Abstract
Evolutionary conservation of the hypothalamus attests to its critical role in the control of fundamental behaviors. However, our knowledge of hypothalamic connections is incomplete, particularly for the lateral hypothalamic area (LHA). Here we present the results of neuronal pathway-tracing experiments to investigate connections of the LHA juxtaventromedial region, which is parceled into dorsal (LHAjvd) and ventral (LHAjvv) zones. Phaseolus vulgaris leucoagglutinin (PHAL, for outputs) and cholera toxin B subunit (CTB, for inputs) coinjections were targeted stereotaxically to the LHAjvd/v.
Results: LHAjvd/v connections overlapped highly but not uniformly. Major joint outputs included: Bed nuc. stria terminalis (BST), interfascicular nuc. (BSTif) and BST anteromedial area, rostral lateral septal (LSr)- and ventromedial hypothalamic (VMH) nuc., and periaqueductal gray. Prominent joint LHAjvd/v input sources included: BSTif, BST principal nuc., LSr, VMH, anterior hypothalamic-, ventral premammillary-, and medial amygdalar nuc., and hippocampal formation (HPF) field CA1. However, LHAjvd HPF retrograde labeling was markedly more abundant than from the LHAjvv; in the LSr this was reversed. Furthermore, robust LHAjvv (but not LHAjvd) targets included posterior- and basomedial amygdalar nuc., whereas the midbrain reticular nuc. received a dense input from the LHAjvd alone. Our analyses indicate the existence of about 500 LHAjvd and LHAjvv connections with about 200 distinct regions of the cerebral cortex, cerebral nuclei, and cerebrospinal trunk. Several highly LHAjvd/v-connected regions have a prominent role in reproductive behavior. These findings contrast with those from our previous pathway-tracing studies of other LHA medial and perifornical tier regions, with different connectional behavioral relations. The emerging picture is of a highly differentiated LHA with extensive and far-reaching connections that point to a role as a central coordinator of behavioral control.
Keywords: lateral hypothalamic area, fundamental behavior, cognitive systems, motor systems, hypothalamus
Introduction
About a century of research on the hypothalamic neuronal network has established its critical role in the control of fundamental behaviors and their supporting homeostatic processes (for historical reviews see Le Gros Clark, 1938; Fulton et al., 1940; Harris, 1955; Nauta and Haymaker, 1969). The early research established a broad functional role for the hypothalamus, and indicated further that different hypothalamic regions could control different functions (or different aspects of the same function). However, despite the profound behavioral and physiological effects of experimental hypothalamic electrolytic lesion and electrical stimulation, nothing definitive was said about the organization of the underlying neuronal connections—although much was hinted at in pioneering neuroanatomical studies using a variety of now classic histological staining techniques (Gurdjian, 1927; Krieg, 1932; Ramon y Cajal, 1995). The major limitation on the acquisition of connection data was an absence of techniques for determining neuronal connections (such as anterograde and retrograde pathway-tracing) and neuronal chemoarchitecture (such as immunohistochemistry—IHC). Prior to the emergence and (beginning in the 1970s) application of these methods to neuroanatomical studies, the available and prevailing techniques were axon degeneration methods and a variety of histological staining techniques (for further perspective and reviews see Haymaker et al., 1969; Morgane and Panksepp, 1979; Swanson, 1987, 1999; Zaborszky et al., 2006).
Initial non-lesion anterograde neuronal pathway-tracing studies employed the autoradiographic method (tritiated amino acids) which, despite problems with interpretability (Swanson, 1981), was applied extensively to investigate central connections, including those of the LHA (Saper et al., 1979; Veening et al., 1982). In parallel with advances in pathway-tracing methods, advances in neuropharmacology led to the availability of receptor-targeted drugs which could replace electrical stimulation methods to identify central sites of functional significance—for example, the determination of central sites from which drinking could be elicited by central injections of either angiotensin II, or the cholinergic agonist carbachol (Swanson and Sharpe, 1973; Swanson et al., 1973; Sharpe and Swanson, 1974).
As neuropharmacological methods enabled more selective targeting than electrical stimulation, so the introduction of non-isotopic retrograde and anterograde neuronal pathway-tracers provided more effective tools than lesion/degeneration methods with which to determine the organization of central neuronal connections. Prominent among the latter is the lectin Phaseolus vulgaris leucoagglutinin (PHAL), which was introduced as an anterograde neuronal tracer in the 1980s (Gerfen and Sawchenko, 1984), and enabled for the first time (with detection by IHC) the qualitative determination of the microscopic morphology and topographic organization of connections between stereotaxically targeted gray matter regions. Similarly, now routinely used retrograde neuronal tracers introduced in the same period include the B subunit of cholera toxin (CTB) (Stoeckel et al., 1977; Dumas et al., 1979; Trojanowski et al., 1981; Luppi et al., 1987, 1990), and hydroxystilbamidine (trade name Fluoro-Gold; FG) (Schmued and Fallon, 1986).
In the past decade, a second revolution in methods for system-level connection analysis has occurred, comparable in impact to that which occurred in the 1970s (Swanson, 2007). The application of molecular genetics methods (such as opto- and pharmacogenetic, and viral pathway-tracing approaches) to investigating hypothalamic structure-function relations is beginning to provide long-sought clarity about the organization of this critical neuronal network. Recent investigations that exemplify the application of these methods include the elucidation of hypothalamic networks for feeding behavior (Betley et al., 2013), and aggression (Lin et al., 2011)—for reviews of the approaches see (Zaborszky et al., 2006; Fenno et al., 2011; Farrell and Roth, 2013; Sternson, 2013). Nevertheless, now classic pathway-tracing techniques, using tracers such as PHAL and CTB, remain useful tools in the continuing quest to determine the basic plan of the hypothalamic neuronal network, and by extension the nervous system (Swanson, 2000, 2007; Zingg et al., 2014). Furthermore, analysis and interpretation of the massive amount of connectional data generated by the application of these techniques is increasingly being aided by computational neuroinformatics approaches (Swanson and Bota, 2010; Brown and Swanson, 2013; Bota et al., 2014).
In two previous studies on the connections of the LHA, we employed a CTB + PHAL co-injection strategy to investigate the macroconnections of LHA regions medially and dorsally adjacent to the column of the fornix: The LHA juxtadorsomedial and juxtaparaventricular regions (LHAjd, and LHAjp), and the LHA suprafornical region (LHAs). Impetus for these studies was generated by a novel provisional cytoarchitectural parcellation schema for the LHA (Swanson, 2004), and they served as starting point for more focused structure-functional analysis of individual components. An earlier PHAL study focused on an LHA region ventral to the fornix-LHA subfornical (LHAsf) (Goto et al., 2005).
A notable finding was the sheer number of gray matter regions connecting to the delineated LHA regions (up to several hundred, far exceeding the first-order connectivity of any other brain region studied similarly to date). In addition, distinct topographic differences were found. Relating the prominent connections of the LHAsf anterior part (LHAsfa), LHAjp, LHAjd, and LHAs to the existing literature, possible behavioral functions were suggested to which these connections may contribute. In very broad terms these hypothesized functions were primarily ingestive behavior (for the LHAs), defensive behavior for LHAjp and LHAjd (Hahn and Swanson, 2010, 2012), and defensive or foraging behavior for the LHAsf (Goto et al., 2005). Two recent functional studies have provided data consistent with these hypotheses: LHAjd neurons are indicated to play a role in the expression of conditioned defensive responses (Faturi et al., 2014), and LHAs neurons in the activation of feeding in response to activation of agouti-related peptide (AGRP)-expressing axons in the LHAs (Betley et al., 2013).
In the present study we focus on the LHA region ventromedial to the fornix (and adjacent to the hypothalamic ventromedial nucleus—VMH), that is divided into two zones: The LHA juxtaventromedial region, dorsal- (LHAjvd) and ventral (LHAjvd) zones. The parcellation of these dorsoventrally contiguous zones, based on the Nissl cytoarchitecture, was described previously (Swanson, 2004). Briefly, their rostral to caudal extent is approximately the same as the VMH (which also provides a medial boundary), and they extend laterally to the LHAsf. The LHAjvd is bounded dorsally by the LHAjd and LHAjp; the LHAjvv is bounded ventrally by the tuberal nucleus. In addition to their cytoarchitectural parcellation, the tract of the post-commissural fornix provides a readily identifiable fiducial marker that corresponds approximately to the dorsolateral boundary of the LHAjvd.
Methods
The methods follow those described previously (Hahn and Swanson, 2010, 2012), and are provided here abridged. Experiments were performed according to the NIH Guidelines for the Care and Use of Laboratory Animals, and all protocols were approved by the University of Southern California Institutional Animal Care and Use Committee. Adult male Sprague–Dawley rats (290–360 g; Harlan) under anesthesia (1 ml/kg body weight of 50 mg/ml ketamine and 10 mg/ml xylazine, intramuscular) received single, iontophoretic injections of a mixture of 2.5% P. vulgaris leucoagglutinin (PHAL; Vector Labs) and 0.25% cholera toxin B subunit (CTB; List Labs) targeted stereotaxically to the LHAjv region (Swanson, 2004). From 12 to 20 days later, the rats were deeply anesthetized with sodium pentobarbital (40 mg/kg body weight, intraperitoneal) and perfused with ice-cold 0.9% saline followed by 4% paraformaldehyde (pH 9.5). Brains were removed and post-fixed overnight at 4°C in the same fixative containing 12% sucrose, and then frozen rapidly in dry-ice cooled hexane. Serial 25 or 30 μm thick transverse-plane frozen sections (4 or 5 series) were obtained with the use of a sliding microtome. One series was processed for immunohistochemical (IHC) detection of PHAL, another for detection of CTB, and an intervening series was stained with cresyl violet (Nissl stain) to reveal cytoarchitecture (Simmons and Swanson, 1993). For IHC detection of tracers, the sections were incubated in primary antibodies directed against either CTB (1:10,000; goat, List BioLabs) or PHAL (1:3000; rabbit, Dako) (3 nights, refrigerated). This was followed by a biotinylated secondary antibody (1:1000; donkey anti-rabbit or goat, Vector Labs) (90 min) and then an avidin-biotin-horseradish peroxidase reagent (1:1000; ABC reagent, Vector Labs) (2 h). The sections were then recycled to the secondary antibody (overnight, refrigerated), and the following day placed in freshly prepared ABC reagent (90 min). Visualization of labeling was accomplished with the use of 3,3′-Diaminobenzidine (DAB, 0.05%) in the presence of hydrogen peroxide (0.005%), with the addition of ammonium nickel (II) sulfate (0.1%) to enhance visualization of CTB. Injection sites (PHAL–containing perikarya, and CTB deposits) were identified along with anterogradely (PHAL) labeled axons, and retrogradely (CTB) labeled perikarya; these were analyzed and plotted (Illustrator CS5/6, Adobe) onto a digital reference series of drawings of the rat brain as described previously (Swanson, 2004; Hahn and Swanson, 2010). Adjacent series of Nissl–stained sections were used for reference. Digital photomicrographs [Microscope: Zeiss AxioImager (Carl Zeiss); Cameras: Orca ER (Hamamatsu), or Retiga 2000R (Q Imaging)] were acquired singly or using stitching software (Volocity, Perkin Elmer) and then composed (Photoshop CS5/6, Adobe).
Results
From a series of PHAL + CTB coinjection experiments targeted to the LHA (n = 93), the spatial extent of nine injection sites resulting from coinjections that included the LHAjvv and/or LHAjvd (LHAjvd/v) is shown in Figure 1. The most comprehensive analysis was performed on two representative datasets, resulting from experiments with injection sites that were the most restricted to (and inclusive of) the LHAjvv (experiment LHA #2) or the LHAjvd (experiment LHA #77) (Figures 1, 2).
Figure 1.
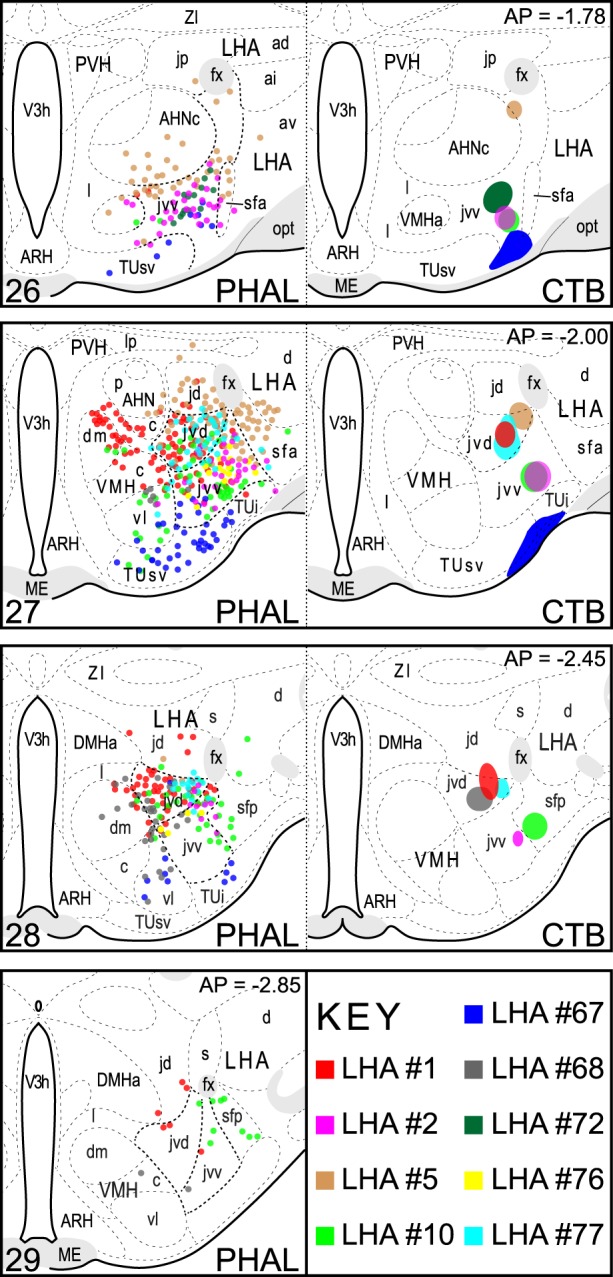
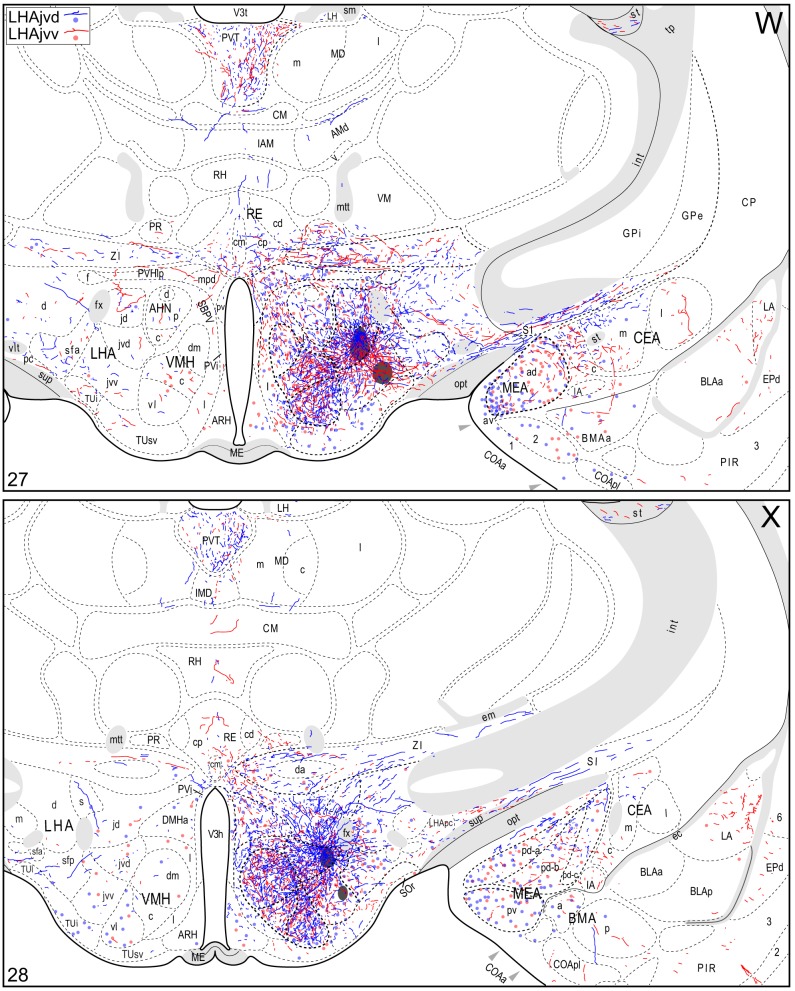
LHAjv region injection site maps. The extent of injection sites for nine CTB + PHAL coinjections are represented. These included the LHAjvd or LHAjvd, and neighboring regions. Data were plotted with the aid of a drawing tube and with reference to adjacent Nissl-stained sections. Numbers in the upper right/lower left corresponding (respectively) to distance caudal Bregma/atlas levels (Swanson, 2004). This figure is also available as a separate vector graphics file (Figure S1).
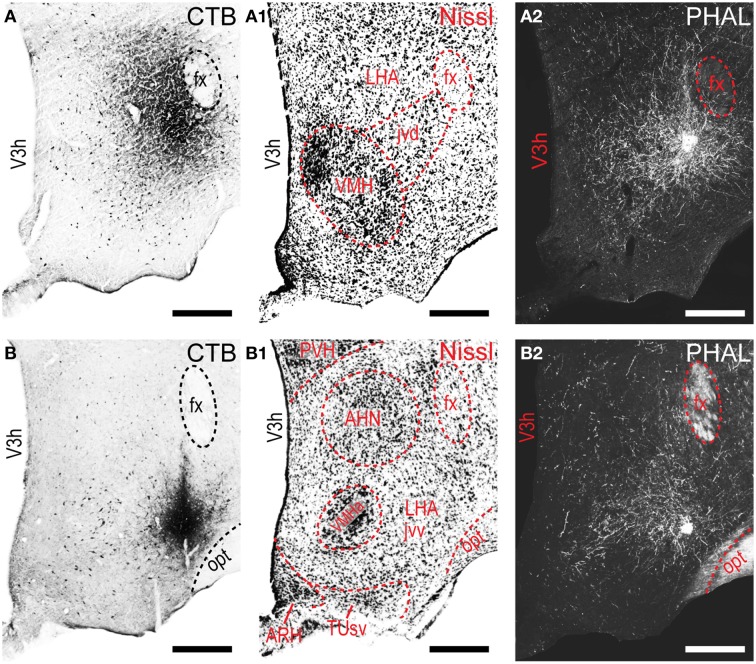
Figure 2.
LHAjvd and LHAjvv representative injection sites. Immunohistochemically detected CTB (brightfield) and PHAL (darkfield) coinjection sites centered in the LHAjvd (A,A2) and LHAjvv (B,B2) at the levels shown. Adjacent Nissl-stained sections are also shown (LHAjvd: A1; LHAjvv: B1). NB. Apparent further extent of CTB injection site shown in “A” compared to “B” is an artifact resulting from partial dispersion of nickel-enhanced DAB reaction product during the time between reaction and section mounting, attributable to weaker fixation—the darker center area of the injection site in “A” is representative of its essential extent at this level (see Figure 1). Scale bars = 250 μM.
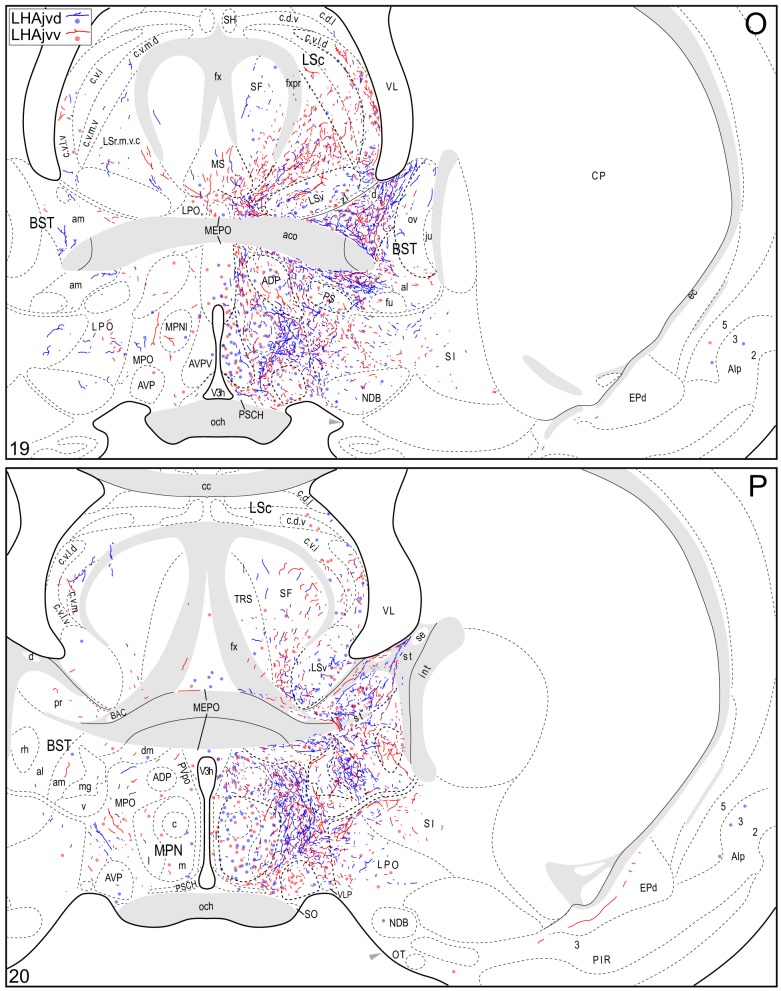
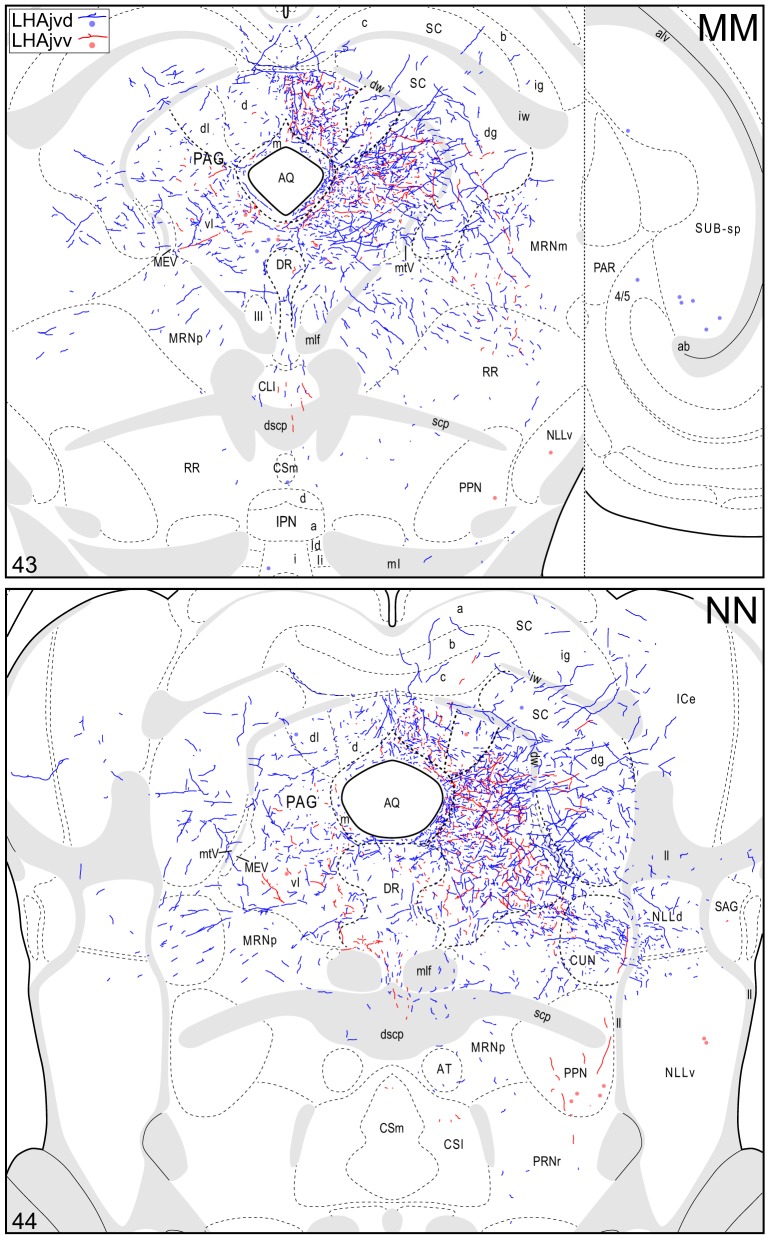
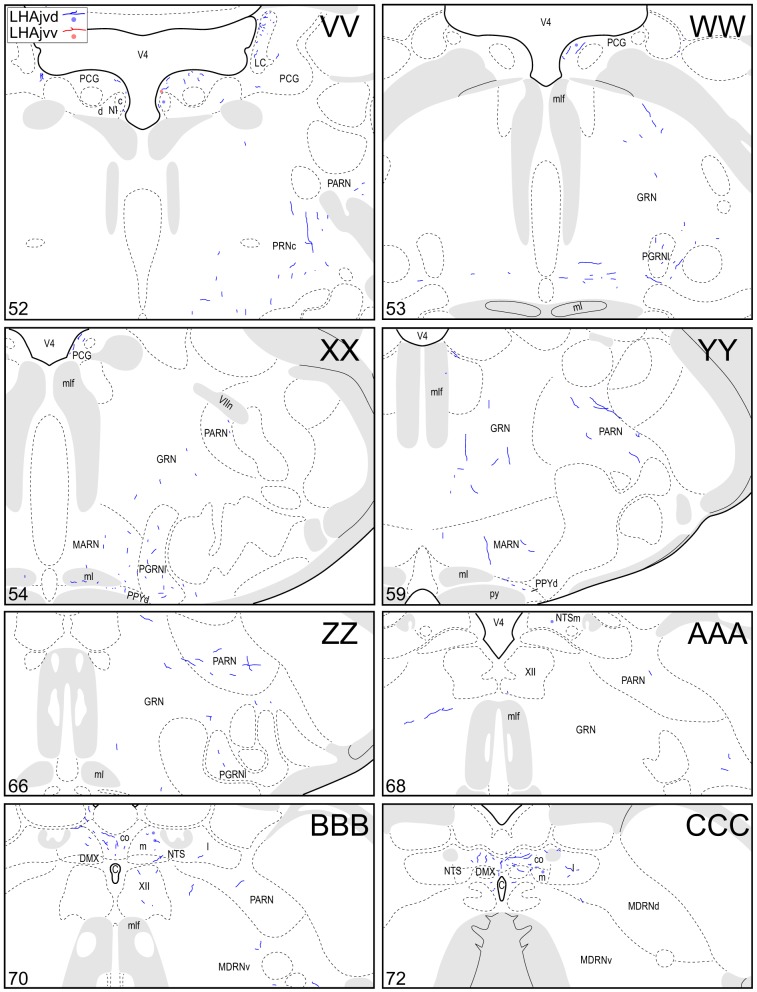
The pattern of LHAjvv and LHAjvd first-order connections was similar (Figure 3)—a similarity accentuated by comparison to the connections of other medial- and perifornical tier LHA regions with which they differed markedly (Goto et al., 2005; Hahn and Swanson, 2010, 2012). However, our analyses also revealed several marked differences, apparent as differences in both the topography (qualitative differences) and relative abundance (quantitative differences) of LHAjvd/v connections. The rostral reach of LHAjvd/v first-order connections was similar, with the outputs of both extending rostrally to about the rostral limit of the infralimbic area (ILA, Figures 4D,E), and sources of input extending slightly farther rostral, to rostral levels of the prelimbic area (PL, Figures 4A,B). In contrast, connections of the LHAjvd were found to extend farther caudally (albeit sparsely) than those of the LHAjvv, to at least as far caudal as the nucleus of the solitary tract (NTS, Figure 4CCC); the caudal-most connections of the LHAjvv were in the pontine central gray (PCG, Figures 4SS–UU).
