify residual neoplasia in patients after endoscopic polypectomy of high risk rectal lesions.
MATERIALS AND METHODS
Study population
This study was approved by the Indiana University Health Office of Research Administration. Patients who underwent transrectal or transcolonic EUS from October 2002 to October 2009 for the evaluation of a polypectomy site were identified from an IRB-approved EUS database that contains data on all EUS procedures performed at our medical center. Medical records were abstracted to identify consecutive patients who underwent endoscopic polypectomy of a high risk rectosigmoid colon lesion (0-25 cm from the anal verge), followed by EUS at our center for evaluation of any residual colonic lesion and/or regional nodal metastases. The following lesions were defined as high risk lesions: Carcinoid, tubulovillous adenoma (TVA), tubular adenoma (TA) with HGD (TA-HGD), carcinoma in-situ or CA. Information concerning the initial lesion morphology, histopathology and resection margins were obtained from endoscopy and pathology reports provided by referring physicians.
Procedures
Sigmoidoscopy was initially performed to evaluate for endoscopic evidence of residual mass, ulcer or scar, using a GIF 140 or GIF 160 endoscope (Olympus America, Inc., Center Valley, PA, US). Forceps biopsies of the polypectomy site were taken at the discretion of the endoscopist. Enhanced optical techniques such as chromoendoscopy, narrow band imaging, magnification and endomicroscopy were not used during WLE in any patient. Irrespective of endoscopic findings, all patients then underwent EUS by 1 of 6 experienced endosonographers using a mechanical or electronic radial echoendoscope (GFUM130, GFUM160 or GFUE160-AL5 (Olympus America, Inc., Center Valley, Pa, US). Water instillation or rotation of the patient was utilized as needed to optimize ultrasound imaging of the polypectomy site. The presence of rectal wall thickening, residual mass or lymphadenopathy was noted. EUS-fine-needle aspiration (FNA) of any residual mass or perirectal/colonic adenopathy was performed at the discretion of the endoscopist using a linear echoendoscope (GF-UC30P or GF-UC140P-AL5, Olympus America, Inc., Center Valley, Pa, US) with a 22- or 25-gauge needle (EUSN-1, EUS-N2, EUSN-3, or Echotip Ultra needle (Cook Medical Inc., Winston-Salem, NC, US). A cytopathologist who was not blinded to the patient's clinical history was available for on-site preliminary interpretation of FNA specimens.
As part of routine care, all patients were contacted by telephone 48 h after EUS to assess for any procedure-related complications. Recommendations about follow-up were made by the endoscopist, but final decisions were made by the referring physicians. Follow-up surgery or endoscopy records were evaluated when performed. Follow-up of all incomplete medical records were requested from all referring physicians’ offices.
Definitions
Residual neoplasia following the previous polypectomy was considered present if WLE/BX, EUS-FNA, subsequent surgical resection or any follow-up endoscopy with biopsy (inside or outside our institution) detected adenomatous or malignant tissue at or near the resection site. A patient was considered not to have residual disease following polypectomy if endoscopy/EUS detected no neoplasia and either:
A second follow-up endoscopy at least 6 months later showing no recurrence or;
A subsequent surgical resection that revealed no evidence of residual adenoma or malignancy. Malignancy was defined as invasive carcinoma that invaded through the muscularis mucosa and into the submucosa.
Polyps without evidence of malignancy, carcinoids, and adenomas with HGD were considered as benign.
White light endoscopy alone was considered positive or negative according to endoscopist's final written impression. If not explicitly stated, documentation of scar, ulcer or normal findings was considered negative. WLE was considered positive if a residual mass or polyp was detected, prompting repeat endoscopic resection or referral for surgery. WLE with mucosal biopsies was considered positive if biopsy of the polypectomy site showed adenoma or malignancy, and negative if these were absent.
Endoscopic ultrasound was positive for residual neoplasia if a mass or malignant appearing LN (with or without FNA) was identified during ultrasound imaging or if the endoscopist believed that referral for therapy (chemoradiation or surgery) was indicated based on EUS findings. A malignant-appearing perirectal or pericolonic LN was defined by the presence of three of the following four features: Size ≥5 mm, round shape, hypoechoic margins and location within 10 mm of the polypectomy site. Aspirated LNs were classified according to final cytology; malignant cytology was considered a true positive. A negative EUS was defined as the absence of any of the above findings. Wall thickening alone following polypectomy was not considered evidence of residual neoplasia since normally occurs following polypectomy with electrocautery. The incremental yield of EUS was defined as:
Residual neoplasm not seen on WLE and biopsy (WLE/BX) and
Abnormal perirectal adenopathy.
