SUMMARY
The serotonergic raphe nuclei of the midbrain are principal centers from which serotonin neurons project to innervate cortical and sub-cortical structures. The dorsal raphe nuclei receive light input from the circadian visual system [1] and indirect input from the biological clock nuclei [2, 3]. Dysregulation of serotonin neurotransmission is implicated in neurobehavioral disorders, such as depression and anxiety [4], and alterations in the serotonergic phenotype of raphe neurons have dramatic effects on affective behaviors in rodents [5]. Here, we demonstrate that day length (photoperiod) during development induces enduring changes in mouse dorsal raphe serotonin neurons—programming their firing rate, responsiveness to noradrenergic stimulation, intrinsic electrical properties, serotonin and norepinephrine content in the midbrain, and depression/anxiety-related behavior in a melatonin receptor 1 (MT1)-dependent manner. Our results establish mechanisms by which seasonal photoperiods may dramatically and persistently alter the function of serotonin neurons.
RESULTS AND DISCUSSION
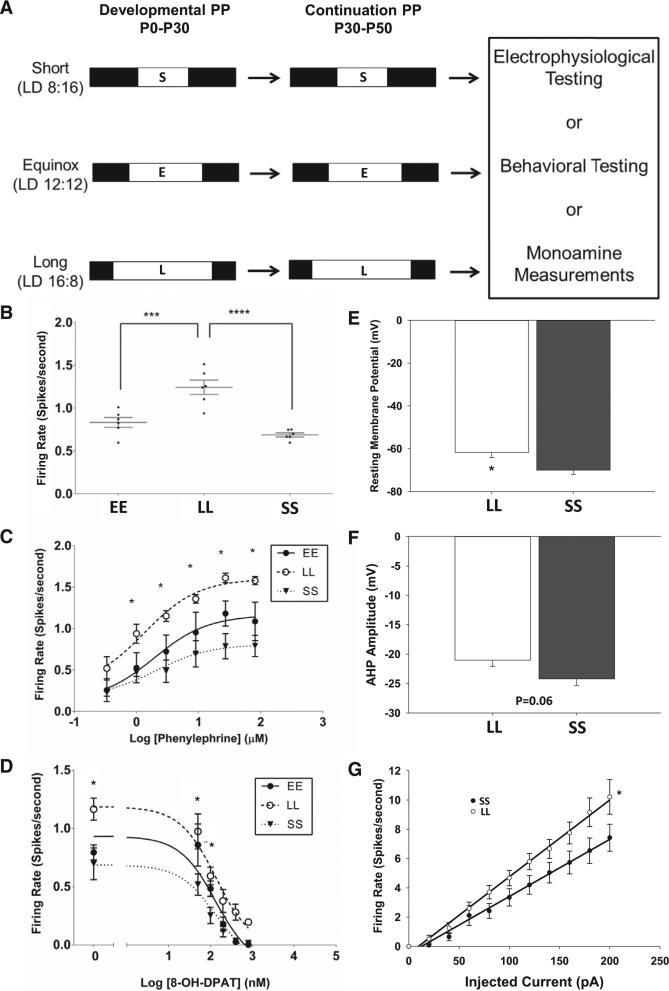
Multielectrode array recordings were performed on acute dorsal raphe nucleus (DRN) slices to measure the firing rate of serotonin neurons in the dorsomedial portion of the DRN (Figure S1). Mice were exposed to different photoperiods during development and then subjected to electrophysiological, neurochemical, or behavioral testing (Figure 1A). Neurons from long (LL) photoperiod mice exhibited significantly increased firing rates, compared to equinox (EE) and short (SS) photoperiod groups (LL = 1.24 ± 0.084 Hz, n = 6 mice, 153 cells; EE = 0.83± 0.058 Hz, n = 6 mice, 92 cells; SS = 0.69 ± 0.024 Hz, n = 6 mice, 70 cells; EE versus LL: p = 0.0005; LL versus SS: p < 0.0001; F(2,15) = 22.75; one-way ANOVA with Holm-Sidak's multiple comparison test; Figure 1B). Noradrenergic excitatory input mediated by ADRA1b receptors and serotonergic auto-inhibition from 5HT1a autoreceptors are critical regulators of raphe neuron spontaneous spike frequency [6]. Dose-response curves performed with the adrenergic agonist phenylephrine (PE) revealed that serotonergic neurons in DRN from LL photoperiod mice (n = 4 mice; 57 cells) exhibited significantly higher firing rates in response to a range of PE concentrations compared to neurons from EE (n = 3 mice; 19 cells) and SS (n = 3 mice; 23 cells) mice (Figure 1C; Table S1). Thus, increased response to the ADRA1b agonist PE, present in the recording medium at 3 μM to simulate the in vivo noradrenergic input that activates serotonin neurons, likely contributes to increased firing rate in serotonin neurons observed in vitro from mice developed in LL photoperiods. In contrast, dose-response curves for 8-OH-DPAT, a 5HT1a agonist that activates the inhibitory 5HT1a autoreceptor, suppressed ongoing spike activity with similar concentration dependence in all groups (Figure 1D). The baseline firing rate before 8-OH-DPAT inhibition was significantly elevated in LL as in Figure 1; however, IC50 values for each photoperiod were not significantly different (Table S2). These data indicate that LL photoperiods increase responsiveness of raphe serotonin neurons to adrenergic stimulation but do not significantly affect the responsiveness to 5HT1a negative feedback.
