Abstract
A 22-year-old male student with no past medical illness, presented with acute onset dysarthria, binocular diplopia, and dysphagia over 10 hours. On examination, he had tachycardia, hypertension, generalized hyper-reflexia, and bilateral pupil sparing oculomotor, troclear, abducens, trigeminal, facial, glossopharyngeal, and vagus nerve palsy. Rest examination was unremarkable. Facial nerve conduction study (NCS) showed decreased amplitude bilaterally and neurogenic pattern on electromyography. Limb NCS, repetitive nerve stimulation, neostigmine test, brain magnetic resonance imaging, cerebrospinal fluid, and biochemical tests were normal. Only positive tests were low thyroid-stimulating hormone (TSH) (<0.01), high free T3 (19.2 pmol/L), and high free T4 (39.2 pmol/L). Thyroid ultrasonography, anti-thyroid peroxidase, and anti-thyroglobulin antibody were normal. Patient was treated with anti-thyroid drugs, with which he completely recovered in 2 months. Though many cases with thyrotoxic myopathy have been reported, only few mention neuropathic cause of dysphagia or polyneuritis cranialis. Getting done thyroid function tests may be helpful in patients with polyneuritis cranialis of uncertain etiology.
Keywords: Bulbar palsy, dysphagia, neuropathy, polyneuritis cranialis, thyrotoxicosis
Introduction
Patients presenting with multiple cranial neuropathies are not uncommon in neurologic clinical practice. The evaluation of these patients can often be overwhelming due to the vast and complicated etiologies as well as the potential for devastating neurologic outcomes. Dysfunction of the cranial nerves can occur anywhere from the brainstem to their peripheral courses. Polyneuritis cranialis is a multiple cranial neuropathy that has been attributed to Lyme disease, herpes zoster, as a Guillain-Barré variant and multiple other causes. We discuss such a case due to an unsuspected etiology.
Case Report
A 22-year-old male student, with no significant past history of any medical illness, presented with a history of acute onset hoarseness of voice and binocular diplopia since 10 hours and severe hypophonia with dysphagia since 4 hours. There was no visual blurring or history of eating canned food or abdominal pain or other gastrointestinal symptoms. Patient denied any recent history of fever or any other infection in the preceding weeks to months. There was no prior such episode of bulbar or limb weakness and the current illness did not show any fluctuations in the symptoms. On examination, tachycardia, hypertension and multiple cranial nerve palsies, including bilateral pupil sparing oculomotor, troclear, abducens, trigeminal (including facial sensory impairment), facial, glossopharyngeal, and vagus nerves was detected [Figure 1]. Patient also had generalized limb hyperreflexia but normal tone and power, absent jaw jerk, and flexor plantar reflex bilaterally. Rest examination was unremarkable. No orthostatic hypotension or other signs of autonomic dysfunction were detected. Investigations showed a normal contrast enhanced magnetic resonance imaging (MRI) of brain and computed tomography (CT) of the base of skull. Nerve conduction study (NCS) of facial nerve showed bilaterally decreased amplitude with a neuropathic pattern on electromyography (EMG). Limb NCS and repetitive nerve stimulation (RNS) tests were normal. Ice pack test and neostigmine tests failed to show any improvement in symptoms. Acetylcholine esterase antibody was also negative. Blood sugar, glycosylated hemoglobin, serum electrolytes including potassium level, electrocardiography, and renal and liver function tests were normal. Muscle enzyme levels including creatinine phosphokinase (CPK) and CPK-NAC were normal. Cerebrospinal fluid (CSF) examination done on second day and repeated on eighth day showed normal proteins, two lymphocytes and normal glucose level. Polymerase chain reaction (PCR) of CSF for herpes simplex-1 and varicella zoster was negative. Patient also tested negative for human immunodeficiency virus (HIV), antinuclear antibody (ANA) by immunofluorescence, venereal disease research laboratory (VDRL) antibody, acetylcholine esterase (ACE), and antibodies against borrelia burgdorferi. There was no history suggestive of diphtheria, sarcoidosis, recent ingestion of canned food or treatment with chemotherapeutic agents. The only positive tests were a low TSH level− < 0.01 (normal range = 0.25-5.0 uIU/mL), high free T3 level−19.2 (normal range = 4.0-8.3 pmol/L), and high free T4 level− 39.2 (normal range = 9.0-20.0 pmol/L). Thyroid ultrasonography was normal and anti-thyroid peroxidase (anti-TPO) and anti-thyroglobulin antibodies were negative. Considering the multiple cranial nerve with facial sensory involvement, a diagnosis of polyneuritis cranialis was made. As CSF analysis was normal, a Guillain Barré Syndrome (GBS) variant was considered less likely. In view of abnormal thyroid function tests, a thyrotoxicosis-associated polyneuritis cranialis was concluded and not idiopathic polyneuritis cranialis. Patient was treated with Carbimazole 10 mg thrice daily and Propranolol 40 mg thrice daily, with which over next 2 weeks, he showed partial recovery in jaw muscles, lower facial muscles, dysphagia, and dysarthria, and almost complete recovery in 8 weeks. As neither GBS nor idiopathic polyneuritis cranialis were considered as the final diagnosis, immunomodulatory drugs like immunoglobulins or plasmapheresis, or corticosteroids were not administered.
Figure 1.