Statistical analysis
Performance characteristics (sensitivity and specificity) for detecting residual pathology were calculated for WLE and EUS using results from WLE/BX, EUS-FNA and histopathology from subsequent surgery as true positive/true negative. For patients without surgical resection or biopsy/FNA-proven persistent disease, a follow-up endoscopy at least 6 months after the index EUS was required for inclusion into evaluation of performance characteristics. Patients who did not have biopsies done at the time of WLE and EUS were excluded from the calculation of sensitivity/specificity of WLE with biopsies. Results were stratified from the original surgical pathology specimen into the presence (CA group) or absence of cancer (non-CA group). Values are presented as means and standard deviations or proportions with associated 95% confidence intervals (CIs).[20] Chi-square and Fisher's exact tests were used to compare categorical variables, and continuous variables were compared with a t-test. P < 0.05 was considered as significant.
RESULTS
Study population
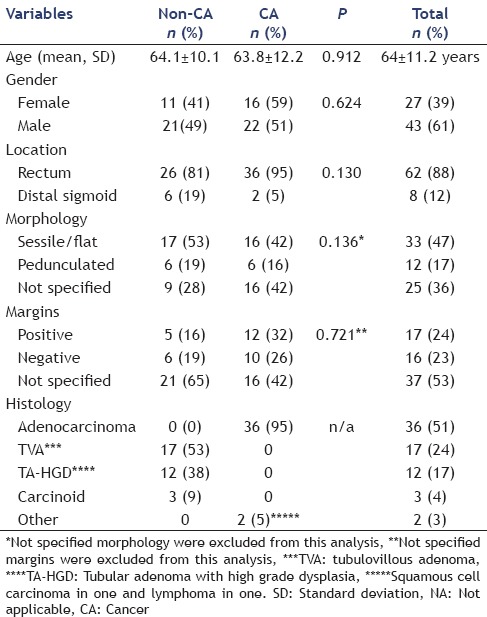
Between October 2002 and October 2009, 1444 patients underwent a lower EUS exam at our institution. Of these, 70 (4.8%) patients (mean age 64 ± 11 years, 61% male) were referred for EUS following endoscopic polypectomy of high risk rectosigmoid colon lesions and comprise the study population. Final diagnoses included the malignancy (CA group) in 38 (54%) and benign neoplasms (non-CA group) in 32 (46%). Histological diagnoses in the CA group included CA in 36 (51%), squamous cell carcinoma in 1 (1.5%) and lymphoma in 1 (1.5%), whereas the non-CA group included TVA in 17 (24%), TA-HGD in 12 (17%), carcinoid in 3 (4%). These are summarized in Table 1
Table 1.
Patient demographics and characteristics of polyps at initial endoscopy
Findings from endoscopy and polypectomy prior to endoscopic ultrasound
The characteristics of the polyps from initial endoscopy prior to EUS are presented in Table 1. Lesion location was in the rectum in 62 (88%) and distal sigmoid in 8 (12%). In 63 (9%), the referring endoscopist reported complete resection in 77% of CA and 67% of non-CA patients (P = 0.58). Pathology reports from polypectomy reported margin status in 33 (47%) and were positive in 17 (12 cancer, 5 benign) and negative in 16 (10 cancer, 6 benign). Resection margin status was unknown in the remaining 37 (16 cancer and 21 non-CA patients). Endoscopic description about pretreatment tumor size was available in 56 (80%) and measured a mean 22 ± 12 mm. Morphology was described in 45 (64%) and was flat/sessile in 33 and pedunculated in 12.
Endoscopic and endoscopic ultrasound findings
Follow-up sigmoidoscopy (WLE) and EUS at our hospital were performed a mean 35 ± 24 days following initial endoscopy. Findings of WLE and EUS at our hospital in the 33 CA and non-CA patients with known resection margins groups are summarized in Figures 1–4.
Figure 1.
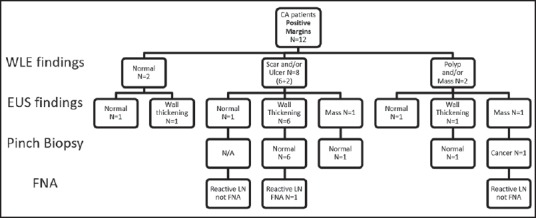
Findings of white light endoscopy and endoscopic ultrasound in cancer patients with positive resection margins
Figure 4.
