Abstract
Background
In continuation of our previously interest in the saccharification of agriculture wastes by Bacillus megatherium in solid state fermentation (SSF), we wish to report an investigation and comparative evaluation among Trichoderma sp. for the saccharification of four alkali-pretreated agricultural residues and production of hydrolytic enzymes, carboxymethyl cellulase (CMCase), filter paperase (FPase), pectinase (PGase) and xylanase (Xylase) in SSF. The optimization of the physiological conditions of production of hydrolytic enzymes and saccharification content from Trichoderma virens using alkali-pretreated wheat bran was the last goal.
Methods
The physico-chemical parameters of SSF include incubation time, incubation temperature, moisture content of the substrate, incubation pH, supplementation with carbon and nitrogen sources were optimized.
Results
Saccharification of different solid state fermentation sources wheat bran, date's seeds, grass and palm leaves, were tested for the production of fermentable sugar by Trichoderma sp. The maximum production of hydrolytic enzymes CMCase, FPase, PGase and Xylase and saccharification content were obtained on wheat bran. Time course, moisture content, optimum temperature, optimum pH, supplementation with carbon and nitrogen sources were optimized to achieve the maximum production of the hydrolytic enzymes, protein and total carbohydrate of T. virens using alkali pre-treated wheat bran. The maximum production of CMCase, FPase, PGase, Xylase, protein and carbohydrate content was recorded at 72 h of incubation, 50-70 % moisture, temperature 25-35 °C and pH 5. The influence of supplementary carbon and nitrogen sources was studied. While lactose and sucrose enhanced the activity of PGase from 79.2 to 582.9 and 632.6 U/g, starch inhibited all other enzymes. This was confirmed by maximum saccharification content. Among the nitrogen sources, yeast extract and urea enhanced the saccharification content and CMCase, PGase and Xylase.
Conclusions
The results of this study indicated that alkali pre-treated wheat bran was a better substrate for saccharification and production of hydrolytic enzymes CMCase, FPase, PGase and xylase by T. virens compared to other alkali-pretreated agricultural residues tested.
Keywords: Trichoderma sp, Saccharification, Hydrolytic enzymes, Agriculture wastes
Background
Agricultural residues, forests and agro industrial practices generally accumulated in the environment and caused pollution problem. Active efforts were being made to convert these organic waste resources into either glucose or alcohol, and use this either as fuel or as a valuable starting material for chemical synthesis [1]. Saccharification of polysaccharides to glucose by microbial hydrolytic enzymes which had attracted the attention of the researchers, as this was the first step of bioconversion of organic material into valuable products such as sugar, fine chemicals and biofuels [2]. As the cost of cellulosic substrates play the central role in determining the economy of the saccharification process, lot of emphasis had been given to the usage of low price substrates and therefore screening of the agricultural wastes for release of sugars as organic wastes from renewable forest and agricultural residues [3]. The saccharification of different agro-wastes had been reported by other workers employing enzymes from different organisms [4–6].
Recently, a significant interest raised in using solid state fermentation (SSF) instead of submerged fermentation (SmF). The advantages of SSF in comparison to traditional SmF were better yields, easier recovery of products, the absence of foam formation and smaller reactor volumes. Moreover, contamination risks were significantly reduced due to the low water contents and, consequently, the volume of effluents decreases [7]. Another very important advantage was that, it permits the use of agricultural and agro-industrial residues as substrates which were converted into products with high commercial value like secondary metabolites, organic acids, pesticides, aromatic compounds, fuels and enzymes [8]. Furthermore, the utilization of these compounds helps in solving pollution, which otherwise caused their disposal [9]. For enzyme production, the costs of these techniques were lower and the production higher than submerged cultures [10,11].
Structural properties of cellulose such as the degree of crystallinity, the degree of polymerization and the surface area, limit accessibility of substrate to enzyme and had been demonstrated [12] to affect the rate of enzymatic hydrolysis of cellulose. Pretreatment methods, which disrupted the highly-ordered cellulose structure and the lignin-carbohydrate complex, remove lignin, and increase the surface area accessible to enzymes, promoted the hydrolysis, and increased the rate and extent of hydrolysis of cellulose in various lignocellulosic residues. The enzymatic hydrolysis of cellulosic materials correlated with the level of cellulose crystallinity [13] complete enzymatic hydrolysis of the polysaccharides of lignocelluloses required a concerted action of a complex array of hydrolases including cellulase, xylanase, pectinase, and other side-group cleavage enzymes [14].
Several cell-decomposing microorganisms produce cellulases which were the most economic and available sources for fermentable sugar production, because these microorganisms could grow on inexpensive media. The genus Trichoderma, filamentous ascomycetes were widely used in industrial applications because of high secretary capacity and inducible promoting characteristics [15]. The structural complexity were often easily degraded by xylanases, mannanases etc. which were present in some cellulase preparations, so that their presence may actually lead to increased production of reducing sugars and greater susceptibility of the residual cellulose [16–18].
In continuation of our interest in the saccharification of agriculture wastes by SSF [19], we wished to report an investigation and comparative evaluation among Trichoderma sp., T. reesei, T. viride, T. harzianum and T. virens for the saccharification of four alkali-pretreated agricultural residues, wheat bran, date’s seeds, wild grass and palm’s leaves under solid state fermentation for the production of hydrolytic enzymes, carboxymethyl cellulase (CMCase), filter paperase (FPase), pectinase (PGase) and xylanase (Xylase). The polysaccharide composition of these agricultural residues included different concentrations from cellulose, hemicelluloses and lignin [20–22]. The optimization of the physiological conditions of production of hydrolytic enzymes and saccharification content from T. virens using alkali-pretreated wheat bran was the last goal.
Methods
Microorganisms
T. reesei, T. viride, T. harzianum and T. virens were obtained from National Research Centre, Cairo, Egypt and maintained on potato dextrose agar. The slants were grown at 28 °C for seven days and stored at 4 °C.
Pretreatment of agricultural wastes
Wheat bran, date's seeds, grass and palm leaves were chosen as the sole nutrient source for solid-state fermentation (SSF). They dried in an oven at 80 °C for 24 h. The dried substrates were then milled in a commercial mill and sieved. The mean diameter of the dried substrates was 1.0 mm. The substrates were pretreated with 1.0 M NaOH at 121 °C and 15 psi pressure for 1 hr at the ratio of 1:10 (w/v) [23,24]. The pretreated materials were washed with tap water until the pH of the filtrate reached 7.0. The washed materials were dried at 60 °C overnight to constant weight and stored at room temperature for further use.
Inoculum medium
The medium used for inoculum of Trichoderma sp. preparation contained (g l−1): KH2PO4, 28; (NH4)2SO4, 19.6; Urea, 4.2; MgSO4. 7H20, 4.2; CoCl2, 4.2; FeSO4. 7H20, 0.07; MnSO4. 7H20, 0.021; ZnSO4 7H20, 0.019; CaC12, 0.028; yeast extract, 7; and glucose, 15; pH 5.0 ± 0.2. The media were sterilized by autoclaving at 121 °C pressure of 15 psi for 15 min. The culture was incubated and shaken at 30 °C for 48 hr in an orbital shaking incubator at 150 rpm before transferring to the production medium [25].
