Abstract
Background:
Conventional microscopy underestimates the burden of malarial infection when compared with molecular diagnosis using polymerase chain reaction (PCR)-based methods. Lower density parasitemias serve as a reservoir for infection. We evaluated the prevalence of submicroscopic infections in an area of unstable malarial transmission in India and determined whether these infections negatively impacted maternal or fetal outcomes.
Methods:
This cross-sectional study (2007–2008) was undertaken in two districts of Chhattisgarh, recruiting women from both antenatal clinics (ANCs) and delivery units (DUs). For ANC/DU subjects, peripheral/placental blood, respectively, was obtained for conventional microscopy and collected onto filter paper for PCR analysis.
Results:
There were 3425 pregnant women, including 2477 ANC subjects and 948 DU subjects who had both microscopic and PCR samples available. Polymerase chain reaction detected significantly more Plasmodium infections than traditional light microscopy both from peripheral (3.4 vs 1.2%; OR 2.9, 95% confidence intervals (CIs) 1.9–4.5) and placental (4.2 vs 1.7%; OR 2.5, 95% CIs 1.4–4.8) blood samples. Submicroscopic infections were not associated with anemia or severe maternal anemia among ANC or DU participants and were not associated with low birth weight (LBW) among DU participants. In contrast, microscopically detected infections were associated with severe anemia and LBW.
Conclusions:
In this area of unstable malarial transmission from India, submicroscopic infections did not identify a set of pregnant women at increased risk for anemia or LBW. Until PCR techniques become much less expensive and available as a point of care test for the field setting, its use will be limited for malarial detection.
Keywords: Malaria, Pregnancy, Subpatent infection, Submicroscopic infection, Plasmodium falciparum, Anemia, India, Polymerase chain reaction
INTRODUCTION
Pregnancy poses specific challenges for malaria diagnosis, as Plasmodium falciparum parasites may be sequestered in the placenta but circulating at very low densities and therefore undetectable in peripheral blood smear.1 Unrecognized malarial infection during pregnancy can have negative consequences for both mother and fetus including maternal anemia, prematurity, low birth weight (LBW), stillbirth, and neonatal mortality.2,3 Even in nonpregnant individuals, conventional light microscopy fails to detect half or more of all malarial infections when compared with molecular diagnosis using polymerase chain reaction (PCR)-based methods; gametocytes and non-falciparum infections are particularly underestimated.4,5 A seasoned microscopist is only able to discern parasitemias above a density of approximately 100 parasites per microliter,6,7 compared with PCR where detection can occur at 1 parasite per microliter or less.8 These low-density parasitemias serve as a reservoir for continued transmission of the disease and may contribute to negative health.9–11
A number of investigators have evaluated the burden of submicroscopic infections during pregnancy in the African subcontinent. These studies have consistently shown that conventional microscopy vastly underestimates infection compared to PCR in both peripheral and placental blood samples.12–22 Nonetheless, the clinical relevance of these submicroscopic infections to the mother and fetus remains unclear. Submicroscopic infections were linked with maternal anemia in some,17,18,20,23 but not all,12,13,15,21 studies. Similarly, the data are conflicting about the impact of submicroscopic infections on LBW; some studies suggest an increased risk,16,24 in the setting of submicroscopic infections and others no association.20–23 Neither study that evaluated preterm birth found an increased risk associated with submicroscopic infection.21,23
The proportion of malarial infections that are submicroscopic appears to be higher in areas of low transmission. A meta-analysis of surveys conducted in endemic areas reported that 88% of all infections went undetected by microscopy in areas of low transmission compared to only 25.5% in areas of high transmission.5 In this study, a low-transmission area was defined as one where the P. falciparum prevalence based on PCR in cross-sectional surveys was <10%; high-transmission areas were characterized by a prevalence >75%. Between these two extremes, there was an inverse relationship between the proportion of submicroscopic infections and the transmission intensity (by PCR prevalence).
Limited information on the epidemiology of submicroscopic malarial infections during pregnancy is available from areas outside of Africa where levels of malarial transmission are much lower. The contribution of these submicroscopic infections to adverse maternal and fetal outcomes may be distinct from settings of higher endemicity. In one study from Northwest Colombia where both P. falciparum and Plasmodium vivax are present, 79% of all infections at the time of delivery were submicroscopic.25 Notably, these submicroscopic infections were associated with placental villitis and intervillitis, inflammatory lesions in the placenta that may contribute to poor birth outcomes.
The clinical relevance of submicroscopic malarial infections in pregnancy has not been evaluated from the Asia-Pacific region where malaria prevalence is much lower than sub-Saharan Africa.26 We had access to antenatal peripheral and placental blood samples from a cross-sectional study conducted in central east India.27 The purpose of our work was to determine the proportion of malarial infections during pregnancy that are submicroscopic in a setting of unstable seasonal transmission. We further aimed to describe the consequences of such submicroscopic infections to both the mother and the newborn as compared with overt microscopic infections. This information will help elucidate the true burden of malaria in pregnancy in India as it may be underappreciated by conventional diagnostic techniques alone.
METHODS
We have previously reported on the prevalence of peripheral and placental parasitemia in an unselected pregnant population enrolled during antenatal care or at the time of delivery.27 We now present further analysis detailing submicroscopic infections identified by PCR from the same cross-sectional study and compare prevalence and impact of these infections with the microscopically detected infections we previously described.