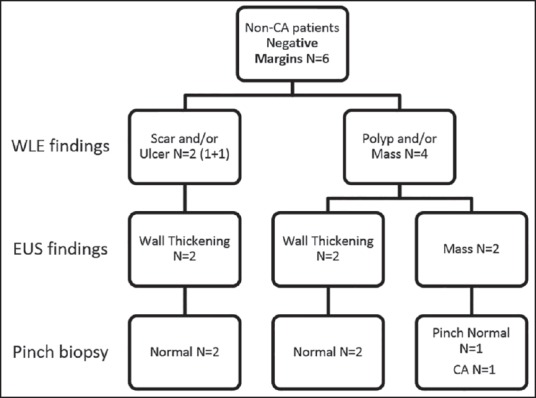
Findings of white light endoscopy and endoscopic ultrasound in noncancer patients with negative resection margins
Figure 2.
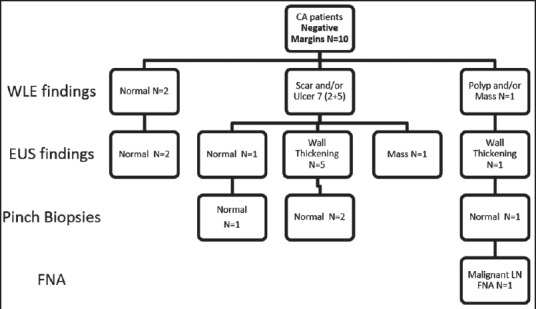
Findings of white light endoscopy and endoscopic ultrasound in cancer patients with negative resection margins
Figure 3.
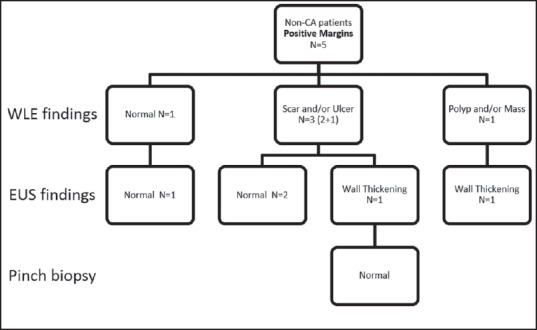
Findings of white light endoscopy and endoscopic ultrasound in noncancer patients with positive resection margins
Of the 16 cancer patients with unknown margins, WLE was normal in 2 but revealed a polyp or mass in 5, scar in 4 and ulcer in 5. In these same 16, EUS was normal in 2 but showed wall thickening in 8, an intramural mass in 6 and a benign appearing LN in one. Of the 21 noncancer patients with unknown margins, WLE was normal in 1, showed polyp/mass in 7, scar in 5 and ulcer in 8. EUS showed mass in 4 and wall thickening in 17.
Incremental yield of endoscopic ultrasound
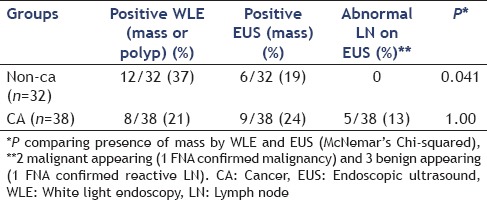
There was no statistically significant difference between the detection of the mass lesion in cancer patients by WLE alone (21%) and EUS (24%; P = 1.0). In non-CA patients WLE alone detected a mass more frequently than EUS (37% vs. 19%, P = 0.041). EUS suggested remaining disease (mass) in the wall in 9/38 (24%) CA and 6/32 (19%) of non-CA patients [Table 2]
Table 2.
Incremental yield of EUS for the detection of mass following endoscopic polypectomy of 32 benign (non-CA) and 38 malignant (CA) lesions
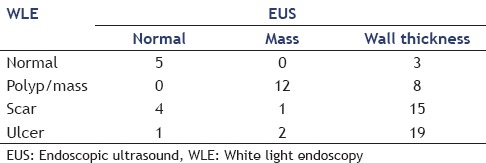
White light endoscopy identified 20 masses and EUS identified 15. In 12 patients, a mass was detected by both WLE and EUS. In the remaining 8 WLE masses, EUS found wall thickening alone in two CA patients and 6 non-CA patients. EUS demonstrated a mass in 3 CA patients in whom WLE found none [Table 3]. Based on EUS findings, those three patients underwent subsequent additional therapy.