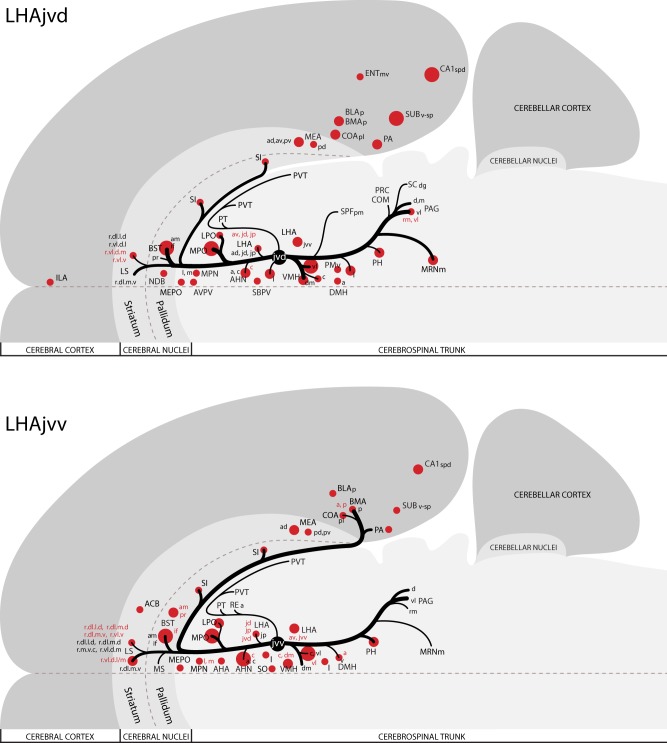
Figure 3.
Summary of major LHAjv region connections. General organization of representative principal connections (moderate or higher in magnitude) of the LHAjvd (experiment LHA #2) and LHAjvv (experiment LHA #77) plotted onto a truncated flatmap representation of the central nervous system (see Swanson, 2004). Axonal outputs are represented by black lines; sites of retrograde labeling (inputs) by red discs. The relative magnitude of each connection is indicated by line thickness/disc diameter. Red text is used to indicate sites of LHAjvd/v input for instances where different subdivisions of the same region have different input and output connections. This figure is also available as a separate vector graphics file (Figure S3).
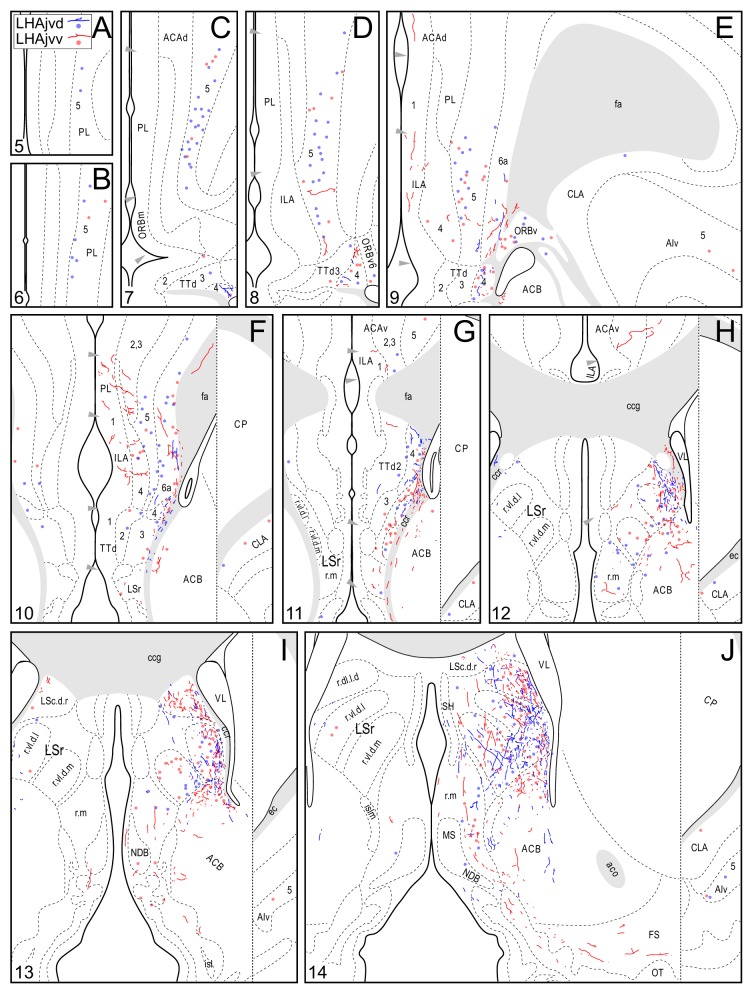
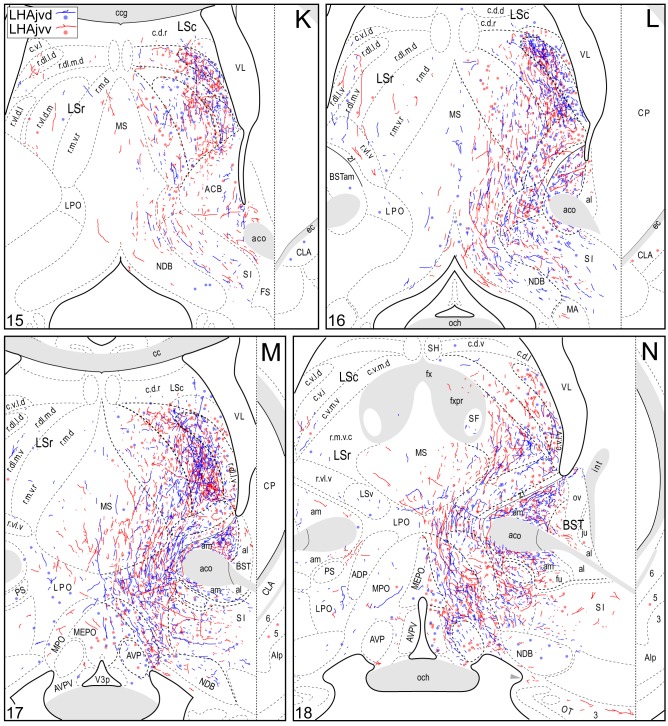
Figure 4.
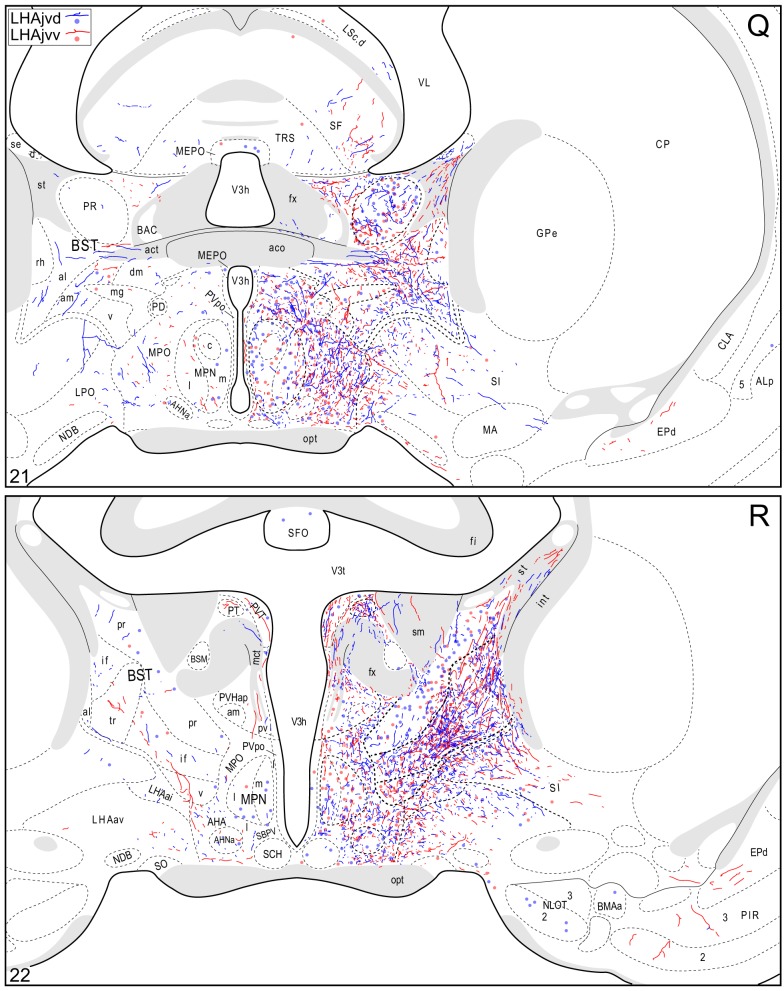
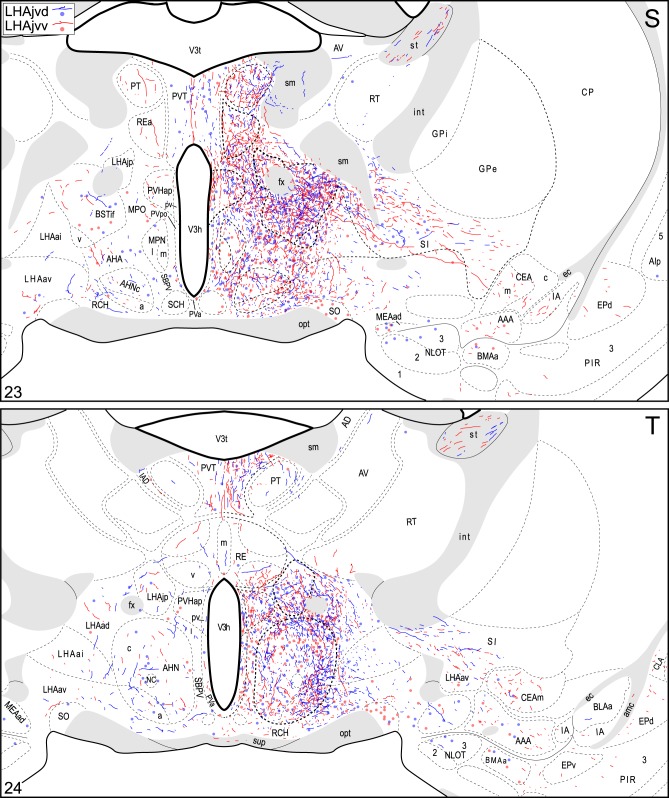
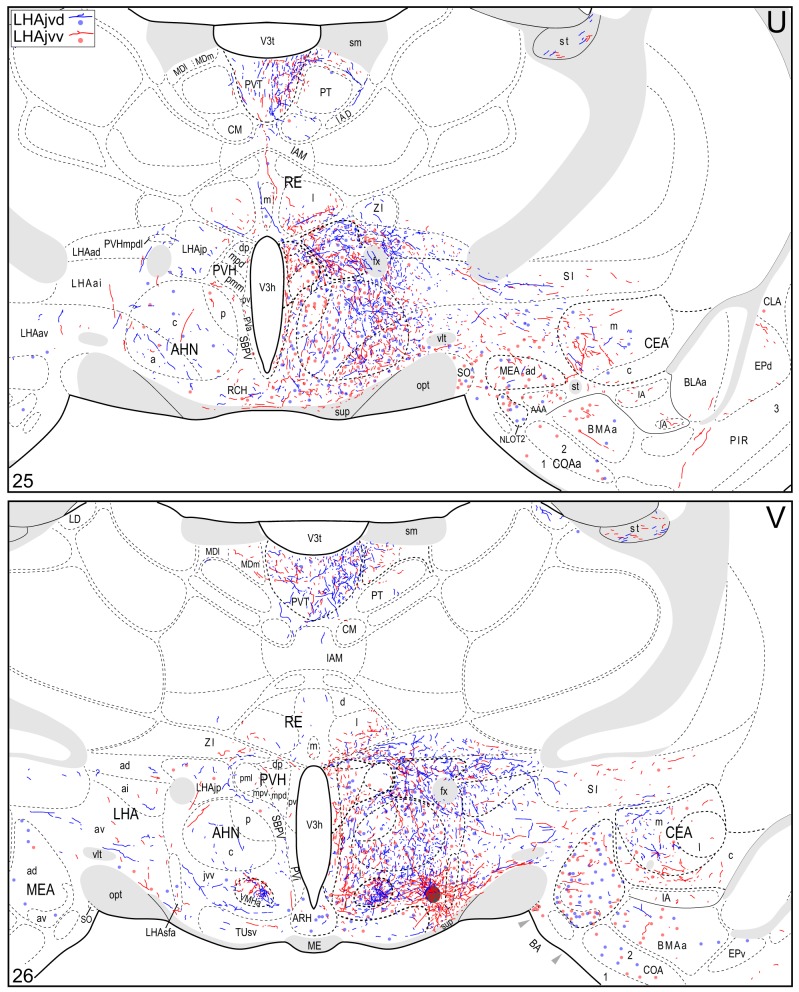
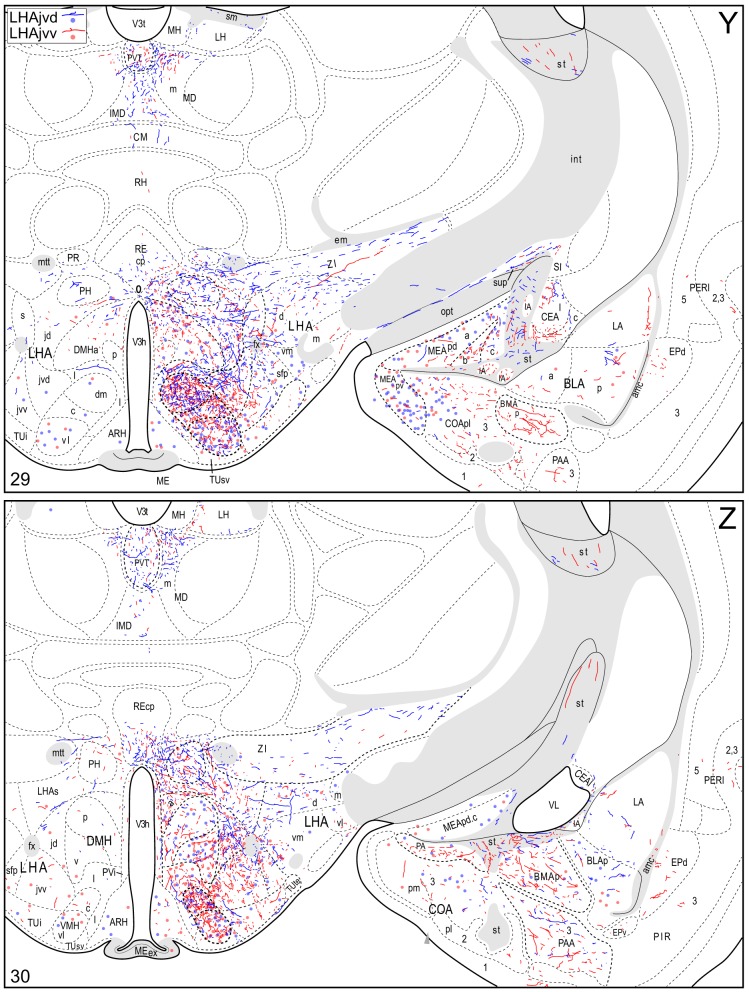
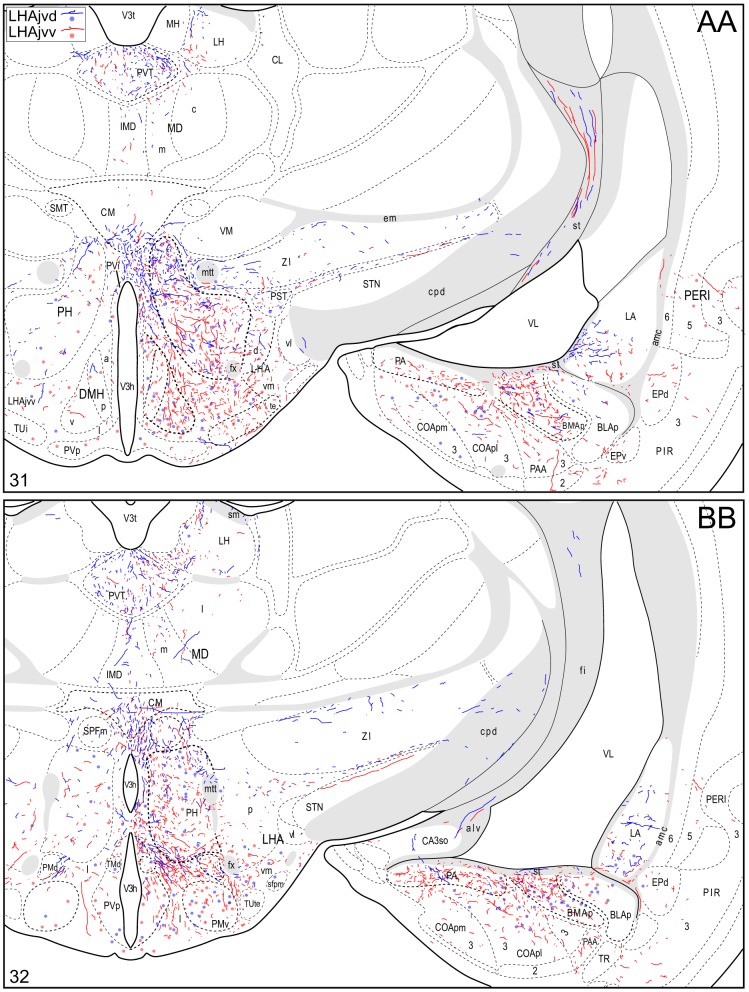
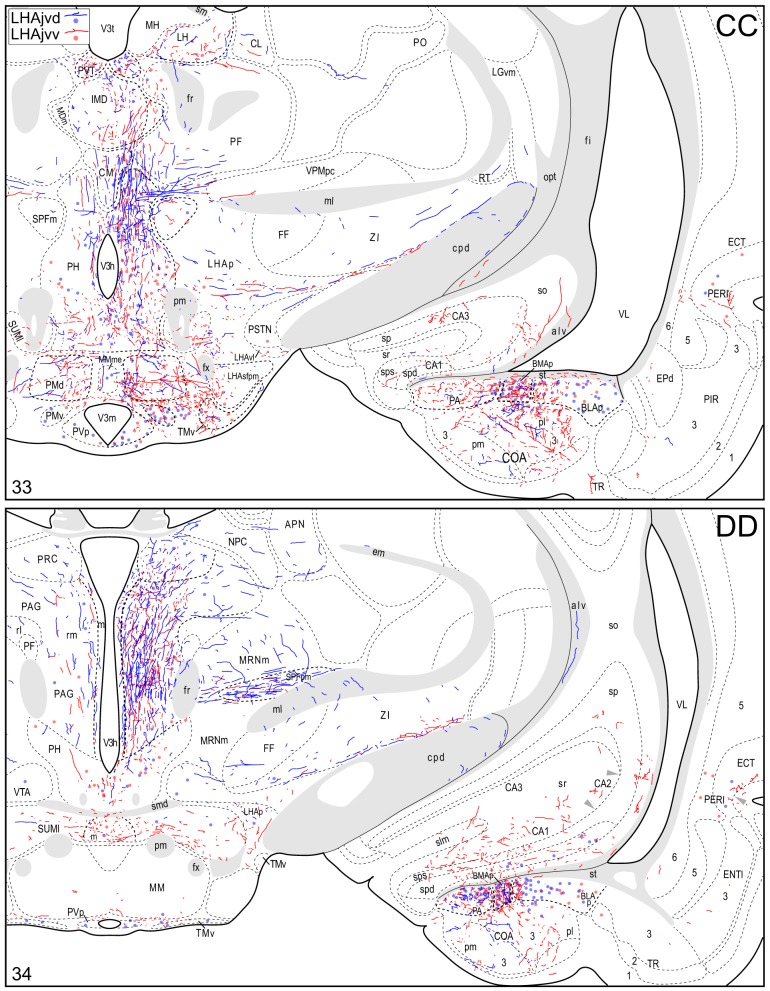
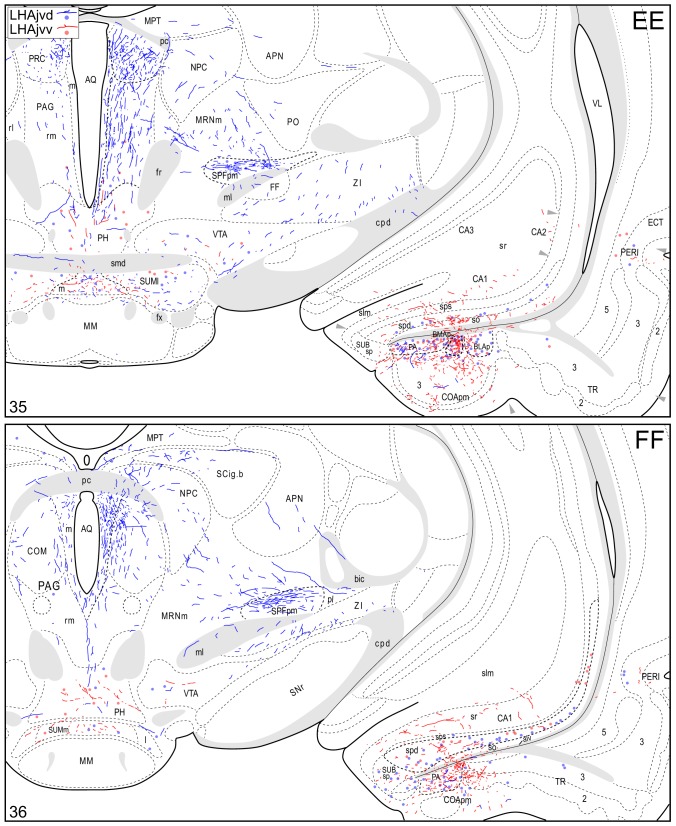
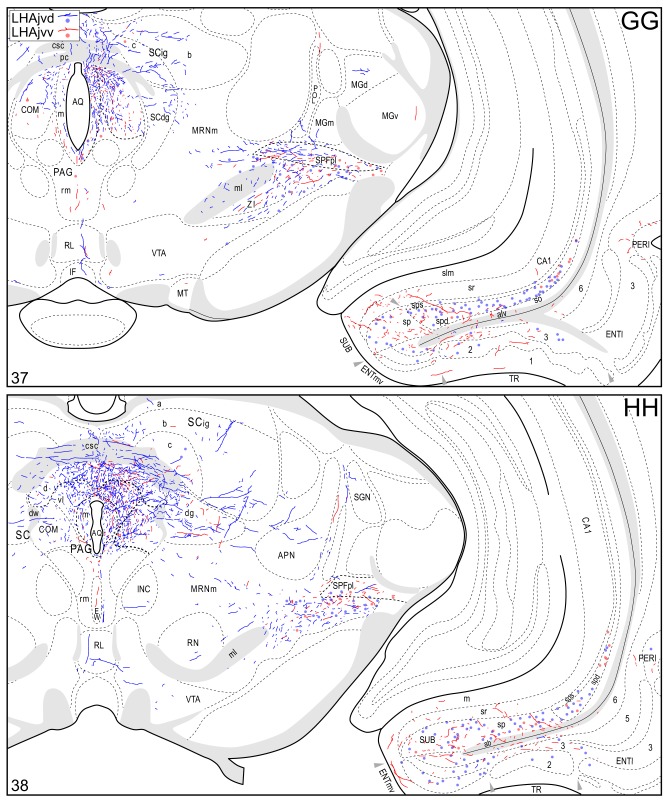
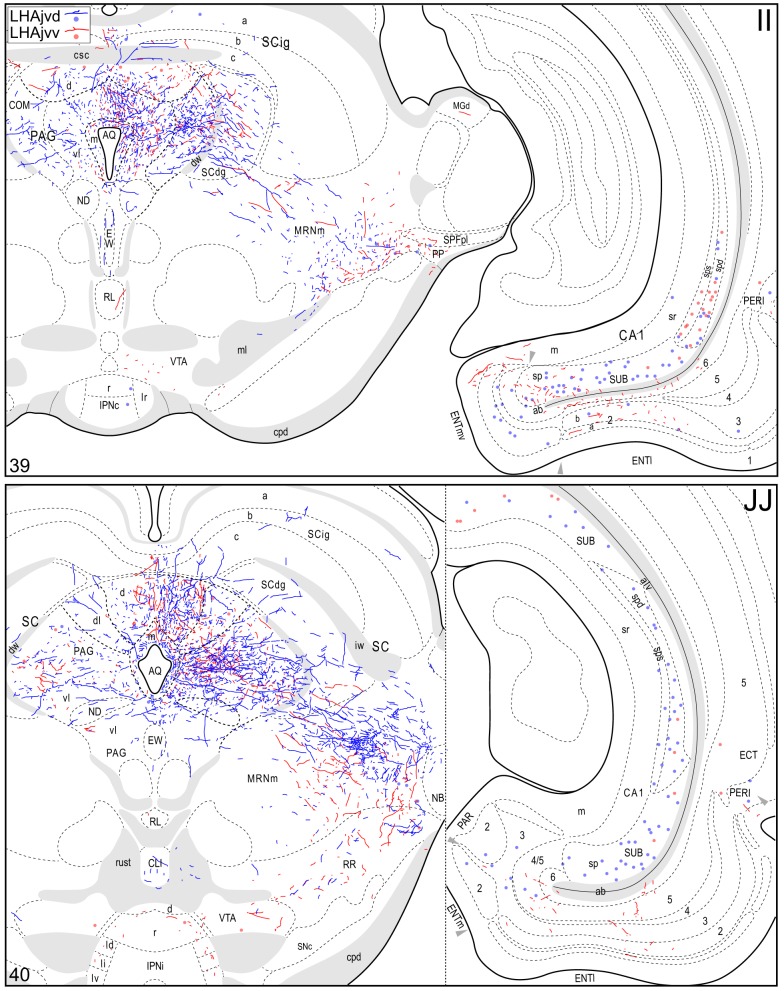
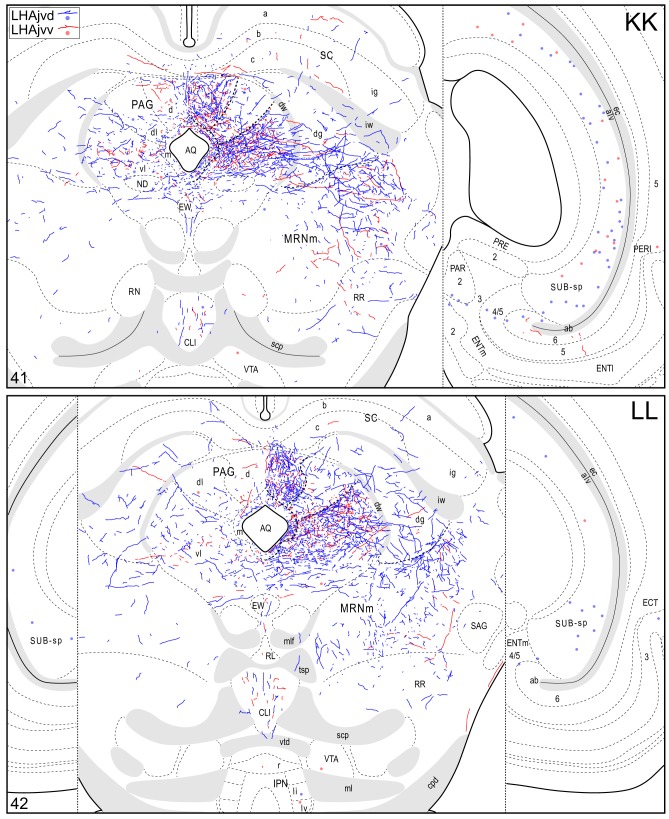
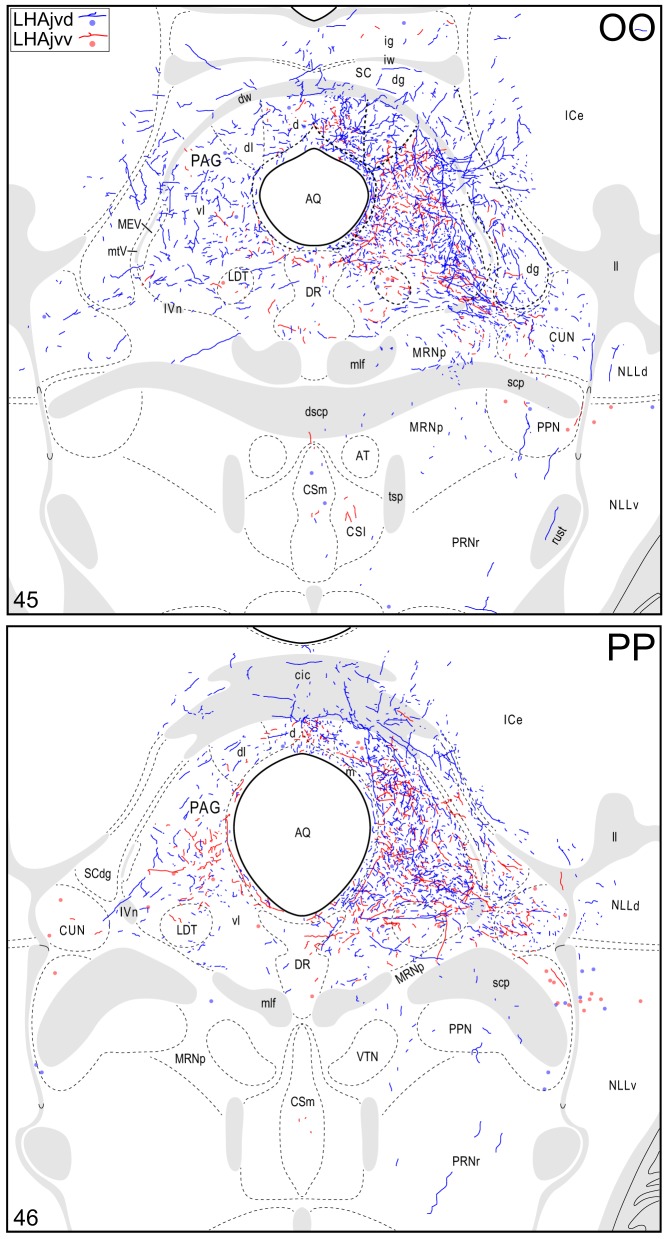
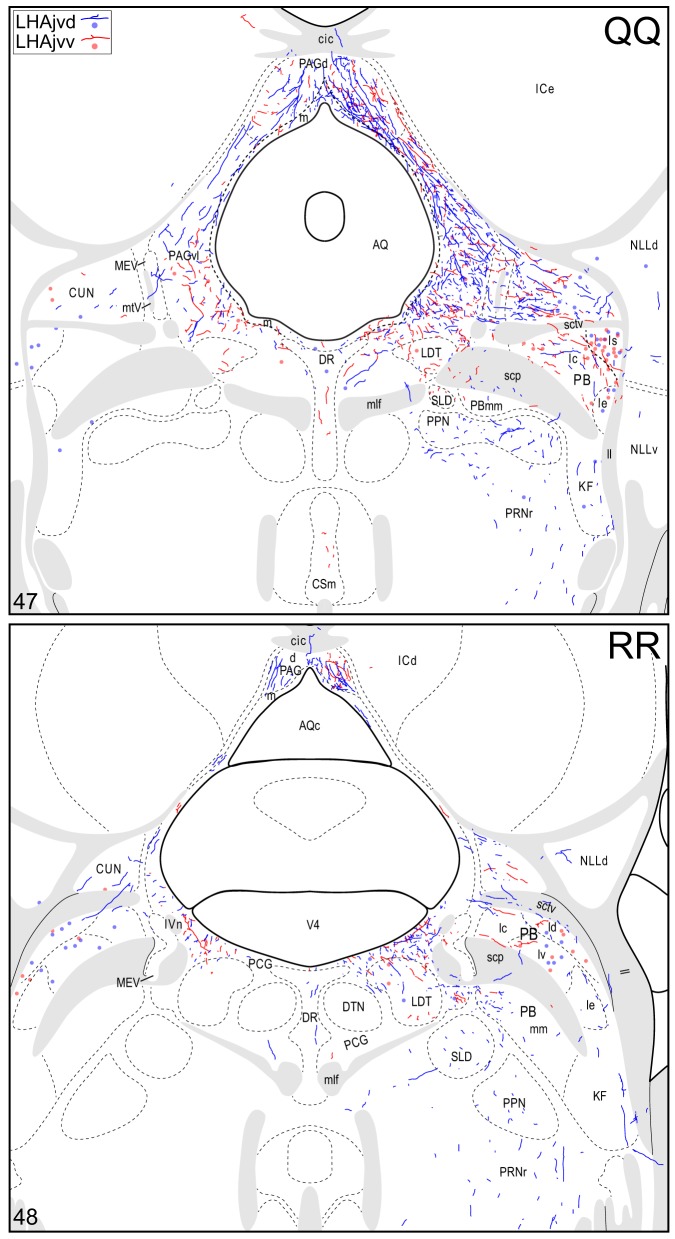
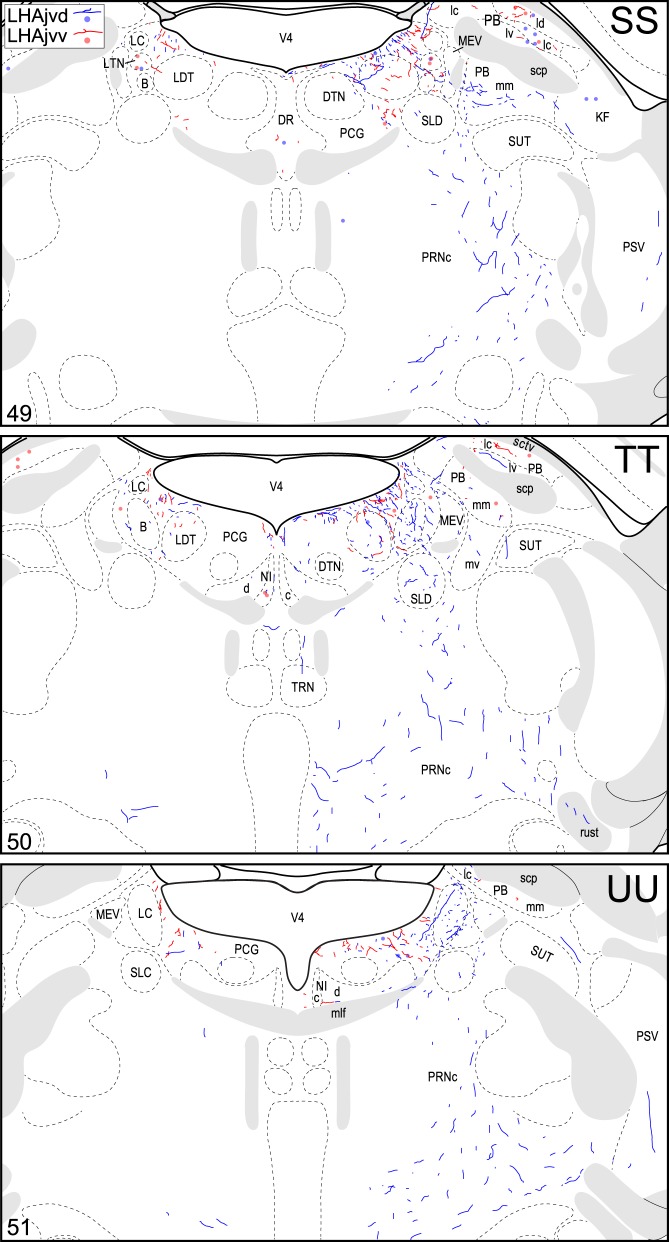
Connection maps of the LHAjvd and LHAjvv. Representative neuronal connections (inputs and outputs) of the LHAjvd (experiment LHA #2) and LHAjvv (experiment LHA #77) mapped to reference atlas levels (Swanson, 2004). PHAL labeled axons and CTB labeled cell bodies were visualized immunohistochemically and mapped with reference to adjacent Nissl-stained sections. Colored lines represent axonal output connections (PHAL); colored dots represent individual retrogradely labeled cell bodies (CTB); blue = LHAjvd, red = LHAjvv. Dots are semi-transparent to facilitate visualization of superimposed cell bodies. NB. During sectioning of brain tissue blocks from experiment LHA #77, a small portion of tissue was lost at the block faces, corresponding to the dorsal half of atlas levels 35 and 36. Numbers in lower left correspond to atlas levels (Swanson, 2004). This figure is also available as a separate vector graphics file (Figure S4).