Study location and participants
This cross-sectional study was undertaken in Chhattisgarh state in central India from June 2007 to May 2008. Chhattisgarh is the third most malarious state after Orissa and Jharkhand contributing 11% of all annually reported malaria cases in India.28 Enrollment occurred in Rajnandgaon and Bastar districts, within both a rural and urban facility in each district. The former had unstable malaria with a slide positivity rate of 1.6% based on symptomatic patients during active surveillance in community surveys while the latter had stable malaria with a slide positivity rate of 13%.29 Both study areas receive two rounds of indoor residual spraying annually, specifically dichlorodiphenyltrichloroethane in Rajnandgaon and a pyrethroid (alpha-cypermethrin) in Bastar.
Screening and enrollment
Pregnant women aged 15 years or older who presented for a routine checkup at the antenatal clinics (ANCs) at study facilities were enrolled after obtaining written informed consent. Similarly, women aged 15 years or older who came to the study delivery units (DU) were enrolled after providing informed consent. These represent two distinct cross-sectional cohorts (ANC and DU); women were not followed longitudinally. The details of screening, enrollment, and study procedures have been described in detail elsewhere.27 Women who had previously participated were excluded from participating again in the ANC component of the study but could participate in the DU component.
Laboratory procedures
From women enrolled during an ANC visit, peripheral blood was obtained by finger-stick for hemoglobin determination, preparation of thick and thin smears, and collected onto filter paper for subsequent PCR analysis. For those enrolled at delivery, in addition to peripheral blood samples, placental blood from the maternal side of placenta was obtained by incision for preparation of thin and thick blood smears and filter paper collection for subsequent PCR. An impression smear was also prepared. Smears from peripheral and placental blood were stained with 3% Giemsa for 30 minutes and examined under 100× oil immersion.30 An experienced microscopist who was blinded to the PCR results and clinical history of the subject examined the blood films. A slide was considered positive if at least one asexual form of parasite was detected in 100 microscopic fields in thick blood films. Blood parasite density was determined from thick films by counting the number of parasites against 200 white blood cells (WBCs) and assuming that each subject had 8000 WBCs per microliter of blood.31 Placental malaria was considered present if either the thick smear or impression smear was positive for asexual parasites. The thin film was used to identify the Plasmodium species. All smears were rechecked by a member of the parasitology laboratory at the National Institute of Malaria Research (NIMR) Field Station in Jabalpur. Because of limited resources, we performed only PCR on peripheral blood samples from ANC subjects and placental blood samples from DU participants. A portable Hemocue machine (Ängelholm, Sweden) was used for hemoglobin estimation using the manufacturers’ internal quality control before each assay.
DNA isolation
The blood spotted area (3 drops of blood, approximately 15–25 μl) was punched and put into a 1.5 ml tube. The blood spots were soaked in 150 μl TE buffer (10 mM Tris, 0.1 mM EDTA, pH 8.0) and incubated for an hour at room temperature. Following this, tubes were placed into a dry bath at 50°C, incubated for 15 minutes, and punched by pipette tips several times. Finally, the tubes were incubated at 97°C for 15 minutes and centrifuged at 8000 RPM for 2 minutes. DNA was eluted in the 80–100 μl TE buffer, stored at −20°C for PCR amplification, and out of this, 6 μL was taken for each PCR assay.
Species-specific nested PCR
Species-specific nested PCR was carried out to diagnose the malarial parasite using the 18s rRNA gene.32 Briefly, a two-step PCR approach was used. Primers used for primary PCR reaction were genus specific. Later, the amplified product was used for species detection in a nested PCR assay. The primary PCR was performed in a volume of 25 μl with 0.175 U of Taq DNA polymerase, 0.2 mM each dNTP, 0.4 μM each primer, and 1.5 mM MgCl2. The reaction was allowed to proceed for 35 cycles after an initial denaturation at 94°C for 1 minute, annealing at 55°C for 1 minute, and extension at 72°C for 1 minute. The final extension was at 72°C for 10 minutes. The species-specific nested PCR was performed in a volume of 25 μl with 0.15 U of Taq DNA polymerase, 0.2 mM each dNTP, 0.4 μM each primer, and 1.0 mM MgCl2. The reaction was allowed to proceed for 25 cycles after an initial denaturation at 94°C for 1 minute, annealing at 58°C for 1 minute, and with extension at 72°C for 1 minute. A final extension for 10 minutes was carried out at 72°C. The amplified products were resolved by 1.5% agarose gel electrophoresis and stained with ethidium bromide for visual detection by ultraviolet transillumination.
An independent research assistant unaware of the participant’s clinical status or the microscopy results performed the PCR on coded samples. All negative slides that tested PCR positive and all positive slides that were PCR negative were reexamined by another expert technician blinded to the results of microscopy, PCR, and clinical status of the participants.
Other antenatal and DU procedures
During ANC visits, gestational age was calculated by fundal height when the fundus was above the umbilicus with estimated weeks corresponding to the centimeter by fundal height. Those below the umbilicus were considered to be less than 20 weeks gestation. At the time of delivery, all neonates were weighed with an electronic digital scale to the nearest 10 g and the gestational ages of all live births estimated within 24 hours of delivery by means of a modified Ballard examination.33 Women with positive blood smear results were referred for treatment. Additional details of delivery (including date and time of delivery, type, complications, and plurality) were abstracted from delivery records.
Study definitions
Peripheral parasitemia: presence of asexual P. falciparum or P. vivax parasitic forms on blood smears. Placental parasitemia: presence of asexual P. falciparum or P. vivax parasitic forms on placental blood/impression smears of the maternal side of placenta. Submicroscopic infection: evidence of parasitemia by PCR with negative microscopy. Anemia: hemoglobin < 11 g/dl. Severe anemia: hemoglobin < 7 g/dl. Low birth weight: birth weight < 2500 g. Prematurity: gestational age < 37 weeks as assessed by Ballard examination. Stillbirth: death of a fetus before delivery in a pregnancy estimated at 28 weeks gestation or greater.