Table 3.
Comparison of WLE and EUS findings
An abnormal-appearing peritumoral LN was identified in 5 (13%) CA patients. Of these, two appeared malignant, while three were described as benign. EUS-FNA confirmed residual malignancy in one and was reactive in the other. No pericolonic LNs were identified in the non-CA patients
Follow-up of patients
Follow-up was available in 26/38 (68%) CA and 22/32 (69%) non-CA patients a median 23 (range: 0-93) months after the EUS exam.
Of the 26 CA patients, follow-up procedures included: Transanal excision in 9, laparotomy in four, chemoradiation in 4, and repeat endoscopy in 9. During this period, residual neoplasia was detected in 11 (42%) CA patients. Of the 9 patients who underwent endoscopic surveillance alone without further treatment, there was no local recurrence during a median follow-up of 67 months (range: 8-68).
In the non-CA group, follow-up procedures included: transanal resection in two, laparotomy in one, and surveillance alone in 19. During this period, residual disease was detected in 6/22 (27%) non-CA patients. In 19 patients who underwent endoscopic surveillance alone (median follow-up 42 months (6-72); two developed recurrent adenomas at the resection site 8 and 17 months after EUS, respectively.
Performance of WLE, WLE/BX and EUS for detection of residual neoplasia
The performance of WLE, WLE/BX and EUS for detection of residual neoplasia is summarized in Table 4. For the 48 patients having adequate follow-up, 17 (35%) had residual disease. The sensitivity and specificity of WLE alone, WLE with biopsy, and EUS for the detection of residual neoplasia were 65% (CI: 38-86) and 84% (CI: 66-95), 71% (CI: 42-92) and 95% (CI: 74-100), 59% (CI: 33-82) and 84% (CI: 66-95), respectively. These values were similar between all groups
Table 4.
Sensitivity, specificity, PPV and NPV of WLE, WLE + biopsies, EUS in the detection of residual high risk lesions after polypectomy
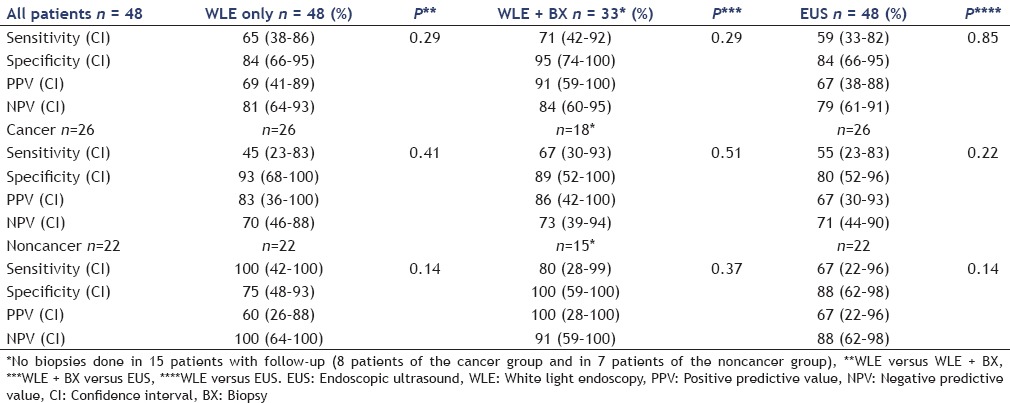
In patients with adequate follow-up, residual neoplasia was identified in 11 (42%) CA and 6 (27%) non-CA patients. The sensitivity and specificity of WLE alone, WLE with biopsy and EUS alone for the detection of residual neoplasia in both groups were similar.
DISCUSSION
The role of transrectal EUS in the locoregional staging of rectal cancer is well established.[6,7,13,14,15,16,17,18] EUS also is useful in detecting local recurrence in the rectosigmoid cancer after surgery.[21,22,23,24] Although assessment of postpolypectomy site is a common reason for referral for EUS, its role in this setting remains uncertain.