Solid state fermentation
SSF was performed to study the effect of various physicochemical parameters required for the optimum production of enzymes and saccharification content by Trichoderm sp. prior to inoculation, the agriculture waste was sterilized in an autoclave for 20 min at 121 °C and 1.2 atmospheres. To each 50 ml Erlenmeyer flask, 5 g of sterilized agriculture waste, 5 × 105 spores/g, and appropriate amount of water (10 % moisture) were added. The physico-chemical parameters included incubation time, incubation temperature (20, 30, 35, 40, 45 °C), moisture content of the substrate (10 %, 20 %, 40 %, 60 %, 100 %) and incubation pH (4 to 8) were optimized. The pH was adjusted using 0.1 M NaOH or HCl. Studies were also performed to evaluate the influence of different carbon sources (glucose, maltose, starch, sucrose, lactose at 1 % w/v) and nitrogen sources (yeast extract, urea, sodium nitrate, ammonium sulphate, ammonium chloride at 1 % w/v) when added to the fermentation medium contained agriculture waste. Each experiment is done in 3 sets.
Enzyme extraction
Crude enzyme was extracted by mixing a 5 g of fermented matter with 50 ml distilled water on a rotary shaker (180 rpm/min) overnight. The suspension was then centrifuged at 12000 rpm for 10 min and the supernatant was designated as a crude extract.
Enzyme assays
Carboxymethylcellulase (CMCase), filter paperase (FPase), pectinase (PGase) and xylanase (Xylase) activities were assayed by determining the liberated reducing end products using glucose, glucose, galacturonic acid and xylose as standards, respectively [26]. Substrates used were CM-cellulose, filter paper, polygalacturonic acid and birchwood xylan for CMCase, FPase, PGase and Xylase, respectively. The reaction mixture (0.5 ml) contained 1 % substrate, 0.05 M sodium acetate buffer pH 5.5 and 0.1 ml crude extract. Assays were carried out at 37 °C for 1 h. Then 0.5 ml dinitrosalicylic acid reagent was added to each tube. Then the reaction mixture was mixed well, and heated in a boiling water bath for 10 min. After cooling to room temperature, the absorbance was measured at 560 nm. One unit of enzyme activity is defined as the amount of enzyme which liberated one μmol of reducing sugar per min under standard assay conditions. All the experimental work was run in triplicates.
Protein determination
Protein concentration was determined according to the dye binding method of Bradford [27] with bovine serum albumin as standard.
Determination of total reducing sugars
Total reducing sugars were determined by the method of Miller [26]. The reaction mixture contained 0.5 ml of crude extract and 0.5 ml dinitrosalicylic acid reagent. The tubes were heated in a boiling water bath for 10 min. After cooling to room temperature, the absorbance was measured at 560 nm. Glucose served as the calibration standard for total reducing sugar determination.
Determination of total soluble carbohydrates
Total soluble carbohydrates were determined by the method of Dubois et al. [28]. The reaction mixture contained 25 μl of a 4:1 mixture of phenol and water, 0.8 ml of crude extract and 2 ml of concentrated sulfuric acid. Then mixed well, and heated in a boiling water bath for 30 min. The absorbance was determined at 480 nm. Glucose served as the calibration standard for total carbohydrate determination.
Statistical analysis
The obtained data were statistically analyzed as a randomized complete block design with three replicates by analysis of variance (ANOVA) using the statistical package software SAS (SAS Institute Inc., 2000, Cary, NC., USA). Comparisons between means were made by F-test and the least significant differences (LSD) at level P = 0.05. Correlations coefficient among the different parameters were also calculated by SAS.
Results and discussion
Production of CMCase, FPase, PGase and Xylase were tested using alkali pretreated wheat bran, date's seeds, wild grass and palm leaves as substrates by T. reesei, T. viride, T. harzianum and T. virens in solid state fermentation (SSF). Figure 1 showed that the maximum production of CMCase, FPase, Xylase were obtained by T. virens (123.26, 49.3 and 348 U/g solid, respectively) in SSF containing alkali pre-treated wheat bran, while maximum PGase activity was obtained by T. reesei (499.9 U/g solid) in SSF containing alkali pre-treated palm leaves. Among the different solid substrates screened, saccharification of alkali pre-treated wheat bran supported maximum yields of total carbohydrates and reducing sugars (45 and 38.92 mg/g solid, respectively) by T. virens compared with other Trichoderma sp. tested (Fig. 2). There was no relation between total carbohydrate/sugar and enzyme contents, except of wild grasses and wheat bran. This may be attributed to some fungi used the total carbohydrate and sugar as nutrients (produced by enzymes act on certain agricultural residues as palm leaves and date seeds) for growth of fungi, resulted in the depletion of the total carbohydrate and sugar. From these results, the optimization of the production of total carbohydrate, reducing sugar, CMCase, FPase, PGase and Xylase of T. virens using alkali pre-treated wheat bran in SSF was performed in the following studies.
Fig. 1.
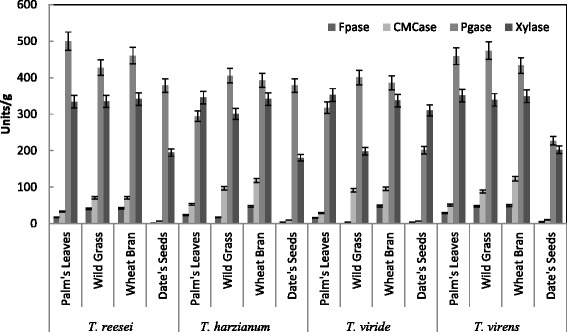
Effect of different organic materials on the production of CMCase, FPase, PGase and Xylase by Trichoderma sp. in SSF. Process conditions: incubation times 72 h, initial moisture content 50 % (by volume per mass) and temperature 30 °C. The data presented were averages of three experiments
Fig. 2.