Data management and analysis
Results of the PCR and microscopy examination were recorded on separate forms. After double data entry, the database was rechecked for inconsistent entries and errors were corrected. Data were then analyzed using Stata/IC 8.2 for Windows (StataCorp, College Station, TX, USA).
All parameters are presented as percentages with 95% confidence intervals (CIs) unless stated otherwise and compared using the chi-square or Fisher’s exact statistic as appropriate. Odds ratios with 95% CIs are also presented in 2 × 2 contingency tables. Using univariate logistic regression models, the odds of parasite detection were compared between PCR and microscopy and between submicroscopic and microscopic detection. Peripheral blood smears were compared with peripheral blood PCR among ANC participants. Separately, placental blood/impression smears were compared with placental blood PCR among DU participants. Peripheral blood smear results from DU participants were not considered in this analysis, as no PCR results were available for comparison. Univariate logistic models were also used to estimate the risk of maternal anemia and adverse birth outcomes with both microscopically and PCR-detected infections. Analysis of variance was used to determine differences between mean hemoglobin levels and mean birth weights by parasitemia group. Adjusted analyses were not performed.
Ethical clearance
The Institutional Review Boards of Boston University and the U.S. Centers for Disease Control and Prevention, the Ethics Committee of the NIMR in India, the Scientific Advisory Committee of the NIMR, and the Health Ministry Screening Committee of the Indian Council of Medical Research (ICMR) reviewed and approved the study protocol and informed consent forms.
RESULTS
A total of 3726 pregnant women (2696 from ANC and 1030 from DU) were enrolled in the parent cross-sectional study. Two eligible women screened at the time of ANC declined participation. Peripheral dried blood spots for PCR were available for comparison with peripheral microscopy for 2477 of the 2696 women recruited from the ANC. Placental dried blood spots for PCR were available for comparison with placental blood microscopy/impression smears in 948 of the 1030 women recruited from DUs. This secondary analysis presents results of the 3425 women with both microscopy and PCR results. Maternal and sociodemographic characteristics, and use of malaria prevention measures are summarized for both ANC and DU participants in Table 1. The majority of the enrolled pregnant women (ANC/DU) were in their first or second pregnancies between the ages of 20 and 34 years, and of lower socio-economic status. Bednet use was fairly common but malaria chemoprophylaxis is rare.
Table 1. Baseline characteristics of pregnant women enrolled at antenatal clinics (ANCs) and delivery units (DUs).
| Characteristic | Antenatal enrollees (n = 2477) | Delivery unit enrollees (n = 948) |
| na (%) | na (%) | |
| Maternal characteristics and sociodemographics | ||
| Age categories (years) | ||
| <20 | 224 (9.1) | 70 (7.4) |
| 20–34 | 2190 (88.4) | 859 (90.6) |
| ≧35 | 63 (2.5) | 19 (2.0) |
| Primigravid/Secundigravidb | 1888 (76.2) | 929 (98.0) |
| Historically disadvantaged castec | 2099 (84.8) | 822 (86.9) |
| No formal schoolingb | 505 (20.4) | 262 (27.6) |
| Owns house | 2169 (87.6) | 850 (89.7) |
| Impermanent roof materialb | 2001 (80.8) | 811 (85.5) |
| Impermanent wall materialb | 1807 (72.9) | 746 (78.7) |
| Malaria prevention measures | ||
| Use of malaria chemoprophylaxis | 4 (0.2) | 3 (0.3) |
| Sleeps under bednet most nightsb | 677 (67.9) | 143 (49.3) |
| Slept under a bednet last nightb | 599 (60.0) | 199 (68.6) |
| House has been sprayed with insecticide | 660 (26.6) | 258 (27.2) |
aNumbers may not add to sample size secondary to missing data.
bSignificant difference (P < 0.05) between ANC subjects and DU subjects.
cHistorically disadvantaged castes include scheduled caste, other backward caste, and scheduled tribes.
Prevalence of microscopic and submicroscopic parasitemia
As anticipated, PCR detected significantly more infections than traditional light microscopy (Table 2). The overall prevalence of parasitemia from peripheral blood samples obtained during antenatal care was 3.4% by PCR compared with 1.2% by microscopy (OR 2.9; 95% CIs 1.9–4.5). Similarly, the odds of detecting parasitemia by PCR were over two-fold higher than microscopy for placental samples (4.2 vs 1.7%, OR 2.5; 95% CIs 1.4–4.8). The increased detection was evident for both P. falciparum and P. vivax in both peripheral and placental samples.
Table 2. Prevalence of peripheral and placental parasitemia by microscopy and PCR.
| P. falciparum | P. vivax | Overall | ||||
| n (%) | OR (95% CIs) | n (%) | OR (95% CIs) | n (%) | OR (95% CIs) | |
| Peripheral parasitemiaa (n = 2477) | ||||||
| Light microscopy | 26 (1.0) | Referent | 4 (0.2) | Referent | 30 (1.2) | Referent |
| PCR | 45 (1.8) | 1.7 (1.0–2.8) | 40 (1.6) | 8.1 (3.2–26.3) | 84b (3.4) | 2.9 (1.9–4.5) |
| Placental parasitemiaa (n = 948) | ||||||
| Light microscopy | 12 (1.3) | Referent | 4 (0.4) | Referent | 16 (1.7) | Referent |
| PCR | 30 (3.2) | 2.6 (1.3–5.6) | 9 (1.0) | 2.5 (0.7–10.8) | 40c (4.2) | 2.5 (1.4–4.8) |
OR: odds ratio; CIs: confidence intervals; PCR: polymerase chain reaction.
aPeripheral samples were obtained from women recruited at antenatal clinics; placental samples were obtained from women recruited at the time of delivery.
b1 case detected as P. malariae and 2 mixed (P. falciparum + P. vivax) by PCR.
c1 mixed (P. falciparum + P. vivax) by PCR.