Figure 1. Photoperiod Shapes the Physiological Properties of 5-HT Neurons.
(A) Photoperiod paradigm.
(B) Firing rate of serotonergic neurons in DRN slices from mice exposed to different photoperiods (EE, equinox; LL, long; SS, short; p < 0.001; one-way ANOVA; EE versus LL: adj. p = 0.0005; LL versus SS: adj. p < 0.0001; Holm-Sidak multiple comparison test).
(C) Dose-response curve to phenylephrine (PE). Neurons from LL mice (open circles) display an increased firing rate compared to SS mice (closed triangle) across most doses of PE (1 μM: p = 0.0379; 3 μM: p = 0.0022; 9 μM: p = 0.0023; 27 μM: p = 0.0002; 81 μM: p = 0.0003; mixed design two-way ANOVA with Tukey's MC test) and compared to EE mice at 81 μM (p = 0.0280).
(D) Dose-response curve to 8-OH-DPAT. Neurons from LL mice display an increased firing rate compared to SS mice at baseline and at two doses of 8-OH-DPAT (0 nM: p = 0.0006; 50 nM: p = 0.0008; 100 nM: p = 0.00151; mixed design two-way ANOVA with Tukey's multiple comparison test).
(E) Resting membrane potential is significantly different across photoperiods (p = 0.01 LL versus SS; t test).
(F) After-hyperpolarization amplitude across photoperiod shows a trend toward reduction in long photoperiod (p = 0.06 LL versus SS; t test).
(G) Neurons from LL mice have increased intrinsic excitability compared to SS mice (p = 0.001). Error bars represent the SEM.
The increase in responsiveness of LL photoperiod raphe neurons to adrenergic stimulation could result from increased adrenergic receptor expression or activation, or from changes in the intrinsic excitability of serotonin neurons that may amplify the effects of adrenergic input. Neither adra1b receptor mRNA expression nor ADRA1b receptor binding nor the EC50 values for PE were found to be different in the midbrain across photoperiods (Figures S2A and S2B; Table S1), although given the widespread expression of this receptor in that region, this does not rigorously exclude the possibility of changes in expression limited to serotonin neurons. To test for changes in intrinsic excitability, we measured electrophysiological variables from LL and SS DRN serotonin neurons by whole-cell recording. No EE group was included here as EE and SS groups were not different in their baseline spike rates (Figure 1B). The resting membrane potential of neurons from LL photoperiod mice was significantly depolarized compared to those from SS photoperiods (LL –61.73 ± 2.42, n = 12 cells; SS –69.99 ± 1.99, n = 13 cells; –mV; p = 0.01; Figure 1E) and neurons from the LL group exhibited a trend toward lower amplitude after-hyperpolarization following action potentials compared to SS (LL –21.03 ± 1.09, n = 12 cells; SS –24.22 ± 1.15, n = 13 cells; mV; p = 0.06; Figure 1F). Serotonin neurons from LL photoperiod also exhibited increased spike rate for a given amount of membrane current injection compared to SS, with the slope of the current/spike relationship being significantly increased in LL (LL slope 0.052; n = 12 cells; SS slope 0.039; n = 13 cells; p = 0.001; Figure 1G). Thus, photoperiod acts to alter the intrinsic properties of serotonin neurons, with LL photoperiods producing changes that increase excitability. The depolarized resting potential and increased excitability of serotonin neurons from LL photoperiod mice are similar to those of the juvenile state of dorsal raphe neurons [7], suggesting that photoperiod is an environmental stimulus that may influence the functional maturation of serotonin neurons, with LL photoperiods preserving an immature-like state that is more highly excitable.