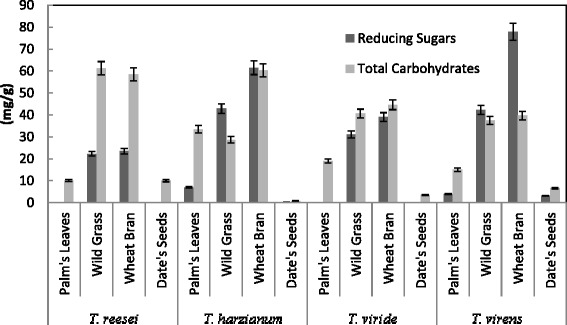
Effect of different organic materials on Saccharification content by Trichoderma sp. in SSF. Process conditions: incubation times 72 h, initial moisture content 50 % (by volume per mass) and temperature 30 °C. The data presented were averages of three experiments
Figure 3 shows the time course experiments of CMCase, FPase, Xylase, PGase and protein production by T. virens grown on alkali pretreated wheat bran in SSF. All enzymes and protein exhibited their maximum activities 58.2, 65.6, 372.4, 474 U/g and 8.5 mg/g at 72 hours. However, time course of enzyme cellulase production by Trichoderma spp. was studied using steamed alkali-treated sugarcane bagasse at 30 °C for six days. CMCase biosynthesis was not detected up to 24 hours of incubation and then the enzyme activity increased sharply up to 72 hours and highest CMCase activity was found 1.31 U/ml at 144 hours. FPase production started after a large lag period (about 72 hours) and thereafter the enzyme synthesis increase sharply. The final FPase activity was 0.110 U/ml at 144 hours. Initially, Xylase production was low and at 96 hours a sharp increase in enzyme production was observed and after that the enzyme activity remained constant. The highest Xylase activity was found after 144 hours of incubation which was 13.250 U/ml [29]. Of all four strains of fungi, T. reesei and T. viride were the best cellulase-producing fungi after 72 h offer mutation. A co-culture of T. reesei and T. viride gave better results with wheat straw; the CMCase activities were 560.9 and 212.7 IU, respectively [30]. Another study reported that PGase and Xylase of T. virens, exhibited their maximum activity at day 4 (132 and 75 units/g solid, respectively) [31]. Similarly, Penicillium decumbens was grown on a mixture of corn straw (90 %) and wheat bran (10 %) the maximum activity of Xylase was measured after 4 days of fermentation [32]. Couri et al. [33] also studied the production of Xylase by A. niger using different agroindustrial residues – mango peel and wheat bran – as the solid substrate. The maximum of xylase activity was reached after 72 h and 24 h of fermentation using wheat brain and mango peel, respectively.
Fig. 3.
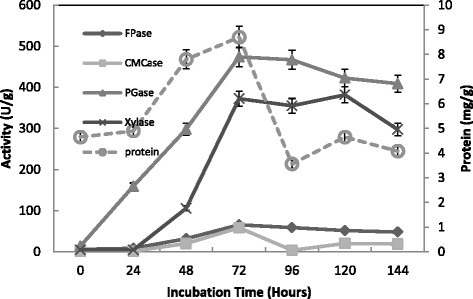
Effect of incubation time on the production of CMCase, FPase, PGase, Xylase and protein by T. virens in SSF using alkali pre-treated wheat bran as a substrate. Process conditions: incubation times 72 h, initial moisture content 50 % (by volume per mass) and temperature 30 °C. The data presented were averages of three experiments
The increase in glucose production depends on availability of cellulose in the medium and also due to the specific binding of the enzymes with substrates [34]. Saccharification of alkali pre-treated wheat bran in SSF was shown in Fig. 4 with a maximum yield 49.1 mg/g reducing sugars and 71.9 mg/g total carbohydrates obtained at 72 h by T. virens. Begum and Alimon [35] obtained the maximum amount of reducing sugar (4.15 mg/g) measured in sugarcane bagasse after alkali pre-treatment at 24 h by Aspergillus oryzae ITCC-4857.01. They also obtained the highest saccharification for alkali-reated sugarcane bagasse at 96 hrs when water hyacinth induced enzyme was used. They concluded that the saccharification rates of alkali pre-treated substrates were higher than those of enzymatic treated substrates. Similarly, Ja’afaru and Fagade [36] recorded the highest reducing sugar 5.55 mg/ml for alkali pre-treated corn cob at 48 hrs. Time course of enzymatic saccharification of alkali pre-treated bagasse showed rapid initial increase of reducing sugar concentration (up to 8 h) and the rate of this increase was substantially reduced at later stages by T. viride [37].
Fig. 4.
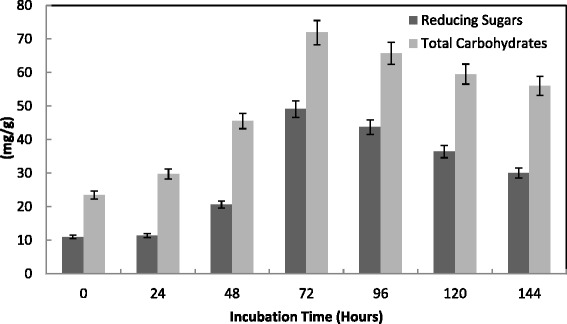
Effect of incubation time on the saccharification content by T. virenss in SSF using alkali pre-treated wheat bran. Process conditions: initial moisture content 50 % (by volume per mass) and temperature 30 °C. The data presented were averages of three experiments
The moisture content was an important factor that influences the growth and product yield in SSF [38]. Moisture was reported to cause swelling of the substrates, thereby facilitating better utilization of the substrate by microorganisms [39,40]. The data presented in the Figs. 5 and 6, clearly indicated a maximum production of the enzymes CMCase, FPase, Xylase and PGase ranged from 50 to 70 % moisture. The highest level of protein production was detected at 50 % moisture. For CMCase production moisture level was optimized and found that 40 % moisture level was best for production by T. viridei [41]. Any further increase in the ratio resulted in the decrease of enzyme yields may be due to clumping of solid particles which results in the decrease of interparticle space leading to decreased diffusion of nutrients [40,42]. In contrast, the low moisture content leads to the decreased solubility of nutrients present in the wheat bran thereby decreases enzyme yields [43]. The optimum moisture contents for Xylase production by T. longibrachiatum and A. tereus were 55 and 75 %, respectively [44]. A high production of Xylase of Aspergillus species was detected at 40–50 % moisture using dry koji as substrate [45]. An initial moisture content of 40 % provided better conditions for production of PGase from A. niger than those of 25, 55, and 70 % [46].
Fig. 5.
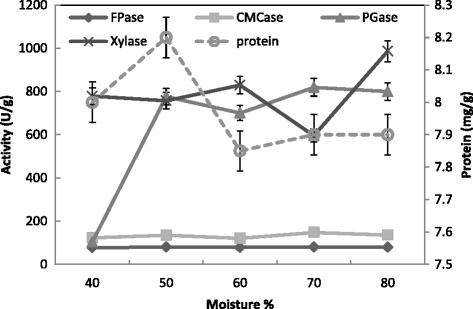
Effect of moisture % on the production of CMCase, FPase, PGase, Xylase and protein by T. virens in SSF using alkali pre-treated wheat bran as a substrate. Process conditions: incubation times 72 h, and temperature 30 °C. The data presented were averages of three experiments
Fig. 6.