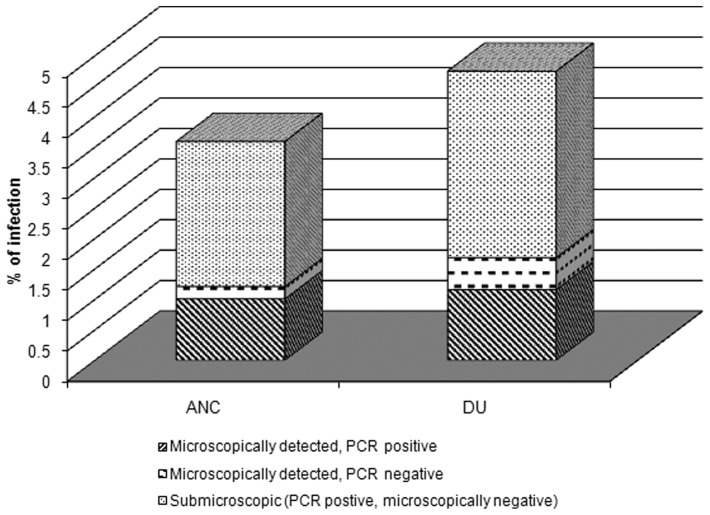
Nearly two-thirds of both peripheral and placental infections identified were submicroscopic (PCR positive, microscopy negative) (Fig. 1). Among antenatal peripheral blood samples, 59 of 89 infections (66.3%; 95% CIs 55.9–75.3) were submicroscopic. The proportion of submicroscopic placental infections was similar to 29 of 45 placental infections undetected by microscopy (64.4%; 95% CIs 49.8–76.8). In ANC samples, the proportion of submicroscopic infections was over 90% for P. vivax (38/42; 90.5%) compared to only half of the P. falciparum infections (26/52; 50.0%; P < 0.001 comparing species). In contrast, there was not a significant difference in submicroscopic infections by Plasmodium species among placental samples (64.7% P. falciparum vs 66.7% P. vivax, P = 0.90). Notably, PCR did not detect 10 of the microscopically detected infections. Five of these missed infections were from peripheral samples (5.6% of peripheral infections), and the remaining five of the infections missed by PCR were from placental samples (11.1% of placental infections). The DNA extracted from these filter paper samples had little blood in them and failed to amplify; this may have contributed to the observed results. In our cohort, neither maternal age nor parity altered the risk for submicroscopic peripheral or placental infections (Supplementary Material 1 http://dx.doi.org/10.1179/2047773215Y.0000000002.s1). Peripheral and placental infections among women who did not sleep under a bednet on most nights were more likely to have a microscopic infection, although reaching significance only for the peripheral infections (P = 0.014). Women whose homes had not been sprayed with insecticide during governmental indoor residual spraying campaigns were more likely to have a microscopically detected placental (P = 0.004), but not peripheral (P = 0.476) malarial infection.
Figure 1.
Prevalence of parasitemia among peripheral and placental blood samples. For peripheral samples, 89 infections were detected of which 25 (28.1%) were detected by both microscopy and PCR, 59 (66.3%) were detected by PCR alone (submicroscopic), and an additional 5 detected by microscopy alone (5.6%). Among placental samples, 45 infections were detected of which 11 (24.4%) were detected by both microscopy and PCR, 29 (64.4%) were submicroscopic, and the remaining 5 (11.1%) detected by microscopy alone.
Anemia
Anemia was common with a prevalence of 67% (1659/2477) among subjects enrolled at ANCs and 75% (709/947) among women enrolled at delivery. Among ANC subjects, the mean hemoglobin was similar in women without any detectable parasites by either microscopy or PCR (10.3 ± 1.5 g/dl) and in those with submicroscopic infections (10.1 ± 1.3 g/dl). Women with microscopically detected infections had significantly lower hemoglobin on average (8.0 ± 2.3 g/dl, P < 0.0001 by analysis of variance). The odds of severe anemia were much higher among women with microscopically detected peripheral malarial infections compared to women without parasites visible on peripheral smear (30.0 vs 3.0%; OR 13.7, 95% CIs 5.3–32.5) (Table 3). All infections detected by PCR, including both microscopic and submicroscopic infections were also associated with an increase in the odds for severe anemia (OR 3.7; 95% CIs 1.6–7.8). This effect size was appreciably lower than when considering microscopic infections only. Women with submicroscopic infections (PCR positive, slide negative) were not more likely to be severely anemic when compared with women who had negative results by both PCR and microscopy (1.7 vs 3.1%). There was no association between peripheral parasitemia and a less severe anemia (Hgb < 11 g/dl) whether the parasites were detected by microscopy, PCR, or both.