Morphological features of labeled axons included substantial branching and distinct terminal arbors of varying density. In addition, labeled axons typically had numerous varicosities—sites of potential synaptic contact (Wouterlood and Groenewegen, 1985; Thomson et al., 1996). Approximately 95% of anterograde and retrograde labeling was ipsilateral to injection sites. Contralateral PHAL and CTB labeling generally mirrored the pattern of ipsilateral labeling in several regions, but its abundance varied, and in several regions where ipsilateral labeling was abundant it was essentially (or entirely) absent: It was present in the hypothalamus, thalamus, midbrain, and dorsal parts of the hindbrain; it was absent from the hippocampal formation, and essentially absent from the amygdalar region, and the lateral half of the rostral midbrain. The results of other injection sites illustrated in Figure 1 are considered below, in the Comparative Analysis Section.
LHAjvd and LHAjvv output connections
LHAjvd/v outputs to the cerebral cortex
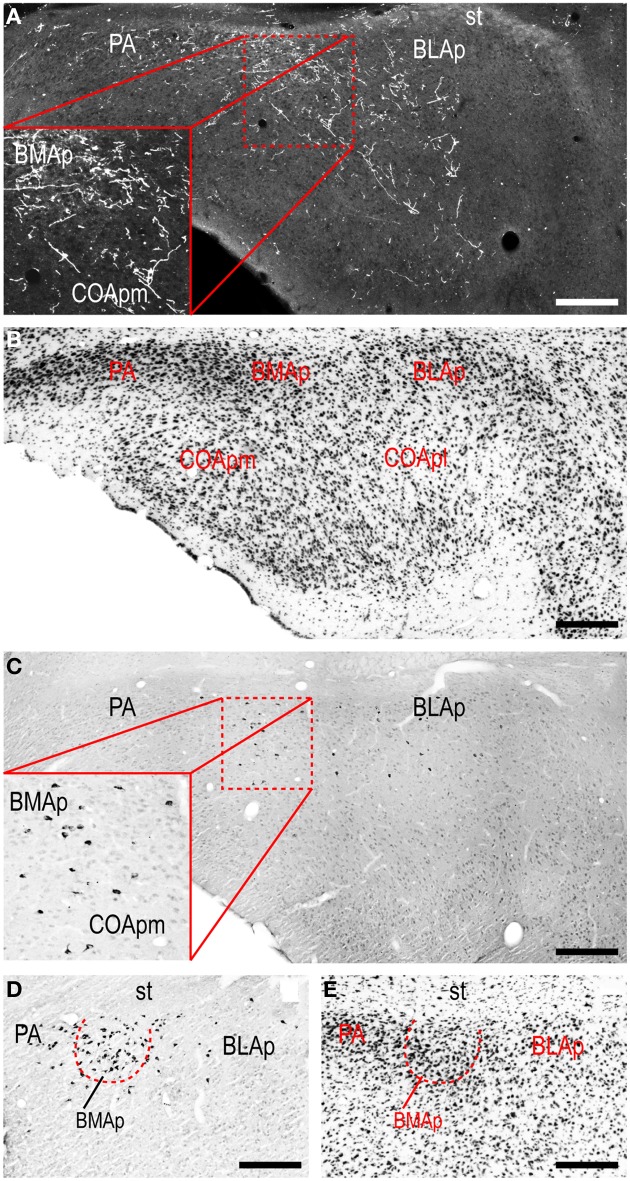
A conspicuous difference was apparent with respect to LHAjvd/v outputs to the cerebral cortex, in that these connections, which target several parts of the cerebral cortex, arise almost exclusively from the LHAjvv. They include inputs to several components of the amygdalar region associated with the cerebral cortex, and foremost among these (in the cortical subplate) are inputs to basomedial- (BMA, posterior part, very dense), and posterior (PA) amygdalar nuclei (Figures 4X–FF, 5A). In the cortical plate, olfactory-related amygdalar components receive a light to moderate input from the LHAjvv, namely the piriform-amygdalar transition area (PAA, Figures 4X–BB), and the posterior part of the cortical amygdalar area (COAp, mostly its lateral zone, Figures 4X–CC). Two other parts of the cerebral cortex also receive a light input from the LHAjvv: The hippocampal formation (HPF, primarily the subiculum and field CA1, Figures 4CC–II), and the prefrontal cortex (a very light input to prelimbic- and infralimbic areas, Figures 4D–G). Where present, input from the LHAjvd to the cerebral cortex includes regions targeted by the LHAjvv (such as ILA, and COAp), but it is of comparatively little amount.
Figure 5.
LHAjvv connections with the cortical parts of the amygdalar region. Photomicrographs of PHAL/anterograde (A, and zoomed inset; darkfield) and CTB/retrograde (C,D and zoomed inset in C; brightfield) labeling in cortical parts of the amygdalar region. Adjacent sections are shown in (A,C). Adjacent Nissl-stained sections are also shown (B,E). Scale bars = 250 μM (A–C), 200 μM (D,E).
LHAjvd/v outputs to the cerebral nuclei
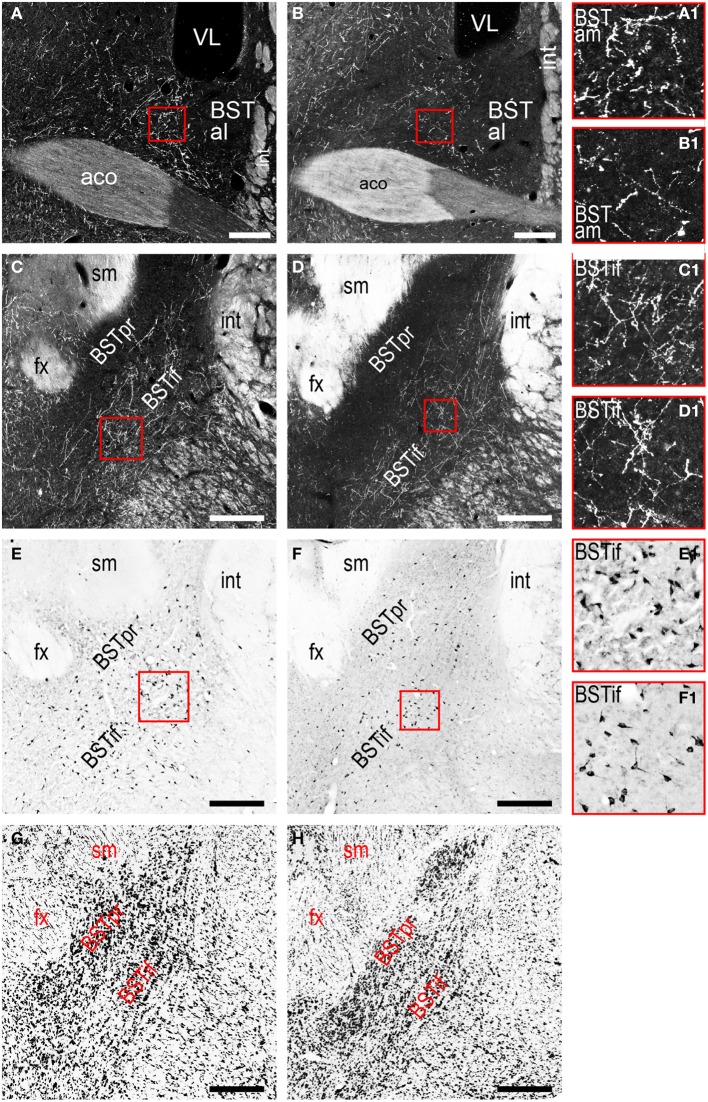
Beginning with cerebral nuclei of the pallidum, the substantia innominata (SI) receives a substantial input from the LHAjvd/v (Figures 4K–X). This input is present mostly at rostral SI levels, and in the medial half; axons of passage were also observed, intermingled with axon terminals in the SI, especially at caudal levels. More impressive than the SI input is a major input to certain regions of the bed nucleus of the stria terminalis (BST); most striking are inputs to the BSTam (Figures 4L–Q, 6) and BSTif (Figures 4R,S, 7A,B); the LHAjvd also provides a light input to rostral levels of the BSTpr (Figures 4P,Q).
Figure 6.
LHAjv region connections with the bed nucleus of the stria terminalis (BST). (A,B) Darkfield photomicrographs of PHAL/anterograde labeling in the BST anterior division from the LHAjvd (A,A1) and LHAjvv (B,B1). Red boxed areas highlight inputs to the BST anteromedial area. (C,D) Darkfield and (E,F) brightfield photomicrographs of PHAL/anterograde- (C,D) and CTB/retrograde (E,F) labeling from the LHAjvd (C,E,C1,E1) and LHAjvv (D,F,D1,F1) in the BST posterior division. Red boxed areas in (C–F) correspond to (C1–F1), and highlight LHAjv region connections with the BST interfascicular nucleus; adjacent sections are shown in (C,E) and (D,F); adjacent Nissl-stained sections are also shown (G,H). Scale bars = 250 μM.
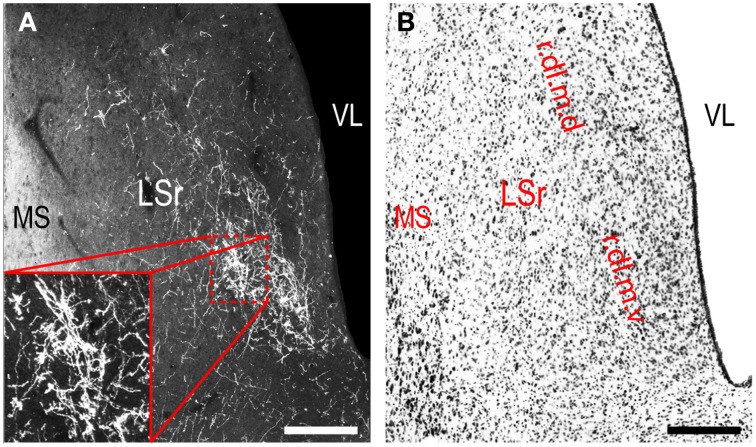
Figure 7.
LHAjvv outputs to the rostral lateral septal nucleus (LSr). (A) Darkfield photomicrograph of PHAL/anterograde labeling in the LSr from the LHAjvv. Zoomed inset highlights input to the LSr.dl.m.v. (B) Adjacent Nissl-stained section. Scale bars = 250 μM.
Continuing rostral from the BST into the striatum, a major LHAjvd/v axonal input reaches the lateral septal nucleus (LS), the vast majority of which targets the rostral part (LSr). Within the LSr, each of its three zones (medial, ventrolateral and dorsolateral) receives an input from both LHAjvd and LHAjvv, although the densest input is to the dorsolateral zone (LSr.dl), and especially from the LHAjvv to the ventral domain of its medial region (LSr.dl.m.v) (Figures 4M, 7). The overall extent of input to the LS from the LHAjvd/v is somewhat greater from the LHAjvv.
While most of the LHAjvd/v output to the striatum is to the LS, additional striatal nuclei are also targeted, including (sparingly) the nucleus accumbens (Figures 4G–K), and several striatal components of the amygdalar region, especially the medial part of the central amygdalar nucleus (Figures 4S,T). It is also worth noting that axons were clearly labeled in the stria terminalis, suggesting at least one route whereby axons originating in the LHAjvd/v reach striatal amygdalar nuclei.
LHAjvd/v outputs to the cerebrospinal trunk
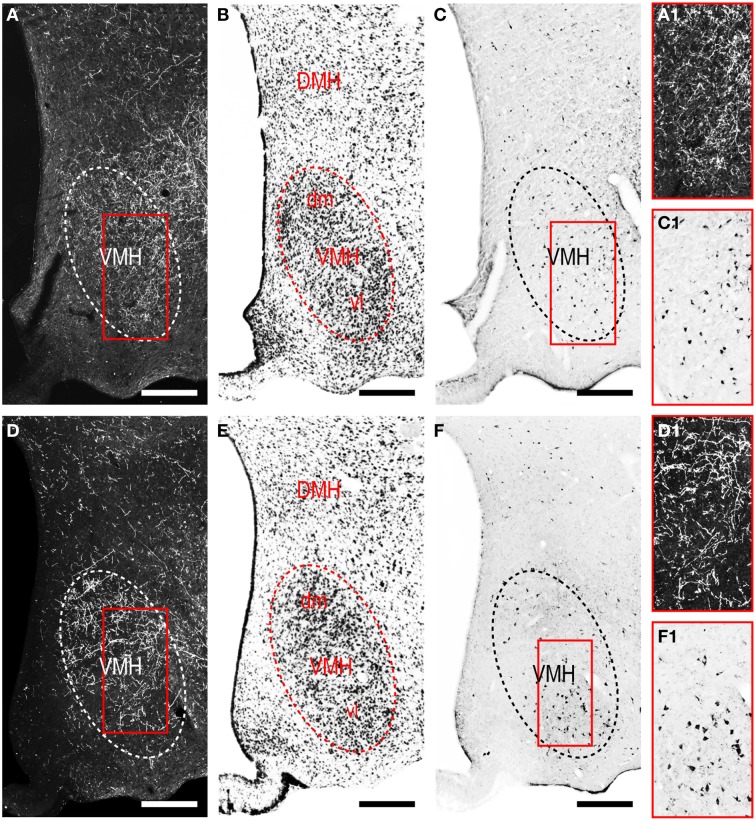
The LHAjvd and LHAjvv both send a major output to the ventromedial hypothalamic nucleus (VMH); this forms a massive terminal field in the VMH that extends across the entire nucleus (Figures 4V–Z, 8), including a dense bilateral input to the VMHa (Figure 4V). In contrast, the dorsomedial hypothalamic nucleus (DMH) receives very little input from the LHAjvd, and a relatively sparse input from the LHAjvv (Figures 4X–AA). LHAjvd/v outputs to other LHA regions are comparable (slightly greater from the LHAjvd); they are extensive, but of generally low abundance, with the most prominent being restricted to the LHA anterior group and LHA medial tier regions. Nevertheless, the LHAjp and LHAjd both receive a moderate input from the LHAjvd/v (Figures 4S–Z); also of note is a bidirectional connection between the LHAjvd and LHAjvv (Figures 4T–X).
Figure 8.
LHAjv region connections with the ventromedial hypothalamic nucleus (VMH). (A,D) Darkfield photomicrographs of PHAL/anterograde labeling from the LHAjvd (A,A1) and LHAjvv (D,D1) in the VMH. Red boxed areas in (A,D) correspond to (A1) and (D1) and highlight LHAjv region inputs to the VMH. (C,F) Brightfield photomicrographs of CTB/retrograde labeling from the LHAjvd (C) and LHAjvv (F) in the VMH. Red boxed areas in (C,F) correspond to (C1,F1) and highlight LHAjv region inputs to the VMH. Adjacent sections are shown in (A,C) and (D,F). Adjacent Nissl-stained sections are also shown (B,E). Scale bars = 250 μM.
Tracing rostrally from the LHAjvd/v, the next hypothalamic site to receive substantial input is the anterior hypothalamic nucleus (AHN); this input is mostly restricted to the anterior and central parts of the AHN (Figures 4S–Q). At similar levels, a sparse (mostly LHAjvv) input to the parvicellular division of the PVH is noteworthy (Figures 4S–V). Additional rostral hypothalamic sites receiving a major input from both LHAjvv and LHAjvd include the lateral- (LPO) and (especially) medial (MPO) preoptic areas (Figures 4K–Q). At the caudal end of the hypothalamus, the posterior hypothalamic nucleus (PH) receives a substantial input from the LHAjvv, in comparison to a rather sparser input from the LHAjvd (Figures 4AA,BB) (despite the presence of substantial LHAjvd-originating axons of passage in the PH).
The LHAjvd and LHAjvv both send a moderate to abundant output to thalamic nuclei within the midline group of the dorsal thalamus, specifically to thalamic paraventricular- (PVT; entire length, and somewhat bilateral, Figures 4R–CC) and paratenial (PT; rostral levels, Figures 4R,S) nuclei, and less so to the nucleus reuniens (RE; mostly from the LHAjvv to RE ventral half, and especially anterior part, Figures 4S–Z). In addition, the LHAjvd/v sends an output to the subparafascicular nucleus of the thalamus (SPF), in particular from the LHAjvd to the medial division of the SPF parvicellular part (SPFpm, Figures 4DD,FF).
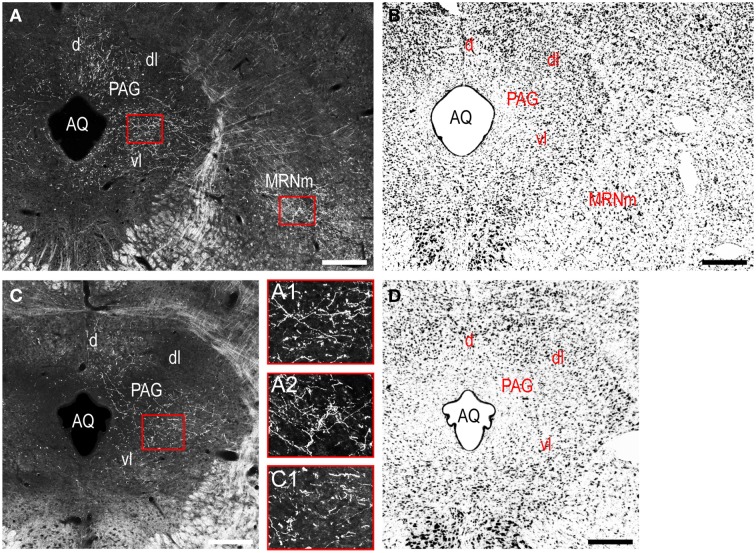
Caudal to the hypothalamus, the major LHAjvd and LHAjvv output targets are the periaqueductal gray (PAG) and the midbrain reticular nucleus (MRN). In addition, the LHAjvd provides a moderate input to the superior colliculus (SC). Reviewing these connections in more detail, the PAGvl receives an extremely dense and extensive input (mostly restricted to its dorsal half), a more moderate input is received by the PAGd; the PAGm and PAGrm also receive a light to moderate input (Figures 4DD–RR, 9). Furthermore, the precommissural and commissural PAG both receive a moderate input from the LHAjvd, but little input from the LHAjvv (Figures 4DD–II). Input to the MRN (reaching there via the PAG) is directed to its magnocellular part, and is especially dense from the LHAjvd (Figures 4JJ–LL). The moderate input to the SC from the LHAjvd targets mostly its deep gray layer, but also includes lightly SC intermediate layers (Figures 4FF–PP).
Figure 9.
LHAjv region outputs to the midbrain. (A,C) Darkfield photomicrographs of PHAL/anterograde labeling in the midbrain from the LHAjvd (A,A1,A2) and LHAjvv (C,C1). Red boxed areas in (A,C) correspond to (A1,A2) and (C2), and delineate inputs to the PAGvl (A1,C1) and MRNm (A2). Adjacent Nissl-stained sections are also shown (B,D). Scale bars = 250 μM.
At caudal levels of the PAG, an output from the LHAjvd (moderate) and LHAjvv (light) to the cuneiform nucleus was apparent (Figures 4NN–PP), as was a lighter LHAjvd/v output to the lateral parabrachial nucleus. Caudal to the PAG, input from the LHAjvd/v was relatively light and arose primarily from the LHAjvd; regions receiving input included the pontine central gray (PCG), lateral dorsal tegmental- (LDT) and Barrington's (B) nuclei, locus ceruleus (LC), pontine- (PRN, caudal part) and parvicellular reticular nuclei (PARN), and the nucleus of the solitary tract (NTS, commissural part, Figures 4BBB,CCC).
LHAjvd and LHAjvv input connections
LHAjvd/v inputs from the cerebral cortex
Cerebral cortical retrograde labeling from the LHAjvd/v was abundant; however, it was more abundant for the LHAjvd than for the LHAjvv (in contrast to cerebral cortical input connections from the LHAjvd/v, which arose almost exclusively from the LHAjvv). The sources of this cortical input were within areas and regions of the sensory-motor- and polymodal association cortices. With respect to the former, a moderate amount of retrograde labeling was found in cortical amygdalar- (COA, especially posterior part, lateral zone, Figures 4Z–DD) and infralimbic (ILA, Figures 4D–G) areas, and to a lesser degree in the tenia tecta (TT, dorsal part), postpiriform transition area (TR), and the nucleus of the lateral olfactory tract (NLOT).