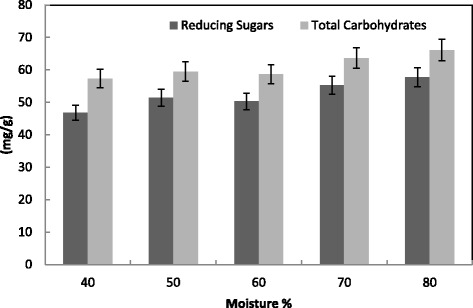
Effect of moisture % on the saccharification content by T. virens in SSF using alkali pre-treated wheat bran as substrate. Process conditions: incubation times 72 h and temperature 30 °C. The data presented were averages of three experiments
The incubation temperature is very important in enzyme production [47]. The usual temperature maintained in SSF systems was in the range of 25-32 °C, depending on the growth kinetics of microorganism employed for fermentation purposes [48]. In the present study, the optimum temperature for maximum production of both CMCase and PGase was 30 °C; and 25 °C for FPase and xylase at 35 °C (Fig. 7). The protein content was ranged from 7 to 8 mg/g for all temperatures tested with high level at 35 °C. This was confirmed by maximum reducing sugar (49.2 mg/g) at 25 °C and total carbohydrates (55.5 mg/g) at 30 °C (Fig. 8). In contrast, maximum hydrolysis of substrates occurred at 50 °C [49]. Other studies had reported that 40 °C was found best for CMCase secretion by T. viride [41]. Optimal PGase and Xylase production (130 and 74 units/g solid, respectively) was obtained at 35 °C and 28 °C for T. harzianum, respectively [31]. On the contrary, the maximum activity of PGase and Xylase (120 and 55 units/g solid, respectively) of T. virens was detected at 28 °C and 35 °C, respectively. Similar optimal temperatures of production of PGase and xylanase from Penicillium decumbens [32], A. niger [33], A. oryzae [47], and A. awamori [50] were ranged from 28 °C to 32 °C. In the steady state operation for production of Xylase by A. niger the optimum temperature was 28 °C. Results showed that higher incubation temperature favors biomass growth and lower temperature favors the biosynthesis of xylanase [51]. The maximal PGase production by mixed culture of A. niger and Saccharomyces cerevisiae was detected at 37 °C [52].
Fig. 7.
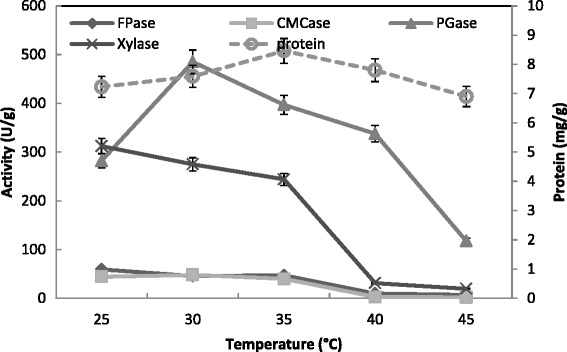
Effect of incubation temperature on the production of CMCase, FPase, PGase, Xylase and protein by T. virens in SSF using alkali pre-treated wheat bran as a substrate. Process conditions: incubation times 72 h and initial moisture content 50 % (by volume per mass). The data presented were averages of three experiments
Fig. 8.
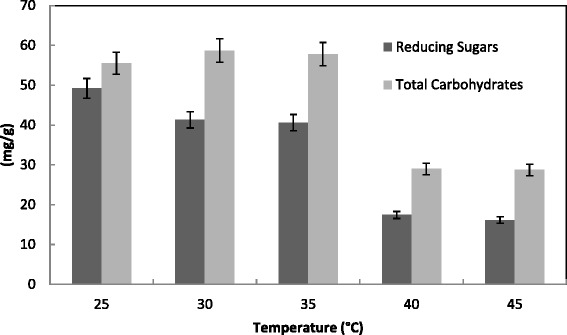
Effect of incubation temperature on the saccharification content by T. virens in SSF using alkali pre-treated wheat bran as substrate. Process conditions: incubation times 72 h and initial moisture content 50 % (by volume per mass).The data presented were averages of three experiments
Figure 9 showed a maximum activity of CMCase (110 U/g), FPase (95 U/g), Xylase (910 U/g), PGase (820 U/g) and protein (9 mg/g) for T. virens at pH 5.0. This was reinforced by a maximum saccharification at the same pH 5 (Fig. 10). Another study reported a maximum degree of saccharification at pH 5.0 by Trichoderma sp. [29]. Maximum CMCase activity (16.2 U/ml) was also obtained at pH 5.5 by T. viride [41]. Optimal PGase and Xylase production of T. harzianum was obtained at pH 7 and 6 with 120 and 70 units/g solid, respectively. On the contrary, the maximum activity of PGase and xylanase of T. virens was detected at pH 6.0 and 7.0 with 140 and 60 units/g solid, respectively [31]. At the medium pH (6.0), the maximal xylanase production by A. terreus under SSF using palm as substrate was reported [53]. Patil and Dayanand [54] reported that pH 5.0 was optimum for the maximum production of pectinases of A. niger using deseeded sunflower head in both submerged and solid state fermentation.
Fig. 9.
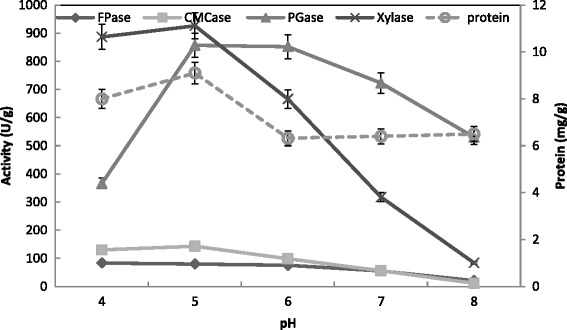
Effect of pH on the production of CMCase, FPase, PGase, Xylase and proein by T. virens in SSF using alkali pre-treated wheat bran as a substrate. Process conditions: incubation times 72 h, initial moisture content 50 % (by volume per mass) and temperature 30 °C. The data presented were averages of three experiments
Fig. 10.
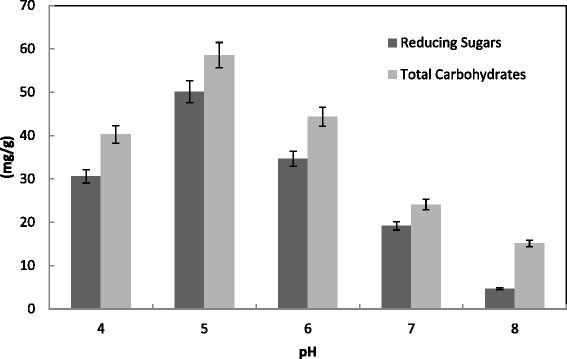
Effect of pH on the saccharification content by T. virens in SSF using alkali-pre-treated wheat bran. Process conditions: incubation times 72 h, initial moisture content 50 % (by volume per mass) and temperature 30 °C. The data presented were averages of three experiments
The influence of supplementary carbon sources such as starch, sucrose, maltose, lactose or glucose at 1 % (by mass) on production of CMCase, FPase, Xylase, PGase and protein was studied. The protein content was slightly increased in presences of the carbon sources. While lactose and sucrose enhanced the activity of PGase from 79.2 to 582.9 and 632.6 U/g solid, respectively, starch inhibited all other enzymes (Fig. 11). This was confirmed by maximum saccharification content of 54.9 and 63.4 mg/g reducing sugars respectively by lactose and sucrose, and minimum reducing sugars of 28.1 mg/g by starch (Fig. 12). However, starch and sucrose enhanced the Xylase activities from 40 to 55–60 units/g solid, while all carbon sources exhibited slightly effect on PGase activities for Trichoderma spp. using cantaloupe and watermelon rinds [31]. Botella et al. [50] reported that when 6 % glucose was added as extra carbon source, the production of Xylase and PGase by A. awamori using grape pomace as substrate increased significantly. However, at 8 % both enzyme activities declined. In contrast, when wheat bran was used as the solid substrate, Xylase by A. tamari was resistant to catabolic repression even at 10 % glucose [55]. Alternatively, D-glucose, D-mannose, maltose, sucrose and cellobiose significantly repressed CMCase formation in case of using rice straw and sugar cane bagasse as carbon sources [56].
