Abstract
Background and Purpose:
Symptomatic intracranial hemorrhage (sICH) is the most serious adverse event in stroke patients who received i.v. rt-PA and is usually associated with poor outcomes. The SEDAN score is built up to predict sICH. We aim to externally validate the SEDAN score in Thai patients from single center in the real world practice.
Materials and Methods:
The SEDAN score of stroke patients treated with intravenous rt-PA at Thammasat University Hospital from January 2010 to June 2012 was calculated. Patients were divided into three groups including symptomatic intracranial hemorrhage (sICH), asymptomatic intracranial hemorrhage (AsICH) and no intracerebral hemorrhage (NoICH). The primary outcome of analyses was sICH. Each parameter of the SEDAN score and correlation between score and sICH were analyzed with univariate and multivariate model.
Results:
295 patients (18.6% of stroke admission) were treated with i.v. rt-PA. 13 patients (4.4%) had sICH and 31 patients (10.4%) had AsICH. Baseline blood sugar >12 mmol/l, early infarction, hyperdense cerebral artery, age >75 years-old and NIHSS ≥10(SEDAN) were associated with sICH by univariate analysis (P value = 0.018, <0.001, <0.001, 0.002 and 0.027 respectively). The rate of sICH occurrence was increased in accordance with the increasing of the SEDAN score. By multivariate analysis, odds ratio of baseline blood sugar >12 mmol/l, early infarction, hyperdense cerebral artery, age >75 years-old and NIHSS ≥10 were 1.248, 2.503, 1.107, 1.532 and 1.263 respectively.
Conclusions:
The SEDAN score was practical to use and predictive in Thai population. Each parameter of the SEDAN score was an independent risk factor for sICH after treatment with i.v. rt-PA.
Keywords: Acute ischemic stroke, intravenous recombinant tissue plasminogen activator, symptomatic intracranial hemorrhage, the SEDAN score
Introduction
Stroke is the leading cause of adult disability particularly in elderly and remains the third most common cause of death in developing world[1] as well as in Thailand.[2,3] Unfortunately, a few interventions have been approved for improving outcomes of acute ischemic stroke.[4,5] Among those interventions, intravenous recombinant tissue plasminogen activator (i.v. rt-PA) given within 4.5 hours after the onset of ischemic stroke is the biggest milestone.[6,7] The benefit of i.v. rt-PA in acute ischemic stroke has also been reported in Thailand[8,9] as well as in other developing countries.[10,11] Symptomatic intracranial hemorrhage (sICH) is the most serious adverse event in acute stroke patients who received i.v. rt-PA and is usually associated with poor outcomes.[12] First introduced by European Cooperative Acute Stroke Study (ECASS) II trial, definition of sICH is “clinical deterioration causing an increase in the National Institutes of Health Stroke Scale (NIHSS) score of ≥4 points and whether the hemorrhage was likely to be the cause of the clinical deterioration”.[13] According to sICH per ECASS II criteria, sICH in Thai population is around 2-6%.[8,9,14]
The SEDAN score is built up to predict sICH per ECASS II consisting of 5 parameters: Baseline blood glucose 8.1 to 12.0 mmol/L = 1 point and >12.0 mmol/L = 2 points, early infarct signs on baseline computed tomography (CT) = 1 point, hyperdense cerebral artery sign = 1point, age >75 years = 1 point, and baseline NIHSS score ≥10 = 1 point.[15] External validation of SEDAN score by retrospective data from Safe Implementation of Treatments in Stroke (SITS)–International Stroke Thrombolysis Register (ISTR) showed moderately predictive value for sICH per ECASS II.[16] We aim to externally validate the SEDAN score in Thai patients from single center in the real world practice.
Materials and Methods
Patients
Thammasat University Hospital is a 460-beds hospital with a Stroke Unit. The acute stroke service with integrated referral network was established in October 2007. There were two major pathways for the acute stroke patients to achieve the acute stroke system: Referred from outside hospitals of acute stroke network or walk-in. There were 1,583 patients admitted with acute ischemic stroke in Stroke Unit of Thammasat University Hospital from January 2010 to June 2012. One thousand two hundred and seventy patients were sorted out because of untreated with i.v. rt-PA. Six patients refused for treatment. Twelve patients were treated by endovascular therapy. Remained 295 patients were treated with i.v. rt-PA during 4.5 hours time window (as shown below) Figure 1.