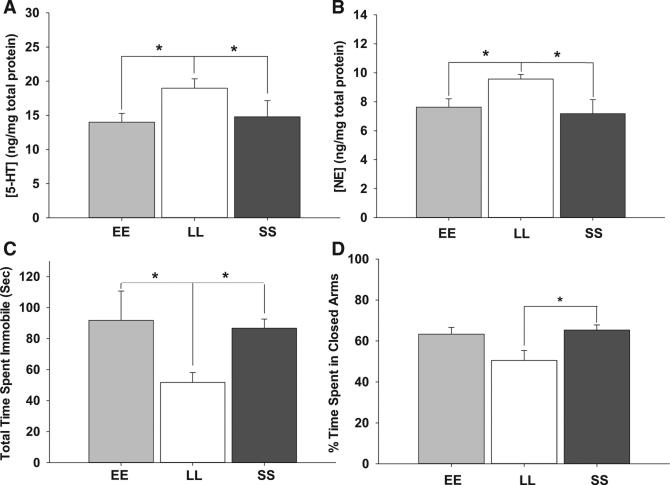
Photoperiod also altered serotonin signaling and affective behavior. Midbrain 5-HT content was increased in LL mice compared to EE and SS groups (LL 18.97 ± 1.38, n = 12; EE 14.00 ± 1.29, n = 12; SS 14.80 ± 2.36, n = 6; ng/mg total protein; p = 0.047; df = 2; F = 3.42; Figure 2A). In addition, midbrain norepinephrine content was also increased in LL mice compared to EE and SS (LL 9.57 ± 0.32, n = 12; EE 7.63 ± 0.58, n = 12; SS 7.18 ± 0.96, n = 6; ng/mg total protein; p = 0.013; df = 2; F = 5.13; Figure 2B). Thus, both the effectiveness of NE on DRNs and the strength of its input are increased in LL photoperiods. Mice from LL photoperiods showed significant reductions in time spent immobile in the forced swim test (EE 91.46 ± 12.57, n = 8; LL 51.89 ± 6.39, n = 15; SS 86.77 ± 5.86, n = 20; p = 0.002; df = 2; F = 7.22; Figure 2C) and in time spent in the closed arm of the elevated z maze (EE 63.34 ± 3.27, n = 12; LL 50.54 ± 5.81, n = 15; SS 65.35 ± 2.50, n = 20; p = 0.047; df = 2; H = 6.10; Figure 2D), whereas no effects of photoperiod were observed in the tail suspension test (Figure S3). Both LL and SS groups showed reductions in thigmotaxis in open field tests compared to EE (Figure S3). A number of previous studies in multiple rodent models have shown that photoperiod manipulation may produce variable results in rodent thigmotaxis in the open field test while displaying consistent results in the forced swim test [8–11]. This suggests the possibility that thigmotaxis may be an insensitive measure of rodent anxiety-related behaviors in photoperiod manipulation or that photoperiod may more consistently impact depression-related behaviors. Taken together, our results here indicate that prior photoperiod influences serotonin content and norepinephrine input to the DRN and reinforce previous findings that photoperiod can drive alterations in depression/anxiety-like behaviors [8, 12].
Figure 2. Long Photoperiod Increases Midbrain Monoamines and Decreases Depression/Anxiety Behaviors.
(A) LL mice exhibit ~20% greater 5-HT concentration in the midbrain over both EE and SS (p = 0.047).
(B) LL mice exhibit an ~25% increase in NE concentration in the midbrain over both EE and SS (p = 0.013).
(C) LL mice display significantly less time spent immobile than EE and SS in the forced swim test (p = 0.002).