Areas of the polymodal association cortex providing input to the LHAjvd/v include the prelimbic- (PL, moderate abundance, Figures 4A–D) and perirhinal (PERI, low abundance) areas, and the hippocampal formation (HPF, high abundance). Within the HPF, a low to moderate amount of retrograde labeling from the LHAjvd (but not the LHAjvv) was found in the entorhinal area, and abundant retrograde labeling (principally from the LHAjvd) was present in the subiculum and field CA1 (Figures 4DD–MM). Cortical subplate retrograde labeling from the LHAjvd/v was copious yet circumscribed, and was localized primarily in basal- and posterior (PA) amygdalar nuclei; more specifically, the basolateral- (BLA, posterior part) and adjacent basomedial [BMA, posterior (mostly) and anterior parts] amygdalar nuclei (Figures 4Z–EE, 5).
LHAjvd/v inputs from the cerebral nuclei
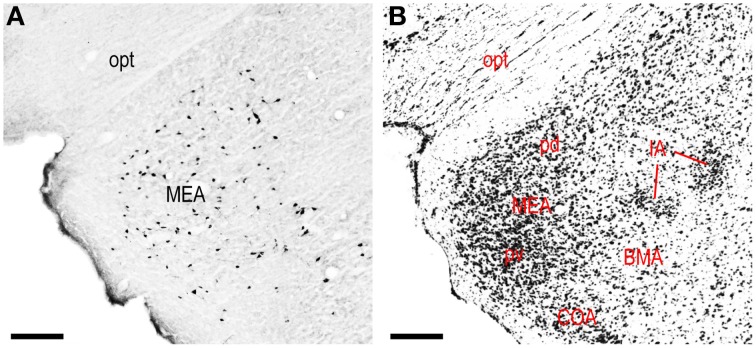
Retrograde labeling from the LHAjvd/v was present in several cerebral nuclei. Starting with striatal nuclei, numerous neurons were retrogradely labeled in the rostral part of the lateral septal nucleus (LSr), and in the medial amygdalar nucleus (MEA). Within the LSr, the highest density of retrogradely labeled neurons was present in the LSr ventrolateral zone, followed by the LSr dorsolateral- and medial zones (Figures 4G–O); a little retrograde labeling was also present in the LS caudal part. Retrograde labeling in the MEA was numerous, included each of its parts, and was mostly more abundant from the LHAjvd (Figures 4S–Z, 10); relatively little retrograde labeling from the LHAjvd/v was present in the CEA as well. A low to moderate abundance of retrogradely labeled neurons (moderate from the LHAjvv) was also present in a dorsomedial region of the nucleus accumbens (Figures 4H–K).
Figure 10.
LHAjvv inputs from the medial amygdalar nucleus (MEA). (A) Photomicrograph of CTB/retrograde labeling in the MEA from the LHAjvd. An adjacent Nissl-stained section is also shown (B). Scale bars = 200 μM.
Among pallidal nuclei, the most numerous retrograde labeling from the LHAjvd/v was in the following nuclei of the bed nucleus of the stria terminalis: Anteromedial- (BSTam), principal- (BSTpr), and interfascicular (BSTif) nucleus (Figures 4L–S, 6). In fact, the BST as a whole (but especially the BSTam, -pr, and -if) had the highest abundance of retrograde labeling obtained for an individual region in this analysis. In relation to this, it is noteworthy that while analysis of anterograde labeling revealed the BSTam and BSTif both receive a robust input from the LHAjvd/v, the BSTpr receives comparatively little. Retrogradely labeled neurons in other BST nuclei were rarely present.
LHAjvd/v inputs from the cerebrospinal trunk
The cerebrospinal trunk was extensively retrogradely labeled from the LHAjvd/v, with the vast majority of retrogradely labeled neurons present in the motor subsystem. Only six regions of the sensory and behavioral state subsystems (three from each) were retrogradely labeled (moderately at most) from the LHAjvd/v; this compares to retrograde labeling at a moderate or higher level in 22 regions of the motor subsystem from the LHAjvd/v. Nevertheless, of the three prominent sensory subsystem input sites, two were retrogradely labeled more numerously from the LHAjvd, and these were in the thalamus: The subparafascicular- (parvicellular part, especially lateral division) and paraventricular thalamic nuclei. The other (and most) notable site of sensory subsystem retrograde labeling from the LHAjvd/v was the parabrachial nucleus, especially its lateral division (Figures 4QQ–TT). Notable sources of behavioral state system input to the LHAjvd/v were the subparventricular zone, LHAd, and the pedunculopontine nucleus (the last mostly retrogradely labeled from LHAjvv).
Motor subsystem retrograde labeling from the LHAjvd/v was very abundant. Most of this input originated in the hypothalamus, and came from hypothalamic medial zone nuclei, the hypothalamic periventricular region, and the reticular formation (including from several other LHA regions). Additional retrograde labeling was present in the central gray (substantial), and in the neuroendocrine motor zone (up to moderate). The pattern of motor subsystem retrograde labeling from the LHAjvd and LHAjvv was essentially similar.
Medial hypothalamic zone nuclei retrogradely labeled abundantly from LHAjvd/v were the AHN (mostly central part, Figures 4R–W) and VMH (all parts, but especially ventrolateral, Figures 4V–Z, 8). Substantial retrograde labeling was also found in the MPN (lateral and medial parts, Figures 4O–S), PMv (Figures 4BB,CC), and the posterior hypothalamic nucleus (PH, Figures 4AA–FF); additional retrograde labeling (up to moderate) was present in all divisions of the PAG (most notably the PAGvl) (Figures 4II–QQ).
Several hypothalamic periventricular region nuclei provide LHAjvd/v input. Numerous retrogradely labeled neurons were found in the medial preoptic area (MPO, Figures 4M–R); a relatively low density (yet cumulatively substantial amount) of retrograde labeling was also present in internuclear areas. In addition, moderate retrograde labeling was present in the median preoptic- (MEPO, Figures 4M–Q), anteroventral periventricular- (AVPV, Figures 4N,O), and dorsomedial hypothalamic (DMH, all parts, but mostly anterior, Figures 4X–AA) nuclei; a less than moderate level of retrograde labeling was found in the anterior hypothalamic area (AHA), and in the anterodorsal/ventral- (ADP/AVP) and periventricular hypothalamic (PV, posterior part) nuclei.
A substantial portion of the reticular formation retrograde labeling from the LHAjvd/v was located in nearby regions of the hypothalamic lateral zone; notably in the LPO (Figures 4K–Q), and in several motor-related LHA regions, including (especially) the LHAav, LHAjp, and LHAjd (Figures 4S–Z). A low to moderate abundance of retrograde labeling was also present in the LHAad, LHAai, LHAsfp, retrochiasmatic area (RCH), and tuberal nucleus (TU). In addition, a moderate abundance of neurons were retrogradely labeled in LHAjvv from the LHAjvd, and vice versa (corroborating the anterograde labeling). Beyond the hypothalamic zone, two retrogradely labeled midbrain nuclei are noteworthy: (1) The midbrain reticular nucleus (MRN, magnocellular part, labeling mostly restricted to a lateral region at rostral levels, Figures 4GG–JJ), which was retrogradely labeled substantially from the LHAjvd (in comparison to substantially fewer MRN neurons retrogradely labeled from the LHAjvv); (2) the cuneiform nucleus, in which a low abundance of retrograde labeling was found from the LHAjvd/v (slightly greater from the LHAjvd) (Figures 4OO–QQ).
Finally, the neuroendocrine motor system was retrogradely labeled to a moderate level of abundance from the LHAjvd/v; the sources of this input were the supraoptic (SO)- hypothalamic paraventricular (PVH)- and arcuate (ARH) nuclei. A substantial cluster of neurons was retrogradely labeled in the SO from the LHAjvv (Figures 4R–T); ARH retrograde labeling from the LHAjvd/v was of low to moderate abundance (Figures 4V–AA); PVH retrograde labeling from the LHAjvd/v was also of low to moderate abundance and was present mostly in the PVH anterior- (PVHap, Figures 4R–T) and medial parvicellular (PVHmpd, Figures 4U–W) parts.
Comparative analysis
Previous studies have investigated the output and/or input connections of every LHAjv-contiguous region in the rat: LHAjd (Hahn and Swanson, 2012), AHN (Risold et al., 1994), VMH (Canteras et al., 1994; Shimogawa et al., 2014), TU (Canteras et al., 1994; Toth et al., 2010), and LHAsf (Goto et al., 2005). It was therefore possible to compare the present data with the previously published work, to which the reader is also referred. In addition to comparing the principal datasets, we also compared data obtained from experiments with injection sites that included the LHAjvd or LHAjvv, but were less restricted to them (that is, including one or more LHAjv-contiguous region) (Figure 1). Although a detailed comparative analysis of these data was not within the purview of this study, a general review confirmed the existence of distinct differences and similarities between the previously reported connections of the LHAjv-contiguous regions and the LHAjv region; it also confirmed that (without exception) the pattern of labeling obtained from tracer injections that included the LHAjvd/v and one or more LHAjvd/v-contiguous regions, was a combination of the labeling (the indicated connections) resulting from the most restricted LHAjvd/v injections, plus additional connections described previously for the LHAjv-contiguous regions. In addition, the present results are in agreement with a previous preliminary analysis of LHAjv region output connections based on data obtained from a single PHAL experiment (Goto et al., 2005); they are also in general agreement with a recent analysis of inputs to a delineated hypothalamic “aggression” area, which overlaps the LHAjv region (Toth et al., 2010).
A potential source of variability in tract-tracing experiments stems from visual approximation of the effective spread of tracer molecules from an injection site. In the present study, restriction of the principal LHAjvd/v injection sites (experiments LHA #2 and #77) to the LHAjv region is supported by data from a PHAL + CTB study of the connections of the nucleus incertus (NI)—a distinct pontine cell group (Goto et al., 2001). The output connections of the NI include a dense terminal input to the LHAjv-contiguous LHAsf; this input specifically delineates the LHAsfa at a rostrocaudal level corresponding to the center of the injection sites for experiments LHA #2 and #77 (compare our Figure 1 to their Figures 8B, 10I) (Goto et al., 2001). The NI to LHAsfa input arises principally from a relatively cell-diffuse lateral differentiation of the NI, referred to as its diffuse part (NId); a less substantial input arises from a cell-compact medial part (NIc). The previous study (Goto et al., 2001) indicated a virtual absence of input to the LHAjv region from the NId (see their Figures 8B,C), and a very light and diffuse input from the NIc (see their Figures 7D,E). Consistent with this data, and consistent with no appreciable spread of CTB into the LHAsf for experiments LHA #2 and #77, it is salient to note we found no retrograde labeling (from experiments LHA #2 and #77) in the NId, and only a few retrogradely labeled neurons in the NIc (Figures 4TT–VV). Similarly, the amount of anterograde labeling we found in the NI from the LHAjv was miniscule, consistent with a general absence of retrograde labeling in the LHAjvd/v following NI CTB injections, even though the latter did result in retrograde labeling of neurons in LHAjv-adjacent regions (Goto et al., 2001).
Discussion
A readily grasped description of the hypothalamus divides it into three longitudinal zones, bilateral to the third ventricle. In this schema, the lateral-most zone includes all LHA regions, whereas progressively narrower medial and periventricular zones contain several well defined nuclei (Swanson, 1987). Accumulated experimental evidence supports the existence of segregated networks within the hypothalamic medial zone that are critical for the control of different types of fundamental behavior (Risold et al., 1997; Swanson, 2000). Collectively, the medial hypothalamic zone nuclei within these networks form the rostral segment of a behavior control column, the caudal segment of which is formed by regions centered in the ventromedial midbrain (Swanson, 2000) (see their Figure 10).
In two previous sister papers, using the methods applied in the present study on the LHAjvd and LHAjvv, we described the connections of the LHAjd (Hahn and Swanson, 2012) and LHAjp (Hahn and Swanson, 2010)—the two other regions of the LHA medial tier (Swanson, 2004); the connections of the LHA perifornical tier regions (LHAs and LHAsf) have been investigated similarly: LHAs (Hahn and Swanson, 2010), and LHAsf (outputs only) (Goto et al., 2005). The connections of these LHA regions with, and in relation to, the behavior control column suggested different LHA regions have primary involvement with different behaviors: LHAs with ingestive behavior (Hahn and Swanson, 2010), LHAsfa with defensive or exploratory/foraging behavior (Goto et al., 2005), LHAjp and LHAjd with defensive behavior (Hahn and Swanson, 2010, 2012). The connections of the LHAjvd and LHAjvv follow this general pattern and, as we discuss here, extend it to include reproductive and also aggressive behaviors.
In our previous paper we showed the LHAjd has robust connections with three highly interconnected hypothalamic medial zone nuclei (and subdivisions) (AHN, VMHdm, PMd) involved in defensive (“fight”-or-“flight”) behavior control (Canteras et al., 1997; Risold et al., 1997; Canteras, 2002). By comparison, in the present study we found the LHAjv region has substantially less direct connection with the PMd, but robust connections with the AHN and VMHdm. Moreover, both dorsal and ventral LHAjv zones have considerable connectivity with the PMv, VMHvl, and MPN. The latter three medial zone nuclei/subdivisions, which are also highly interconnected, are prominently sexually dimorphic and central to the control of reproductive behavior (Canteras et al., 1997; Risold et al., 1997; Canteras, 2002).
Retrograde labeling from the LHAjv region (up to moderate for the LHAjvd) in the AVPV continues this theme—the AVPV is another prominently sexually dimorphic nucleus which in female rats has a critical role in reproductive function (Wiegand and Terasawa, 1982). A more direct link to reproductive function is suggested by the moderate retrograde (and light anterograde) labeling within regions adjacent to the AVPV that contain a preponderance of gonadotropin-releasing hormone (GnRH) perikarya (Baker et al., 1975; Witkin et al., 1982; Merchenthaler et al., 1984; Wray and Hoffman, 1986; Silverman et al., 1987). The finding of LHAjvd/v connections with the AVPV and GnRH cell body region in the male rat accords with previous retrograde (Hahn and Coen, 2006) and anterograde (Gu and Simerly, 1997) tracing studies in the female rat. In addition, GnRH-immunopositive axons have been reported in the vicinity of the LHAjvd/v (Merchenthaler et al., 1984).
An association of LHAjvd/v connections with reproductive and defensive behaviors extends beyond the hypothalamus, as evidenced by substantial LHAjvd/v retrograde labeling in the BSTpr and all parts of the MEA. In the rat the MEA and BSTpr are both major recipient sites for olfactory information, notably defensive (or aggressive) and reproductive behavior-relevant pheromonal information relayed from the accessory olfactory bulb (AOB) (Scalia and Winans, 1975; Risold et al., 1997; Simerly, 2002; Mohedano-Moriano et al., 2007). Furthermore, the present data indicate a substantial input to the LHAjvd/v from cerebral cortical components of the amygdalar region (BLAp, BMAp, COApl, COApm, PA) that also receive olfactory input from the main and/or accessory olfactory bulbs (Swanson and Petrovich, 1998; Dong et al., 2001a; Petrovich et al., 2001; Pro-Sistiaga et al., 2007). In addition, feedback modulation of this input is suggested by a striking LHAjvv (but not the LHAjvd) input to the BMAp and PA, and a moderate input to the COA.
Olfactory sensory processing in relation to agonistic and reproductive behaviors is prominent for a macrosmatic animal like the rat (Barnett, 1963); nevertheless, reviewing the amygdalar region components connected to the LHAjvd/v it is pertinent to note also associations with non-olfactory sensory processing, and additional behavioral control. For example, conveyance of auditory and visual information to the BMAp and BLAp (and thence to the LHAjvd/v) may be inferred from a massive input to the BMAp (and substantial input to the BLA) from the lateral amygdalar nucleus (LA) (Pitkanen et al., 1995)1, which is a major recipient of thalamic and cerebral cortical inputs involved in visual and auditory processing (McDonald, 1998)1. Likewise, there are inputs to the LA, BLA, and BMA from thalamic and cerebral cortical regions centrally involved in the processing of somatosensory, viscerosensory, and gustatory sensory information (McDonald, 1998)1. Therefore, polymodal information may be relayed to the LHAjvd/v from several striatal and cortical parts of the amygdalar regions.
In addition to having potential relevance for agonistic and reproductive behaviors, conveyance of polymodal information from the amygdalar region to the LHAjvd/v may also be relevant to ingestive behavior. This possibility is illustrated by a series of recent experiments that employed a behavioral model in which feeding is potentiated by an auditory stimulus previously paired with feeding (Holland et al., 2002). In this model, excitotoxic (NMDA) lesion of the BLA is reported to abolish increased feeding to the conditioned stimulus (CS) (Holland et al., 2002)2. In addition, a more recent study using the same behavioral model found a significant increase in CS-associated immediate early gene (IEG) expression in basal amygdalar nuclei retrogradely labeled from the LHA (Petrovich et al., 2005). In the latter study, Fluoro-Gold (FG) injections were targeted to the ventral half of the LHA, but were not restricted to particular LHA regions; nevertheless, partial inclusion of the LHAjv region is suggested by a pattern of amygdalar FG labeling similar to the present data (compare their Figure 4A with our Figures 4AA,BB) (Petrovich et al., 2005). Two related and more recent reports examining IEG expression in this model indicated significant involvement of all basal amygdalar regions (Cole et al., 2013), and also the LHAjv region (plus several other LHA regions) (Cole et al., 2015).
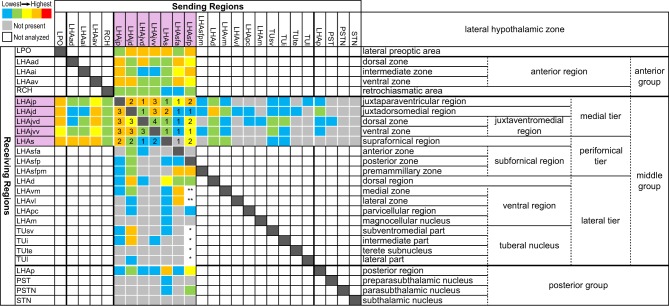
More direct LHAjv region links to ingestive behavior (and metabolism) are indicated by several other LHAjv region connections. One in particular stands out, and is indicated by moderate LHAjvd/v retrograde labeling in the hypothalamic arcuate nucleus (Figures 4V–AA) (Atasoy et al., 2012; Keen-Rhinehart et al., 2013; Sohn et al., 2013). For a consideration of this and other of LHAjv region connections in relation to one particular mode of ingestive behavior, namely drinking, see (Swanson, 2000), and note regions identified in their Figure 11 that are shown here to have connections with the LHAjv region (Table 1 and Figure 3).
Table 1.
Connections of the LHAjv region.
| Extrinsic connections of the LHAjv region | Outputs | Inputs | ||
|---|---|---|---|---|
| (PHAL) | (CTB) | |||
| LHAjvv | LHAjvd | LHAjvv | LHAjvd | |
| (LHA#2) | (LHA#77) | (LHA#2) | (LHA#77) | |
| CELL GROUP OR REGION | ||||
| 1. CEREBRUM | ||||
| 1.1. CEREBRAL CORTEX | ||||
| 1.1.1. Cortical Plate | ||||
| Sensory-motor cortex | ||||
| Somatomotor areas | ||||
| Visceral sensory-motor areas | ||||
| infralimbic area (ILA) | ++ | + | ++ | +++ |
| Olfactory areas tenia tecta | ||||
| Dorsal part (TTd) | − | + | ++ | ++ |
| Postpiriform transition area (TR) | − | − | + | ++ |
| Piriform-amygdala transition area (PAA) | ++ | − | − | − |
| Nucleus of the lateral olfactory tract (NLOT) | − | − | + | ++ |
| Cortical amygdalar area | ||||
| Anterior part (COAa) | − | − | ++ | ++ |
| Posterior part | ||||
| Lateral zone (COApl) | +++ | + | +++ | ++++ |
| Medial zone (COApm) | ++ | + | ++ | ++ |
| Polymodal association cortex | ||||
| Prelimbic area (PL) | + | − | ++ | +++ |
| Orbital area | ||||
| Ventral part (ORBv) | − | − | + | + |
| Agranular insular area | ||||
| Dorsal part (Ald) | − | − | + | + |
| Posterior part (Alp) | − | − | + | + |
| Ectorhinal area (ECT) | − | − | + | + |
| Perirhinal area (PERI) | + | − | ++ | + |
| Hippocampal formation | ||||
| Retrohippocampal region | ||||
| Entorhinal area | ||||
| Lateral part (ENTl) | + | − | − | + |
| Medial part, dorsal zone (ENTm) | − | − | − | ++ |
| Medial part, ventral zone (ENTmv) | − | − | − | +++ |
| Subiculum | ||||
| Pyramidal layer (SUB-sp) | ++ | − | +++ | +++++ |
| Hippocampal region | ||||
| Ammon's horn | ||||
| Field CA1 | ||||
| Stratum radiatum (CA1sr) | + | − | − | − |
| Pyramidal layer | ||||
| Deep (CA1spd) | + | − | ++++ | +++++ |
| Superficial (CA1sps) | − | − | + | ++ |
| Stratum oriens (CA1so) | ++ | − | − | − |
| 1.1.2. Cortical Subplate | ||||
| Claustrum (CLA) | − | − | + | + |
| Endopiriform nucleus | ||||
| Dorsal part (EPd) | + | − | − | − |
| Lateral amygdalar nucleus (LA) | + | + | + | + |
| Basolateral amygdalar nucleus | ||||
| Anterior part (BLAa) | − | − | + | − |
| Posterior part (BLAp) | + | + | +++ | ++++ |
| Basomedial amygdalar nucleus | ||||
| Anterior part (BMAa) | − | − | +++ | ++ |
| Posterior part (BMAp) | +++++ | + | +++ | ++++ |
| Posterior amygdalar nucleus (PA) | ++++ | + | +++ | ++++ |
| 1.2. CEREBRAL NUCLEI | ||||
| 1.2.1. Striatum | ||||
| Nucleus accumbens (ACB) | + | + | +++ | + |
| Olfactory tubercle (OT) | − | − | + | − |
| Lateral septal complex | ||||
| Lateral septal nucleus | ||||
| Caudal (caudodorsal) part | ||||
| Dorsal zone | ||||
| Rostral region (LSc.d.r) | + | + | − | + |
| Lateral region (LSc.d.l) | − | − | + | − |
| Ventral region (LSc.d.v) | − | − | + | − |
| Ventral zone | ||||
| Medial region | ||||
| Dorsal domain (LSc.v.m.d) | − | − | + | − |
| Ventral domain (LSc.v.m.v) | − | − | + | + |
| Intermediate region (LSc.v.i) | + | − | + | + |
| Lateral region | ||||
| Dorsal domain (LSc.v.l.d) | + | − | ++ | ++ |
| Ventral domain (LSc.v.l.v) | + | − | + | ++ |
| Rostral (rostroventral) part | ||||
| Medial zone (LSr.m) | + | − | + | ++ |
| Dorsal region (LSr.m.d) | − | − | ++ | ++ |
| Ventral region | ||||
| Rostral domain (LSr.m.v.r) | + | ++ | +++ | ++ |
| Caudal domain (LSr.m.v.c) | +++ | ++ | ++ | + |
| Ventrolateral zone | ||||
| Dorsal region | ||||
| Medial domain (LSr.vl.d.m) | +++ | + | ++++ | +++ |
| Lateral domain (LSr.vl.d.l) | + | +++ | ++++ | +++ |
| Ventral region (LSr.vl.v) | + | + | ++ | − |
| Dorsolateral zone | ||||
| Medial region | ||||
| Dorsal domain (LSr.dl.m.d) | +++ | ++ | +++ | ++ |
| Ventral domain (LSr.dl.m.v) | ++++ | ++++ | +++ | ++ |
| Lateral region | ||||
| Dorsal domain (LSr.dl.l.d) | +++ | +++ | +++ | +++ |
| Ventral domain (LSr.dl.l.v) | − | + | ++ | ++ |
| Ventral part (LSv) | + | ++ | ++ | +++ |
| Septofimbrial nucleus (SF) | + | + | + | − |
| Anterior amygdalar area (AAA) | + | − | + | + |
| Central amygdalar nucleus | ||||
| Medial part (CEAm) | ++ | + | + | ++ |
| Capsular part (CEAc) | + | − | + | − |
| Medial amygdalar nucleus | ||||
| Anterodorsal part (MEAad) | − | + | ++++ | ++++ |
| Anteroventral part (MEAav) | − | − | ++ | ++++ |
| Posterodorsal part | ||||
| Sublayer a (MEApd-a) | − | − | +++ | +++ |
| Sublayer b (MEApd-b) | + | − | ++ | ++ |
| Sublayer c (MEApd-c) | − | + | + | ++ |
| Posteroventral part (MEApv) | − | − | +++ | ++++ |
| Intercalated amygdalar nuclei (IA) | + | − | − | + |
| 1.2.2. Pallidum | ||||
| Substantia innominata (SI) | ++++ | ++++ | +++ | +++ |
| Medial septal complex | ||||
| Medial septal nucleus (MS) | +++ | + | ++ | ++ |
| Diagonal band nucleus (NDB) | + | + | + | +++ |
| Bed nuclei of the stria terminalis | ||||
| Anterior division | ||||
| Anterolateral area (BSTal) | + | + | + | + |
| Anteromedial area (BSTam) | ++++ | +++++ | ++++ | +++++ |
| Rhomboid nucleus (BSTrh) | + | − | − | + |
| Dorsomedial nucleus (BSTdm) | + | + | + | − |
| Fusiform nucleus (BSTfu) | − | − | + | |
| Ventral nucleus (BSTv) | ++ | + | + | ++ |
| Magnocellular nucleus (BSTmg) | − | − | + | + |
| Posterior division | ||||
| Principal nucleus (BSTpr) | + | +++ | ++++ | +++++ |
| Interfascicular nucleus (BSTif) | ++++ | +++++ | +++++ | +++++ |
| Transverse nucleus (BSTtr) | + | + | + | ++ |
| 2. CEREBELLUM | − | − | − | − |
| 3. CEREBROSPINAL TRUNK | ||||
| 3.1. SENSORY SYSTEM | ||||
| 3.1.1. Thalamus | ||||
| Sensory-motor cortex related | ||||
| Ventral group of the dorsal thalamus | ||||
| Subparafascicular nucleus thalamus | ||||
| Parvicellular part | ||||
| Medial division (SPFpm) | + | +++ | − | + |
| Lateral division (SPFpl) | + | + | + | ++ |
| Polymodal association cortex related | ||||
| Lateral group of the dorsal thalamus | ||||
| Medial group of the dorsal thalamus | ||||
| Mediodorsal nucleus thalamus | ||||
| Medial part (MDm) | + | + | − | − |
| Intermediodorsal nucleus thalamus (IMD) | − | + | − | + |
| Midline group of the dorsal thalamus | ||||
| Paraventricular nucleus thalamus (PVT) | +++ | ++++ | + | ++ |
| Paratenial nucleus (PT) | +++ | +++ | − | + |
| Nucleus reuniens | ||||
| Rostral division | ||||
| Anterior part (REa) | +++ | + | − | − |
| Ventral part (REv) | + | − | − | − |
| Lateral part (REl) | ++ | − | − | − |
| Median part (REm) | − | − | − | + |
| Caudal division | ||||
| Caudal part (REcp) | + | + | − | − |
| Intralaminar group of the dorsal thalamus | ||||
| Central medial nucleus thalamus (CM) | − | + | − | − |
| 3.1.2. Visual | − | − | − | − |
| 3.1.3. Somatosensory | − | − | − | − |
| 3.1.4. Auditory | − | − | − | − |
| Nucleus of the lateral lemniscus | ||||
| Dorsal part (NLLd) | − | + | − | + |
| Ventral part (NLLv) | − | − | + | + |
| 3.1.5. Gustatory | − | − | − | − |
| 3.1.6. Visceral | − | − | − | − |
| Nucleus of the solitary tract | ||||
| Commissural part (NTSco) | − | + | − | − |
| Medial part, caudal zone (NTSm) | − | − | − | + |
| Parabrachial nucleus | ||||
| Lateral division | ||||
| Central lateral part (PBlc) | + | − | + | + |
| Dorsal lateral part (PBld) | − | − | + | + |
| External lateral part (PBle) | − | − | − | + |
| Superior lateral part (PBls) | − | − | ++ | + |
| Ventral part (PBlv) | − | − | + | + |
| Kölliker-Fuse subnucleus | − | − | − | + |
| Medial division | ||||
| Medial medial part (PBmm) | − | + | − | − |
| 3.1.7. Humerosensory | ||||
| Subfornical organ (SFO) | − | − | − | + |
| 3.2. BEHAVIORAL STATE SYSTEM | ||||
| Suprachiasmatic nucleus (SCH) | − | − | − | + |
| Subparaventricular zone (SBPV) | ++ | + | ++ | +++ |
| Hypothalamic lateral zone, state related | ||||
| Lateral hypothalamic area, Dorsal region (LHAd) | − | + | ++ | ++ |
| Tuberomammillary nucleus, ventral part (TMv) | − | − | − | + |
| Supramammillary nucleus | ||||
| Medial part (SUMm) | + | − | − | + |
| Lateral part (SUMl) | + | − | + | + |
| Pedunculopontine nucleus (PPN) | − | + | ++ | + |
| Pontine reticular nucleus, rostral part (PRNr) | − | + | − | |
| Raphé nuclei | ||||
| Superior central nucleus raphé, medial part (CSm) | − | − | − | + |
| Dorsal nucleus raphé (DR) | + | + | − | + |
| Laterodorsal tegmental nucleus (LDT) | − | + | + | + |
| Locus ceruleus (LC) | − | + | − | − |
| 3.3. MOTOR SYSTEM | ||||
| 3.3.1. Behavior Control Column | ||||
| Medial preoptic nucleus | ||||
| Lateral part (MPNl) | + | + | +++ | +++ |
| Medial part (MPNm) | − | − | +++ | +++ |
| Central part (MPNc) | − | − | − | + |
| Anterior hypothalamic nucleus | ||||
| Anterior part (AHNa) | +++ | +++ | ++ | ++ |
| Central part (AHNc) | +++ | +++ | +++++ | ++++ |
| Posterior part (AHNp) | − | + | ++ | + |
| Paraventricular nucleus hypothal., descending division | ||||
| Dorsal parvicellular part (PVHdp) | − | − | − | + |
| Lateral parvicellular part (PVHlp) | − | − | + | + |
| Ventromedial hypothalamic nucleus | ||||
| Anterior part (VMHa) | ++ | ++ | ++ | + |