Table 3. Association between microscopic and submicroscopic malarial infections with maternal anemia and severe anemia.
| Anemia | Severe anemia | |||
| n/d (%) | OR (95% CIs) | n/d (%) | OR (95% CIs) | |
| Peripheral parasitemiaa | ||||
| Microscopic infections | ||||
| Positive | 26/30 (86.7%) | 1.8 (1.0.–3.3) | 9/30 (30.0%) | 13.7 (5.3–32.5) |
| Negative | 1633/2447 (66.7%) | Referent | 74/2447 (3.0%) | Referent |
| PCR-detected infectionsb | ||||
| Positive | 64/84 (76.2%) | 1.6 (0.9–2.8) | 9/84 (10.7%) | 3.7 (1.6–7.8) |
| Negative | 1595/2393 (66.7%) | Referent | 74/2393 (3.1%) | Referent |
| Submicroscopic infections | ||||
| PCR positive/slide negative | 43/59 (72.9%) | 1.4 (0.7–2.6) | 1/59 (1.7%) | 0.6 (0.01–3.3) |
| PCR negative/slide negative | 1590/2388 (66.6%) | Referent | 73/2388 (3.1%) | Referent |
| Placental parasitemiaa | ||||
| Microscopic infections | ||||
| Positive | 15/16 (93.8%) | 5.0 (0.8–212.2) | 4/15 (26.7%) | 10.6 (2.3–38.7) |
| Negative | 694/931 (74.5%) | Referent | 24/694 (3.5%) | Referent |
| PCR-detected infectionsb | ||||
| Positive | 32/40 (80.0%) | 1.4 (0.6–3.5) | 5/32 (15.6%) | 5.3 (1.5–15.9) |
| Negative | 677/907 (74.3%) | Referent | 23/677 (3.4%) | Referent |
| Submicroscopic infections | ||||
| PCR positive/slide negative | 22/29 (75.9%) | 1.1 (0.4–3.0) | 2/22 (9.1%) | 3.0 (0.3–13.5) |
| PCR negative/slide negative | 672/902 (74.5%) | Referent | 22/672 (3.3%) | Referent |
n/d: numerator/denominator; OR: odds ratio; CIs: confidence intervals; PCR: polymerase chain reaction.
aPeripheral samples were obtained from women recruited at antenatal clinics (ANCs); peripheral samples were obtained from women recruited at the time of delivery.
bPCR-detected infections include infections that were microscopic (PCR positive, slide positive) and those that are submicroscopic (PCR positive, slide negative).
Similar to the antenatal evaluations, hemoglobin levels among mothers at the time of delivery differed by the presence of placental parasitemia. As with ANC subjects, women without any evidence of malaria by microscopy or PCR and those with submicroscopic infections had similar mean hemoglobin levels (10.1 ± 1.6 and 9.9 ± 2.1 g/dl, respectively). These levels were significantly higher than the mean hemoglobin among women with microscopically detected infections (8.3 ± 2.4 g/dl, P = 0.0002 by analysis of variance). Microscopically detected placental infections were associated with an increased risk for severe maternal anemia as were PCR-detected infections (Table 3). Similar to ANC results, the effect size was higher for microscopically detected infections compared with PCR-identified infections. Again, submicroscopic placental infections did not confer an increased risk of severe anemia. Neither positive microscopy nor positive PCR from placental samples was associated with a lesser degree of anemia (Hgb < 11 g/dl).
Birth outcomes
The association of microscopic and submicroscopic placental infections with birth weight is shown in Table 4. Women with microscopically detected placental infections were more likely to deliver a LBW infant (OR 5.8, 95% CIs 1.4–33.7) compared to women with negative smears. On average, these mothers with positive placental microscopy delivered infants that were 310 g lighter than women without microscopically detected infections (P = 0.004). In contrast, no association was found between PCR-detected infections and LBW. Notably, the prevalence of LBW among women with positive PCR samples (36.4%) was similar to those with negative PCR samples (34.9%) and with negative microscopy (34.0%) and much lower than women with positive microscopy (75.0%). Submicroscopic placental infections were also not associated with an increase in the risk for LBW. Furthermore, the average birth weights of infants born to women with submicroscopic infections were not significantly different than women without parasitemia by PCR or microscopy (2656.7 g vs 2576.0 g, P = 0.31). Having a malarial infection detected by either microscopy or PCR did not significantly increase the odds for stillbirth; however, the study was underpowered to assess this outcome given its rarity in our study population. Preterm birth was also not more common among women infected with malaria detected either by microscopy or PCR.
Table 4. Association of microscopic and submicroscopic malarial infections with birth weight.
| Low birth weight (LBW) (<2500 g) | Birth weight (g) | |||
| n/d (%) | Effect sizea | Mean (±SD) | Effect sizea | |
| Microscopic infections | ||||
| Positive | 9/12 (75.0%) | 5.8 (1.4–33.7) | 2268.7 ± 361.6 | −310.3 (−97.4, −523.2) |
| Negative | 229/674 (34.0%) | Referent | 2579.0 ± 417.6 | Referent |
| PCR-detected infectionsb | ||||
| Positive | 12/33 (36.4%) | 1.1 (0.5–2.3) | 2540.1 ± 427.4 | −35.3 (−168.1, 97.4) |
| Negative | 226/653 (34.6%) | Referent | 2575.5 ± 418.2 | Referent |
| Submicroscopic infections | ||||
| PCR positive/slide negative | 5/24 (20.8%) | 0.5 (0.1–1.4) | 2656.7 ± 386.3 | 80.3 (−74.3, 234.9) |
| PCR negative/slide negative | 224/650 (34.5%) | Referent | 2576.4 ± 418.5 | Referent |
n/d: numerator/denominator; OR: odds ratio; CIs: confidence intervals; PCR: polymerase chain reaction; SD: standard deviation.
aEffect size for low birth weight represents the unadjusted odds ratio (95% CIs). Effect size for birth weight represents the unadjusted difference in grams (95% CIs).
bPCR-detected infections includes infections that were microscopic (PCR positive, slide positive) and those that are submicroscopic (PCR positive, slide negative).
DISCUSSION
We undertook this investigation as the clinical relevance of submicroscopic P. falciparum infections in pregnancy is not well established for the Asia-Pacific region where malarial transmission is unstable and where both P. vivax and P. falciparum are prevalent. To the best of our knowledge, this is the first study of South Asia to address this gap.