(D) LL mice display significantly less time spent in the closed arms of the elevated zero maze than EE and SS (p = 0.047).
Error bars represent the SEM.
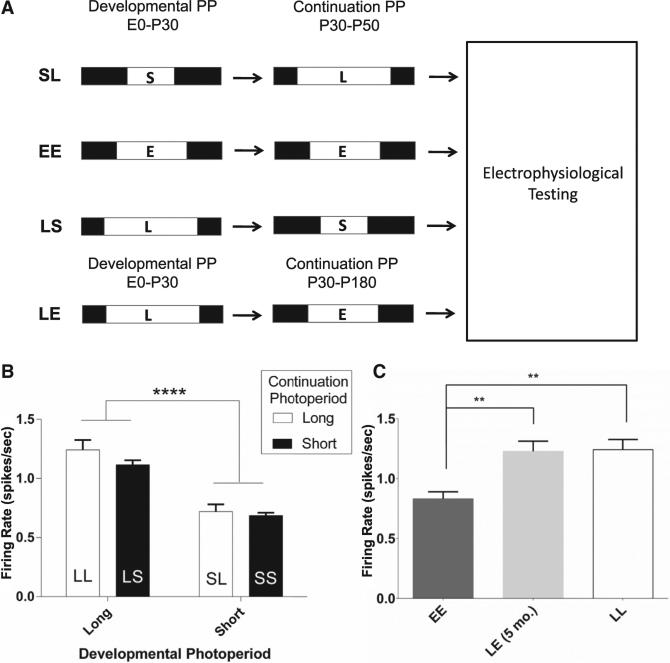
The first 3 or 4 weeks of postnatal life have been proposed to be a critical window for environmental input and plasticity governing the physiological maturation of DRN serotonin neurons in rodents [7]. To test whether photoperiodic effects on dorsal raphe neuron neurophysiology are due to enduring effects of photoperiod exposure during development and maturation, cohorts of mice were switched from long to short (LS) or short to long (SL) photoperiods at P30 until testing at P50–P90 (Figure 3A). DRN neuron spike rates from LS and SL mice were compared with those from mice that were continued on the same photoperiod from P0 to P50–P90 (LL and SS; Figure 1). Effects of developmental photoperiod on neuronal firing rate were sustained despite reversal of the photoperiod for 3–7 weeks. The firing rate of raphe neurons from LS mice was similar to LL mice (LS: 1.12 ± 0.09, n = 6 mice, 54 cells; LL: 1.24 ± 0.08; Hz ± SEM), and the firing rate of SL neurons was similar to SS (SL: 0.72 ± 0.06, n = 6 mice, 63 cells; SS: 0.69 ± 0.02; Hz ± SEM). Analysis of the effects of developmental and continuation photoperiod revealed a significant effect of developmental photoperiod on firing rate, with mice raised on a long developmental photoperiod (LL and LS) having an increased firing rate compared to mice raised on a short developmental photoperiod (SS and SL), regardless of continuation photoperiod (development: p < 0.0001; continuation: p = 0.17; interaction: p = 0.42; two-way ANOVA; Figure 3B). To test the duration of developmental programming of dorsal raphe neurons, a cohort of long developmental photoperiod mice was switched to EE photoperiod at P30 and maintained in that photoperiod for 5 months (LE). The spontaneous firing rate of DRN neurons from these mice was essentially identical to LL mice (LL: 1.24 ± 0.08, n = 6 mice, 153 cells; LE: 1.21 ± 0.07, n = 6 mice, 35 cells; EE = 0.84 ± 0.06, n = 6 mice, 92 cells; Figure 3C), well elevated from EE (p = 0.0053; one-way ANOVA). Thus, developmental photoperiod can have enduring effects on the activity of dorsal raphe neurons that are not reversed by weeks, or even months, of subsequent photoperiod change.
Figure 3. Switched Photoperiod Paradigm Reveals Persistence of Developmental Photoperiod Effects on Serotonergic Neurons.
(A) Switched photoperiod paradigm. Mice raised on short or long photoperiods were switched at P30 (LS, long to short; SL, short to long; LE, long to equinox).