| Dorsomedial part (VMHdm) | ++++ | +++++ | +++ | ++++ |
| Central part (VMHc) | +++ | +++ | +++ | +++ |
| Ventrolateral part (VMHvl) | +++ | ++++ | +++++ | +++++ |
| Ventral premammillary nucleus (PMv) | − | − | ++ | +++ |
| Dorsal premammillary nucleus (PMd) | ++ | − | − | + |
| Medial mammillary nucleus, median part (MMme) | − | − | − | + |
| Ventral tegmental area (VTA) | − | − | + | + |
| Midbrain reticular nucleus, retrorubral area (RR) | − | + | − | − |
| Midbrain reticular nucleus, parvicellular part (MRNp) | − | ++ | − | − |
| 3.3.2. Superior Colliculus, motor related | ||||
| Intermediate gray layer | ||||
| sublayer b (SCig-b) | − | + | − | − |
| sublayer c (SCig-c) | − | + | − | + |
| Deep gray layer (SCdg) | − | +++ | − | − |
| 3.3.3. Postcerebellar and Precerebellar Nuclei | − | − | − | − |
| 3.3.4. Vestibulomotor regions | − | − | − | − |
| 3.3.5. Central Gray | ||||
| Epithalamus | ||||
| Lateral habenula (LH) | + | − | − | + |
| Posterior hypothalamic nucleus (PH) | ++++ | ++ | ++++ | ++++ |
| Periaqueductal gray | ||||
| Precommissural nucleus (PRC) | + | +++ | − | + |
| Commissural nucleus (COM) | ++ | +++ | + | + |
| Rostromedial division (PAGrm) | +++ | ++ | + | ++ |
| Medial division (PAGm) | + | ++ | − | + |
| Dorsal division (PAGd) | ++++ | ++++ | + | ++ |
| Dorsolateral division (PAGdl) | + | + | + | + |
| Ventrolateral division (PAGvl) | +++++ | +++++ | ++ | +++ |
| Pontine central gray, general | ||||
| Pontine central gray (PCG) | + | ++ | + | + |
| Lateral tegmental nucleus (LTN) | − | − | + | − |
| Barrington's nucleus (B) | − | + | − | − |
| 3.3.6. Hypothalamic Periventricular Region | ||||
| Median preoptic nucleus (MEPO) | − | + | +++ | +++ |
| Anteroventral periventricular nucleus (AVPV) | − | − | ++ | +++ |
| Preoptic periventricular nucleus (PVpo) | − | − | + | + |
| Anterodorsal preoptic nucleus (ADP) | − | − | ++ | ++ |
| Anteroventral preoptic nucleus (AVP) | + | + | ++ | ++ |
| Parastrial nucleus (PS) | − | − | + | + |
| Medial preoptic area (MPO) | ++++ | +++++ | +++++ | +++++ |
| Anterior hypothalamic area (AHA) | ++ | ++ | +++ | ++ |
| Dorsomedial hypothalamic nucleus | ||||
| Anterior part (DMHa) | ++ | + | +++ | +++ |
| Posterior part (DMHp) | + | − | + | ++ |
| ventral part (DMHv) | ++ | − | ++ | ++ |
| Periventricular hypothal. nuc., posterior part (PVp) | − | − | ++ | ++ |
| Internuclear area, hypothal. periventricular region (I) | +++ | +++ | ++++ | ++++ |
| 3.3.7 Reticular Formation | ||||
| Hypothalamic lateral zone, motor related | ||||
| Lateral preoptic area (LPO) | +++ | +++ | ++++ | +++ |
| Lateral hypothalamic area, motor related (LHAmo) | ||||
| Juxtaparaventricular region (LHAjp) | +++ | +++ | +++ | +++ |
| Juxtadorsomedial region (LHAjd) | ++ | +++ | +++ | +++ |
| Juxtaventromedial region | ||||
| Dorsal zone (LHAjvd) | ++ | SITE | +++ | SITE |
| Ventral zone (LHAjvv) | SITE | ++ | SITE | ++++ |
| anterior region | ||||
| Dorsal zone (LHAad) | ++ | +++ | ++ | + |
| Intermediate zone (LHAai) | + | + | ++ | ++ |
| Ventral zone (LHAav) | ++ | ++ | ++++ | +++ |
| Retrochiasmatic area (RCH) | ++ | ++ | ++ | ++ |
| Tuberal nucleus (TU) | ||||
| Subventromedial part (TUsv) | − | − | ++ | ++ |
| Intermediate part (TUi) | + | − | + | ++ |
| Suprafornical region (LHAs) | + | + | + | + |
| Subfornical region | ||||
| Anterior zone (LHAsfa) | − | + | + | + |
| Posterior zone (LHAsfp) | − | − | + | ++ |
| Premammillary zone (LHAsfpm) | − | − | + | − |
| Magnocellular nucleus (LHAm) | − | − | − | + |
| Parvicellular region (LHApc) | − | − | − | + |
| Ventral region | ||||
| Medial zone (LHAvm) | − | − | + | + |
| Lateral zone (LHAvl) | − | − | − | − |
| Posterior region (LHAp) | + | + | + | + |
| Preparasubthalamic nucleus (PST) | − | − | − | + |
| Zona incerta, general | ||||
| Zona incerta (ZI) | + | ++ | + | + |
| Pretectal region | ||||
| Anterior pretectal nucleus (APN) | − | + | − | − |
| Midbrain reticular nuc., magnocellular part, general | ||||
| Midbrain reticular nucleus, magnocellular part (MRNm) | +++ | +++++ | ++ | ++++ |
| Cuneiform nucleus (CUN) | ++ | +++ | + | ++ |
| Pontine reticular nucleus, caudal part (PRNc) | − | + | − | − |
| Gigantocellular reticular nucleus (GRN) | − | ++ | − | − |
| Magnocellular reticular nucleus (MARN) | − | + | − | − |
| Parvicellular reticular nucleus (PARN) | − | + | − | − |
| 3.3.8. Motoneuron Groups | ||||
| Neuroendocrine motor zone | ||||
| Magnocellular | ||||
| Supraoptic nucleus, general | ||||
| Supraoptic nucleus, proper (SO) | − | − | +++ | + |
| Paraventricular nuc. hypothal., magnocellular division | ||||
| Posterior magnocellular part | ||||
| Lateral zone (PVHpml) | − | − | + | − |
| Medial zone (PVHpmm) | − | − | + | − |
| Parvicellular | ||||
| Paraventricular nuc. Hypothal., parvicellular division | ||||
| Anterior parvicellular part (PVHap) | ++ | + | ++ | ++ |
| Medial parvicellular part, dorsal zone (PVHmpd) | ++ | + | + | ++ |
| Periventricular part (PVHpv) | + | − | + | + |
| Periventricular hypothalamic nucleus, anterior part (PVa) | − | − | − | + |
| Periventricular hypothal. nuc., intermediate part (PVi) | − | − | + | + |
| Arcuate hypothalamic nucleus (ARH) | − | + | ++ | ++ |
Connections of the LHAjvd and LHAjvv determined from analysis of CTB + PHAL coinjection experiments. Data from two representative experiments are shown from injections that were centered within the LHAjvd (experiment LHA #77) or LHAjvv (experiment LHA #2). The relative magnitude of output connections (PHAL) is represented by the following semi-quantitative grading schema: - = absence of labeling; + = very low; ++ = low; +++ = moderate; ++++ = high; +++++ = very high. The relative magnitude of input connections (CTB) is represented by the following grading schema: - = absence of labeling; + = 2–8; ++ = 9–22; +++ = 23–44; ++++ = 45–79; +++++ ≥ 80. Regions that contained only PHAL-labeled axons with the appearance of axons of passage (that is, presumptive non-synapse forming), or regions that contained only a single CTB-labeled neuron, or single PHAL-labeled axon terminal, are not included in the table (for these details see Figure 4). The brain region hierarchy follows Swanson (2004).
Distinct brain regions with demonstrated involvement in the processing of polymodal information relevant to ingestive, agonistic, and reproductive behaviors are therefore linked by their common connection to the LHAjvd/v. This assertion prompts reiteration of a few related conceptual points that serve as a sort of leitmotif to this discussion. Firstly, experimental evidence associating a given brain region with one type of behavior is not evidence of its preclusion from other behaviors; secondly, ultimately this is because all behaviors result from patterned sequential activation of the motor system, which is evidenced by the fact that different behaviors may involve similar motor patterns. For example, the motor pattern necessary for locomotion may be engaged during ingestive, reproductive or agonistic goal-directed behaviors: Approaching a food source (ingestion), a mate (reproduction), or approaching/avoiding a prey/threat (aggression or defense).
Given that behavior generally serves to support survival in a dynamic environment (external and internal), this favors motor patterns that are attuned to environmental context, either through innate or learned mechanisms. In this regard substantial LHAjvd/v connections with the hippocampal formation (HPF; specifically the ventral part of hippocampal field CA1 and the subiculum) are noteworthy. The HPF has a role in episodic memory and spatial navigation (O'Keefe and Nadel, 1978; Squire, 1992; Morris, 2006; Buzsaki and Moser, 2013), with potential relevance to multiple behaviors. For example, and with respect to the current data, this is evidenced by indicated involvement of ventral field CA1 in ingestive (Kanoski et al., 2011; Hsu et al., 2014; Cole et al., 2015) and defensive (Kjelstrup et al., 2002; Pentkowski et al., 2006; Markham et al., 2010; Wang et al., 2013) behaviors; hippocampal involvement in reproductive behaviors is also suggested (Weiland et al., 1997; Woolley et al., 1997; Pawluski and Galea, 2006).
Complexity and diversity of hippocampal function is reflected in its underlying neural connections (intrinsic and extrinsic), which have an intricate topography (Groenewegen et al., 1987; Risold and Swanson, 1996; Witter, 2006; Cenquizca and Swanson, 2007). Especially relevant to the present data are hippocampal output connections to the lateral septal nucleus (LS) (Swanson and Cowan, 1976, 1977; Risold and Swanson, 1997b; Cenquizca and Swanson, 2007). The LS rostral part (LSr) has robust LHAjvd/v connections (present data); furthermore, a continuity of topographic organization exists such that discrete regions of the LS receiving from discrete hippocampal regions connect with discrete regions of the hypothalamus (Risold and Swanson, 1996, 1997b); this is also reflected in regional differences of LS chemoarchitecture (Risold and Swanson, 1997a). The present data extend the continuity of topographical relations to include direct hippocampal connections with the LHAjvd/v—as was also reported in our previously described connections of the LHAjd, LHAjp and LHAs (Hahn and Swanson, 2010, 2012).
Adding another layer of structural organization are recent genetic data indicating that distinct gene expression domains are superimposed on hippocampal connectional topography (Thompson et al., 2008; Dong et al., 2009; Fanselow and Dong, 2010). A model for how these domains relate to existing knowledge of structural and functional hippocampal organization was provided recently (Strange et al., 2014). In general terms the LHAjvd/v connections with the LSr and ventral field CA1/subiculum are consistent with their putative involvement in social behaviors—for example, social defensive behavior (Faturi et al., 2014); however, a deeper understanding will evidently require experimental investigations that take full account of the new genetic data.
Returning to a more focused consideration of LHAjvd/d connections in relation to specific behaviors, of additional note is the moderate bidirectional LHAjvd/v connection with the parvicellular part of the thalamic subparafascicular nucleus (SPFp). This connection was predominantly with the LHAjvd, and primarily an output from the LHAjvd to the SPFp medial division (SPFpm), although it also involved the SPFp lateral division. The SPFpm is indicated to play a role in sexual behavior, as demonstrated (for example) by elevated SPFpm Fos expression in female rats following intromission, and in male rats following ejaculation (Coolen et al., 1996, 1998)—a suggested interpretation is that the SPFp may play a role in post-copulation inhibition of sexual behavior (sexual satiety) (Veening and Coolen, 2014). Other brain regions showing a pronounced increase in Fos expression in rats following ejaculation include the MPN, BSTpr, and MEApd (Veening and Coolen, 1998). Given that these nuclei were substantially retrogradely labeled from the LHAjvd/v, it reinforces potential (and perhaps primary) involvement of the LHAjv region in reproductive behavior control.
In relation to this, it is relevant to review the generally light LHAjvd/v connection with the nucleus accumbens (ACB); this was essentially restricted to the caudal half of the ACB, mostly dorsomedial (ACBdm), and included a low to moderate amount of retrograde labeling from the LHAjvv. A current view of the ACBdm indicates rostral and caudal structure/function differences associated respectively with behavioral expressions of “liking/pleasure” (rostral) and “disliking/displeasure” (caudal) (Richard et al., 2013; Ho and Berridge, 2014). Moreover, a recent study reported a post-ejaculatory change in the electrophysiological profile of medial ACB neurons, consistent with their involvement in behavioral inhibition, although a rostral-caudal distinction was not drawn (Matsumoto et al., 2012). More generally, the need for careful correlation of ACB functional and structural data is emphasized by fine-grained topographic differences highlighted in recent reappraisals of ACB connections (Thompson and Swanson, 2010; Zahm et al., 2013).
Gene expression data and more recent optogenetic studies indicate a role for the LHAjvd/v in the broad control of social behaviors. Notably, androgen and/or estrogen receptors are highly expressed in each of the aforementioned reproductive-behavior related sites that connect with the LHAjvd/v (Simerly et al., 1990; Shughrue et al., 1997). Furthermore, it was shown recently that a member of the LIM/homeobox (Lhx) gene family (Lhx6) is expressed robustly in LHAjvd/v-connected AOB recipient sites, and in nuclei of the hypothalamic medial zone reproductive behavior related network (Choi et al., 2005); whereas other members of the Lhx gene family are expressed in regions of the LHAjvd/v-connected medial hypothalamic zone defensive behavior related network (Choi et al., 2005). More recently, optogenetic manipulation of estrogen receptor 1-expressing neurons in the VMHvl of male mice identified these neurons as an integrative locus for attack and mounting behavior (Lee et al., 2014). Together, these data support a putative role for the LHAjvd/v in the control of defensive/aggressive (agonistic), and especially reproductive behaviors.
With respect to the robust connections of the LHAjvd/v with the VMH, the dendritic morphology of the latter's neurons raises a salient point. In an elegant study, Eugene Millhouse described the morphology of VMH neurons labeled with the rapid Golgi method (Millhouse, 1973). The dendrites of VMH neurons were found to extend well beyond the boundary of the VMH, particularly ventrolateral (through the tuberal nucleus, to reach the pial surface), and also lateral (into the LHAjv region, extending to about the fornix) (Millhouse, 1973). A corollary of this topography is that synaptic input to the LHAjvd/v may be either to LHAjvd/v neurons, to the dendrites of VMH neurons, or to both. Moreover, this consideration applies elsewhere, not least elsewhere in the LHA, as is finely illustrated in other studies by Millhouse (1969, 1973, 1979) that show dendrites of LHA neurons (including those in the LHAjv region) extending beyond the boundaries of their parent regions (as defined by the current LHA parcellation). In addition, the spatial arrangement of VMH dendrites reported by Millhouse (1973) is supported in the current study by the presence of at least a few PHAL-labeled neuronal cell bodies in the VMH and LHAjv region neighbors following LHAjvd/v-targeted injections, even when the injection site was restricted to the LHAjvd/v (Figure 1). These considerations draw attention to a previous PHAL study of the VMH, not only because of the possible inclusion of the neighboring LHA, but also because of several similarities in the outputs of the VMHvl and the LHAjv region (e.g., to the BSTpr, MPO, and parts of the CEA, MRN, and SPFp) (Canteras et al., 1994). Moreover, in addition to the discussed association with reproductive behaviors, it is noteworthy that the VMHvl and LHAjv regions constitute a substantial portion of a hypothalamic region whose stimulation is associated with aggressive behavioral responses (Hrabovszky et al., 2005), giving additional weight to a possible role for the LHAjv region in control of the aggressive component of agonistic behavior.
The foundational relation of neuronal architecture to function is given further emphasis by another LHAjv region hypothalamic connection, as it relates to axon morphology. As noted earlier, axon varicosities can be sites of synaptic terminals of passage (boutons en passant) (Wouterlood and Groenewegen, 1985; Thomson et al., 1996). Apropos of this point, and deserving of extended discussion, is a cluster of retrograde labeling in the supraoptic nucleus (SO) from the LHAjvv (but not the LHAjvd) (Figures 4R–V); incidentally, an injection site restricted mostly to the tuberal nucleus (our experiment LHA #67, Figure 1) also resulted in substantial SO retrograde labeling (not shown)—these results are consistent with a recent CTB retrograde tracing study in the rat (Toth et al., 2010).
Almost (if not) all SO neurons in the rat are neuroendocrine and express either vasopressin (VAS) or oxytocin (OXY) (Swanson, 1986; Markakis and Swanson, 1997). Axon collaterals of SO neurons with numerous varicosities are reported to exist (Mason et al., 1984), but most are oriented dorsal and dorsolateral to the SO and do not extend far from it (rarely reaching more than a few hundred microns beyond the nucleus) (Mason et al., 1984). Nevertheless, longer non-branching axons of SO neurons en route to the posterior pituitary are also reported to possess numerous varicosities, giving them a beaded appearance (Bargmann and Scharrer, 1951; Randle et al., 1986). Beaded VAS-expressing axons coursing along the same pathway (including through the LHAjvv and tuberal nucleus) have also been reported (DeVries et al., 1985). It is of additional note that neurosecretory axons within the posterior pituitary are beaded too (Bargmann and Scharrer, 1951), and form synaptic terminals of passage (Tweedle et al., 1989). Taken together, these data are consistent with magnocellular neuroendocrine axon varicosities as sites of potential synaptic contact, and support the existence of a little-investigated non-neuroendocrine connection of the supraoptic nucleus.
Of related note are two previous retrograde-tracing studies which indicate that a lateral region of the retrochiasmatic area (just dorsal to the optic tract) has bidirectional SO connections (Thellier et al., 1994a,b); in addition, in one of these studies (Thellier et al., 1994a), following separate injections of four different retrograde tracers into the SO, tracer-labeled neurons were not reported in the LHA region corresponding to the LHAjvv, consistent with the present PHAL data (in that we found no evidence of LHAjvv (or LHAjvd) input to the SO). It is not known at this time whether the neurons we found retrogradely labeled in the SO from the LHAjvv expressed VAS or OXY. However, existing knowledge of the relative distribution VAS- and OXY-expressing SO neurons indicates in general terms that dorsomedial and ventrolateral SO neurons appear to mostly express VAS at rostral and caudal levels respectively (vice versa for OXY), with more intermingling at mid levels (Hou-Yu et al., 1986). On this basis, it's possible that both OXY and VAS expressing neurons were retrogradely labeled from the LHAjvv (Figures 4R–V).
Additional perspective is provided by recalling that neuroendocrine neurons projecting to the posterior pituitary are present in (in addition to the PVH and SO) several sites not thought of as classically neuroendocrine, including the BST, MPO, LPO, ZI, and LHA regions lateral to the fornix (Kelly and Swanson, 1980). This highlights the potential for non-neuroendocrine signaling of otherwise neuroendocrine neurons. In the present study this possibility is supported not only by SO retrograde labeling from the LHAjvv, but also by retrograde labeling from the LHAjvd/v in several divisions of the PVH (Figures 4R–W); we also found PVH retrograde labeling in our previous analysis of LHAs, LHAjp, and LHAjd connections (Hahn and Swanson, 2010, 2012). Exemplifying further the potential contribution to behavioral control of dual neuroendocrine/non-neuroendocrine signaling is a recent study reporting retrograde labeling of VAS-expressing neurons in the PVH and SO following injections of the retrograde tracer Fluoro-Gold into dorsal HPF fields CA1-3 (Zhang and Hernandez, 2013).
To round out this section of the discussion, it is fitting to consider the possible functional role of putative SO input to LHAjvv neurons. An interesting possibility is raised by studies indicating a significant increase in the release of OXY (but not VAS) from SO neurons in subordinate male rats encountering dominant aggressive conspecifics (Wotjak et al., 1996; Engelmann et al., 1999). This finding is made more intriguing by OXY microdialysis data obtained in one of the studies, which revealed a significant encounter-associated increase in OXY in the subordinates in a hypothalamic region just medial to the SO and dorsal to the optic tract (Engelmann et al., 1999)—a region adjacent to the LHAjvv, and within the path of OXY axons en route to the posterior pituitary (Armstrong, 1995); moreover, the increase in OXY release in this region was up to ~320% over basal levels (about double the maximum increase measured from within the SO) and, strikingly, was not associated with an increase in plasma levels of OXY (Engelmann et al., 1999). In the same model, a significant increase in VAS but not OXY release in the PVH was measured (also dissociated from neuroendocrine release) (Wotjak et al., 1996). Neurons we found retrogradely labeled in the PVH from the LHAjvv included some within the magnocellular PVH divisions in which OXY and VAS neurons predominate (Figures 4U,V) (Simmons and Swanson, 2009).
Combining these data elucidates a novel potential coordinating link between defensive and reproductive behaviors consisting of VAS/OXY PVH/SO neurons activated in association with defensive behavioral responses, and their downstream connection to the LHAjvv, which is well placed via its connections to influence reproductive behavior. This possibility is enhanced by evidence in the rat of a direct input from the AOB to a region immediately lateral to the SO in which a high density of SO-neuron dendrites are present (Smithson et al., 1992); in the same study no AOB input to PVH was found (Smithson et al., 1992).
In this context and in relation to the earlier discussion of LHAjv region connections with sites activated in response to sexual behavior (notably ejaculation), established links between OXY release and male sexual behavior are noteworthy. Thus, it is suggested that central actions of OXY may include post-ejaculatory inhibition of sexual behavior (Stoneham et al., 1985). Consistent with this suggestion, OXY-expressing SO neurons show elevated post-ejaculatory levels of Fos, correlated to the rapidity of ejaculation (Pattij et al., 2005); also consistent with this suggestion is increased excitation of OXY-expressing SO neurons in male rats by electrical stimulation of the penile nerve (Honda et al., 1999). However, a dichotomous note is struck by a positive association of neuroendocrine OXY release and sexual behavior (Stoneham et al., 1985), and a putative role for OXY-expressing PVH neurons in penile erection (Veronneau-Longueville et al., 1999).
Shifting focus to discussion of other LHAjvd/v connections in relation to their putative involvement in control of fundamental behaviors, several LHAjvd/v-midbrain connections are worth noting. Foremost is a dense and extensive (mostly LHAjvd) input to the PAGvl (predominantly to its dorsal half), and a substantial input to the PAGd. A current structure/function model of the neuronal network underlying defensive behavioral responses to stimuli indicative of a threat (that is, “fear” responses) co-relates specific PAG regions with behavioral responses to particular threat stimuli (Gross and Canteras, 2012; Canteras and Graeff, 2014). In this model, which is based largely on experimental data from male rats, the PAGd and dorsal half of the PAGvl are indicated to play a role in mediating defensive responses to interoceptive and social threat stimuli (for example and respectively, visceral pain and an aggressive conspecific); whereas the PAGvl (but not the PAGd) and PAGdl are indicated (respectively) to mediate defensive responses to non-visceral pain (for example footshock; PAGvl), and existential threats (for example a predator; PAGdl) (Gross and Canteras, 2012; Canteras and Graeff, 2014).
Such a PAG division of function in relation to defensive behavior could be seen from a Darwinian perspective to align with an indicated role for the LHAjvd/v in control of reproductive behavior, as follows: If activation of LHAjvd/v to PAGvl and PAGd connections increases the threshold for activation of defensive behavioral responses to non-life threatening painful, interoceptive, or social stimuli (which require activation of the PAGvl and/or PAGd) concurrently with activation of other LHAjvd/v connections that promote reproductive behavior, conceivably this could increase the probability of reproduction; whereas, when LHAjvd/v connections involved in promoting reproductive behavior are not active, a concurrent reduction in the threshold for activation of the same defensive responses could benefit survival. Furthermore, inhibition of defensive responses to the existential threat of a predator would have less obvious benefit, whether or not an animal was engaging in reproductive behavior, which is consistent with a very sparse LHAjvd/v to PAGdl connection. Similar hypotheses might also be developed in relation to indicated LHAjvd/v involvement in aggressive behavior (Hrabovszky et al., 2005; Toth et al., 2010).
Also in the midbrain, the LHAjv region (mostly LHAjvd) targets extensively the magnocellular part of the midbrain reticular nucleus (MRNm), and the deep gray layer of the superior colliculus (SCdg); fairly extensive LHAjvd connections with the cuneiform nucleus were also apparent, as was a circumscribed LHAjvd/v connection with the nucleus of the lateral lemniscus (NLL). The LHAjvd/v-MRNm connection is very heterogeneous in terms of its distribution and directionality. At rostral MRNm levels, retrograde labeling from the LHAjvd/v was present in a far lateral MRNm region, ventral to the SPFpl, and intermingled with sparsely distributed of PHAL-labeled axons (Figures 4GG–II). At a mid-rostrocaudal MRNm level, a very dense LHAjvd input to a more central region of the MRNm was evident (Figure 4JJ), surrounded by a less dense but more extensive input that extended caudally (Figures 4KK–MM).
In a previous paper, we discussed the MRNm in relation to its light connections with the LHAjd, which are distinct from and less substantial than MRMm-LHAjvd/v connections (Hahn and Swanson, 2012). As a component of the reticular formation within the motor system, the MRN shares the same classification as the entire hypothalamic lateral zone. This close association is reflected in the diversity of indicated MRN structure and function, and also by a general lack of understanding and absence of consensus regarding both (Rye et al., 1987, 1988; Steininger et al., 1992; Inglis and Winn, 1995; Jhou et al., 2009; Kita and Kita, 2011). The present data underscore the need for investigation of subregional MRN differences.
For the LHAjvd connections with the SCdg, CUN and NLL, somewhat more elaborated associations to control of fundamental behaviors can be drawn. The SC has an established role in orienting movements (notably of the eye and head) to various stimuli (Dean et al., 1989); these include visual stimuli conveyed directly to superficial layers of the SC from retinal ganglion cells (Simon and O'Leary, 1992; Hattar et al., 2006; Morin and Studholme, 2014), and indirectly from visual areas of the cerebral cortex (Kasper et al., 1994); deeper SC layers receive and integrate sensory information from multiple sensory modalities, including visual, auditory, and somatosensory (Meredith and Stein, 1986; Stein et al., 2009).