We found a marked underestimation of malarial infection in pregnant women when diagnosed by standard microscopy in peripheral or placental blood compared with PCR, as has been reported previously in other settings outside South Asia.12–22 Indeed, the majority of infections was submicroscopic (66.3% of peripheral and 64.4% of placental infections) in line with a meta-analysis suggesting higher proportions of submicroscopic infections in areas of lower malaria prevalence.5 The association of malaria prevention measures with submicroscopic infections was inconsistent in our data. The use of these measures may have been less in more impoverished women; therefore, any association between preventive measures and a reduced risk of microscopic infections may reflect the underlying sociodemographics rather than protection against malaria itself. Maternal characteristics such as age and parity did not alter the proportion of submicroscopic infections in our analysis.
In contrast to many studies in sub-Saharan Africa linking submicroscopic infections with adverse maternal,17,18,20,23 or infant,16,24 outcomes, we did not identify an association between submicroscopic infections and either maternal anemia of any degree or LBW. Women with submicroscopic infections were no more likely to suffer anemia of any severity or to deliver LBW babies than women in whom no parasites were detected by PCR or microscopy. This differs from the microscopically detected infections, which were associated with both severe anemia and LBW. When evaluating all PCR-detected infections including both microscopic and submicroscopic infections, parasitemia was associated with severe maternal anemia and LBW but the effect size was diminished compared with microscopic infections considered alone. This likely reflects the ability of PCR to detect both higher density infections, which would be identified by microscopy, and lower density infections.
Microscopy and PCR detect infections in different ways. One advantage of PCR is that a larger volume of blood can be tested, several microliters compared to a tenth of a microliter by microscopy. Microscopy visualizes viable whole parasites in erythrocytes, while PCR detects parasite DNA.34 The DNA detected by PCR may be from a viable asexual parasite, a gametocyte, or represent nonviable DNA from past infections following treatment.35 This detection of recent past infections may account for why the submicroscopic infections are not associated with concurrent anemia or severe anemia in our subjects although mouse models suggest rapid clearance of plasmodial DNA from the circulation. More likely, submicroscopic infections reflect lower density parasitemias, which may not be as strongly linked with anemia in a population with a fairly high degree of anemia at baseline. Past infections of the placenta, which can be better detected by placental histology, have nonetheless been associated with adverse birth outcomes. However, tissue sections were not collected for histology in our study. Furthermore, PCR techniques are unlikely to identify past infections that occurred remote from delivery given the clearance of nonviable DNA.
Recruitment of an unselected population of pregnant women receiving antenatal care or at the time of delivery rather than women with symptoms of malaria was a strength of this study. Not withstanding, this study has several limitations. First, this was a cross-sectional facility based study rather than a prospective longitudinal study. A longitudinal design would have allowed us to determine whether submicroscopic infections persist over time to be detected eventually by microscopy at some time during pregnancy. Further, we lacked matched peripheral and placental samples, which might have answered whether peripheral PCR detects placental infections that are often missed on peripheral blood smears. Microscopic readings for initial detection of parasitemia were limited to 100 microscopic fields as per World Health Organization standards for microscopic diagnosis of malaria.31 The sensitivity of microscopy for detecting parasitemia might have been slightly higher if 200 or more fields had been read in the thick blood films for initial identification of positive specimens.
While we did not identify adverse maternal or fetal outcomes associated with submicroscopic infections detected by PCR, our ability to entirely exclude this possibility was limited by both the low prevalence of parasitemia and the rarity of some outcomes. A meta-analysis with other studies may help shed additional light on whether and/or when PCR should be included in the armamentarium for malaria control. That said, almost one quarter of the infections among DU participants in this setting was caused by P. vivax, which are less strongly linked with adverse pregnancy outcomes further limiting our power to detect an impact on pregnancy outcome. This is distinct from malarial epidemiology in much of Africa where P. falciparum is the predominant species; comparison of studies between continents may thus be challenging.
CONCLUSION
This study confirms, in a South Asian setting of lower malarial transmission, that PCR is a more sensitive method than microscopy in detecting malarial infections in pregnant women. However, since PCR techniques are expensive and labor intensive, require sophisticated equipment and personnel, and do not appear to identify a group of women at risk for adverse maternal or infant outcome, we do not recommend their use in clinical practice for routine diagnosis of malaria during pregnancy in India at this time. Nonetheless, PCR has several distinct advantages over microscopy and may have utility in certain circumstances. These include malaria elimination initiatives or in campaigns aimed at identifying reservoirs of continued transmission. In research settings, PCR may be better suited to monitor the full impact of any intervention measure such as any new drugs or a program of intermittent screening and treatment.36 However, until a PCR technique becomes cost-effective and available as a point-of-care test for field work, it is unlikely to be used routinely in malarial programs in field settings.
DISCLAIMER STATEMENTS
Contributors NS, VU and DHH conceived the study; NS, VU, MD, KYA, BJW and DHH designed the study protocol; RS and PKB carried out the sample collection and molecular experiments; NS, BJW, MPS, MM and DHH carried out the data analysis and interpretation of results; NS, VU, BJW, PKB, MPS and DHH wrote the manuscript. All authors read and approved the final manuscript.
Funding This work was supported by the Indo-U.S. Collaborative Network with funding from the ICMR and the National Institute of Child Health and Development (1 R03 HD52167-01). The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the ICMR or the National Institute of Health. BJW was supported by the National Institutes of Health (NIH K23 ES021471).
Conflicts of interest No conflict of interest.