(B) Effects of developmental photoperiod. Left bars, developmental long photoperiod groups; right bars, developmental short photoperiod groups; black bars, long photoperiod continuation photoperiod; open bars, short continuation photoperiod (developmental effect: p < 0.0001; continuation effect: p = 0.1744; interaction: p = 0.4167; two-way ANOVA).
(C) Persistence of developmental photoperiod effects on firing rate. Firing rate in LE neurons (right) is similar to LL, but not EE (LE versus EE; middle; p = 0.0028; one-way ANOVA with Holm-Sidak multiple comparison test) and LL versus EE mice (right; p = 0.0037).
Error bars represent the SEM.
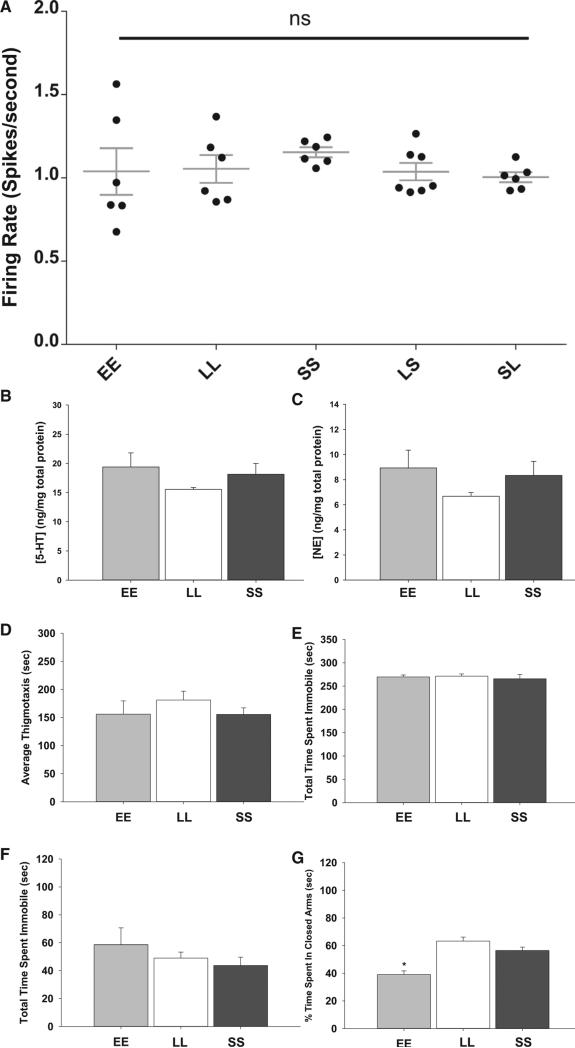
The hormone melatonin is a key photoperiodic signal that can acutely inhibit the activity of dorsal raphe neurons through melatonin receptor 1 (MT1) receptor signaling [13]. In addition, maternal-fetal melatonin signaling of photoperiod in late gestation can program gonadal development later in life in rodents, suggesting that melatonin signaling during development can have enduring effects [14]. We tested whether melatonin signaling was involved in photoperiodic programming of raphe neurons using MT1 receptor knockout mice. In the absence of MT1 receptor signaling, there were no photoperiod-specific differences in spike rates, and the spontaneous firing rate of serotonin neurons was elevated above 1 Hz across all photoperiods (Figure 4A), similar to LL photoperiod wild-type mice. In addition, there were no differences across photoperiods in 5-HT or NE concentrations in the midbrain in MT1 KO mice (Figures 4B and 4C). Finally, MT1 KO mice displayed no changes in depression- and anxiety-related behaviors across photoperiods in the OFT, TST, or FST (Figures 4D–4F). In the EZM, MT1 KO EE mice spent significantly less time in the closed arm of the maze compared to MT1 KO LL and MT1 KO SS mice (p < 0.001; df = 2; F = 14.91; one-way ANOVA; Figure 4G) but also showed significantly elevated distance traveled in the OFT. Therefore, the MT1 receptor plays a critical role in photoperiodic programming of the serotonergic neurons as well as depression- and anxiety-related behaviors in mice. The effects of the MT1 receptor on serotonin neuron programming could be direct or could be mediated indirectly through melatonin effects on the development of the adrenal [15] or gonadal [14] systems, which have hormonal input to the raphe.