Figure 1.
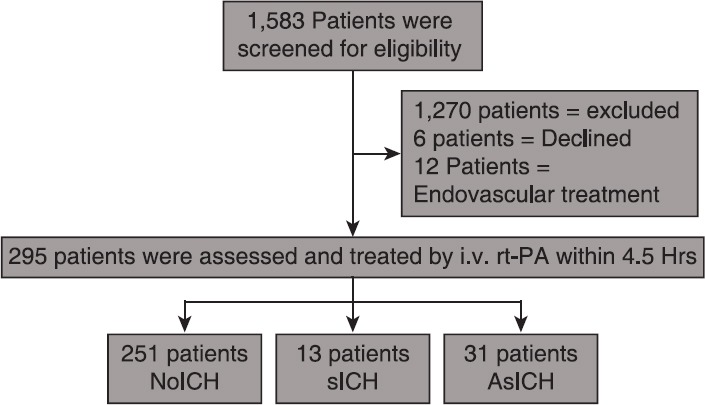
Study design
Definition
Symptomatic intracranial hemorrhage (sICH) was defined by patient who had clinical deterioration causing an increase in the NIHSS score of more than or equal to 4 points and if the hemorrhage was likely to be the cause of the clinical deterioration (per the ECASS, II).
Early infarct signs included hypoattenuation comprising <1/3 of middle cerebral artery or other area which was the cause of ischemic stroke or obscuration of the lentiform nucleus or loss of insular ribbon or obscuration of the Sylvian fissure or cortical sulcal effacement.
Statistical analysis
Data were recorded in computer with Microsoft excel, analyzed with software Statistical Package for the Social Sciences (SPSS) version 16.0, data of the participating patients were kept confidentially in accordance with the rule of human research ethic. The variables of this study had the form of continuous variables and discrete variables. Compiling demographic data, risk factors, and co-morbidity, CT scan of patients prior to treatment with rt-PA and assessing NIHSS score for the purpose to calculate SEDAN score were collected with descriptive statistic. Patients were divided into three groups including sICH, asymptomatic intracranial hemorrhage (AsICH), and no intracerebral hemorrhage (NoICH). Univariate associations of parameters in SEDAN score among groups were evaluated by Pearson chi-square test. Multivariate logistic regression model were performed for calculating the value of absolute risk, odds ratio, and 95% confidence interval of each parameter. Data were analyzed by SPSS version 16.0 for studying the relation of variables by using linear regression for correlation at the significant level P-value < 0.05. Area under the receiver operating characteristic curve (AUC-ROC) was done to describe how well our values matched the original SEDAN score.
Results
The i.v. rt-PA rate was 18.6% of patients admitted with acute ischemic stroke. Of 295 patients treated with i.v. rt-PA, there were 252 (85.4%) infarct location in anterior circulation and 43 (14.5%) in posterior circulation. There were 44 ICH (14.8%) with 13 sICH (4.4%) and 31 AsICH (10.4%).
The average age of NoICH, sICH, and AsICH were 69, 72, and 71 years old, respectively. Patients in sICH group were statistically significant older than those in NoICH group. Diabetes, atrial fibrillation, and ischemic heart disease were more found in patients with ICH. Baseline characteristics of our population were shown in Table 1. Characteristics of all sICH patients were shown in Table 2.
Table 1.