The diagnosis of a malignant rectal polyp has different implications for a patient as compared with a malignant colonic polyp,[8] defining the role of EUS for assessment of polypectomy site is important for several reasons. First, early rectal cancers can be removed by full thickness transanal resection of the polypectomy site, an approach not applicable to colonic lesions. Second, radical proctectomy with total mesorectal resection for rectal cancer has a greater morbidity compared to comparable surgery for colon cancer.[8] Finally, local recurrence rates of rectal cancer can be 10 or more times higher than the known 2-4% recurrence rate of colon cancer at the anastomosis.[1]
In our study, we found no difference between the sensitivity and specificity of WLE alone (65% and 84%), WLE with biopsy (71% and 95%), and EUS (59% and 84%) for the detection of residual neoplasia. Overall, there was a slight incremental diagnostic yield of EUS after endoscopic polypectomy of high risk rectal lesions in the CA group. Specifically, EUS identified 3 residual masses missed by WLE in CA patients, which led to subsequent therapy. In addition, abnormal LNs were seen in 13% of patients with CA group including one with residual cancer detected by EUS-FNA. In the non-CA group, EUS did not detect any additional masses or LNs. Therefore, EUS is not necessary for short-term (<3 months) surveillance after resection of benign rectal lesions, but appears to be useful for follow-up of malignant polyps. These data furthermore support consensus guidelines,[1] which recommend follow-up endoscopic exam 3-6 months after the initial resection to inspect and biopsy the resection site. However, this guideline[1] does not clearly endorse the utilization of EUS exams for surveillance and recent ASGE guidelines also do not endorse the use of EUS after resection of colorectal cancer.[7]
García-Aguilar et al.[8] proposes a follow-up algorithm for malignant rectal polyps removed endoscopically, undergoing EUS evaluation within 1-month of the polypectomy. Patients with findings consistent with perirectal LN should be strongly considered for a radical abdominal surgery and for neoadjuvant therapy. Patients without perirectal LN should undergo full thickness transanal excision of the polypectomy site, regardless of EUS images of the rectal wall. Only patients with pedunculated polyps, negative resection margins and normal can be observed and entered in a follow-up protocol. Based on the findings in the CA group of our study, we do agree with the described algorithm. EUS has well established role in staging of rectal cancer, and which includes the finding of malignancy in a polyp.
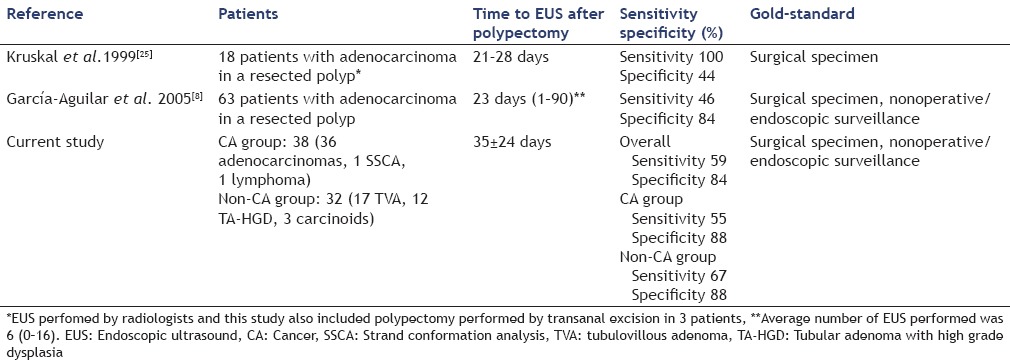
Table 5 summarizes published studies evaluating usefulness of EUS for detection of residual rectal tumor after polypectomy. Kruskal et al.[25] have reported their experience with staging rectal cancer in 18 consecutive patients who underwent EUS after CA was discovered in a polypectomy specimen, benign-appearing at time of endoscopy, EUS had a sensitivity of 100%, specificity of 44%, positive predictive value (PPV) of 64% and negative predictive value (NPV) of 100% for detection of residual tumor. The precise staging was correctly predicted 44% of patients. García-Aguilar et al.[8] subsequently studied 63 patients with malignant rectal polyps removed by snare who underwent successive EUS exams. Polyp and EUS findings were compared with histology in the group who underwent surgery or for recurrence in the patients who had nonoperative surveillance. Overall EUS had a sensitivity of EUS 46%, specificity 84%, PPV 43%, NPV 86% and accuracy of 91%. EUS was more useful than polyp morphologic or histologic criteria to determine the presence of residual cancer in the rectal wall. In the CA group of our study, EUS showed a sensitivity of 55%, specificity 80%, PPV 67%, NPV 71%. EUS appears useful following endoscopic resection of a malignant rectal polyp, however it cannot definitely exclude the possibility of residual tumor in the rectal wall and that decisions regarding definitive management of these patients cannot be based solely on EUS images
Table 5.