Ethics approval The Institutional Review Boards of Boston University and the U.S. Centers for Disease Control and Prevention, the Ethics Committee of the National Institute of Malaria Research (NIMR) in India, the Scientific Advisory Committee of the NIMR, and the Health Ministry Screening Committee of the Indian Council of Medical Research (ICMR) reviewed and approved the study protocol and informed consent forms. This paper was approved by the NIMR Publications Committee (020/2014).
Acknowledgments
We thank the Chief Medical Officer and medical specialists of district Baster and Rajnandgaon for providing space for laboratories in the hospitals. Grateful thanks are due to the Chhattisgarh Government for administrative clearance of the study.
The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Uneke CJ. Diagnosis of Plasmodium falciparum malaria in pregnancy in sub-Saharan Africa: the challenges and public health implications. Parasitol Res. 2008;102:333–42. doi: 10.1007/s00436-007-0782-6. [DOI] [PubMed] [Google Scholar]
- 2.Desai M, ter Kuile FO, Nosten F, McGready R, Asamoa K, Brabin B, et al. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. 2007;7:93–104. doi: 10.1016/S1473-3099(07)70021-X. [DOI] [PubMed] [Google Scholar]
- 3.Singh N, Shukla MM, Sharma VP. Epidemiology of malaria in pregnancy in central India. Bull World Health Organ. 1999;77:567–72. [PMC free article] [PubMed] [Google Scholar]
- 4.Okell LC, Bousema T, Griffin JT, Ouedraogo AL, Ghani AC, Drakeley CJ. Factors determining the occurrence of submicroscopic malaria infections and their relevance for control. Nat Comm. 2012;3:237. doi: 10.1038/ncomms2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okell LC, Ghana AC, Lyons E, Drakely CJ. Submicroscopic infection in Plasmodium-falciparum-endemic populations: a systematic review and meta-analysis. J Infect Dis. 2009;200:1509–17. doi: 10.1086/644781. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Malaria diagnosis: memorandum from a WHO meeting. Bull World Health Organ. 1988;66:575–94. [PMC free article] [PubMed] [Google Scholar]
- 7.Dowling MA, Shute FT. A comparative study of thick and think blood films in the diagnosis of scanty malaria parasitaemia. Bull World Health Organ. 1966;34:249–67. [PMC free article] [PubMed] [Google Scholar]
- 8.Kamau E, Alemayehu S, Feghali KC, Saunders D, Ockenhouse CF. Multiplex qPCR for detection and absolute quantification of malaria. PLoS One. 2013;8:e71539. doi: 10.1371/journal.pone.0071539. [eCollection] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giha HA, A-Elbasit IE, A-Elgadir TM, Adam I, Berzins K, Elghazali G, et al. Cerebral malaria is frequently associated with latent parasitaemia among the semi-immune population of eastern Sudan. Microbes Infect. 2005;7:1196–203. doi: 10.1016/j.micinf.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Mosha JF, Sturrock HJ, Greenhouse B, Greenwood B, Sutherland CJ, Gadalla N, et al. Epidemiology of subpatent Plasmodium falciparum infection: implications for detection of hotspots with imperfect diagnostics. Malar J. 2013;12:221. doi: 10.1186/1475-2875-12-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mens P, Spieker N, Omar S, Heijnen M, Schallig H, Kager PA. Is molecular biology the best alternative for diagnosis of malaria to microscopy? A comparison between microscopy, antigen detection and molecular tests in rural Kenya and urban Tanzania. Trop Med Int Health. 2007;12:238–44. doi: 10.1111/j.1365-3156.2006.01779.x. [DOI] [PubMed] [Google Scholar]
- 12.Mockenhaupt FP, Rong B, Till H, Eggelte TA, Beck S, Gyasi-Sarpong C, et al. Submicroscopic Plasmodium falciparum infections in pregnancy in Ghana. Trop Med Int Health. 2000;5:167–73. doi: 10.1046/j.1365-3156.2000.00532.x. [DOI] [PubMed] [Google Scholar]
- 13.Saute F, Menendez C, Mayor A, Aponte J, Gomez-Olive X, Dgedge M, et al. Malaria in pregnancy in rural Mozambique: the role of parity, submicroscopic and multiple Plasmodium falciparum infections. Trop Med Int Health. 2002;7:19–28. doi: 10.1046/j.1365-3156.2002.00831.x. [DOI] [PubMed] [Google Scholar]
- 14.Mayengue PI, Rieth H, Khattab A, Issifou S, Kremsner PG, Klinkert MQ, et al. Submicroscopic Plasmodium falciparum infections and multiplicity of infection in matched peripheral, placental and umbilical cord blood samples from Gabonese women. Trop Med Int Health. 2004;9:949–58. doi: 10.1111/j.1365-3156.2004.01294.x. [DOI] [PubMed] [Google Scholar]
- 15.Walker-Abbey A, Djokam RR, Eno A, Leke RF, Titanji VP, Fogako J, et al. Malaria in pregnant Cameroonian women: the effect of age and gravidity on submicroscopic and mixed-species infections and multiple-parasite genotypes. Am J Trop Med Hyg. 2005;72:229–35. [PubMed] [Google Scholar]
- 16.Adegnika AA, Verweij JJ, Agnandji ST, Chai SK, Breitling LP, Ramharter M, et al. Microscopic and submicroscopic Plasmodium falciparum infection but not inflammation caused by infection is associated with low birth weight. Am J Trop Med Hyg. 2006;75:798–803. [PubMed] [Google Scholar]