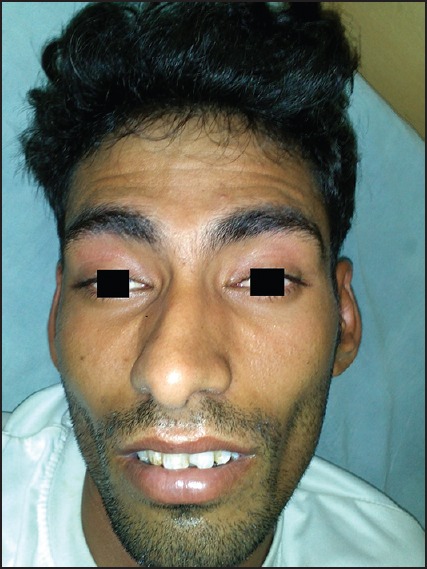
Picture showing bilateral ptosis and facial palsy
Discussion
Our patient presented with polyneuritis cranialis with limb hyperreflexia. After ruling out other causes, we diagnosed it as thyrotoxicosis induced polyneuritis cranialis. The same was confirmed by the response to treatment with anti-thyroid drugs. Hyperthyroidism can explain the systemic features of tachycardia and hypertension. Hyperthyroidism is known to cause myopathy as well as polyneuropathy. Described since the last century, “Basedow's paraplegia” is a polyneuropathy that has been well characterized and is due to a direct effect of hyperthyroidism.[1] It presents as a subacute lower motor neuron type of paraparesis affecting proximal and distal muscles with areflexia and without sensory or bladder involvement. Many studies also mention a subacute to chronic polyneuropathy involving the limbs in hyperthyroid patients.[2,3,4] The prevalence of the same on electrophysiological testing has been mentioned from 19% to 65% of patients.[2,3,4] Most of the patients with neuropathy were however asymptomatic. Also few mention entrapment neuropathies like carpal tunnel syndrome in hyperthyroid patients too, in addition to that seen commonly in hypothyroid patients. Duyff et al.,[4] observed that hyperthyroidism-associated neuropathy had a very good response to anti-thyroid treatment. Many patients of thyrotoxic myopathy presenting with isolated bulbar palsy have been reported.[5,6] However, thyrotoxic neuropathy causing dysphagia is rare and has been reported by Chiu et al.[7] Literature search shows one more case mentioning polyneuritis cranialis in a patient of psychiatric illness, who was previously hypothyroid and well-controlled with treatment, but developed acute onset relative hyperthyroidism, which coincided with the onset of polyneuritis cranialis.[8] The pathogenesis of hyperthyroidism induced neuropathy is debatable. Mechanisms proposed include either a direct effect of the excessive thyroxin, immune-mediated destruction, or due to the hyper metabolic state causing nutritional deficiency to the nerves. It has also been suggested that “thyrotoxic myopathy” is actually a neuropathic disorder in its early stages of denervation.[9] Neuron soma is the site of the primary lesion ultimately affecting peripheral nerve, dorsal root ganglion, and anterior horn cell.[10]
Our patient presented with polyneuritis cranialis, which is seen quite commonly in neurology practice, and is often concluded as a GBS variant or idiopathic. We would like to add that thyrotoxicosis may be a rare but easily diagnosable and treatable cause of the polyneuritis cranialis, and screening for the same should be done in patients in whom no other etiology can be detected in rest of the tests.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Charcot J. Nouveaux signes de la maladie de Basedow. Bull Méd. 1889;3:147–9. [Google Scholar]
- 2.Ludin HP, Spiess H, Koenig MP. Neuromuscular dysfunction associated with thyrotoxicosis. Eur Neurol. 1969;2:269–78. doi: 10.1159/000113803. [DOI] [PubMed] [Google Scholar]
- 3.Sozay S, Gokce-Kutsal Y, Celiker R, Erbas T, Basgoze O. Neuroelectrophysiological evaluation of untreated hyperthyroid patients. Thyroidology. 1994;6:55–9. [PubMed] [Google Scholar]
- 4.Duyff RF, Van den Bosch J, Laman DM, van Loon BJ, Linssen WH. Neuromuscular findings in thyroid dysfunction: A prospective clinical and electrodiagnostic study. J Neurol Neurosurg Psychiatry. 2000;68:750–5. doi: 10.1136/jnnp.68.6.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathew B, Devasia AJ, Ayyar V, Thyagaraj V, Francis GA. Thyrotoxicosis presenting as acute bulbar palsy. J Assoc Physicians India. 2011;59:386–7. [PubMed] [Google Scholar]
- 6.Okada H, Yoshioka K. Thyrotoxicosis complicated with dysphagia. Intern Med. 2009;48:1243–5. doi: 10.2169/internalmedicine.48.2202. [DOI] [PubMed] [Google Scholar]
- 7.Chiu WY, Yang CC, Huang IC, Huang TS. Dysphagia as a manifestation of thyrotoxicosis: Report of three cases and literature review. Dysphagia. 2004;19:120–4. doi: 10.1007/s00455-003-0510-z. [DOI] [PubMed] [Google Scholar]
- 8.Fountoulakis KN, Andreoulakis E, Iacovides A. Possible polyneuritis cranialis in a psychotic patient: Diagnostic and therapeutic dilemmas. J Neuropsychiatry Clin Neurosci. 2012;4:E26–7. doi: 10.1176/appi.neuropsych.11100250. [DOI] [PubMed] [Google Scholar]
- 9.McComas AJ, Sica RE, McNabb AR, Goldberg WM, Upton AR. Neuropathy in thyrotoxicosis. N Engl J Med. 1973;289:219–20. doi: 10.1056/nejm197307262890420. [DOI] [PubMed] [Google Scholar]
- 10.McComas AJ, Sica RE, McNabb AR, Goldberg WM, Upton AR. Evidence for reversible motoneuron dysfunction in thyrotoxicosis. J Neurol Neurosurg Psychiatry. 1974;37:548–58. doi: 10.1136/jnnp.37.5.548. [DOI] [PMC free article] [PubMed] [Google Scholar]