Figure 4. MT1KO Negates Circadian Photoperiod's Effects.
(A) No difference in spontaneous firing rate across the five photoperiods tested. EE: 64 cells; LL: 66 cells; SS: 81 cells; SL: 56 cells; LS: 98 cells; n = 6 mice for each photoperiod (p = 0.7183).
(B) No difference in 5-HT concentration in the midbrain. EE: 5 mice; LL: 6 mice; SS: 6 mice (p = 0.355).
(C) No difference in NE concentration in the midbrain. n = 6 for each photoperiod (p = 0.281).
(D) No difference in thigmotaxis in the OFT (p = 0.466).
(E) There is no difference in time spent immobile in the TST (p = 0.761).
(F) No difference in time spent immobile in the FST (p = 0.399).
(G) EE mice spend significantly less time in the closed arm of the EZM than LL or SS mice but also exhibit increased overall locomotor activity (p < 0.001; F = 14.91; all tests were compared via a one-way ANOVA).
Error bars represent the SEM.
Seasonal photoperiods can acutely impact depression/anxiety behaviors in rodents and in humans, as seen in seasonal affective disorder [16]. Although our experiments used mice, which are nocturnal rodents, the photoperiodic effects on depression and anxiety behaviors was similar to that in humans, with LL photoperiods having anti-depressive and anxiolytic effects compared to SS photoperiods [17]. In addition, 5-HT signaling was increased by prior summer-like photoperiods in mice, and 5-HT turnover in the human brain is lowest in the winter and rises in response to the duration of bright light [18]. Our results indicate that, in mice, circadian light cycles experienced during development can have enduring effects on serotonin neuron activity that persist even after substantial intervals of photoperiod reversal. There is also accumulating epidemiological evidence of increased risk of mood and affective disorders in human seasonal birth cohorts [17, 19], with a variety of potential seasonal signals proposed, including photoperiod, exposure to flu virus, and vitamin D deficiency. Our results establish mechanisms by which seasonal photoperiods may dramatically and persistently alter the function of midbrain serotonin neurons through melatonin signaling and institute an experimental model for exploring the role of neural circuit programming by circadian light cycles in the seasonality of neurobehavioral disorders.
EXPERIMENTAL PROCEDURES
Animals and Housing
Group-housed male and female C3Hf+/+ mice were used in these studies. Sex differences were analyzed for all tests, and no significant differences were observed. C3Hf+/+ mice are melatonin producing and lack the retinal degeneration alleles of the parent C3H strain [20]. They were developed and raised to maturity on photoperiods designated EE (12 hr of light and 12 hr of darkness), SS (8 hr of light and 16 hr of darkness), or LL (16 hr of light and 8 hr of darkness). At P30, some mice were switched to the opposing photoperiod—SL, LS, or long to equinox (LE) photoperiod. Experimental assays were performed at P50–P90, with the exception of the long-to-equinox cohort, in which the animals were tested 5 months after switching.
Multielectrode Array Electrophysiological Recording
DRN slices were placed on perforated electrode arrays and immobilized with a harp for recording. 40 μM tryptophan and 3 μM PE were added to the recording solution (in μM: 124 NaCl, 3.5 KCl, 1 NaH2PO4, 1.3 MgSO4, 2.5 CaCl2,10 D(+)-glucose, and 20 NaHCO3), which was perfused (1.3 ml/min) over the slice once in the recording chamber. Serotonin neurons were identified by eliciting 5HT1a-mediated suppression of spontaneous firing rate. Serotonin at a concentration of 40 μM (for all general firing rate measurements) or 8-OH-DPAT at a concentration of 1 μM (for dose-response experiments) was perfused (1.3 ml/min) over the slice for 5 min after 4–6 min of recording. After 5 min of serotonin or 8-OH-DPAT, normal ACSF was started again and recording continued until recovery was observed. For more details, see Supplemental Experimental Procedures.
Dose-Response Experiments
Dose-Response Curve to PE
DRN slices were prepared and placed on the multielectrode array as stated above. The baseline perfusing medium contained 40 μM tryptophan but no PE. The first dose of PE (333 nM) was perfused over the slice for at least 3 min. The slice was then switched back to ACSF without PE until the firing rate silenced once more before perfusing subsequent doses of PE. This procedure was repeated for each subsequent dose.