- 17.Mayor A, Serra-Casas E, Bardají A, Sanz S, Puyol L, Cisteró P, et al. Submicroscopic infections and long-term recrudescence of Plasmodium falciparum in Mozambican pregnant women. Malar J. 2009;8:9. doi: 10.1186/1475-2875-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mockenhaupt FP, Ulmen U, von Gaertner C, Bedu-Addo G, Bienzle U. Diagnosis of placental malaria. J Clinical Microbiol. 2002;40:306–8. doi: 10.1128/JCM.40.1.306-308.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Omer S, Khalil E, Ali H, Sharief A. Submicroscopic and multiple Plasmodium falciparum infections in pregnant Sudanese women. N Am J Med Sci. 2011;2:137–41. doi: 10.4297/najms.2011.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayor A, Moro L, Aguilar R, Bardají A, Cisteró P, Serra-Casas E, et al. How hidden can malaria be in pregnant women? Diagnosis by microscopy, placental histology, polymerase chain reaction and detection of histidine-rich protein 2 in plasma. Clin Infect Dis. 2012;54:1561–8. doi: 10.1093/cid/cis236. [DOI] [PubMed] [Google Scholar]
- 21.Cohee LM, Kalilani-Phiri L, Boudova S, Joshi S, Mukadam R, Seydel KB, et al. Submicroscopic malaria infection during pregnancy and the impact of intermitted preventive treatment. Malar J. 2014;13:274. doi: 10.1186/1475-2875-13-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rantala AM, Taylor SM, Trottman PA, Luntamo M, Mbewe B, Maleta K, et al. Comparison of real-time PCR and microscopy for malaria parasite detection in Malawian pregnant women. Malar J. 2010;9:269. doi: 10.1186/1475-2875-9-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mockenhaupt FP, Bedu-Addo G, von Gaertner C, Boyé R, Fricke K, Hannibal I, et al. Detection and clinical manifestation of placental malaria in southern Ghana. Malar J. 2006;5:119. doi: 10.1186/1475-2875-5-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohammed AH, Salih MM, Elhassan EM, Mohmmed AA, Elzaki SE, El-Sayed BB, et al. Submicroscopic Plasmodium falciparum malaria and birth weight in an area of unstable malaria transmission in Central Sudan. Malar J. 2013;12:172. doi: 10.1186/1475-2875-12-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arango EM, Samuel R, Agudelo OM, Carmona-Fonseca J, Maestre A, Yanow SK. Molecular detection of malaria at delivery reveals a high frequency of submicroscopic infections and associated placental damage in pregnant women from Northwest Colombia. Am J Trop Med Hyg. 2013;89:176–83. doi: 10.4269/ajtmh.12-0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rijken MJ, McGready R, Boel ME, Poespoprodjo R, Singh N, Syafruddin D, et al. Malaria in pregnancy in the Asia-Pacific region. Lancet Infect Dis. 2012;12:75–88. doi: 10.1016/S1473-3099(11)70315-2. [DOI] [PubMed] [Google Scholar]
- 27.Singh N, Singh MP, Wylie BJ, Hussain M, Kojo YA, Shekhar C, et al. Malaria prevalence among pregnant women in two districts with differing endemicity in Chhattisgarh, India. Malar J. 2012;11:274. doi: 10.1186/1475-2875-11-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.NVBDCP. NVBDCP malaria situation in India; 2012. Available from: http://www.nvbdcp.gov.in/Doc/mal-situation-June13.pdf (accessed 2013 August 16) [Google Scholar]
- 29.Anon, 2008–10 State vector borne disease control programme: malaria control programme annual report: 2000–2010, state vector borne disease control programme in Raipur Chhattisgarh. Chhattisgarh, India: Directorate of Health services; 2010. [Google Scholar]
- 30.World Health Organization. Basic malaria microscopy part 1: learners guide. 2nd edn. Geneva; 2010. Available from: http://whqlibdoc.who.int/publications/2010/9789241547826_eng.pdf (accessed 2013 August 16) [Google Scholar]
- 31.World Health Organization. Parasitological confirmation of malaria diagnosis; 2009. Available from: http://whqlibdoc.who.int/publications/2010/9789241599412_eng.pdf (accessed 2013 August 16) [Google Scholar]
- 32.Snounou G, Viriyakosol S, Zhu XP, Jarra W, Pinheiro L, do Rosario VE, et al. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. 1993;61:315–20. doi: 10.1016/0166-6851(93)90077-b. [DOI] [PubMed] [Google Scholar]
- 33.Ballard JL, Khoury JC, Wedig K, Wang L, Eilers-Walsman BL, Lipp R. New Ballard Score, expanded to include extremely premature infants. J Pediatr. 1991;119:417–23. doi: 10.1016/s0022-3476(05)82056-6. [DOI] [PubMed] [Google Scholar]
- 34.Singer LM, Newman RD, Diarra A, Moran AC, Huber CS, Stennies G, et al. Evaluation of a malaria rapid diagnostic test for assessing the burden of malaria during pregnancy. Am J Trop Med Hyg. 2004;70:481–5. [PubMed] [Google Scholar]
- 35.Kain KC, Kyle DE, Wongsrichanalai C, Brown AE, Webster HK, Vanijanonta S, et al. Qualitative and semiquantitative polymerase chain reaction to predict Plasmodium falciparum treatment failure. J Infect Dis. 1994;170:1626–30. doi: 10.1093/infdis/170.6.1626. [DOI] [PubMed] [Google Scholar]
- 36.Hamer DH, Singh MP, Wylie BJ, Yeboah-Antwi K, Tuchman J, Desai M, et al. Burden of malaria in pregnancy in Jharkhand State, India. Malar J. 2009;8:210. doi: 10.1186/1475-2875-8-210. [DOI] [PMC free article] [PubMed] [Google Scholar]