Demographic data
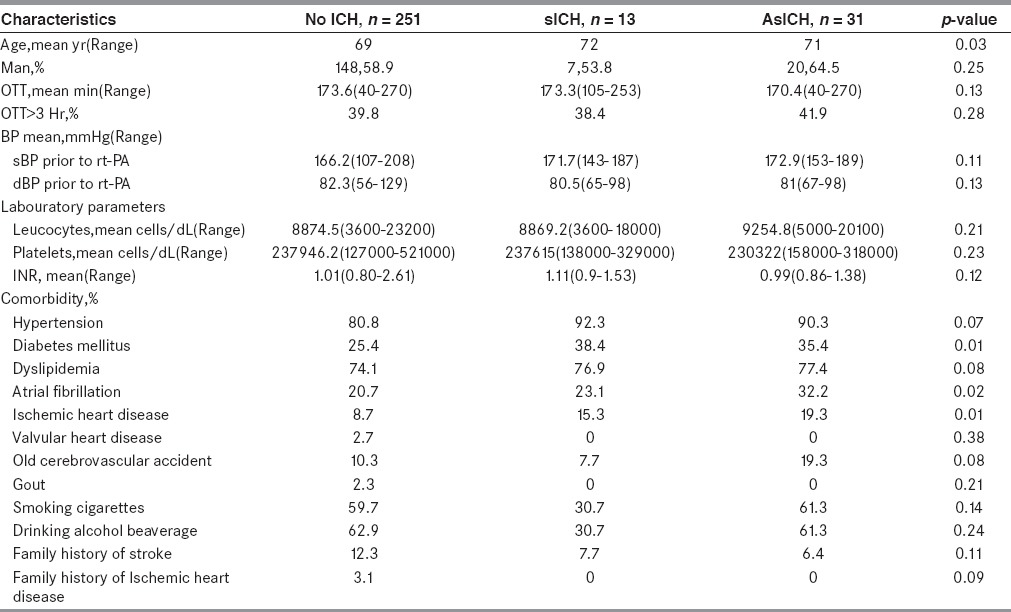
Table 2.
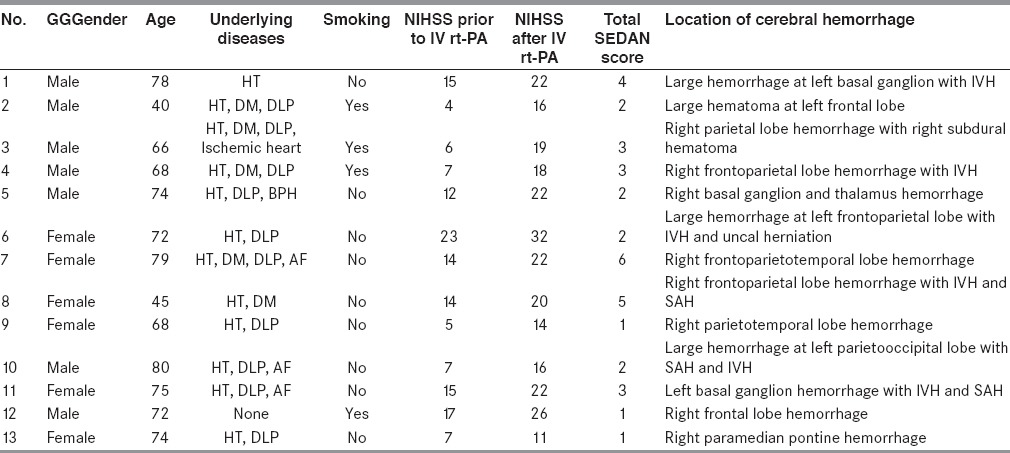
Characteristics of all patients with sICH
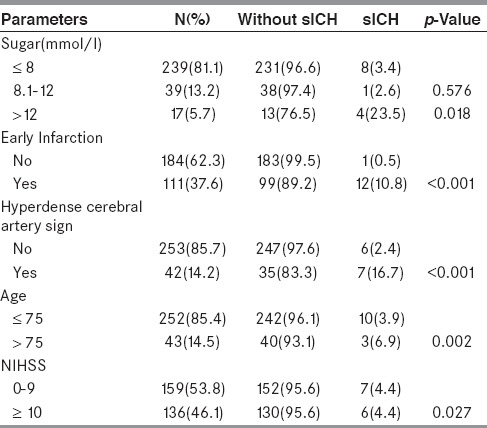
Univariate evaluation of each parameter in SEDAN score showed that:
Three tiers of baseline blood sugar (continuous variables) consist of ≤8, 8.1-12, and >12 mmol/l. From13 patients with sICH, eight patients had baseline blood sugar ≤8 mmol/l and one patient had baseline blood sugar between 8.1 and 12 mmol/l. From 282 patients without sICH, 231patients had baseline blood sugar ≤8 mmol/l and 38 patients had baseline blood sugar between 8.1 and 12 mmol/l. There was no significantly difference between sICH and without sICH for the sugar level at ≤8 and 8.1–12 mmol/l. Considering the sugar level >12 mmol/l, there were four out of 13 patients with sICH while there were 13 out of 282 patients without sICH. There was significantly difference between sICH and without sICH for the sugar level at >12 mmol/l with P-value of 0.018.