Dose-Response Curve to 8-OH-DPAT
DRN slices were prepared and placed on the multielectrode array as previously stated. ACSF containing 40 μM tryptophan and 3 μM PE was perfused over the slice for initial firing rate. The lowest dose of 8-OH-DPAT (50 nM) was perfused over the slice after at least 4 min of recording the firing rate. Each subsequent dose was added in a stepwise fashion after at least 2 min exposure to the previous dose. Spikes were categorized and sorted as stated above.
Whole-Cell Electrophysiological Recordings
Whole-cell current clamp was performed with the same ex vivo culture procedure as stated above. The external solution contained: 124 NaCl; 2.8 KCl; 2 CaCl2; 2 MgSO4; 1.25 NaH2PO4; 26 NaHCO3; 10 glucose (in mM); 40 μM tryptophan (pH 7.4); and 300 mmol/kg Osm. Putative serotonergic neurons were selected based on 8-OH-DPAT (1 μM) response. Spontaneous excitatory and inhibitory inputs were inhibited by 10 μM CNQX and 10 μM gabazine, respectively, during the recording. Recording pipettes (5 MΩ) were filled with 135 K gluconate, 5 NaCl, 10 HEPES, 1 EGTA, 2 MgCl2, 2.5 Na2ATP, 0.2 Na2GTP, 10 sucrose (in mM; pH 7.4), and 290 mmol/kg Osm. Perfusion rate was 1 ml/min at 32°C. The access resistance was limited to less than 50 MΩ. The calculated liquid junction potential (15 mV) was adjusted. Series resistance was 90% compensated with 60 μs lag time. The data were treated with unpaired two-tailed t test.
Behavioral Testing
Male and female mice were group housed and transferred from our housing facility to light-controlled boxes in the Vanderbilt Neurobehavioral Core. After 1 week of acclimation, the elevated zero maze was conducted on the first day of testing and then the open field test, the tail suspension test, and the forced swim test, each conducted on successive days with ca. 24 hr between each test. They were tested in the middle of their light phase (1100–1500).
For additional and detailed methods, please see Supplemental Experimental Procedures. Experiments were performed in accordance with the Vanderbilt University Institutional Animal Care and Use Committee and NIH guidelines.
Supplementary Material
Highlights.
Summer-like long photoperiods increase serotonin neuron excitability and firing
Long photoperiods increase serotonin and norepinephrine levels in the midbrain
Long photoperiods during development induce lasting increases in firing rate
Knockout of the MT1 receptor negates the observed photoperiodic changes
ACKNOWLEDGMENTS
This work was supported by NIH R01 EY015815 (to D.G.M.), P50 MH096972 (Randy D. Blakely), US National Science Foundation Graduate Research Fellowship 0909667 (to M.C.T.), and Vanderbilt Conte Pilot Grant (to C.R.J.). The authors also thank the Vanderbilt Neurochemistry Core, BioAnalytical Core (Drs. Raymond Johnson, Benlian Gao, and Paul Gresch), and the Vanderbilt Silvio S. Conte Center for Neuroscience Research for all their help and expertise.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures, three figures, and two tables and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2015.03.050.