Fig. 11.
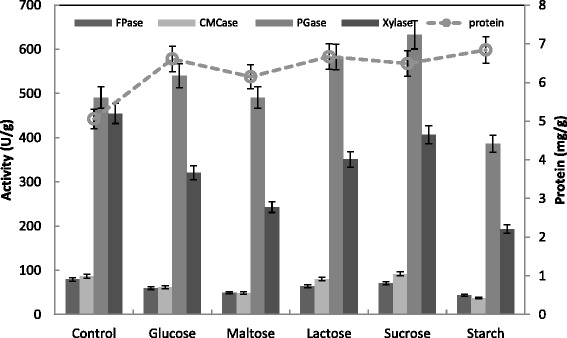
Effect of carbon source (1 %) supplementation on the production of CMCase, FPase, PGase, Xylase and protein by T. virens in SSF using alkali pre-treated wheat bran as a substrate. Process conditions: incubation times 72 hr, initial moisture content 50 % (by volume per mass) and temperature 30 °C. The data presented were averages of three experiments
Fig. 12.
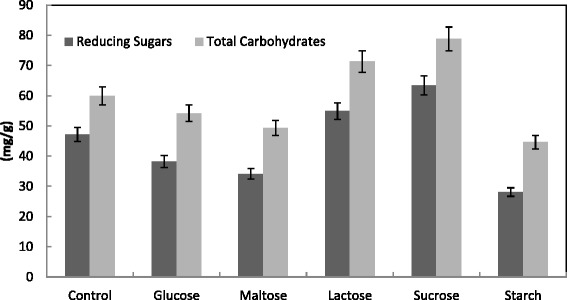
Effect of carbon source (1 %) supplementation on the saccharification content by T. virens in SSF using alkali pre-treated wheat bran. Process conditions: incubation times 72 h, initial moisture content 50 % (by volume per mass) and temperature 30 °C. The data presented were averages of three experiments
Studies on supplementation of nitrogen sources such as ammonium sulphate, ammonium nitrate, ammonium chloride, yeast extract or urea at 1 % concentration to the solid substrates showed various effects on CMCase, FPase, Xylase, PGase and protein production by T. virens (Fig. 13). All nitrogen sources enhanced the saccharification enzymes with significant enhancement for CMCase (from 86.9 to 204 U/g), PGase (from 491.1 to 939.4 U/g) and Xylase (from 455 to 1064 U/g) by yeast extract and urea. This was confirmed by maximum saccharification from 47.2 to 157.1 and 162.1 by yeast extract and urea respectively (Fig. 14). However, ammonium sulphate, ammonium nitrate, yeast extract and urea increased PGase activities of T. harzianum from 90 to 110–113 units/g solid and decreased PGase activities of T. virens. The high level of protein was detected in presence of KNO3. However, urea increased Xylase activities of T. harzianum and T. virens [30]. Among nitrogen sources yeast extract was the best to enhance the enzyme activity FPase (0.281 ± 0.13 IU/g) and CMCase (3.66 ± 0.02 IU/g) by A. fumigatus grown on alkali-pretreated sawdust [57]. Another study reported the highest level of enzyme formation expressed in terms of specific activity with ammonium chloride with both rice straw and sugar cane bagasse by A. terreus DSM 826. Other nitrogen sources namely ammonium sulphate, potassium nitrate and ammonium oxalate gave also considerable amounts of CMCase with both wastes as compared with sodium nitrate [56].
Fig. 13.
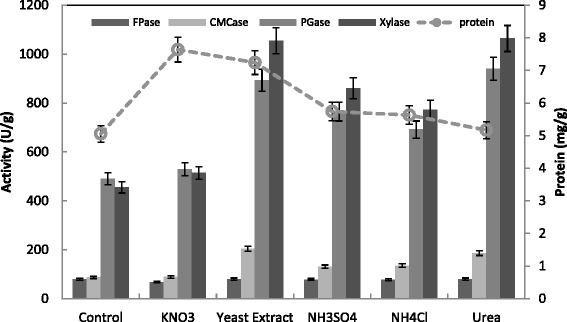
Effect of nitrogen source (1 %) supplementation on the production of CMCase, FPase, PGase, Xylase and protein by T. virens in SSF using alkali pre-treated wheat bran as a substrate. Process conditions: incubation times 72 h, initial moisture content 50 % (by volume per mass) and temperature 30 °C. The data presented were averages of three experiments
Fig. 14.
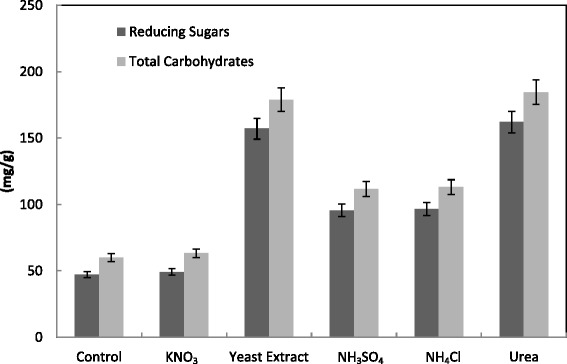
Effect of nitrogen source (1 %) supplementation on the saccharification content by T. virens in SSF using alkali-pre-treated wheat bran. Process conditions: incubation times 72 h, initial moisture content 50 % (by volume per mass) and temperature 30 °C. The data presented were averages of three experiments
Comparison of CMCase, FPase, PGase and xylase production pattern by T. virens used in this study and other microorganisms confirmed the potential of T. virens for economic hydrolytic enzymes production (Table 1). In fact, the present microorganism was better producer of FPase, CMCase, Xylase and PGase (approximately 49.3, 123.26, 348 and 499.9 units/g solid, respectively) compared to other microorganisms.
Table 1.