From 13 patients with sICH, 12 of them had early infarction (discrete variables) in the initial CT brain. While, from 282 patients without sICH, 99 of them had early infarction. There was significantly difference between sICH and without sICH for early infarction in initial CT brain with P-value <0.001.
From 13 patients with sICH, 12 of them had hyperdense cerebral artery sign (discrete variables) in initial CT brain. While, from 282 patients without sICH, 35 of them had this sign. There was significantly difference for hyperdense cerebral artery sign (discrete variables) in initial CT brain with P-value <0.001.
From 13 patients with sICH, three of them were more than 75 years old (continuous variables). While, from 282 patients without sICH, 40 of them were more than 75 years old. There was significantly difference for age more than 75 year old with P-value of 0.002.
From 13 patients with sICH, six of them had initial NIHSS as 10 or more (continuous variables). While, from 282 patients without sICH, 130 of them had initial NIHSS as 10 or more. There was significantly difference for initial NIHSS as 10 or more with P-value of 0.027.
The univariate evaluation was shown in Table 3.
Table 3.
Univariate Comparison of sICH and without sICH
The rate of sICH occurrence was increased in accordance with the increasing of the SEDAN score in our study. The SEDAN score was ranking from 0 to 6 point. All 87 patients with 0-point showed no either sICH or AsICH at all. While the only one patient with 6-point had sICH. The rate of sICH was correlated with increasing total score: 0% at total score 0, 3.6% at total score 1, 5.4% at total score 2, 8.6% at total score 3, 9.2% at total score 4, 33.3% at total score 5, and 100% at total score 6. Moreover, the rate of AsICH was also correlated with increasing total score: 0% at total score 0, 3.6% at total score 1, 17.6% at total score 2, 22.8% at total score 3, 45.4% at total score 4, and 66.7% at total score 5. The correlation of the SEDAN score with sICH and AsICH was showed in Table 4 and Figure 2.
Table 4.
Proportion of patients in each parameter of SEDAN score
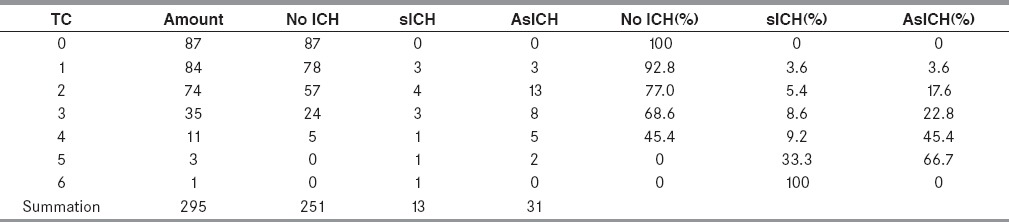
Figure 2.