REFERENCES
- 1.Morin LP. Serotonin and the regulation of mammalian circadian rhythmicity. Ann. Med. 1999;31:12–33. doi: 10.3109/07853899909019259. [DOI] [PubMed] [Google Scholar]
- 2.Morin LP. Neuroanatomy of the extended circadian rhythm system. Exp. Neurol. 2013;243:4–20. doi: 10.1016/j.expneurol.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gooley JJ, Lu J, Fischer D, Saper CB. A broad role for melanopsin in nonvisual photoreception. J. Neurosci. 2003;23:7093–7106. doi: 10.1523/JNEUROSCI.23-18-07093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anguelova M, Benkelfat C, Turecki G. A systematic review of association studies investigating genes coding for serotonin receptors and the serotonin transporter: I. Affective disorders. Mol. Psychiatry. 2003;8:574–591. doi: 10.1038/sj.mp.4001328. [DOI] [PubMed] [Google Scholar]
- 5.Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B, Silver J, Weeber EJ, Sweatt JD, Deneris ES. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–247. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- 6.Levine ES, Jacobs BL. Neurochemical afferents controlling the activity of serotonergic neurons in the dorsal raphe nucleus: microiontophoretic studies in the awake cat. J. Neurosci. 1992;12:4037–4044. doi: 10.1523/JNEUROSCI.12-10-04037.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rood BD, Calizo LH, Piel D, Spangler ZP, Campbell K, Beck SG. Dorsal raphe serotonin neurons in mice: immature hyperexcit-ability transitions to adult state during first three postnatal weeks suggesting sensitive period for environmental perturbation. J. Neurosci. 2014;34:4809–4821. doi: 10.1523/JNEUROSCI.1498-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pyter LM, Nelson RJ. Enduring effects of photoperiod on affective behaviors in Siberian hamsters (Phodopus sungorus). Behav. Neurosci. 2006;120:125–134. doi: 10.1037/0735-7044.120.1.125. [DOI] [PubMed] [Google Scholar]
- 9.Prendergast BJ, Kay LM. Affective and adrenocorticotrophic responses to photoperiod in Wistar rats. J. Neuroendocrinol. 2008;20:261–267. doi: 10.1111/j.1365-2826.2007.01633.x. [DOI] [PubMed] [Google Scholar]
- 10.Prendergast BJ, Nelson RJ. Affective responses to changes in day length in Siberian hamsters (Phodopus sungorus) Psychoneuroendocrinology. 2005;30:438–452. doi: 10.1016/j.psyneuen.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Einat H, Kronfeld-Schor N, Eilam D. Sand rats see the light: short photoperiod induces a depression-like response in a diurnal rodent. Behav. Brain Res. 2006;173:153–157. doi: 10.1016/j.bbr.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Ciarleglio CM, Resuehr HES, McMahon DG. Interactions of the serotonin and circadian systems: nature and nurture in rhythms and blues. Neuroscience. 2011;197:8–16. doi: 10.1016/j.neuroscience.2011.09.036. [DOI] [PubMed] [Google Scholar]
- 13.Domínguez-López S, Mahar I, Bambico FR, Labonté B, Ochoa-Sánchez R, Leyton M, Gobbi G. Short-term effects of melatonin and pinealectomy on serotonergic neuronal activity across the light-dark cycle. J. Psychopharmacol. (Oxford) 2012;26:830–844. doi: 10.1177/0269881111408460. [DOI] [PubMed] [Google Scholar]
- 14.Goldman BD. Pattern of melatonin secretion mediates transfer of photoperiod information from mother to fetus in mammals. Sci. STKE 2003. 2003:PE29.. doi: 10.1126/stke.2003.192.pe29. [DOI] [PubMed] [Google Scholar]
- 15.Torres-Farfan C, Richter HG, Germain AM, Valenzuela GJ, Campino C, Rojas-García P, Forcelledo ML, Torrealba F, Serón-Ferré M. Maternal melatonin selectively inhibits cortisol production in the primate fetal adrenal gland. J. Physiol. 2004;554:841–856. doi: 10.1113/jphysiol.2003.056465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magnusson A, Boivin D. Seasonal affective disorder: an overview. Chronobiol. Int. 2003;20:189–207. doi: 10.1081/cbi-120019310. [DOI] [PubMed] [Google Scholar]
- 17.Foster RG, Roenneberg T. Human responses to the geophysical daily, annual and lunar cycles. Curr. Biol. 2008;18:R784–R794. doi: 10.1016/j.cub.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Lambert GW, Reid C, Kaye DM, Jennings GL, Esler MD. Effect of sunlight and season on serotonin turnover in the brain. Lancet. 2002;360:1840–1842. doi: 10.1016/s0140-6736(02)11737-5. [DOI] [PubMed] [Google Scholar]
- 19.Disanto G, Morahan JM, Lacey MV, DeLuca GC, Giovannoni G, Ebers GC, Ramagopalan SV. Seasonal distribution of psychiatric births in England. PLoS ONE. 2012;7:e34866. doi: 10.1371/journal.pone.0034866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Contreras-Alcantara S, Baba K, Tosini G. Removal of melatonin receptor type 1 induces insulin resistance in the mouse. Obesity. 2010;18:1861–1863. doi: 10.1038/oby.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.