Summary of available studies evaluating usefulness of EUS for detection of residual tumor after polypectomy of high risk rectal polyps
Recently, Holinga et al. reported a single center experience with EUS follow-up of 24 patients with incidental primary rectal carcinoids <10 mm, 58% of which were removed by band-assisted EMR. Out of the 71% patients who underwent EUS surveillance, 8.3% patients had a metastatic perirectal LN identified. The authors recommend surveillance EUS examinations after the removal of low-risk rectal carcinoid tumors, and propose a surveillance protocol consisting of follow-up EUS 3 months after endoscopic or local resection to ensure complete removal, followed by EUS every 6 months for 3 years.[26] Our study included three carcinoids, however none had positive EUS findings or LN. Follow-up was obtained in two patients and no recurrence occurred in those. Our findings don’t support conclusions by Holinga et al., however we included a limited number of patients with carcinoids.
Our study has limitations inherent to its retrospective design. Furthermore, surgical follow-up was not available in the majority of patients and long-term follow-up was available in only 69% of patients. We only included in the sensitivity/specificity calculation patients with a minimum endoscopic follow-up of 6 months, however ideally surgical pathology gold standard should have been used in all patients. Also, length of follow-up was not homogeneous in different patients and it is possible that local recurrences could have been missed. Of note, as a limitation of the retrospective design of our study, we lack information of use of NBI in our series. The lack of NBI and endomicroscopy may underestimate use of WLE or other non-EUS testing.
In a busy EUS practice, assessment of polypectomy site is a common referral. Our results suggest that in this setting EUS has a limited role, especially in patients in which initial resection did not show malignancy. In our opinion, EUS should be performed in follow-up and staging of malignant polyps resected endoscopically, but not of benign lesions. Further prospective evaluations would be required to further define usefulness of EUS in this setting.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Rex DK, Kahi CJ, Levin B, et al. Guidelines for colonoscopy surveillance after cancer resection: A consensus update by the American Cancer Society and the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2006;130:1865–71. doi: 10.1053/j.gastro.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 2.Winawer SJ, Zauber AG, Fletcher RH, et al. Guidelines for colonoscopy surveillance after polypectomy: A consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. Gastroenterology. 2006;130:1872–85. doi: 10.1053/j.gastro.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Winawer SJ, Zauber AG, Fletcher RH, et al. Guidelines for colonoscopy surveillance after polypectomy: A consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. CA Cancer J Clin. 2006;56:143–59. doi: 10.3322/canjclin.56.3.143. [DOI] [PubMed] [Google Scholar]
- 4.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: A joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130–60. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 5.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: A joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–95. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Davila RE, Rajan E, Adler D, et al. ASGE guideline: The role of endoscopy in the diagnosis, staging, and management of colorectal cancer. Gastrointest Endosc. 2005;61:1–7. doi: 10.1016/s0016-5107(04)02391-0. [DOI] [PubMed] [Google Scholar]
- 7.ASGE Standards of Practice Committee. Fisher DA, Shergill AK, et al. Role of endoscopy in the staging and management of colorectal cancer. Gastrointest Endosc. 2013;78:8–12. doi: 10.1016/j.gie.2013.04.163. [DOI] [PubMed] [Google Scholar]
- 8.García-Aguilar J, Hernández de Anda E, Rothenberger DA, et al. Endorectal ultrasound in the management of patients with malignant rectal polyps. Dis Colon Rectum. 2005;48:910–6. doi: 10.1007/s10350-004-0903-6. [DOI] [PubMed] [Google Scholar]