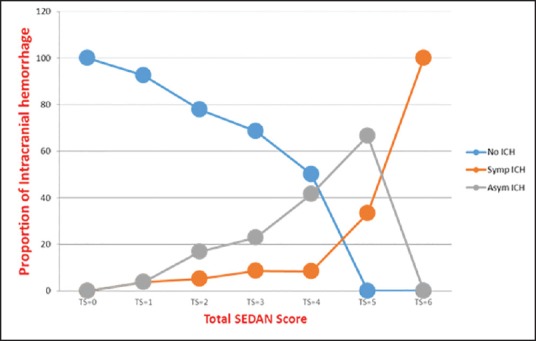
Correlation of no intracerebral hemorrhage (NoICH), asymptomatic intracranial hemorrhage (AsICH), and symptomatic intracranial hemorrhage (sICH) with SEDAN score
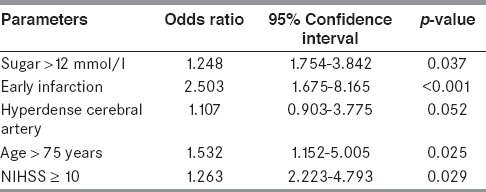
To analyze the variable factors between sICH and without sICH group, the multivariate analysis was performed. The age over 75 years old, early infarction, hyperdense cerebral artery, baseline blood sugar more than 12 mmol/l, and NIHSS as 10 or more were the risk factors to develop sICH after treated with rt-PA at 1.532, 2.503, 1.107, 1.248, and 1.263 times, respectively. All parameters of SEDAN score, except hyperdense cerebral artery, showed significant prediction of sICH (shown in Table 5). The performance of the SEDAN score by AUC-ROC for our data was 0.76 (95% CI, 0.66-0.86).
Table 5.
Multivariate logistic regression modeling procedures of each parameter
Discussion
The intravenous thrombolytic rate is 18.5% of patients who admitted with acute ischemic stroke in this study, which is a bit lower from 21% in our previous report 2 years earlier.[9] However, this rate is still considerably high as compared to which reported from most centers from many countries.[8,17,18,19] The proportion of patients received treatment within 3 to 4.5 hours time-window in this study is higher than those from previous study. The sICH rate in this study is 4.4%, which is higher than the rate of 2% reported in previous study.[9] However, it is still comparable with the rate reported from most centers including the centers in Thailand.[8,14,20]
The SEDAN score is used to predict the risk of symptomatic intracerebral hemorrhage among patients receiving i.v. rt-PA.[15] Easy to use is the advantage. The five variables including age, baseline blood glucose, NIHSS, early infarct, and hyperdense artery by CT, are able to acquire in every primary stroke center. The SEDAN score had the highest predictive power with moderate performance as compared with others including MSS (Multicenter Stroke Survey); HAT (Hemorrhage After Thrombolysis), glucose at presentation, race (Asian), age, sex (male), systolic blood pressure at presentation, and severity of stroke at presentation (NIH Stroke Scale (GRASPS); SITS; and stroke prognostication using age and NIH Stroke Scale (SPAN)-100.[21] The SEDAN score could be helpful in assessing the risk of symptomatic intracranial hemorrhage. There has been the first external validation study of the SEDAN score reported in the literature by Mazya and colleagues.[16] Mazya's study employed the retrospective data from the International Stroke Thrombolysis Register for validation.[16] Our study should represent the validation of the SEDAN score in real world practice.
Comparison with original study of the SEDAN score, the number of patients with sICH in our study was less.[15] However, most of baseline characteristics of both studies were comparable (shown in Table 6). Score by score comparison with original study shows almost similar trend and rate of sICH (shown in Figure 3).[15] The rate of sICH in Thai stroke patients with i.v. rt-PA treatment was correlated with increasing score. Early infarction in initial CT brain was the strongest independent risk factor in our study. This should put emphasis on the value of data gathering of CT brain before decision of thrombolytic treatment.[5]
Table 6.
Baseline characteristics comparison between this study and original the SEDAN score study
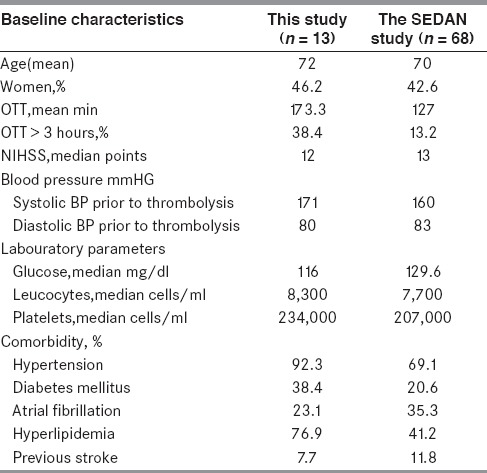
Figure 3.