Sacchrification enzyme activities of different fungal isolates grown on lignocellulosic substrates under solid state fermentation
| Enzyme source | Carbon source | Enzyme activities (U/g dry substrate) | References | |||
|---|---|---|---|---|---|---|
| FPase | CMCase | Xylase | PGase | |||
| Aspergillus ustus | Rice straw | 5.82 | 12.58 | 740 | - | Shamala and Sreekantiah [58] |
| Wheat bran | 3.78 | 11.84 | 615.26 | - | ||
| Aspergillus soja | Crushed maize | - | - | - | 30 | Ustok et al. [59] |
| Aspergillus awamori | Grape pomace | - | - | 40 | 25 | Botella et al. [60] |
| Aspergillus terreus M11 | Corn stover | 243 | 581 | - | - | Gao et al. [61] |
| Aspergillus niger KK2 | Rice straw | 19.5 | 129 | 5070 | - | Kang et al. [62] |
| Aspergillus niger | Ordos Plateau | - | - | - | 36 | Debing et al. [63] |
| Aspergillus niger | Citrus peel | - | - | 65 | 18 | Rodriguez-fernandez et al. [64] |
| Aspergillus niger | Deseeded sunflower | - | - | - | 34 | Patil and Dayanand [54] |
| Myceliophthora sp. IMI 387099 | Rice straw | 2.44 | 32.9 | 900.2 | - | Badhan et al. [65] |
| Wheat straw | 1.37 | 30.8 | 656.6 | - | ||
| Bagasse | 0.7 | 6.62 | 620.1 | - | ||
| Corn cob | 0.31 | 11.38 | 411.6 | - | ||
| Wheat bran | 0.74 | 26.6 | 128.9 | - | ||
| Thermoascus aurantiacus | Wheat straw | 4.3 | 956 | 2973 | - | Kalogeris et al. [66] |
| Trichoderma harzianum and Trichoderma virens | cantaloupe and watermelon | - | - | 80 | 140 | Mohamed et al. [31] |
| Trichoderma harzianum SNRS3 | Rice straw | 6.25 | 111.31 | 433.75 | - | Rahnama et al. [67] |
| Trichoderma reesei MCG77 | Rice bran | 2.314 | - | - | - | Latifan et al. [68] |
| Trichoderma virens | Wheat bran | 49.3 | 123.26 | 348 | 499.9 | Present study |
Conclusion
The present study revealed the saccharification potential of T. virens on alkali pre-treated wheat bran as an agricultural waste in SSF. The optimal conditions for production of CMCase, FPase, PGase and xylase and sccharification content utilizing alkali pre-treated wheat bran as the solid substrate in SSF included incubation for 72 h, temperature at 25-35 °C, substrate moisture content of 50-70 % and pH at 5.0.
Acknowledgement
This project was funded by the National Plan for Science, Technology and Innovation (MAARIFAH) – King Abdulaziz City for Science and Technology - the Kingdom of Saudi Arabia – award number (11-ENE1527-03). The authors also, acknowledge with thanks Science and Technology Unit, King Abdulaziz University for technical support.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
El-R, MS, AA, GM, II and AL-H performed all experiments and read and approved the final manuscript.
Contributor Information
Reda M. El-Shishtawy, Email: elshishtawy@hotmail.com
Saleh A. Mohamed, Email: saleh38@hotmail.com
Abdullah M. Asiri, Email: aasiri2@gmail.com
Abu-bakr M. Gomaa, Email: abgomaa@yahoo.com
Ibrahim H. Ibrahim, Email: ihseada@yahoo.com
Hasan A. Al-Talhi, Email: atttalhi@hotmail.com
References
- 1.Kumakura M, Kasai N, Tameda M, Kaetsu I. Method of pretreatment in saccharification and fermentation of waste cellulose resource. US Pat. 1988;4:769–082. [Google Scholar]
- 2.Howard RL, Abotsi E, Jansen Van REL, Howard S. Lignocellulose biotechnology: issues of bioconversion and enzyme production. Afric J Biotechnol. 2003;2:602–19. doi: 10.5897/AJB2003.000-1115. [DOI] [Google Scholar]
- 3.Heck JX, Hertz PF, Ayub MAZ. Cellulase and xylanase production by isolated Amazon Bacillus strains using soybean industrial residue based solid state cultivation. Braz J Microbiol. 2002;33:213–8. doi: 10.1590/S1517-83822002000300005. [DOI] [Google Scholar]
- 4.Baig MMV, Baig MLB, Baig MIA, Yasmeen M. Saccharification of banana agro-waste by cellulolytic enzymes. Afric J Biotechnol. 2004;3:447–50. doi: 10.5897/AJB2004.000-2088. [DOI] [Google Scholar]
- 5.Katzen R, Fowler DE. Ethanol of lignocellulosic waste with utilization of recombinant bacteria. Appl Biochem Biotechnol. 1994;45:697–707. doi: 10.1007/BF02941841. [DOI] [PubMed] [Google Scholar]
- 6.van Wyk JPH, Leogale PB. Saccharification of wastepaper mixtures with cellulase from Penicillium funiculosum. Biotechnol Lett. 2001;23:1849–52. doi: 10.1023/A:1012706708176. [DOI] [Google Scholar]
- 7.Raimbault M. General and microbiological aspects of solid substrate fermentation. Elec J Biotech. 1998;1:1–15. doi: 10.2225/vol1-issue3-fulltext-9. [DOI] [Google Scholar]
- 8.Martins ES, Silva D, Da Silva R, Gomes E. Solid state production of thermostable pectinases from thermophilic Thermoascusurantiacus. Process Biochem. 2002;37:949–54. doi: 10.1016/S0032-9592(01)00300-4. [DOI] [Google Scholar]
- 9.Couto SR, Sanroman MA. Application of solid-state fermentation to food industry – a review. J Food Eng. 2005;22:211–9. [Google Scholar]
- 10.Pandey A. Aspects of fermenter design for solid-state fermentations. Process Biochem. 1991;26:355–61. doi: 10.1016/0032-9592(91)85026-K. [DOI] [Google Scholar]
- 11.Sukumaran RK, Singhania RR, Mathew GM, Pandey A. Cellulase production using biomass feed stock and its applicationin lignocellulose saccharification for bio-ethanol production. Renew Energ. 2009;34:421–4. doi: 10.1016/j.renene.2008.05.008. [DOI] [Google Scholar]
- 12.Fan LT, Lee YH, Gharpuray MM. The nature of lignocellulosics and their pretreatments for enzymatic hydrolysis. Advan Biochem Eng. 1982;23:157–87. [Google Scholar]
- 13.Weimer P, Weston W. Relationship between the fine structure of native cellulose and cellulose degradability by the cellulase complexes of Trichoderma reesei and Clostridium thermocellum. Biotechnol Bioeng. 1985;27:1540–7. doi: 10.1002/bit.260271104. [DOI] [PubMed] [Google Scholar]
- 14.Broda P, Birch PRJ, Brooks PR, Sims PFG. Lignocellulose degradation by Phanerochaete chrysosporium: gene families and gene expression for a complex process. Mol Microbiol. 1996;19:923–32. doi: 10.1046/j.1365-2958.1996.474966.x. [DOI] [PubMed] [Google Scholar]