A recent SC retrograde tracing study in rats suggested the existence of a medial and lateral SC division that the authors correlated respectively to behaviors associated with avoidance (such as defensive avoidance of a predator) and approach (such as aggressive approach of a prey, or social approach) (Comoli et al., 2012); these differences were also correlated to somatosensory and visual sensory inputs, such that input from the upper visual field was correlated to avoidance/medial SC, and input from the lower visual field, and somatosensory input from vibrissae, to approach/lateral SC (Comoli et al., 2012). However, the connections of the specific SCdg region that we have identified here as an LHAjvd-recipient site, were not investigated (Comoli et al., 2012). For comparison, a recent study in macaques reported defensive avoidance behaviors evoked in response to microinjection of the GABAA receptor antagonist bicuculline methiodide at medial and lateral intermediate- and deep layer SC sites (DesJardin et al., 2013). Additional cross-species comparative analysis of topographic differences in SC circuitry may help to elucidate further its functions in relation to behavior.
Several studies indicate a role for the cuneiform nucleus in relation to defensive behavioral responses. For example, rats that engage in defensive “freezing” when placed in a context previously paired with a painful footshock show markedly elevated levels of cuneiform nucleus c-Fos expression compared to controls (Carrive et al., 1997). Similarly, a significant increase in cuneiform nucleus c-Fos expression is seen in rats exhibiting defensive behavior in response to predator-associated odor exposure (Dielenberg et al., 2001). With respect to the earlier discussion concerning the PAG, it is relevant to note a major cuneiform nucleus to PAG connection, with the rostral part targeting primarily the PAGdl, and the caudal part primarily the PAGd and dorsal half PAGvl (Netzer et al., 2011)—our data indicate the LHAjv region (primarily LHAjvd) sends an output to all levels of cuneiform nucleus.
The cuneiform nucleus to PAG connection is also indicated to play an essential role in the physiological changes associated with acute defensive responses, which include increased heart rate and blood pressure, related to inhibition of the baroreceptor reflex. For example, rostral cuneiform nucleus microinjection of the GABAA receptor antagonist bicuculline methiodide results in elevated blood pressure and heart rate, baroreceptor reflex inhibition, and elevated c-Fos in the PAGdl (Netzer et al., 2011); whereas, blockade of GABAA receptors in cuneiform nucleus combined with activation of GABAA receptors in the PAGdl prevents the baroreceptor reflex inhibition, and the cardiovascular changes (Netzer et al., 2011). Another central component of the circuitry involved in these responses is the DMH. Thus, pharmacological DMH disinhibition with bicuculline generates cardiovascular effects comparable to those associated with similar disinhibition of the cuneiform nucleus (McDowall et al., 2006; Netzer et al., 2011). It is noteworthy that these effects are also produced by bicuculline disinhibition of a region corresponding to the LHAs (McDowall et al., 2006). In addition, and as noted for the cuneiform nucleus, DMH c-Fos expression in rats is significantly elevated following predator-associated odor exposure that also generates defensive behavioral responses (Dielenberg et al., 2001).
Previous investigation of DMH outputs with discrete PHAL injections elucidated a very light connection to the cuneiform nucleus (Thompson et al., 1996); in the same study, the presence of numerous DMH-originating axons were noted within the LHA, including in the LHAjv region, but their input to the LHAjv region was considered to be sparse (Thompson et al., 1996); in contrast, the presence of extensive DMH retrograde labeling from the LHAjv region in the present study is indicative of a rather substantial DMH to LHAjv connection. Nevertheless, a dense DMH to perifornical region connection described previously (Thompson et al., 1996) does concur with our previous finding of substantial DMH retrograde labeling from the LHAs (Hahn and Swanson, 2010).
In the earlier discussion of LHAjv-PAG connections, we mentioned a recent model that ascribes different roles to different PAG divisions in relation to different threat stimuli (Gross and Canteras, 2012; Canteras and Graeff, 2014). In the most recent iteration (Canteras and Graeff, 2014), the DMH is included in this model as a mediator of interoceptive threats. This is supported by converging lines of experimental evidence. For example, pronounced DMH c-Fos expression occurs in rats exposed briefly to an environment containing hypercarbic gas (20% CO2, 21%O2, 59% N2) (Johnson et al., 2011). In addition, rats receiving an innocuous infusion of sodium lactate after receiving DMH injection of a GABA synthesis inhibitor (l-allylglycine) show elevated heart rate and blood pressure (Shekhar et al., 1996; Johnson et al., 2008), and also reduced social interaction (Johnson et al., 2008); and reduced exploratory behavior (Shekhar et al., 1996).
Comparisons have been drawn between the effects of DMH disinhibition paired with sodium lactate infusion in rats, and the tendency of sodium lactate infusion to precipitate comparable physiological changes in humans predisposed to panic attacks (Cowley and Arana, 1990). It is noteworthy that pharmacological inhibition of DMH GABAergic signaling in rats without infusion of sodium lactate also reduces social interaction (Johnson et al., 2008) and exploratory behavior (Shekhar, 1993), and increases heart rate and blood pressure (Shekhar, 1993; Keim and Shekhar, 1996; Shekhar et al., 1996); furthermore, DMH injection of bicuculline also increases plasma levels of ACTH, corticosterone and noradrenalin; whereas DMH injection of the GABAA agonist muscimol has opposite physiological and behavioral effects (Shekhar, 1993; Shekhar et al., 1993).
The DMH forms a highly interconnected network with five additional hypothalamic nuclei located at preoptic levels of the hypothalamus [AVPV, median preoptic (MEPO)-, anterodorsal/ventral preoptic (ADP/AVP)-, and parastrial (PS) nuclei] (Thompson and Swanson, 2004) (see their Figures 2, 12). It is suggested these six nuclei may constitute a central neural substrate for the generation of visceromotor patterns. Aside from their striking interconnectedness, this is critically supported by their uniquely differentiated output connections to visceromotor-related cell groups: These include neuroendocrine neurons within the hypothalamic neuroendocrine motor zone, and preautonomic PVH neurons (Thompson and Swanson, 2004) (see their Figure 11). Collectively, inputs to these “visceromotor” nuclei are prominent from the rostral hypothalamic behavior control column, and from extrahypothalamic regions notable for their involvement in the processing of interoceptive (viscerosensory and humerosensory) information (Thompson and Swanson, 2004) (see their Figure 15). Interestingly, all six nuclei also provide an input to the LHAjvd/v (most notably the DMH, AVPV and MEPO) but only one receives appreciable LHAjvd/v input: The DMH (Table 1). An additional and more direct influence of interoceptive information on the LHAjv region is evidenced by sparse retrograde labeling from the LHAjvd in the NTS (viscerosensory, Figures 4AAA,BBB) and the SFO (humerosensory, Figure 4R), and moderate retrograde labeling from the LHAjvd/v in the lateral division of the parabrachial nucleus (viscerosensory, Figures 4QQ–TT) (see also Table 1).
These considerations lead to the recognition that the LHAjvd/v appears well placed to integrate polymodal interoceptive information (via its input connections from the DMH and the other “visceromotor” nuclei), and to convey this information (via its output connections) not only to the behavior control column, but also to midbrain regions associated with behavioral initiation (PAG, cuneiform nucleus). Given the prevailing association of LHAjvd connections with reproductive behavior in the discussion so far, and the earlier-developed hypothesis suggesting circumstances in which defensive behavior might be suppressed for the sake of reproductive behavior; the presentation here of a route whereby elevated viscerosensory threats (homeostatic challenges) could inhibit the LHAjvd/v presents an interesting opposite possibility (which might apply also in regard to the earlier discussed LHAjv inputs associated with ingestive behavior). Furthermore, it was noted earlier that LHAs and DMH disinhibition with bicuculline appears to generate similar cardiovascular and behavioral effects (McDowall et al., 2006; Netzer et al., 2011). This association was recently noted and incorporated into a model for detection and response to interoceptive and social threat stimuli (Canteras and Graeff, 2014) (see their Figure 1); possible LHAjd involvement (which receives DMH input) (Thompson and Swanson, 1998; Hahn and Swanson, 2012) was also suggested. The present data argue for inclusion of the LHAjv region in this model.
The final LHAjvd/v-midbrain connection to be discussed here is the aforementioned connection with the NLL; this is mostly with the LHAjvd and includes an output to the NLL dorsal part (NLLd, Figures 4NN–PP) and an input from the NLL ventral part (NLLv), mostly from a cluster of neurons located dorsally in the NLLv (Figures 4MM–QQ). In light of the discussion so far, this connection, despite its relative lightness, is especially interesting. The NLL is central to auditory processing, receiving major input from the cochlear nucleus and sending a major output to the inferior colliculus (Gonzalez-Hernandez et al., 1996; Cant and Benson, 2003; Pollak et al., 2003). A role for the NLL in reflex acoustic startle is indicated, but the precise NLL region contributing to this reflex is a matter of debate (Davis et al., 1982; Lee et al., 1996). Furthermore, a link to social behavior is indicated by the presence of androgen receptors in the rat NLLv (Simerly et al., 1990). It therefore seems relevant to consider further investigation into the role of the NLL in relation to auditory processing associated with social behaviors, and in relation to the specific topography and neurochemistry of NLL connections.
The extrinsic connections of the LHAjv region are remarkably numerous. In terms of the number of LHAjv-connected gray matter regions, the only comparable examples of this level of connectivity are for the LHAjp, LHAjd, and LHAs (Table 2). Notwithstanding the discussion so far, this exceptional level of connectivity cautions against prediction of functional roles—a caution reinforced by the existence as well of extensive LHA intrinsic connections (Figure 11). In addition, although we have highlighted in this discussion some of the more evident functional associations of the LHAjv region network, a comprehensive model will require synthesis of the available knowledge on each of the several hundred identified LHAjvd/v connections—a non-trivial task. Toward this goal, a high-level perspective is provided by picturing the ratio of LHAjv region connections within the framework of a foundational four subsystem structure-function network model of the nervous system (Swanson, 2000). Placing the present data into this network model makes it apparent that the vast majority of LHAjvd and LHAjvv connections are with the motor and cognitive subsystems (Figure 12), as we also found for the LHAjp, LHAjd, and LHAs (Hahn and Swanson, 2010, 2012). The findings represented in Figure 12 also highlight another general characteristic of LHAjv region connectivity, namely abundant bidirectionality, which is also reflected at the level of individual gray matter regions (Table 1, Figures 3, 4).
Table 2.
Connectivity comparison of selected LHA regions.
| Number of connected regions | |||
|---|---|---|---|
| Outputs | Inputs | Total | |
| LHAjp | 123 | 114 | 237 |
| LHAjd | 154 | 165 | 319 |
| LHAs | 128 | 130 | 258 |
| LHAjvd | 104 | 155 | 259 |
| LHAjvv | 104 | 137 | 241 |
| ACBdmt | 2 | 5 | 7 |
The number of regional extrinsic input and output connections of selected LHA regions is shown. The data was obtained in the current study, and from previously published works (Hahn and Swanson, 2010, 2012); it is representative of the connections of the selected LHA regions as determined from the following experiments: LHAjp (Exp. #22), LHAjd (Exp. #34), LHAs (Exp. 11), LHAjvd (Exp. #77), and LHAjvv (Exp. #2). For comparison (and contrast) the number of connected regions of the ACBdm dorsal tip (ACBdmt) is also shown (Thompson and Swanson, 2010).
Figure 11.
Matrix of lateral hypothalamic zone connections for all regions of the LHA medial and perifornical tiers in the rat. Data were obtained from the present and previous studies (Goto et al., 2005; Hahn and Swanson, 2010, 2012). The analysis is restricted to PHAL & CTB data that were mapped directly to the current LHA parcellation. The relative magnitude of output connections for the LHAjp, −jd, −jvd, −jvv, −s, −sfa, and −sfp (PHAL data) and output connections for other parts of the lateral hypothalamic zone (CTB data) are represented by colored squares, according to the five-grade color scale shown, from lowest (blue) to highest (red) (following the scale used in Table 1). Numbered squares indicate the magnitude of retrograde (CTB) labeling (1 = lowest to 5 = highest) for the same direction of connection identified anterogradely with PHAL. Gray squares indicate that analysis was performed but no connection was found. White squares indicate the analysis has not been done. The symbols “*” or “**” indicate input (PHAL) was weak (*) or moderate (**), but the subregional distribution was not described.
Figure 12.
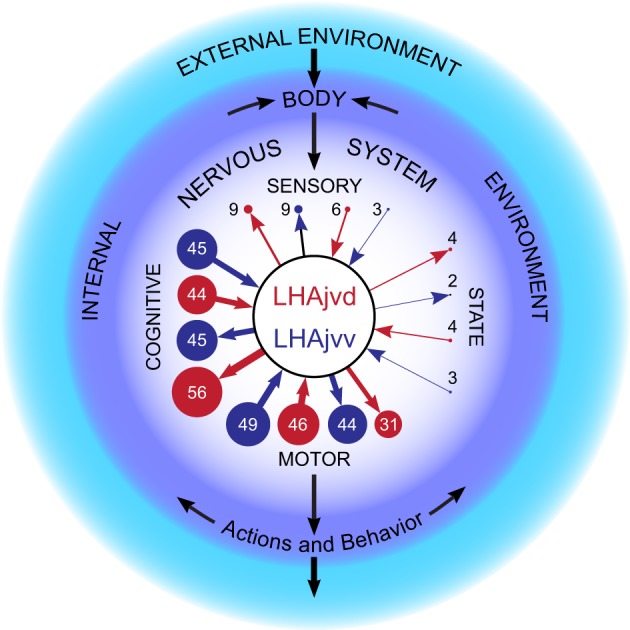
LHAjv region subsystem connections. The relative magnitude of LHAjvd (red) and LHAjvv (blue) connections with the sensory, state, cognitive and motor subsystems of the nervous system, presented within in the context of a basic schematic for nervous system functional organization (adapted from Swanson, 2000). Numbers are the percentage for each subsystem (within the central nervous system) of total inputs and outputs (in terms of summated magnitude) (Table 11). The percentages are also represented by the diameter of the discs, and more approximately by arrow line thickness. Note the preponderance of connections with the cognitive and motor systems, and also the bidirectional nature of connections with all subsystems. This figure is also available as a separate vector graphics file (Figure S12).
Behavioral expression involves the entire nervous system, but it ultimately depends on input from the motor subsystem, which in turn receives input from sensory, cognitive, and behavioral state subsystems (Swanson, 2000). Given that the vast majority of LHAjvd/v connections are with the cognitive and motor subsystems (Figure 12), it follows that LHAjvd/v neurons may play a direct integrating role for information relating to both. Moreover, the extensive bidirectionality of the connections suggests an immediate feedback mechanism. An additional perspective, which also speaks to underlying organizing principles, may be gained by viewing LHAjvd/v connections in the context of an already alluded to structural model for cerebral hemisphere control of motivated behavior (Swanson, 2000).
In relation to the present discussion, the crux of this model is threefold: Firstly, hypothalamic medial zone nuclei form a control column for fundamental goal-oriented behaviors (ingestive, defensive, and reproductive); secondly, the cerebral hemisphere supplies a tripartite descending input to the control column from the cerebral cortex, striatum, and pallidum; thirdly, control column nuclei output to lower levels of the motor subsystem and also (primarily via the thalamus) to the cerebral cortex (Swanson, 2000). In terms of its connections, the LHAjv region conforms to this general model (Table 1, Figure 3). In addition, suggested or indicated functional differentiations for the LHAjvd/v and other medial- and perifornical tier LHA regions (Goto et al., 2005; Hahn and Swanson, 2010, 2012; Betley et al., 2013; Faturi et al., 2014) are congruent with similar functional differentiations for the hypothalamic medial zone behavior control column nuclei (Canteras et al., 1997; Risold et al., 1997; Swanson, 2000; Canteras, 2002). Collectively, these comparisons place the LHA firmly within an existing structure-function model, and point to an indicated role as a coordinator of the behavior control column, and by extension a control-coordinator of fundamental behaviors.
An integrative “high level” behavior coordinating role for the LHAjv region is suggested further by its major bidirectional connections with the BSTif and BSTam (Figure 3). Thorough analysis of the output connections of both indicate they play a controlling or coordinating role in a wide variety of fundamental processes, including (for the BSTif) the control of social behavior (Dong and Swanson, 2004b), and (for the BSTam) the coordination of motor output (neuroendocrine, autonomic, and somatic) relating to energy homeostasis (Dong and Swanson, 2006a). In fact, the outputs of the BST as a whole are practically as diverse as those of the LHA (Dong et al., 2001b; Dong and Swanson, 2003, 2004a,b, 2006a,b,c). To this list might also be added the DMH that is similarly diverse in terms of its inputs, outputs, and implicated functional roles (see earlier discussion) (Thompson et al., 1996; Thompson and Swanson, 1998, 2003). Future retrograde tracing studies of the BST, using the same reference atlas and approach we have applied to the LHA would enable (in combination with existing and present connectivity data) a comprehensive network comparison of the BST and LHA—two similarly diverse and highly differentiated regions.
In addition to knowledge of connections, functional modeling of neuronal networks clearly also requires knowledge of the underlying neurochemistry—in particular the neurotransmitters and their receptors. While it is clear that GABA is the predominant neurotransmitter for extrinsic output connections of the cerebral nuclei (BST included), and glutamate (GLU) for the cerebral cortex (Swanson, 2000), the picture for the LHA is (perhaps unsurprisingly) more complex (Ziegler et al., 2002; Meister, 2007). The challenging complexity is exemplified by the largely LHA-expressed neuropeptides melanin-concentrating hormone (MCH) and hypocretin/orexin (H/O), which are present in intermingled populations of LHA neurons that extend over several LHA regions, including the LHAjvd/v (Swanson et al., 2005; Hahn, 2010). Similarly, neuronal expression of GABA and glutamate is indicated in intermingled populations of LHA neurons within the LHAjv region (although GABA appears to predominate) (Hrabovszky et al., 2005) (see their Figures 2H,I).
Furthermore, MCH and H/O neurons both appear to coexpress either GABA or GLU, but not uniformly: Most (but not all) MCH neurons are indicated to co-release GABA (Elias et al., 2001; Del Cid-Pellitero and Jones, 2012; Jego et al., 2013), but not GLU (Del Cid-Pellitero and Jones, 2012); whereas H/O neurons are indicated to co-release GLU but not GABA (Henny et al., 2010). In addition, a subpopulation of GABAergic (glutamic acid decarboxylase 65-expressing) LHA neurons appear to coexpress neither MCH nor H/O (Karnani et al., 2013). The picture is further complicated by coexpression of neuropeptides. For instance, most (but not all) MCH neurons appear to coexpress cocaine/amphetamine-regulated transcript (CART) (Broberger, 1999; Elias et al., 2001; Cvetkovic et al., 2004), and most (if not all) coexpress the satiety-related neuropeptide nesfatin-1 (Fort et al., 2008); whereas H/O neurons coexpress neither neuropeptide (Elias et al., 2001; Foo et al., 2008; Fort et al., 2008).
Moreover, the neuropeptide gene expression landscape of the LHA is highly pliable. For example, dehydration in rats (brought about by hypertonic saline) results in the neuronal expression of corticotropin-releasing hormone (CRH) in several LHA regions that do not express CRH in euhydrated rats (Kelly and Watts, 1996, 1998; Kay-Nishiyama and Watts, 1999); these include LHA regions in which MCH and H/O are expressed (Swanson et al., 2005; Hahn, 2010), but there is very little co-expression of CRH with either neuropeptide (Watts and Sanchez-Watts, 2007). However, under the same conditions there is a high level of LHA co-expression of CRH and neurotensin (Watts and Sanchez-Watts, 2007). Moreover, most of the dehydration-associated LHA CRH expression occurs in a restricted dorsomedial subregion of the LHAd, with no appreciable CRH expression in the LHAjv region (Watts and Sanchez-Watts, 2007) (see their Figure 2); nevertheless, at least some neurons in this region of the LHAd send an input to the LHAjv region (Figures 4W,X), and overall the LHAd provides a moderate input to the LHAjvd/v (Table 1; compare also our Figure 11 with Figure 2 in Watts and Sanchez-Watts (2007) for review of other dehydration associated CRH-expressing LHA regions in relation to intra-LHA connections).
Concluding remarks
For a defined gray matter region, the level of LHAjv region extrinsic connectivity is unprecedented. Nevertheless, the connections of the LHAjvd and LHAjvv evidently have a distinct organization (Figure 3). From a systems perspective, they are dominated by bidirectional connections with the motor and cognitive subsystems (Figure 12). At a finer level, they receive substantial input from the cerebral cortex, and cerebral nuclei, as well as providing to both a lesser input (but more substantial for the LHAjvv). They also both have substantial bidirectional connections with medial hypothalamic behavior control column nuclei, and also receive input from hypothalamic nuclei associated with neuroendocrine and visceromotor control. Furthermore, both the LHAjvd and LHAjvv have substantial connections with midbrain regions associated with motor pattern initiation. In essence, the LHAjv region interfaces multiple neural networks necessary for control of voluntary and innate behavior.
With regard to specific behaviors, involvement of LHAjv region connections in control of reproductive behaviors is strongly suggested, but this appears to be integrated with subsidiary involvement in agonistic (defensive/aggressive) and ingestive behaviors. The extent of this involvement appears to be very broad, encompassing somatic, autonomic, and endocrine components of behavior. Similar considerations were raised in our previous investigations of LHAjp, LHAjd, and LHAs connections (Hahn and Swanson, 2010, 2012). These considerations lead us to hypothesize the LHAjv region may constitute part of an LHA hub for controlling the coordination of fundamental behaviors. Integral to this model, in addition to LHA extrinsic connections, are intra-LHA connections (Figure 11). In relation to this, a relevant comparison is with cerebral cortical hubs identified in a very recent informatics analysis of the cerebral cortical association connectome (Bota et al., 2015).
Clearly there is much that remains to be discovered about the structure, organization, and function of LHA neuron populations, and about how the dynamic interplay of these neuron populations within the nervous system as a whole contributes to coordination and control of the rich diversity of behaviors that enable animals to survive and reproduce within their changing and often challenging environments. Rather than attempting an exhaustive and all-inclusive treatment of the several hundred LHAjv region connections identified in this study, we have endeavored to provide a representative overview. Consequently, we realize there may be certain LHAjvd/v connections described in the Results that are of particular interest to individual readers that we have discussed only in passing, or not at all. Nevertheless, the above discussion encompasses a range of perspectives. We hope it sparks associations we may have missed or not addressed, and by the connections we have drawn, illuminates paths to further fruitful research and understanding of the LHA, the brain, and the nervous system.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by NIH grant NS16686.