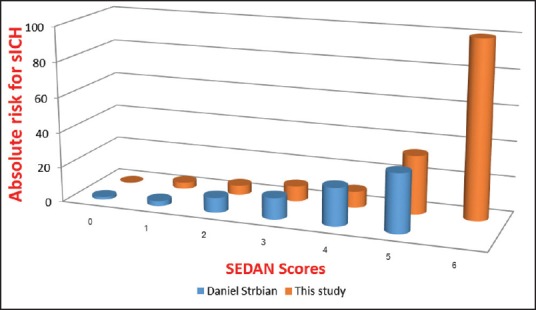
Comparison between our study and original the SEDAN score study
Symptomatic intracranial hemorrhage is the most serious adverse event in stroke patients after treated with i.v. rt-PA.[12] Clinical deteriorations from symptomatic intracranial hemorrhage lead to poor overall outcomes.[12,22] Risk identification of symptomatic intracranial hemorrhage is important. High risk factors may not be contraindications for treatment with i.v. rt-PA.[5] But additional treatment should apply to these high risk patients to prevent occurrence of intracranial hemorrhage. In the future, there may be some treatments to prevent symptomatic intracranial hemorrhage after i.v. rt-PA. For example, the recent study from Korea showed that therapeutic hypothermia after recanalization with endovascular treatment prevent occurrence of intracerebral hemorrhage.[23] Therapeutic hypothermia may also be effective in prevention of intracranial hemorrhage after i.v. rt-PA. The initial study of symptomatic intracranial hemorrhage prevention therapy should focus on the high-risk group. The SEDAN score should facilitate selection of high risk patients for the study.
In conclusion, the SEDAN score was convenient to use in clinical practice. The score was predictive for sICH in Thai stroke patients with i.v. rt-PA treatment. Each parameter of the SEDAN score was an independent risk factor for sICH after treatment with i.v. rt-PA.
Acknowledgement
This work was supported by the National Research University Project of Thailand Office of Higher Education Commission.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: Systematic analysis of population health data. Lancet. 2006;367:1747–57. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 2.Stroke epidemiological data of nine Asian countries. Asian Acute Stroke Advisory Panel (AASAP) J Med Assoc Thai. 2000;83:1–7. [PubMed] [Google Scholar]
- 3.Poungvarin N. Burden of stroke in Thailand. Int J Stroke. 2007;2:127–8. doi: 10.1111/j.1747-4949.2007.00104.x. [DOI] [PubMed] [Google Scholar]
- 4.European Stroke Organisation (ESO) Executive Committee; ESO Writing Committee. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis. 2008;25:457–507. doi: 10.1159/000131083. [DOI] [PubMed] [Google Scholar]
- 5.Jauch EC, Saver JL, Adams HP, Jr, Bruno A, Connors JJ, Demaerschalk BM, et al. Guidelines for the early management of patients with acute ischemic stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 6.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581–7. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 7.Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, et al. ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–29. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 8.Suwanwela NC, Phanthumchinda K, Likitjaroen Y. Thrombolytic therapy in acute ischemic stroke in Asia: The first prospective evaluation. Clin Neurol Neurosurg. 2006;108:549–52. doi: 10.1016/j.clineuro.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Muengtaweepongsa S, Dharmasaroja P, Kummark U. Outcomes of intravenous thrombolytic therapy for acute ischemic stroke with an integrated acute stroke referral network: Initial experience of a community-based hospital in a developing country. J Stroke Cerebrovasc Dis. 2012;21:42–6. doi: 10.1016/j.jstrokecerebrovasdis.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 10.Durai Pandian J, Padma V, Vijaya P, Sylaja PN, Murthy JM. Stroke and thrombolysis in developing countries. Int J Stroke. 2007;2:17–26. doi: 10.1111/j.1747-4949.2007.00089.x. [DOI] [PubMed] [Google Scholar]