- 15.Mach RL, Zeilinger S. Regulation of gene expression in industrial fungi: Trichoderma. Appl Microbiol Biotechnol. 2003;60:515–22. doi: 10.1007/s00253-002-1162-x. [DOI] [PubMed] [Google Scholar]
- 16.Tolan JS, Finn RK. Fermentation of D-xylose to ethanol by genetically modified Klebsiella planticola. Appl Environ Microbiol. 1987;53:2039–44. doi: 10.1128/aem.53.9.2039-2044.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beck MJ. Fermentation of pentoses from wood hydrolysates. Wallingford, UK: CAB International; 1993. [Google Scholar]
- 18.Hahn-Hgerdal B, Hallborn J, Jeppsson H, Olsson L, Skoog K, Walfridsson M. Pentose fermentation to alcohol. In: Saddler JN, editor. Bioconversion of forest and agricultural plant residues. Wallingford: CAB International; 1993. pp. 231–90. [Google Scholar]
- 19.El-Shishtawy RM, Mohamed SA, Asiri AM, Gomaa AM, Ibrahim IH, Al-Talhi HA. Solid fermentation of wheat bran for hydrolytic enzymes production and saccharification content by a local isolate Bacillus megatherium. BMC Biotechnol. 2014;14:29. doi: 10.1186/1472-6750-14-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brilluet JM, Mercier C. Fractionation of wheat bran carbohydrates. J Sci Food Agric. 1981;32:243–51. doi: 10.1002/jsfa.2740320307. [DOI] [Google Scholar]
- 21.Hui YH. Fruit and fruit processing. Iowa: Black Publishing. Anes; 2006. pp. 391–441. [Google Scholar]
- 22.Okia Y, Saito T, Isogai A. TEMPO-mediated oxidation of soft wood thermomechanical pulp. Holzforschung. 2009;63:529–35. [Google Scholar]
- 23.Detroy RW, Cunningham RL, Bothast RJ, Bagby MO, Herman A. Bioconversion of wheat straw cellulose/hemicellulose to ethanol by Saccharomyces uvarum and Pachysolen tannophilus. Biotechnol Bioeng. 1982;24:1105–13. doi: 10.1002/bit.260240507. [DOI] [PubMed] [Google Scholar]
- 24.Lynd LR, Wolkin RH, Grethlein HE. Continuous fermentation of pretreated hardwood and avicel by Clostridium thermocellum. Biotechnol Bioeng Symposium. 1987;17:265–74. [Google Scholar]
- 25.Duenas R, Tengerdy RP, Correa MG. Cellulase production by mixed fungi in solid-substrate fermentation of bagasse. World J Microbiol Biotechnol. 1995;11:333–7. doi: 10.1007/BF00367112. [DOI] [PubMed] [Google Scholar]
- 26.Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–9. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- 27.Bradford MM. A rapid sensitive method of quantitation micro gram quantities of proteins utilizing the principles of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 28.Dubois M, Gilles K, Hamilton J, Rebers P, Smith F. Colorimetric methodfor determination of sugars and related substances. Anal Chem. 1956;28:350–6. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- 29.Mahamudand MR, Gomes DJ. Enzymatic saccharification of sugar cane bagasse by the crude enzyme from indigenous fungi. J Sci Res. 2012;4:227–38. [Google Scholar]
- 30.Zia-ullah Khokhar QS, Nadeem M, Irfan M, Wu J, Samra ZQ, Gul I, et al. Enhanced Production of Cellulase by Trichodermareesei Using Wheat Straw as a Carbon Source. World Appl Sci J. 2014;30:1095. [Google Scholar]
- 31.Mohamed SA, Al-Malki AL, Khan JA, Kabli SA, Al-Garni SM. Solid state production of polygalacturonase and xylanase by Trichoderma species using cantaloupe and watermelon rinds. J Microbiol. 2013;51:605–11. doi: 10.1007/s12275-013-3016-x. [DOI] [PubMed] [Google Scholar]
- 32.Yang X, Chen H, Gao H, Li Z. Bioconversion of corn straw by coupling ensiling and solid-state fermentation. Biores Technol. 2001;78:277–80. doi: 10.1016/S0960-8524(01)00024-4. [DOI] [PubMed] [Google Scholar]
- 33.Couri S, Terzi S, Pinto GS, Freitas S, Da Costa ACA. Hydrolytic enzyme production in solid state fermentation by Aspergillus niger 3T5B8. Process Biochem. 2000;36:255–61. doi: 10.1016/S0032-9592(00)00209-0. [DOI] [Google Scholar]
- 34.Omojasola PF, Jilani OP, Ibiyemi SA. Cellulase production by some fungi cultured on pineapple waste. Nature. 2008;6:64–79. [Google Scholar]
- 35.Begum MF, Alimon AR: Bioconversion and saccharification of some lignocellulosic wastes by Aspergillus oryzae ITCC-4857.01 for fermentable sugar production. Elec J Biotechnol 2011;14(5). www.ejbiotechnology.info/index.php/ejbiotechnology/article/view/v14n53/1355.
- 36.Ja’afaru MI, Fagade OE. Cellulase production and enzymatic hydrolysis of some selected local lignocellulosic substrates by a strain of Aspergillus niger. Res J Biol Sci. 2007;2:13–6. [Google Scholar]
- 37.Ahmed FM, Rahman SR, Gomes DJ. Saccharification of sugarcane bagasse by enzymatic treatment for bioethanol production. Malays J Microbiol. 2012;8:97–103. [Google Scholar]
- 38.Ramesh MV, Lonsane BK. Criticalimportance of moisture content of the medium in α-amylase by Bacillus licheniformis M27 in a solid-state fermentation system. Appl Microbiol Biotechnol. 1990;33:501–5. [Google Scholar]
- 39.Kim JH, Hosobuchi M, Kishimoto M, Seki T, Ryu DDY. Cellulase production by a solidstateculture system. Biotechnol Bioeng. 1985;27:1445–50. doi: 10.1002/bit.260271008. [DOI] [PubMed] [Google Scholar]
- 40.Nagendra PG, Chandrasekharan M. Lglutaminase production by marine Vibrio costicola under solid-state fermentation using different substrates. J Marine Biotechnol. 1996;4:176–9. [Google Scholar]
- 41.Irfan M, Syed Q, Yousaf M, Nadeem M, Baig S, Jafri SA. Studies on the Pretreatment of wheat straw for improve production of Carboxymethyl Cellulase by Trichoderma viride FBL1 in Solid State fermentation. Acad Arena. 2010;2:18–30. [Google Scholar]
- 42.Babu KR, Satyanarayana T. Production of bacterial enzymes by solid-state fermentation. J Sci Ind Res. 1996;55:464–7. [Google Scholar]
- 43.Feniksova RV, Tikhomirova AS, Rakhleeva BE. Conditions for forming amylase andproteinase in surface culture of Bacillus subtilis. Mikrobiol. 1960;29:745–8. [PubMed] [Google Scholar]
- 44.Gervais P, Molin P. The role of the water in solid state fermentation. Biochem Eng J. 2003;13:85–101. doi: 10.1016/S1369-703X(02)00122-5. [DOI] [Google Scholar]