Glossary
Abbreviations
- AAA
anterior amygdalar area
- ab
angular bundle
- ACAd
anterior cingulate area, dorsal part
- ACAv
anterior cingulate area, ventral part
- ACB
nucleus accumbens
- aco
anterior commissure, olfactory limb
- act
anterior commissure, temporal limb
- AD
anterodorsal nucleus, thalamus
- ADP
anterodorsal preoptic nucleus
- AHA
anterior hypothalamic area
- AHNa
anterior hypothalamic nucleus, anterior part
- AHNc
anterior hypothalamic nucleus, central part
- AHNd
anterior hypothalamic nucleus, dorsal part
- AHNp
anterior hypothalamic nucleus, posterior part
- Alp
agranular insular area, posterior part
- alv
alveus
- Alv
agranular insular area, ventral part
- amc
amygdalar capsule
- AMd
anteromedial nucleus, thalamus, dorsal part
- AMv
anteromedial nucleus, thalamus, ventral part
- APN
anterior pretectal nucleus
- AQ
cerebral aqueduct
- AQc
cerebral aqueduct, collicular recess
- ARH
arcuate hypothalamic nucleus
- AT
anterior tegmental nucleus
- AV
anteroventral nucleus, thalamus
- AVP
anteroventral preoptic nucleus
- AVPV
anteroventral periventricular nucleus
- B
Barrington's nucleus
- BA
bed nucleus accessory olfactory tract
- BAC
bed nucleus anterior commissure
- bic
brachium of the inferior colliculus
- BLAa
basolateral amygdalar nucleus, anterior part
- BLAp
basolateral amygdalar nucleus, posterior part
- BMAa
basomedial amygdalar nucleus, anterior part
- BMAp
basomedial amygdalar nucleus, posterior part
- BSM
bed nucleus stria medullaris
- BSTal
bed nuclei stria terminalis, anterior division, anterolateral area
- BSTam
bed nuclei stria terminalis, anterior division, anteromedial area
- BSTd
bed nuclei stria terminalis, posterior division, dorsal nucleus
- BSTdm
bed nuclei stria terminalis, anterior division, dorsomedial nucleus
- BSTfu
bed nuclei stria terminalis, anterior division, fusiform nucleus
- BSTif
bed nuclei stria terminalis, posterior division, interfascicular nucleus
- BSTju
bed nuclei stria terminalis, anterior division, juxtacapsular nucleus
- BSTmg
bed nuclei stria terminalis, anterior division, magnocellular nucleus
- BSTov
bed nuclei stria terminalis, anterior division, oval nucleus
- BSTpr
bed nuclei stria terminalis, posterior division, principal nucleus
- BSTrh
bed nuclei stria terminalis, anterior division, rhomboid nucleus
- BSTse
bed nuclei stria terminalis, posterior division, strial extension
- BSTtr
bed nuclei stria terminalis, posterior division, transverse nucleus
- BSTv
bed nuclei stria terminalis, anterior division, ventral nucleus
- CA1
field CA1, Ammon's horn
- CA1slm
field CA1, lacunosum-moleculare
- CA1so
field CA1, stratum oriens
- CA1sr
field CA1, stratum radiatum
- CA1spd
field CA1, pyramidal layer, deep
- CA1sps
field CA1, pyramidal layer, superficial
- CA2so
field CA2, stratum oriens
- CA2sp
field CA2, pyramidal layer
- CA3so
field CA3, stratum oriens
- CA3sp
field CA3, pyramidal layer
- cc
corpus callosum
- ccg
corpus callosum, genu
- ccr
corpus callosum, rostrum
- CEAc
central amygdalar nucleus, capsular part
- CEAl
central amygdalar nucleus, lateral part
- CEAm
central amygdalar nucleus, medial part
- cic
inferior colliculus commissure
- CL
central lateral nucleus, thalamus
- CLA
claustrum
- CLI
caudal linear nucleus, raphe
- CM
central medial nucleus, thalamus
- COAa
cortical amygdalar area, anterior part
- COApl
cortical amygdalar area, posterior part, lateral zone
- COApm
cortical amygdalar area, posterior part, medial zone
- COM
periaqueductal gray, commissural nucleus
- CP
caudoputamen
- cpd
cerebral peduncle
- CSl
superior central nucleus raphé, lateral part
- CSm
superior central nucleus raphé, medial part
- csc
superior colliculus commissure
- CUN
cuneiform nucleus
- DMHa
dorsomedial hypothalamic nucleus, anterior part
- DMHp
dorsomedial hypothalamic nucleus, posterior part
- DMHv
dorsomedial hypothalamic nucleus, ventral part
- DMX
Dorsal motor nucleus of the vagus nerve
- DR
dorsal nucleus, raphe
- dscp
superior cerebellar peduncle decussation
- DTN
dorsal tegmental nucleus
- ec
external capsule
- ECT
ectorhinal area
- em
external medullary lamina, thalamus
- ENTl
entorhinal area, lateral part
- ENTm
entorhinal area, medial part, dorsal zone
- ENTmv
entorhinal area, medial part, ventral zone
- EPd
endopiriform nucleus, dorsal part
- EPv
endopiriform nucleus, ventral part
- EW
Edinger-Westphal nucleus
- fa
corpus callosum, anterior forceps
- FF
fields of Forel
- fi
fimbria
- fr
fasciculus retroflexus
- FS
striatal fundus
- fx
columns of the fornix
- fxpr
precommissural fornix
- GPe
globus pallidus, external segment
- GPi
globus pallidus, internal segment
- GRN
gigantocellular reticular nucleus
- I
internuclear area, hypothalamic periventricular region
- IA
amygdalar nuclei (intercalated)
- IAD
interanterodorsal nucleus, thalamus
- IAM
interanteromedial nucleus, thalamus
- ICd
inferior colliculus, dorsal nucleus
- Ice
inferior colliculus, external nucleus
- IF
interfascicular nucleus, raphe
- III
oculomotor nucleus
- ILA
infralimbic area
- IMD
intermediodorsal nucleus, thalamus
- INC
interstitial nucleus of Cajal
- int
internal capsule
- IPNa
interpeduncular nucleus, apical subnucleus
- IPNc
interpeduncular nucleus, central subnucleus
- IPNd
interpeduncular nucleus, dorsomedial subnucleus
- IPNi
interpeduncular nucleus, intermediate subnucleus, IPNld, interpeduncular nucleus, lateral subnucleus, dorsal part
- IPNli
interpeduncular nucleus, lateral subnucleus, intermediate part
- IPNlr
interpeduncular nucleus, lateral subnucleus, rostral part
- IPNr
interpeduncular nucleus, rostral subnucleus
- isl
islands of Calleja (olfactory tubercle)
- IVn
trochlear nerve
- KF
Kölliker-Fuse subnucleus (of parabrachial nucleus)
- LA
lateral amygdalar nucleus
- LC
locus ceruleus
- LDT
laterodorsal tegmental nucleus
- LGvm
lateral geniculate complex, ventral part, medial zone
- LH
lateral habenula
- LHAad
lateral hypothalamic area, anterior region, dorsal zone
- LHAai
lateral hypothalamic area, anterior region, intermediate zone
- LHAav
lateral hypothalamic area, anterior region, ventral zone
- LHAd
lateral hypothalamic area, dorsal region
- LHAjd
lateral hypothalamic area, juxtadorsomedial region
- LHAjp
lateral hypothalamic area, juxtaparaventricular region
- LHAjvd
lateral hypothalamic area, juxtaventromedial region, dorsal zone
- LHAjvv
lateral hypothalamic area, juxtaventromedial region, ventral zone
- LHAm
lateral hypothalamic area, magnocellular nucleus
- LHAp
lateral hypothalamic area, posterior region
- LHApc
lateral hypothalamic area, parvicellular region
- LHAs
lateral hypothalamic area, suprafornical region
- LHAsfa
lateral hypothalamic area, subfornical region, anterior zone
- LHAsfp
lateral hypothalamic area, subfornical region, posterior zone
- LHAsfpm
lateral hypothalamic area, subfornical region, premammillary zone
- LHAvl
lateral hypothalamic area, ventral region, lateral zone
- LHAvm
lateral hypothalamic area, ventral region, medial zone
- ll
lateral lemniscus
- LPO
lateral preoptic area
- LSc
lateral septal nucleus, caudal part
- LSc.d
lateral septal nucleus, caudal part, dorsal zone
- LSc.d.d
lateral septal nucleus, caudal part, dorsal zone, dorsal region
- LSc.d.l
lateral septal nucleus, caudal part, dorsal zone, lateral region
- LSc.d.r
lateral septal nucleus, caudal part, dorsal zone, rostral region
- LSc.d.v
lateral septal nucleus, caudal part, dorsal zone, ventral region
- LSc.v.i
lateral septal nucleus, caudal part, ventral zone, intermediate region
- LSc.v.l
lateral septal nucleus, caudal part, ventral zone, lateral region
- LSc.v.l.d
lateral septal nucleus, caudal part, ventral zone, lateral region, dorsal domain
- LSc.v.l.v
lateral septal nucleus, caudal part, ventral zone, lateral region, ventral domain
- LSc.v.m.d
lateral septal nucleus, caudal part, ventral zone, medial region, dorsal domain
- LSc.v.m.v
lateral septal nucleus, caudal part, ventral zone, medial region, ventral domain
- LSr
lateral septal nucleus, rostral part
- LSr.dl.l.d
lateral septal nucleus, rostral part, dorsolateral zone, lateral region, dorsal domain
- LSr.dl.l.v
lateral septal nucleus, rostral part, dorsolateral zone, lateral region, ventral domain
- LSr.dl.m.d
lateral septal nucleus, rostral part, dorsolateral zone, medial region, dorsal domain
- LSr.dl.m.v
lateral septal nucleus, rostral part, dorsolateral zone, medial region, ventral domain
- LSr.m
lateral septal nucleus, rostral part, medial zone
- LSr.m.d
lateral septal nucleus, rostral part, medial zone, dorsal region
- LSr.m.v.c
lateral septal nucleus, rostral part, medial zone, ventral region, caudal domain
- LSr.m.v.r
lateral septal nucleus, rostral part, medial zone, ventral region, rostral domain
- LSr.vl.d.l
lateral septal nucleus, rostral part, ventrolateral zone, dorsal region, lateral domain
- LSr.vl.d.m
lateral septal nucleus, rostral part, ventrolateral zone, dorsal region, medial domain
- LSr.vl.v
lateral septal nucleus, rostral part, ventrolateral zone, ventral region
- LSv
lateral septal nucleus, ventral part
- LTN
lateral tegmental nucleus
- MA
magnocellular nucleus
- MARN
magnocellular reticular nucleus
- mct
medial corticohypothalamic tract
- MDc
mediodorsal nucleus, thalamus, central part
- MDl
mediodorsal nucleus, thalamus, lateral part
- MDm
mediodorsal nucleus, thalamus, medial part
- MDRNd
medullary reticular nucleus, dorsal part
- MDRNv
medullary reticular nucleus, ventral part
- ME
median eminence
- MEex
median eminence, external lamina
- MEAad
medial amygdalar nucleus, anterodorsal part
- MEAav
medial amygdalar nucleus, anteroventral part
- MEApd.a-c
medial amygdalar nucleus, posterodorsal part, sublayer a–c
- MEApv
medial amygdalar nucleus, posteroventral part
- MEPO
median preoptic nucleus
- MEV
midbrain trigeminal nucleus
- MGd
medial geniculate complex, dorsal part
- MGm
medial geniculate complex, medial part
- MGv
medial geniculate complex, ventral part
- MH
medial habenula
- ml
medial lemniscus
- mlf
medial longitudinal fascicle
- MM
medial mammillary nucleus, body
- MMme
medial mammillary nucleus, median part
- MPNc
medial preoptic nucleus, central part
- MPNl
medial preoptic nucleus, lateral part
- MPNm
medial preoptic nucleus, medial part
- MPO
medial preoptic area
- MPT
medial pretectal area
- MRNm
midbrain reticular nucleus, magnocellular part
- MRNp
midbrain reticular nucleus, parvicellular part
- MS
medial septal nucleus
- MT
medial terminal nucleus, accessory optic tract
- mtV
midbrain tract of the trigeminal nerve
- mtt
mammillothalamic tract
- NB
nucleus of the brachium, inferior colliculus
- NC
nucleus circularis
- ND
nucleus of Darkschewitsch
- NDB
diagonal band nucleus
- NIc
nucleus incertus, compact part
- NId
nucleus incertus, diffuse part
- NLOT
nucleus of the lateral olfactory tract
- NLLd
nucleus of the lateral lemniscus, dorsal part
- NLLv
nucleus of the lateral lemniscus, ventral part
- NPC
nucleus of the posterior commissure
- NTSco
nucleus of the solitary tract, commissural part
- NTSl
nucleus of the solitary tract, lateral part
- NTSm
nucleus of the solitary tract, medial part
- och
optic chiasm
- opt
optic tract
- ORBm
orbital area, medial part
- ORBv
orbital area, ventral part
- OT
olfactory tubercle
- PA
posterior amygdalar nucleus
- PAA
piriform-amygdala transition area
- PAGd
periaqueductal gray, dorsal division
- PAGdl
periaqueductal gray, dorsolateral division
- PAGm
periaqueductal gray, medial division
- PAGrl
periaqueductal gray, rostrolateral division
- PAGrm
periaqueductal gray, rostromedial division
- PAGvl
periaqueductal gray, ventrolateral division
- PAR
parasubiculum
- PARN
parvicellular reticular nucleus
- PBlc
parabrachial nucleus, central lateral part
- PBld
parabrachial nucleus, dorsal lateral part
- PBle
parabrachial nucleus, external lateral part
- PBls
parabrachial nucleus, superior lateral part
- PBlv
parabrachial nucleus, ventral lateral part
- PBmm
parabrachial nucleus, medial medial part
- PBmv
parabrachial nucleus, ventral medial part
- pc
posterior commissure
- PCG
pontine central gray
- PD
posterodorsal preoptic nucleus
- PERI
perirhinal area
- PF
parafascicular nucleus
- PGRNl
paragigantocellular reticular nucleus, lateral part
- PH
posterior hypothalamic nucleus
- PIR
piriform area
- PL
prelimbic area
- pm
principal, ammillary tract
- PMd
dorsal premammillary nucleus
- PMv
ventral premammillary nucleus
- PO
posterior complex thalamus
- POL
posterior limiting nucleus, thalamus
- PP
peripeduncular nucleus
- PPN
pedunculopontine nucleus
- PPYd
parapyramidal nucleus, deep part
- PR
perireuniens nucleus
- PRC
periaqueductal gray, precommissural nucleus
- PRE
presubiculum
- PRNc
pontine reticular nucleus, caudal part
- PRNr
pontine reticular nucleus, rostral part
- PS
parastrial nucleus
- PSCH
suprachiasmatic preoptic nucleus
- PST
preparasubthalamic nucleus
- PSTN
parasubthalamic nucleus
- PSV
principal sensory nucleus of the trigeminal
- PT
paratenial nucleus
- PVa
periventricular hypothalamic nucleus, anterior part
- PVHam
paraventricular nucleus hypothalamus, magnocellular division, anterior magnocellular part
- PVHap
paraventricular nucleus hypothalamus, anterior parvicellular part
- PVHdp
paraventricular nucleus hypothalamus, dorsal parvicellular part
- PVHf
paraventricular nucleus hypothalamus, forniceal part
- PVHlp
paraventricular nucleus hypothalamus, lateral parvicellular part
- PVHmpd
paraventricular nucleus hypothalamus, medial parvicellular part, dorsal zone
- PVHmpdl
paraventricular nucleus hypothalamus, medial parvicellular part, dorsal zone, lateral wing
- PVHpml
paraventricular nucleus hypothalamus, posterior magnocellular part, lateral zone
- PVHpmm
paraventricular nucleus hypothalamus, posterior magnocellular part, medial zone
- PVHpv
paraventricular nucleus hypothalamus, periventricular part
- PVi
periventricular nucleus hypothalamus, intermediate part
- PVp
periventricular nucleus hypothalamus, posterior part
- PVpo
preoptic periventricular nucleus
- PVT
paraventricular nucleus, thalamus
- py
pyramidal tract
- RCH
retrochiasmatic area
- Rea
nucleus reuniens, rostral division, anterior part
- REcd
nucleus reuniens, caudal division, dorsal part
- REcm
nucleus reuniens, caudal division, median part
- REcp
nucleus reuniens, caudal division, posterior part
- Red
nucleus reuniens, rostral division, dorsal part
- REl
nucleus reuniens, rostral division, lateral part
- Rem
nucleus reuniens, rostral division, median part
- Rev
nucleus reuniens, rostral division, ventral part
- RH
rhomboid nucleus
- RL
rostral linear nucleus, raphe
- RN
red nucleus
- RR
midbrain reticular nucleus, retrorubral area
- RT
reticular nucleus, thalamus
- rust
rubrospinal tract
- SAG
nucleus sagulum
- SBPV
subparaventricular zone
- SCdg
superior colliculus, deep gray layer
- SCdw
superior colliculus, deep white layer
- SCH
suprachiasmatic nucleus
- SCig.a–c
superior colliculus, intermediate gray layer, sublayer a–c
- SCiw
superior colliculus, intermediate white layer
- scp
superior cerebellar peduncle
- sctv
ventral spinocerebellar tract
- SF
septofimbrial nucleus
- SFO
subfornical organ
- SGN
suprageniculate nucleus, thalamus
- SH
septohippocampal nucleus
- SI
substantia innominate
- SLC
subceruleus nucleus
- SLD
sublaterodorsal nucleus
- sm
stria medullaris
- smd
supramammillary decussation
- SMT
submedial nucleus, thalamus
- SNr
substantia nigra, reticular part
- SNc
substantia nigra, compact part
- SO
supraoptic nucleus, proper
- SOr
supraoptic nucleus, retrochiasmatic part
- SPFm
subparafascicular nucleus thalamus, magnocellular part
- SPFpl
subparafascicular nucleus thalamus, parvicellular part, lateral division
- SPFpm
subparafascicular nucleus thalamus, parvicellular part, medial division
- st
stria terminalis
- STN
subthalamic nucleus
- SUB
subiculum
- SUB-m
subiculum, molecular layer
- SUB–sp
subiculum, pyramidal layer
- SUMl
supramammillary nucleus, lateral part
- SUMm
supramammillary nucleus, medial part
- sup
supraoptic commissures
- SUT
supratrigeminal nucleus
- TMd
tuberomammillary nucleus, dorsal part
- TMv
tuberomammillary nucleus, ventral part
- tp
thalamic peduncles
- TR
postpiriform transition area
- TRN
tegmental reticular nucleus
- TRS
triangular nucleus septum
- tsp
tectospinal pathway
- TTd
tenia tecta, dorsal part
- TUi
tuberal nucleus, intermediate part
- TUl
tuberal nucleus, lateral part
- TUsv
tuberal nucleus, subventromedial part
- TUte
tuberal nucleus, terete subnucleus
- V3h
third ventricle, hypothalamic part
- V3m
third ventricle, mammillary recess
- V3p
third ventricle, preoptic recess
- V3t
third ventricle, thalamic part
- V4
fourth ventricle proper
- VIIn
facial nerve
- VL
lateral ventricle
- VLP
ventrolateral preoptic nucleus
- vlt
ventrolateral hypothalamic tract
- VM
ventral medial nucleus, thalamus
- VMHa
ventromedial nucleus hypothalamus, anterior part
- VMHc
ventromedial nucleus hypothalamus, central part
- VMHdm
ventromedial nucleus hypothalamus, dorsomedial part
- VMHvl
ventromedial nucleus hypothalamus, ventrolateral part
- VPMpc
ventral posteromedial nucleus thalamus, parvicellular part
- VTA
ventral tegmental area
- vtd
ventral tegmental decussation
- VTN
ventral tegmental nucleus
- XII
hypoglossal nucleus
- ZI
zona incerta
- ZIda
zona incerta, dopaminergic group
- zl
zona limitans
Footnotes
1In the cited article a nomenclature different to that used here is employed, such that approximate correspondence may be drawn between BMA and “accessory basal nucleus (AB)”; BLAa/p and “basal nucleus, magnocellular/parvocellular divisions (Bmg/pc).”
2In the cited article it is noted that NMDA lesions also partially included adjacent amygdalar regions.
References
- Armstrong W. E. (1995). Hypothalamic supraoptic and paraventricular nuclei, in The Rat Nervous System, ed Paxinos G. (San Diego, CA: Academic Press; ), 377–390. [Google Scholar]
- Atasoy D., Betley J. N., Su H. H., Sternson S. M. (2012). Deconstruction of a neural circuit for hunger. Nature 488, 172–177. 10.1038/nature11270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker B. L., Dermody W. C., Reel J. R. (1975). Distribution of gonadotropin-releasing hormone in the rat brain as observed with immunocytochemistry. Endocrinology 97, 125–135. 10.1210/endo-97-1-125 [DOI] [PubMed] [Google Scholar]
- Bargmann W., Scharrer E. (1951). The site of origin of the hormones of the posterior pituitary. Am. Sci. 39, 255–259. [PubMed] [Google Scholar]
- Barnett S. A. (1963). The Rat: A Study In Behavior. Chicago, IL: Aldine Publishing Company. [Google Scholar]
- Betley J. N., Cao Z. F., Ritola K. D., Sternson S. M. (2013). Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell 155, 1337–1350. 10.1016/j.cell.2013.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bota M., Spoorns O., Swanson L. W. (2015). Architecture of the cerebral cortical association connectome underlying cognition. Proc. Natl. Acad. Sci. U.S.A. 112, E2093–E2101. 10.1073/pnas.1504394112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bota M., Talpalaru S., Hintiryan H., Dong H. W., Swanson L. W. (2014). BAMS2 workspace: a comprehensive and versatile neuroinformatic platform for collating and processing neuroanatomical connections. J. Comp. Neurol. 522, 3160–3176. 10.1002/cne.23592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broberger C. (1999). Hypothalamic cocaine- and amphetamine-regulated transcript (CART) neurons: histochemical relationship to thyrotropin-releasing hormone, melanin-concentrating hormone, orexin/hypocretin and neuropeptide Y. Brain Res. 848, 101–113. 10.1016/S0006-8993(99)01977-0 [DOI] [PubMed] [Google Scholar]
- Brown R. A., Swanson L. W. (2013). Neural systems language: a formal modeling language for the systematic description, unambiguous communication, and automated digital curation of neural connectivity. J. Comp. Neurol. 521, 2889–2906. 10.1002/cne.23348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G., Moser E. I. (2013). Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat. Neurosci. 16, 130–138. 10.1038/nn.3304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cant N. B., Benson C. G. (2003). Parallel auditory pathways: projection patterns of the different neuronal populations in the dorsal and ventral cochlear nuclei. Brain Res. Bull. 60, 457–474. 10.1016/S0361-9230(03)00050-9 [DOI] [PubMed] [Google Scholar]
- Canteras N. S. (2002). The medial hypothalamic defensive system: hodological organization and functional implications. Pharmacol. Biochem. Behav. 71, 481–491. 10.1016/S0091-3057(01)00685-2 [DOI] [PubMed] [Google Scholar]
- Canteras N. S., Chiavegatto S., DoValle L. E. R., Swanson L. W. (1997). Severe reduction of rat defensive behavior to a predator by discrete hypothalamic chemical lesions. Brain Res. Bull. 44, 297–305. 10.1016/S0361-9230(97)00141-X [DOI] [PubMed] [Google Scholar]
- Canteras N. S., Graeff F. G. (2014). Executive and modulatory neural circuits of defensive reactions: implications for panic disorder. Neurosci. Biobehav. Rev. 46(Pt 3), 352–364. 10.1016/j.neubiorev.2014.03.020 [DOI] [PubMed] [Google Scholar]
- Canteras N. S., Simerly R. B., Swanson L. W. (1994). Organization of projections from the ventromedial nucleus of the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J. Comp. Neurol. 348, 41–79. 10.1002/cne.903480103 [DOI] [PubMed] [Google Scholar]
- Carrive P., Leung P., Harris J., Paxinos G. (1997). Conditioned fear to context is associated with increased Fos expression in the caudal ventrolateral region of the midbrain periaqueductal gray. Neuroscience 78, 165–177. 10.1016/S0306-4522(97)83047-3 [DOI] [PubMed] [Google Scholar]
- Cenquizca L. A., Swanson L. W. (2007). Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Res. Rev. 56, 1–26. 10.1016/j.brainresrev.2007.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi G. B., Dong H. W., Murphy A. J., Valenzuela D. M., Yancopoulos G. D., Swanson L. W., et al. (2005). Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron 46, 647–660. 10.1016/j.neuron.2005.04.011 [DOI] [PubMed] [Google Scholar]
- Cole S., Hobin M. P., Petrovich G. D. (2015). Appetitive associative learning recruits a distinct network with cortical, striatal, and hypothalamic regions. Neuroscience 286, 187–202. 10.1016/j.neuroscience.2014.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S., Powell D. J., Petrovich G. D. (2013). Differential recruitment of distinct amygdalar nuclei across appetitive associative learning. Learn. Mem. 20, 295–299. 10.1101/lm.031070.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comoli E., Das Neves Favaro P., Vautrelle N., Leriche M., Overton P. G., Redgrave P. (2012). Segregated anatomical input to sub-regions of the rodent superior colliculus associated with approach and defense. Front. Neuroanat. 6:9. 10.3389/fnana.2012.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coolen L. M., Peters H. J., Veening J. G. (1996). Fos immunoreactivity in the rat brain following consummatory elements of sexual behavior: a sex comparison. Brain Res. 738, 67–82. 10.1016/0006-8993(96)00763-9 [DOI] [PubMed] [Google Scholar]
- Coolen L. M., Peters H. J., Veening J. G. (1998). Anatomical interrelationships of the medial preoptic area and other brain regions activated following male sexual behavior: a combined fos and tract-tracing study. J. Comp. Neurol. 397, 421–435. [DOI] [PubMed] [Google Scholar]
- Cowley D. S., Arana G. W. (1990). The diagnostic utility of lactate sensitivity in panic disorder. Arch. Gen. Psychiatry 47, 277–284. 10.1001/archpsyc.1990.01810150077012 [DOI] [PubMed] [Google Scholar]
- Cvetkovic V., Brischoux F., Jacquemard C., Fellmann D., Griffond B., Risold P. Y. (2004). Characterization of subpopulations of neurons producing melanin-concentrating hormone in the rat ventral diencephalon. J. Neurochem. 91, 911–919. 10.1111/j.1471-4159.2004.02776.x [DOI] [PubMed] [Google Scholar]
- Davis M., Gendelman D. S., Tischler M. D., Gendelman P. M. (1982). A primary acoustic startle circuit: lesion and stimulation studies. J. Neurosci. 2, 791–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean P., Redgrave P., Westby G. W. (1989). Event or emergency? Two response systems in the mammalian superior colliculus. Trends Neurosci. 12, 137–147. 10.1016/0166-2236(89)90052-0 [DOI] [PubMed] [Google Scholar]
- Del Cid-Pellitero E., Jones B. E. (2012). Immunohistochemical evidence for synaptic release of GABA from melanin-concentrating hormone containing varicosities in the locus coeruleus. Neuroscience 223, 269–276. 10.1016/j.neuroscience.2012.07.072 [DOI] [PubMed] [Google Scholar]
- DesJardin J. T., Holmes A. L., Forcelli P. A., Cole C. E., Gale J. T., Wellman L. L., et al. (2013). Defense-like behaviors evoked by pharmacological disinhibition of the superior colliculus in the primate. J. Neurosci. 33, 150–155. 10.1523/JNEUROSCI.2924-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries G. J., Buijs R. M., Van Leeuwen F. W., Caffe A. R., Swaab D. F. (1985). The vasopressinergic innervation of the brain in normal and castrated rats. J. Comp. Neurol. 233, 236–254. 10.1002/cne.902330206 [DOI] [PubMed] [Google Scholar]
- Dielenberg R. A., Hunt G. E., McGregor I. S. (2001). “When a rat smells a cat”: the distribution of Fos immunoreactivity in rat brain following exposure to a predatory odor. Neuroscience 104, 1085–1097. 10.1016/S0306-4522(01)00150-6 [DOI] [PubMed] [Google Scholar]
- Dong H. W., Petrovich G. D., Swanson L. W. (2001a). Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res. Brain Res. Rev. 38, 192–246. 10.1016/S0165-0173(01)00079-0 [DOI] [PubMed] [Google Scholar]
- Dong H. W., Petrovich G. D., Watts A. G., Swanson L. W. (2001b). Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J. Comp. Neurol. 436, 430–455. 10.1002/cne.1079 [DOI] [PubMed] [Google Scholar]
- Dong H. W., Swanson L. W. (2003). Projections from the rhomboid nucleus of the bed nuclei of the stria terminalis: implications for cerebral hemisphere regulation of ingestive behaviors. J. Comp. Neurol. 463, 434–472. 10.1002/cne.10758 [DOI] [PubMed] [Google Scholar]
- Dong H. W., Swanson L. W. (2004a). Organization of axonal projections from the anterolateral area of the bed nuclei of the stria terminalis. J. Comp. Neurol. 468, 277–298. 10.1002/cne.10949 [DOI] [PubMed] [Google Scholar]
- Dong H. W., Swanson L. W. (2004b). Projections from bed nuclei of the stria terminalis, posterior division: implications for cerebral hemisphere regulation of defensive and reproductive behaviors. J. Comp. Neurol. 471, 396–433. 10.1002/cne.20002 [DOI] [PubMed] [Google Scholar]
- Dong H. W., Swanson L. W. (2006a). Projections from bed nuclei of the stria terminalis, anteromedial area: cerebral hemisphere integration of neuroendocrine, autonomic, and behavioral aspects of energy balance. J. Comp. Neurol. 494, 142–178. 10.1002/cne.20788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H. W., Swanson L. W. (2006b). Projections from bed nuclei of the stria terminalis, dorsomedial nucleus: implications for cerebral hemisphere integration of neuroendocrine, autonomic, and drinking responses. J. Comp. Neurol. 494, 75–107. 10.1002/cne.20790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H. W., Swanson L. W. (2006c). Projections from bed nuclei of the stria terminalis, magnocellular nucleus: implications for cerebral hemisphere regulation of micturition, defecation, and penile erection. J. Comp. Neurol. 494, 108–141. 10.1002/cne.20789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H. W., Swanson L. W., Chen L., Fanselow M. S., Toga A. W. (2009). Genomic-anatomic evidence for distinct functional domains in hippocampal field CA1. Proc. Natl. Acad. Sci. U.S.A. 106, 11794–11799. 10.1073/pnas.0812608106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas M., Schwab M. E., Thoenen H. (1979). Retrograde axonal transport of specific macromolecules as a tool for characterizing nerve terminal membranes. J. Neurobiol. 10, 179–197. 10.1002/neu.480100207 [DOI] [PubMed] [Google Scholar]
- Elias C. F., Lee C. E., Kelly J. F., Ahima R. S., Kuhar M., Saper C. B., et al. (2001). Characterization of CART neurons in the rat and human hypothalamus. J. Comp. Neurol. 432, 1–19. 10.1002/cne.1085 [DOI] [PubMed] [Google Scholar]