- 11.Brainin M, Teuschl Y, Kalra L. Acute treatment and long-term management of stroke in developing countries. Lancet Neurol. 2007;6:553–61. doi: 10.1016/S1474-4422(07)70005-4. [DOI] [PubMed] [Google Scholar]
- 12.Al-Senani FM, Grotta JC. Scott EK, Philip BG, editors. Thrombolytic Therapies. Blue Books of Practical Neurology: Butterworth-Heinemann. 2004;(Chapter 13):267–81. [Google Scholar]
- 13.Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998;352:1245–51. doi: 10.1016/s0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 14.Chindaprasirt J, Sawanyawisuth K, Chattakul P, Limpawattana P, Tiamkao S, Aountri P, et al. Age predicts functional outcome in acute stroke patients with rt-PA treatment. ISRN Neurol 2013. 2013 doi: 10.1155/2013/710681. 710681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strbian D, Engelter S, Michel P, Meretoja A, Sekoranja L, Ahlhelm FJ, et al. Symptomatic intracranial hemorrhage after stroke thrombolysis: The SEDAN score. Ann Neurol. 2012;71:634–41. doi: 10.1002/ana.23546. [DOI] [PubMed] [Google Scholar]
- 16.Mazya MV, Bovi P, Castillo J, Jatuzis D, Kobayashi A, Wahlgren N, et al. External validation of the SEDAN score for prediction of intracerebral hemorrhage in stroke thrombolysis. Stroke. 2013;44:1595–600. doi: 10.1161/STROKEAHA.113.000794. [DOI] [PubMed] [Google Scholar]
- 17.Grotta JC, Burgin WS, El-Mitwalli A, Long M, Campbell M, Morgenstern LB, et al. Intravenous tissue-type plasminogen activator therapy for ischemic stroke: Houston experience 1996 to 2000. Arch Neurol. 2001;58:2009–13. doi: 10.1001/archneur.58.12.2009. [DOI] [PubMed] [Google Scholar]
- 18.van Wijngaarden JD, Dirks M, Huijsman R, Niessen LW, Fabbricotti IN, Dippel DW. Promoting Acute Thrombolysis for Ischaemic Stroke (PRACTISE) Investigators. Hospital rates of thrombolysis for acute ischemic stroke: The influence of organizational culture. Stroke. 2009;40:3390–2. doi: 10.1161/STROKEAHA.109.559492. [DOI] [PubMed] [Google Scholar]
- 19.Katzan IL, Hammer MD, Hixson ED, Furlan AJ, Abou-Chebl A, Nadzam DM. Utilization of intravenous tissue plasminogen activator for acute ischemic stroke. Arch Neurol. 2004;61:346–50. doi: 10.1001/archneur.61.3.346. [DOI] [PubMed] [Google Scholar]
- 20.Seet RCS, Rabinstein AA. Symptomatic intracranial hemorrhage following intravenous thrombolysis for acute ischemic stroke: A critical review of case definitions. Cerebrovasc Dis. 2012;34:106–14. doi: 10.1159/000339675. [DOI] [PubMed] [Google Scholar]
- 21.Strbian D, Michel P, Seiffge DJ, Saver JL, Numminen H, Meretoja A, et al. Symptomatic intracranial hemorrhage after stroke thrombolysis: Comparison of prediction scores. Stroke. 2014;45:752–8. doi: 10.1161/STROKEAHA.113.003806. [DOI] [PubMed] [Google Scholar]
- 22.Muengtaweepongsa S, Dharmasaroja P, Maungboon P, Wattanaruangkowit P. Feasibility and safety of remote radiology interpretation with telephone consultation for acute stroke in Thailand. Neurol India. 2010;58:740–2. doi: 10.4103/0028-3886.72162. [DOI] [PubMed] [Google Scholar]
- 23.Hong JM, Lee JS, Song HJ, Jeong HS, Choi HA, Lee K. Therapeutic hypothermia after recanalization in patients with acute ischemic stroke. Stroke. 2014;45:134–40. doi: 10.1161/STROKEAHA.113.003143. [DOI] [PubMed] [Google Scholar]