- 9.Engstrom PF, Arnoletti JP, Benson AB, 3rd, et al. NCCN Clinical Practice Guidelines in Oncology: Rectal cancer. J Natl Compr Canc Netw. 2009;7:838–81. doi: 10.6004/jnccn.2009.0057. [DOI] [PubMed] [Google Scholar]
- 10.Morson BC, Whiteway JE, Jones EA, et al. Histopathology and prognosis of malignant colorectal polyps treated by endoscopic polypectomy. Gut. 1984;25:437–44. doi: 10.1136/gut.25.5.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nascimbeni R, Burgart LJ, Nivatvongs S, et al. Risk of lymph node metastasis in T1 carcinoma of the colon and rectum. Dis Colon Rectum. 2002;45:200–6. doi: 10.1007/s10350-004-6147-7. [DOI] [PubMed] [Google Scholar]
- 12.Muller S, Chesner IM, Egan MJ, et al. Significance of venous and lymphatic invasion in malignant polyps of the colon and rectum. Gut. 1989;30:1385–91. doi: 10.1136/gut.30.10.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhutani MS. Recent developments in the role of endoscopic ultrasonography in diseases of the colon and rectum. Curr Opin Gastroenterol. 2007;23:67–73. doi: 10.1097/MOG.0b013e328011630b. [DOI] [PubMed] [Google Scholar]
- 14.Siddiqui AA, Fayiga Y, Huerta S. The role of endoscopic ultrasound in the evaluation of rectal cancer. Int Semin Surg Oncol. 2006;3:36. doi: 10.1186/1477-7800-3-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puli SR, Bechtold ML, Reddy JB, et al. How good is endoscopic ultrasound in differentiating various T stages of rectal cancer? Meta-analysis and systematic review. Ann Surg Oncol. 2009;16:254–65. doi: 10.1245/s10434-008-0231-5. [DOI] [PubMed] [Google Scholar]
- 16.Kav T, Bayraktar Y. How useful is rectal endosonography in the staging of rectal cancer? World J Gastroenterol. 2010;16:691–7. doi: 10.3748/wjg.v16.i6.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berton F, Gola G, Wilson SR. Perspective on the role of transrectal and transvaginal sonography of tumors of the rectum and anal canal. AJR Am J Roentgenol. 2008;190:1495–504. doi: 10.2214/AJR.07.3188. [DOI] [PubMed] [Google Scholar]
- 18.Bhutani MS. Endoscopic ultrasound in the diagnosis, staging and management of colorectal tumors. Gastroenterol Clin North Am. 2008;37:215–27. doi: 10.1016/j.gtc.2007.12.001. viii. [DOI] [PubMed] [Google Scholar]
- 19.Harewood GC. Hawes R. H, Fockens P, Varadarajulu S. Endosonography. Philadelphia, USA: Elsevier Saunders; 2011. EUS in rectal cancer; pp. 205–10. [Google Scholar]
- 20.Hawass NE. Comparing the sensitivities and specificities of two diagnostic procedures performed on the same group of patients. Br J Radiol. 1997;70:360–6. doi: 10.1259/bjr.70.832.9166071. [DOI] [PubMed] [Google Scholar]
- 21.Löhnert MS, Doniec JM, Henne-Bruns D. Effectiveness of endoluminal sonography in the identification of occult local rectal cancer recurrences. Dis Colon Rectum. 2000;43:483–91. doi: 10.1007/BF02237191. [DOI] [PubMed] [Google Scholar]
- 22.Hünerbein M, Totkas S, Moesta KT, et al. The role of transrectal ultrasound-guided biopsy in the postoperative follow-up of patients with rectal cancer. Surgery. 2001;129:164–9. doi: 10.1067/msy.2001.110428. [DOI] [PubMed] [Google Scholar]
- 23.Woodward T, Menke D. Diagnosis of recurrent rectal carcinoma by EUS-guided fine-needle aspiration. Gastrointest Endosc. 2000;51:223–5. doi: 10.1016/s0016-5107(00)70426-3. [DOI] [PubMed] [Google Scholar]
- 24.Gleeson FC, Larson DW, Dozois EJ, et al. Local recurrence detection following transanal excision facilitated by EUS-FNA. Hepatogastroenterology. 2012;59:1102–7. doi: 10.5754/hge11898. [DOI] [PubMed] [Google Scholar]
- 25.Kruskal JB, Sentovich SM, Kane RA. Staging of rectal cancer after polypectomy: Usefulness of endorectal US. Radiology. 1999;211:31–5. doi: 10.1148/radiology.211.1.r99ap1231. [DOI] [PubMed] [Google Scholar]
- 26.Holinga J, Khalid A, Fasanella K, et al. Metastatic risk of diminutive rectal carcinoid tumors: A need for surveillance rectal ultrasound? Gastrointest Endosc. 2012;75:913–6. doi: 10.1016/j.gie.2011.11.032. [DOI] [PubMed] [Google Scholar]