- 45.Lu W, Li D, Wu Y. Influence of water activity and temperature on xylanase biosynthesis in pilot-scale solid state fermentation by Aspergillus sulphurous. Enzyme Microb Technol. 2003;32:305–11. doi: 10.1016/S0141-0229(02)00292-2. [DOI] [Google Scholar]
- 46.Castilho LR, Medronho RA, Alves TLM. Production and extraction of pectinases obtained by solid state fermentation of agroindustrial residues with Aspergillus niger. Biores Technol. 2000;71:45–50. doi: 10.1016/S0960-8524(99)00058-9. [DOI] [Google Scholar]
- 47.Smits JP, Rinzema A, Tramper J, van Sonsbeek HM, Knol W. Solid-state fermentation of wheat bran by Trichoderma reeseiQM9414: Substrate composition changes, C balance, enzyme production, growth and kinetics. Appl Microbiol Biotechnol. 1996;46:489–96. doi: 10.1007/s002530050849. [DOI] [Google Scholar]
- 48.Lonsane BK, Ghildyal NP, Budiatman S, Ramakrishna SV. Engineering aspects of solid state fermentation. Enzyme Microb Technol. 1985;7:258–65. doi: 10.1016/0141-0229(85)90083-3. [DOI] [Google Scholar]
- 49.Yamane Y, Fujita J, Shimizu R, Hiyoshi A, Fukuda H, Kizaki Y, et al. Production of cellulose- and xylan-degrading enzymes by a koji mold, Aspergillus oryzae, and their contribution to the maceration of rice endosperm cell wall. J Biosci Bioeng. 2002;93:9–14. doi: 10.1016/S1389-1723(02)80046-9. [DOI] [PubMed] [Google Scholar]
- 50.Botella C, Diaz A, de Ory I, Webb C, Blandino A. Xylanase and pectinase production by Aspergillusawamoriongrape pomace in solid state fermentation. Process Biochem. 2007;42:98–101. doi: 10.1016/j.procbio.2006.06.025. [DOI] [Google Scholar]
- 51.Yuan QP, Wang JD, Zhang H, Qian ZM. Effect of temperature shift on production of xylanase by Aspergillus niger. Process Biochem. 2005;40:3255–7. doi: 10.1016/j.procbio.2005.03.020. [DOI] [Google Scholar]
- 52.Zhou JM, Ge XY, Zhang WG. Improvement of polygalacturonase production at high temperature by mixed culture of Aspergillus niger and Saccharomyces cerevisiae. Biores Technol. 2011;102:10085–8. doi: 10.1016/j.biortech.2011.08.077. [DOI] [PubMed] [Google Scholar]
- 53.Lakshmi GS, Rao CS, Rao RS, Hobbs PJ, Prakasham RS. Enhanced production of xylanase by a newly isolated Asperigullus terreus under solid state fermentation using palm industrial optimization. Biochem Eng J. 2009;48:51–7. doi: 10.1016/j.bej.2009.08.005. [DOI] [Google Scholar]
- 54.Patil SR, Dayanand A. Optimization of process for the production of fungal pectinases from deseeded sunflower head in submerged and solid-state conditions. Bioresour Technol. 2006;97:2340–4. doi: 10.1016/j.biortech.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 55.Farani De Souza D, Marques De Souza CG, Peralta RM. Effect of easily metabolizable sugars in the production of xylanase by Aspergillus tamarii in solid state fermentation. Process Biochem. 2001;36:835–8. doi: 10.1016/S0032-9592(00)00295-8. [DOI] [Google Scholar]
- 56.Abdel-Fatah OM, Hassan MM, Elshafei AM, Haroun BM, Atta HM, Othman AM: Physiological studies on carboxymethyl cellulase formation by Aspergillus terreus DSM 826. Braz J Microbiol 2012;43:01–11. [DOI] [PMC free article] [PubMed]
- 57.Gilna VV, Khaleel KM. Cellulase enzyme activity of aspergillus fumigatus from mangrove soil on lignocellulosic substrate. Recent Res Sci Technol. 2011;3:132–4. [Google Scholar]
- 58.Shamala TR, Sreekantiah KR. Production of cellulases and D-xylanase by some selected fungal isolates. Enzyme Microb Technol. 1986;8:178–82. doi: 10.1016/0141-0229(86)90109-2. [DOI] [Google Scholar]
- 59.Ustok FI, Canan TC, Gogus N. Solid-state production of polygalacturonase by Aspergillus sojae ATCC 20235. J Biotechnol. 2007;127:322–34. doi: 10.1016/j.jbiotec.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 60.Botella C, de Ory I, Webb C, Cantero D, Blandino A. Hydrolytic enzyme production by Aspergillus awamori on grape pomace. Biochem Eng J. 2005;26:100–6. doi: 10.1016/j.bej.2005.04.020. [DOI] [Google Scholar]
- 61.Gao J, Weng H, Zhu D, Yuan M, Guan F, Xi Y. Production and characterization of cellulolytic enzymes from thermoacidophilic fungal Aspergillus Terreus M11 under solid state cultivation of corn stover. Biores Technol. 2008;99:7623–9. doi: 10.1016/j.biortech.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 62.Kang SW, Park YS, Lee JS, Hong SI, Kim SW. Production of cellulose and hemicellulases by Aspergillus niger KK2 from lignocellulosic biomass. Biores Technol. 2004;91:151–6. doi: 10.1016/S0960-8524(03)00172-X. [DOI] [PubMed] [Google Scholar]
- 63.Debing J, Peijun L, Stagnitti F, Xianzhe X, Li L. Pectinase production by solid fermentation from Aspergillus niger by a new prescription experiment. Ecotoxicol Environ Safety. 2006;64:244–50. doi: 10.1016/j.ecoenv.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 64.Rodriguez-Fernndez DE, Rodriguez-Len JA, de Carvalho JC, Sturm W, Soccol CR. The behavior of kinetic parameters in production of pectinaseand xylanase by solidstate fermentation. Biores Technol. 2011;102:10657–62. doi: 10.1016/j.biortech.2011.08.106. [DOI] [PubMed] [Google Scholar]
- 65.Badhan AK, Chadha BS, Kaur Saini HS, Bhat MK. Production of multiple xylanolytic and cellulolytic enzymes by thermophilic fungus Myceliophthora sp. IMI 387099. Biores Technol. 2007;98:504–10. doi: 10.1016/j.biortech.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 66.Kalogeris E, Fountoukides G, Kekos D, Macris BJ. Design of a solid state bioreactor for thermophilic microorganisms. Biores Technol. 1999;67:313–5. doi: 10.1016/S0960-8524(98)00124-2. [DOI] [Google Scholar]
- 67.Nooshin R, Suhaila M, Kalsom MSU, Hooi LF, Nor Aini AR, Arbakariya AB. Effect of Alkali Pretreatment of Rice Straw on Cellulase and Xylanase Production By Local Trichoderma harzianum SNRS3 Under Solid State Fermentation. BioRes. 2013;8:2881–96. [Google Scholar]
- 68.Latifian M, Hamidi-Esfahani Z, Barzegar M. Evaluation of culture conditions for cellulose production by two Trichoderma reesei mutants under solid state fermentation conditions. Biores Technol. 2007;9:3634–7. doi: 10.1016/j.biortech.2006.11.019. [DOI] [PubMed] [Google Scholar]