- Engelmann M., Ebner K., Landgraf R., Holsboer F., W C.T. (1999). Emotional stress triggers intrahypothalamic but not peripheral release of oxytocin in male rats. J. Neuroendocrinol. 11, 867–872. 10.1046/j.1365-2826.1999.00403.x [DOI] [PubMed] [Google Scholar]
- Fanselow M. S., Dong H. W. (2010). Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65, 7–19. 10.1016/j.neuron.2009.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell M. S., Roth B. L. (2013). Pharmacosynthetics: reimagining the pharmacogenetic approach. Brain Res. 1511, 6–20. 10.1016/j.brainres.2012.09.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faturi C. B., Rangel M. J., Jr., Baldo M. V., Canteras N. S. (2014). Functional mapping of the circuits involved in the expression of contextual fear responses in socially defeated animals. Brain Struct. Funct. 219, 931–946. 10.1007/s00429-013-0544-4 [DOI] [PubMed] [Google Scholar]
- Fenno L., Yizhar O., Deisseroth K. (2011). The development and application of optogenetics. Annu. Rev. Neurosci. 34, 389–412. 10.1146/annurev-neuro-061010-113817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo K. S., Brismar H., Broberger C. (2008). Distribution and neuropeptide coexistence of nucleobindin-2 mRNA/nesfatin-like immunoreactivity in the rat CNS. Neuroscience 156, 563–579. 10.1016/j.neuroscience.2008.07.054 [DOI] [PubMed] [Google Scholar]
- Fort P., Salvert D., Hanriot L., Jego S., Shimizu H., Hashimoto K., et al. (2008). The satiety molecule nesfatin-1 is co-expressed with melanin concentrating hormone in tuberal hypothalamic neurons of the rat. Neuroscience 155, 174–181. 10.1016/j.neuroscience.2008.05.035 [DOI] [PubMed] [Google Scholar]
- Fulton J. F., Ranson S. W., Frantz A. M. (1940). The Hypothalamus and Central Levels of Autonomic Function. Baltimore, MD: The Williams and Wilkins Co. [Google Scholar]
- Gerfen C. R., Sawchenko P. E. (1984). An anterograde neuroanatomical tracing method that shows the detailed morphology of neurons, their axons and terminals: immunohistochemical localization of an axonally transported plant lectin, Phaseolus vulgaris leucoagglutinin (PHA-L). Brain Res. 290, 219–238. 10.1016/0006-8993(84)90940-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Hernandez T., Mantolan-Sarmiento B., Gonzalez-Gonzalez B., Perez-Gonzalez H. (1996). Sources of GABAergic input to the inferior colliculus of the rat. J. Comp. Neurol. 372, 309–326. [DOI] [PubMed] [Google Scholar]
- Goto M., Canteras N. S., Burns G., Swanson L. W. (2005). Projections from the subfornical region of the lateral hypothalamic area. J. Comp. Neurol. 493, 412–438. 10.1002/cne.20764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto M., Swanson L. W., Canteras N. S. (2001). Connections of the nucleus incertus. J. Comp. Neurol. 438, 86–122. 10.1002/cne.1303 [DOI] [PubMed] [Google Scholar]
- Groenewegen H. J., Vermeulen-Van der Zee E., te K. A., Witter M. P. (1987). Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of Phaseolus vulgaris leucoagglutinin. Neuroscience 23, 103–120. 10.1016/0306-4522(87)90275-2 [DOI] [PubMed] [Google Scholar]
- Gross C. T., Canteras N. S. (2012). The many paths to fear. Nat. Rev. Neurosci. 13, 651–658. 10.1038/nrn3301 [DOI] [PubMed] [Google Scholar]
- Gu G. B., Simerly R. B. (1997). Projections of the sexually dimorphic anteroventral periventricular nucleus in the female rat. J. Comp. Neurol. 384, 142–164. [PubMed] [Google Scholar]
- Gurdjian E. S. (1927). The diencephalon of the albino rat. Studies on the brain of the rat. No. 2. J. Comp. Neurol. 43, 1–114. 10.1002/cne.900430102 [DOI] [Google Scholar]
- Hahn J. D. (2010). Comparison of melanin-concentrating hormone and hypocretin/orexin peptide expression patterns in a current parceling scheme of the lateral hypothalamic zone. Neurosci. Lett. 468, 12–17. 10.1016/j.neulet.2009.10.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn J. D., Coen C. W. (2006). Comparative study of the sources of neuronal projections to the site of gonadotrophin-releasing hormone perikarya and to the anteroventral periventricular nucleus in female rats. J. Comp. Neurol. 494, 190–214. 10.1002/cne.20803 [DOI] [PubMed] [Google Scholar]
- Hahn J. D., Swanson L. W. (2010). Distinct patterns of neuronal inputs and outputs of the juxtaparaventricular and suprafornical regions of the lateral hypothalamic area in the male rat. Brain Res. Rev. 64, 14–103. 10.1016/j.brainresrev.2010.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn J. D., Swanson L. W. (2012). Connections of the lateral hypothalamic area juxtadorsomedial region in the male rat. J. Comp. Neurol. 520, 1831–1890. 10.1002/cne.23064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris G. W. (1955). Neural Control of the Pituitary Gland. London: Arnold. [Google Scholar]
- Hattar S., Kumar M., Park A., Tong P., Tung J., Yau K. W., et al. (2006). Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J. Comp. Neurol. 497, 326–349. 10.1002/cne.20970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haymaker W., Anderson E., Nauta W. J. H. (1969). The Hypothalamus. Illinois, IL: Charles C Thomas. [Google Scholar]
- Henny P., Brischoux F., Mainville L., Stroh T., Jones B. E. (2010). Immunohistochemical evidence for synaptic release of glutamate from orexin terminals in the locus coeruleus. Neuroscience 169, 1150–1157. 10.1016/j.neuroscience.2010.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho C. Y., Berridge K. C. (2014). Excessive disgust caused by brain lesions or temporary inactivations: mapping hotspots of the nucleus accumbens and ventral pallidum. Eur. J. Neurosci. 40, 3556–3572. 10.1111/ejn.12720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland P. C., Petrovich G. D., Gallagher M. (2002). The effects of amygdala lesions on conditioned stimulus-potentiated eating in rats. Physiol. Behav. 76, 117–129. 10.1016/S0031-9384(02)00688-1 [DOI] [PubMed] [Google Scholar]
- Honda K., Yanagimoto M., Negoro H., Narita K., Murata T., Higuchi T. (1999). Excitation of oxytocin cells in the hypothalamic supraoptic nucleus by electrical stimulation of the dorsal penile nerve and tactile stimulation of the penis in the rat. Brain Res. Bull. 48, 309–313. 10.1016/S0361-9230(98)00180-4 [DOI] [PubMed] [Google Scholar]
- Hou-Yu A., Lamme A. T., Zimmerman E. A., Silverman A. J. (1986). Comparative distribution of vasopressin and oxytocin neurons in the rat brain using a double-label procedure. Neuroendocrinology 44, 235–246. 10.1159/000124651 [DOI] [PubMed] [Google Scholar]
- Hrabovszky E., Halasz J., Meelis W., Kruk M. R., Liposits Z., Haller J. (2005). Neurochemical characterization of hypothalamic neurons involved in attack behavior: glutamatergic dominance and co-expression of thyrotropin-releasing hormone in a subset of glutamatergic neurons. Neuroscience 133, 657–666. 10.1016/j.neuroscience.2005.03.042 [DOI] [PubMed] [Google Scholar]
- Hsu T. M., Hahn J. D., Konanur V. R., Lam A., Kanoski S. E. (2014). Hippocampal GLP-1 Receptors influence food intake, meal size, and effort-based responding for food through volume transmission. Neuropsychopharmacology 40, 327–337. 10.1038/npp.2014.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis W. L., Winn P. (1995). The pedunculopontine tegmental nucleus: where the striatum meets the reticular formation. Prog. Neurobiol. 47, 1–29. 10.1016/0301-0082(95)00013-L [DOI] [PubMed] [Google Scholar]
- Jego S., Glasgow S. D., Herrera C. G., Ekstrand M., Reed S. J., Boyce R., et al. (2013). Optogenetic identification of a rapid eye movement sleep modulatory circuit in the hypothalamus. Nat. Neurosci. 16, 1637–1643. 10.1038/nn.3522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou T. C., Geisler S., Marinelli M., Degarmo B. A., Zahm D. S. (2009). The mesopontine rostromedial tegmental nucleus: a structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J. Comp. Neurol. 513, 566–596. 10.1002/cne.21891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P., Lowry C., Truitt W., Shekhar A. (2008). Disruption of GABAergic tone in the dorsomedial hypothalamus attenuates responses in a subset of serotonergic neurons in the dorsal raphe nucleus following lactate-induced panic. J. Psychopharmacol. 22, 642–652. 10.1177/0269881107082900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. L., Fitz S. D., Hollis J. H., Moratalla R., Lightman S. L., Shekhar A., et al. (2011). Induction of c-Fos in 'panic/defence'-related brain circuits following brief hypercarbic gas exposure. J. Psychopharmacol. 25, 26–36. 10.1177/0269881109353464 [DOI] [PubMed] [Google Scholar]
- Kanoski S. E., Hayes M. R., Greenwald H. S., Fortin S. M., Gianessi C. A., Gilbert J. R., et al. (2011). Hippocampal leptin signaling reduces food intake and modulates food-related memory processing. Neuropsychopharmacology 36, 1859–1870. 10.1038/npp.2011.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnani M. M., Szabo G., Erdelyi F., Burdakov D. (2013). Lateral hypothalamic GAD65 neurons are spontaneously firing and distinct from orexin- and melanin-concentrating hormone neurons. J. Physiol. 591, 933–953. 10.1113/jphysiol.2012.243493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper E. M., Larkman A. U., Lubke J., Blakemore C. (1994). Pyramidal neurons in layer 5 of the rat visual cortex. I. Correlation among cell morphology, intrinsic electrophysiological properties, and axon targets. J. Comp. Neurol. 339, 459–474. 10.1002/cne.903390402 [DOI] [PubMed] [Google Scholar]
- Kay-Nishiyama C., Watts A. G. (1999). Dehydration modifies somal CRH immunoreactivity in the rat hypothalamus: an immunocytochemical study in the absence of colchicine. Brain Res. 822, 251–255. 10.1016/S0006-8993(99)01130-0 [DOI] [PubMed] [Google Scholar]
- Keen-Rhinehart E., Ondek K., Schneider J. E. (2013). Neuroendocrine regulation of appetitive ingestive behavior. Front. Neurosci. 7:213. 10.3389/fnins.2013.00213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keim S. R., Shekhar A. (1996). The effects of GABAA receptor blockade in the dorsomedial hypothalamic nucleus on corticotrophin (ACTH) and corticosterone secretion in male rats. Brain Res. 739, 46–51. 10.1016/S0006-8993(96)00810-4 [DOI] [PubMed] [Google Scholar]
- Kelly A. B., Watts A. G. (1996). Mediation of dehydration-induced peptidergic gene expression in the rat lateral hypothalamic area by forebrain afferent projections. J. Comp. Neurol. 370, 231–246. [DOI] [PubMed] [Google Scholar]
- Kelly A. B., Watts A. G. (1998). The region of the pontine parabrachial nucleus is a major target of dehydration-sensitive CRH neurons in the rat lateral hypothalamic area. J. Comp. Neurol. 394, 48–63. [DOI] [PubMed] [Google Scholar]
- Kelly J., Swanson L. W. (1980). Additional forebrain regions projecting to the posterior pituitary - preoptic region, bed nucleus of the stria terminalis, and zona incerta. Brain Res. 197, 1–9. 10.1016/0006-8993(80)90430-8 [DOI] [PubMed] [Google Scholar]
- Kita T., Kita H. (2011). Cholinergic and non-cholinergic mesopontine tegmental neurons projecting to the subthalamic nucleus in the rat. Eur. J. Neurosci. 33, 433–443. 10.1111/j.1460-9568.2010.07537.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelstrup K. G., Tuvnes F. A., Steffenach H. A., Murison R., Moser E. I., Moser M. B. (2002). Reduced fear expression after lesions of the ventral hippocampus. Proc. Natl. Acad. Sci. U.S.A. 99, 10825–10830. 10.1073/pnas.152112399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg W. J. S. (1932). The hypothalamus of the albino rat. J. Comp. Neurol. 55, 19–89. 10.1002/cne.900550104 [DOI] [Google Scholar]
- Le Gros Clark W. E. (1938. The hypothalamus, morphological, functional, clinical and surgical aspects, in The Hypothalamus, eds Le Gros Clark W. E., Beattie J., Riddoch G., Dott N. M. (Edinburgh: Oliver and Boyd; ), 1–68. [Google Scholar]
- Lee H., Kim D. W., Remedios R., Anthony T. E., Chang A., Madisen L., et al. (2014). Scalable control of mounting and attack by Esr1 neurons in the ventromedial hypothalamus. Nature 509, 627–632. 10.1038/nature13169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Lopez D. E., Meloni E. G., Davis M. (1996). A primary acoustic startle pathway: obligatory role of cochlear root neurons and the nucleus reticularis pontis caudalis. J. Neurosci. 16, 3775–3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D., Boyle M. P., Dollar P., Lee H., Lein E. S., Perona P., et al. (2011). Functional identification of an aggression locus in the mouse hypothalamus. Nature 470, 221–226. 10.1038/nature09736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppi P. H., Fort P., Jouvet M. (1990). Iontophoretic application of unconjugated cholera toxin B subunit (CTb) combined with immunohistochemistry of neurochemical substances: a method for transmitter identification of retrogradely labeled neurons. Brain Res. 534, 209–224. 10.1016/0006-8993(90)90131-T [DOI] [PubMed] [Google Scholar]
- Luppi P. H., Sakai K., Salvert D., Fort P., Jouvet M. (1987). Peptidergic hypothalamic afferents to the cat nucleus raphe pallidus as revealed by a double immunostaining technique using unconjugated cholera toxin as a retrograde tracer. Brain Res. 402, 339–345. 10.1016/0006-8993(87)90041-2 [DOI] [PubMed] [Google Scholar]
- Markakis E. A., Swanson L. W. (1997). Spatiotemporal patterns of secretomotor neuron generation in the parvicellular neuroendocrine system. Brain Res. Rev. 24, 255–291. 10.1016/S0165-0173(97)00006-4 [DOI] [PubMed] [Google Scholar]
- Markham C. M., Taylor S. L., Huhman K. L. (2010). Role of amygdala and hippocampus in the neural circuit subserving conditioned defeat in Syrian hamsters. Learn. Mem. 17, 109–116. 10.1101/lm.1633710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason W. T., Ho Y. W., Hatton G. I. (1984). Axon collaterals of supraoptic neurones: anatomical and electrophysiological evidence for their existence in the lateral hypothalamus. Neuroscience 11, 169–182. 10.1016/0306-4522(84)90221-5 [DOI] [PubMed] [Google Scholar]
- Matsumoto J., Urakawa S., Hori E., de Araujo M. F., Sakuma Y., Ono T., et al. (2012). Neuronal responses in the nucleus accumbens shell during sexual behavior in male rats. J. Neurosci. 32, 1672–1686. 10.1523/JNEUROSCI.5140-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald A. J. (1998). Cortical pathways to the mammalian amygdala. Prog. Neurobiol. 55, 257–332. 10.1016/S0301-0082(98)00003-3 [DOI] [PubMed] [Google Scholar]
- McDowall L. M., Horiuchi J., Killinger S., Dampney R. A. (2006). Modulation of the baroreceptor reflex by the dorsomedial hypothalamic nucleus and perifornical area. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290, R1020–R1026. 10.1152/ajpregu.00541.2005 [DOI] [PubMed] [Google Scholar]
- Meister B. (2007). Neurotransmitters in key neurons of the hypothalamus that regulate feeding behavior and body weight. Physiol. Behav. 92, 263–271. 10.1016/j.physbeh.2007.05.021 [DOI] [PubMed] [Google Scholar]
- Merchenthaler I., Gorcs T., Setalo G., Petrusz P., Flerko B. (1984). Gonadotropin-releasing hormone (GnRH) neurons and pathways in the rat brain. Cell Tissue Res. 237, 15–29. 10.1007/BF00229195 [DOI] [PubMed] [Google Scholar]
- Meredith M. A., Stein B. E. (1986). Visual, auditory, and somatosensory convergence on cells in superior colliculus results in multisensory integration. J. Neurophysiol. 56, 640–662. [DOI] [PubMed] [Google Scholar]
- Millhouse O. E. (1969). A Golgi study of the desending medial forebrain bundle. Brain Res. 15, 341–363. 10.1016/0006-8993(69)90161-9 [DOI] [PubMed] [Google Scholar]
- Millhouse O. E. (1973). The organization of the ventromedial hypothalamic nucleus. Brain Res. 55, 71–87. 10.1016/0006-8993(73)90489-7 [DOI] [PubMed] [Google Scholar]
- Millhouse O. E. (1979). A Golgi anatomy of the rodent hypothalamus, in Anatomy of the Hypothalamus, Vol. 1 eds Morgane P. J., Panksepp J. (New York, NY: Dekker; ), 221–265. [Google Scholar]
- Mohedano-Moriano A., Pro-Sistiaga P., Ubeda-Banon I., Crespo C., Insausti R., Martinez-Marcos A. (2007). Segregated pathways to the vomeronasal amygdala: differential projections from the anterior and posterior divisions of the accessory olfactory bulb. Eur. J. Neurosci. 25, 2065–2080. 10.1111/j.1460-9568.2007.05472.x [DOI] [PubMed] [Google Scholar]
- Morgane P. J., Panksepp J. (1979). Anatomy of the Hypothalamus. New York, NY: Dekker; ). [Google Scholar]
- Morin L. P., Studholme K. M. (2014). Retinofugal projections in the mouse. J. Comp. Neurol. 522, 3733–3753. 10.1002/cne.23635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. (2006). Theories of hippocampal function, in The Hippocampus Book, eds Andersen P., Morris R., Amaral D., Bliss T., O'Keefe J. (Oxford: Oxford University Press; ), 581–714. [Google Scholar]
- Nauta W. J. H., Haymaker W. (1969). Hypothalamic nuclei and fiber connections, in The Hypothalamus, eds Haymaker W., Anderson E., Nauta W. J. H. (Illinois, IL: Charles C Thomas; ), 136–209. [Google Scholar]
- Netzer F., Bernard J. F., Verberne A. J., Hamon M., Camus F., Benoliel J. J., et al. (2011). Brain circuits mediating baroreflex bradycardia inhibition in rats: an anatomical and functional link between the cuneiform nucleus and the periaqueductal grey. J. Physiol. 589, 2079–2091. 10.1113/jphysiol.2010.203737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe J., Nadel L. (1978). The Hippocampus as a Cognitive Map. Oxford: Oxford University Press. [Google Scholar]
- Pattij T., de Jong T. R., Uitterdijk A., Waldinger M. D., Veening J. G., Cools A. R., et al. (2005). Individual differences in male rat ejaculatory behaviour: searching for models to study ejaculation disorders. Eur. J. Neurosci. 22, 724–734. 10.1111/j.1460-9568.2005.04252.x [DOI] [PubMed] [Google Scholar]
- Pawluski J. L., Galea L. A. (2006). Hippocampal morphology is differentially affected by reproductive experience in the mother. J. Neurobiol. 66, 71–81. 10.1002/neu.20194 [DOI] [PubMed] [Google Scholar]
- Pentkowski N. S., Blanchard D. C., Lever C., Litvin Y., Blanchard R. J. (2006). Effects of lesions to the dorsal and ventral hippocampus on defensive behaviors in rats. Eur. J. Neurosci. 23, 2185–2196. 10.1111/j.1460-9568.2006.04754.x [DOI] [PubMed] [Google Scholar]
- Petrovich G. D., Canteras N. S., Swanson L. W. (2001). Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res. Brain Res. Rev. 38, 247–289. 10.1016/S0165-0173(01)00080-7 [DOI] [PubMed] [Google Scholar]
- Petrovich G. D., Holland P. C., Gallagher M. (2005). Amygdalar and prefrontal pathways to the lateral hypothalamus are activated by a learned cue that stimulates eating. J. Neurosci. 25, 8295–8302. 10.1523/JNEUROSCI.2480-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkanen A., Stefanacci L., Farb C. R., Go G. G., LeDoux J. E., Amaral D. G. (1995). Intrinsic connections of the rat amygdaloid complex: projections originating in the lateral nucleus. J. Comp. Neurol. 356, 288–310. 10.1002/cne.903560211 [DOI] [PubMed] [Google Scholar]
- Pollak G. D., Burger R. M., Klug A. (2003). Dissecting the circuitry of the auditory system. Trends Neurosci. 26, 33–39. 10.1016/S0166-2236(02)00009-7 [DOI] [PubMed] [Google Scholar]
- Pro-Sistiaga P., Mohedano-Moriano A., Ubeda-Banon I., Mar Arroyo-Jimenez M., Marcos P., Artacho-Perula E., et al. (2007). Convergence of olfactory and vomeronasal projections in the rat basal telencephalon. J. Comp. Neurol. 504, 346–362. 10.1002/cne.21455 [DOI] [PubMed] [Google Scholar]
- Ramon y Cajal S. (1995). Histology of the Nervous System of Man and Vertebrates. New York, NY: Oxford University Press. [Google Scholar]
- Randle J. C., Bourque C. W., Renaud L. P. (1986). Serial reconstruction of Lucifer yellow-labeled supraoptic nucleus neurons in perfused rat hypothalamic explants. Neuroscience 17, 453–467. 10.1016/0306-4522(86)90259-9 [DOI] [PubMed] [Google Scholar]
- Richard J. M., Castro D. C., Difeliceantonio A. G., Robinson M. J., Berridge K. C. (2013). Mapping brain circuits of reward and motivation: in the footsteps of Ann Kelley. Neurosci. Biobehav. Rev. 37, 1919–1931. 10.1016/j.neubiorev.2012.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risold P. Y., Canteras N. S., Swanson L. W. (1994). Organization of projections from the anterior hypothalamic nucleus - a Phaseolus-Vulgaris-Leukoagglutinin study in the rat. J. Comp. Neurol. 348, 1–40. 10.1002/cne.903480102 [DOI] [PubMed] [Google Scholar]
- Risold P. Y., Swanson L. W. (1996). Structural evidence for functional domains in the rat hippocampus. Science 272, 1484–1486. 10.1126/science.272.5267.1484 [DOI] [PubMed] [Google Scholar]
- Risold P. Y., Swanson L. W. (1997a). Chemoarchitecture of the rat lateral septal nucleus. Brain Res. Brain Res. Rev. 24, 91–113. 10.1016/S0165-0173(97)00008-8 [DOI] [PubMed] [Google Scholar]
- Risold P. Y., Swanson L. W. (1997b). Connections of the rat lateral septal complex. Brain Res. Brain Res. Rev. 24, 115–195. 10.1016/S0165-0173(97)00009-X [DOI] [PubMed] [Google Scholar]
- Risold P. Y., Thompson R. H., Swanson L. W. (1997). The structural organization of connections between hypothalamus and cerebral cortex. Brain Res. Brain Res. Rev. 24, 197–254. 10.1016/S0165-0173(97)00007-6 [DOI] [PubMed] [Google Scholar]
- Rye D. B., Lee H. J., Saper C. B., Wainer B. H. (1988). Medullary and spinal efferents of the pedunculopontine tegmental nucleus and adjacent mesopontine tegmentum in the rat. J. Comp. Neurol. 269, 315–341. 10.1002/cne.902690302 [DOI] [PubMed] [Google Scholar]
- Rye D. B., Saper C. B., Lee H. J., Wainer B. H. (1987). Pedunculopontine tegmental nucleus of the rat: cytoarchitecture, cytochemistry, and some extrapyramidal connections of the mesopontine tegmentum. J. Comp. Neurol. 259, 483–528. 10.1002/cne.902590403 [DOI] [PubMed] [Google Scholar]
- Saper C. B., Swanson L. W., Cowan W. M. (1979). An autoradiographic study of the efferent connections of the lateral hypothalamic area in the rat. J. Comp. Neurol. 183, 689–706. 10.1002/cne.901830402 [DOI] [PubMed] [Google Scholar]
- Scalia F., Winans S. S. (1975). The differential projections of the olfactory bulb and accessory olfactory bulb in mammals. J. Comp. Neurol. 161, 31–55. 10.1002/cne.901610105 [DOI] [PubMed] [Google Scholar]
- Schmued L. C., Fallon J. H. (1986). Fluoro-Gold: a new fluorescent retrograde axonal tracer with numerous unique properties. Brain Res. 377, 147–154. 10.1016/0006-8993(86)91199-6 [DOI] [PubMed] [Google Scholar]
- Sharpe L. G., Swanson L. W. (1974). Drinking induced by injections of angiotensin into forebrain and mid-brain sites of monkey. J. Physiol. 239, 595–622. 10.1113/jphysiol.1974.sp010584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar A. (1993). GABA receptors in the region of the dorsomedial hypothalamus of rats regulate anxiety in the elevated plus-maze test. I. Behavioral measures. Brain Res. 627, 9–16. 10.1016/0006-8993(93)90742-6 [DOI] [PubMed] [Google Scholar]
- Shekhar A., Keim S. R., Simon J. R., McBride W. J. (1996). Dorsomedial hypothalamic GABA dysfunction produces physiological arousal following sodium lactate infusions. Pharmacol. Biochem. Behav. 55, 249–256. 10.1016/S0091-3057(96)00077-9 [DOI] [PubMed] [Google Scholar]
- Shekhar A., Sims L. S., Bowsher R. R. (1993). GABA receptors in the region of the dorsomedial hypothalamus of rats regulate anxiety in the elevated plus-maze test. II. Physiological measures. Brain Res. 627, 17–24. 10.1016/0006-8993(93)90743-7 [DOI] [PubMed] [Google Scholar]
- Shimogawa Y., Sakuma Y., Yamanouchi K. (2014). Efferent and afferent connections of the ventromedial hypothalamic nucleus determined by neural tracer analysis: implications for lordosis regulation in female rats. Neurosci Res. 91, 19–33. 10.1016/j.neures.2014.10.016 [DOI] [PubMed] [Google Scholar]
- Shughrue P. J., Lane M. V., Merchenthaler I. (1997). Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J. Comp. Neurol. 388, 507–525. [DOI] [PubMed] [Google Scholar]
- Silverman A. J., Jhamandas J., Renaud L. P. (1987). Localization of luteinizing hormone-releasing hormone (LHRH) neurons that project to the median eminence. J. Neurosci. 7, 2312–2319. [PMC free article] [PubMed] [Google Scholar]
- Simerly R. B. (2002). Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu. Rev. Neurosci. 25, 507–536. 10.1146/annurev.neuro.25.112701.142745 [DOI] [PubMed] [Google Scholar]
- Simerly R. B., Chang C., Muramatsu M., Swanson L. W. (1990). Distribution of androgen and estrogen-receptor messenger rna-containing cells in the rat-brain - an Insitu hybridization study. J. Comp. Neurol. 294, 76–95. 10.1002/cne.902940107 [DOI] [PubMed] [Google Scholar]
- Simmons D. M., Swanson L. W. (1993). The nissl stain, in Neuroscience Protocols, ed Wouterlood F. G. (Amsterdam: Elsevier; ), 1–7. [Google Scholar]
- Simmons D. M., Swanson L. W. (2009). Comparison of the spatial distribution of seven types of neuroendocrine neurons in the rat paraventricular nucleus: toward a global 3D model. J. Comp. Neurol. 516, 423–441. 10.1002/cne.22126 [DOI] [PubMed] [Google Scholar]
- Simon D. K., O'Leary D. D. (1992). Development of topographic order in the mammalian retinocollicular projection. J. Neurosci. 12, 1212–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithson K. G., Weiss M. L., Hatton G. I. (1992). Supraoptic nucleus afferents from the accessory olfactory bulb: evidence from anterograde and retrograde tract tracing in the rat. Brain Res. Bull. 29, 209–220. 10.1016/0361-9230(92)90028-V [DOI] [PubMed] [Google Scholar]
- Sohn J. W., Elmquist J. K., Williams K. W. (2013). Neuronal circuits that regulate feeding behavior and metabolism. Trends Neurosci. 36, 504–512. 10.1016/j.tins.2013.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire L. R. (1992). Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol. Rev. 99, 195–231. 10.1037/0033-295X.99.2.195 [DOI] [PubMed] [Google Scholar]
- Stein B. E., Stanford T. R., Rowland B. A. (2009). The neural basis of multisensory integration in the midbrain: its organization and maturation. Hear. Res. 258, 4–15. 10.1016/j.heares.2009.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steininger T. L., Rye D. B., Wainer B. H. (1992). Afferent projections to the cholinergic pedunculopontine tegmental nucleus and adjacent midbrain extrapyramidal area in the albino rat. I. Retrograde tracing studies. J. Comp. Neurol. 321, 515–543. 10.1002/cne.903210403 [DOI] [PubMed] [Google Scholar]
- Sternson S. M. (2013). Hypothalamic survival circuits: blueprints for purposive behaviors. Neuron 77, 810–824. 10.1016/j.neuron.2013.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel K., Schwab M., Thoenen H. (1977). Role of gangliosides in the uptake and retrograde axonal transport of cholera and tetanus toxin as compared to nerve growth factor and wheat germ agglutinin. Brain Res. 132, 273–285. 10.1016/0006-8993(77)90421-8 [DOI] [PubMed] [Google Scholar]
- Stoneham M. D., Everitt B. J., Hansen S., Lightman S. L., Todd K. (1985). Oxytocin and sexual behaviour in the male rat and rabbit. J. Endocrinol. 107, 97–106. 10.1677/joe.0.1070097 [DOI] [PubMed] [Google Scholar]
- Strange B. A., Witter M. P., Lein E. S., Moser E. I. (2014). Functional organization of the hippocampal longitudinal axis. Nat. Rev. Neurosci. 15, 655–669. 10.1038/nrn3785 [DOI] [PubMed] [Google Scholar]
- Swanson L. W. (1981). Tracing central pathways with the autoradiographic method. J. Histochem. Cytochem. 29, 117–124. 10.1177/29.1A_Suppl.7288150 [DOI] [PubMed] [Google Scholar]
- Swanson L. W. (1986). Organization of the mammalian neuroendocrine system in Handbook of Physiology, The Nervous System, Vol. 4, ed Bloom F. E. (Baltimore: Waverly Press; ), 169–190. [Google Scholar]
- Swanson L. W. (1987). The hypothalamus, in Handbook of Chemical Neuroanatomy, Vol. 5, eds Bjorklund A., Hokfelt T., Swanson L. W. (Amsterdam: Elsevier; ), 1–124. [Google Scholar]
- Swanson L. W. (1999). The neuroanatomy revolution of the 1970s and the hypothalamus. Brain Res. Bull. 50, 397. 10.1016/S0361-9230(99)00163-X [DOI] [PubMed] [Google Scholar]
- Swanson L. W. (2000). Cerebral hemisphere regulation of motivated behavior. Brain Res. 886, 113–164. 10.1016/S0006-8993(00)02905-X [DOI] [PubMed] [Google Scholar]
- Swanson L. W. (2004). Brain Maps: Structure of the Rat Brain, 3rd Edn. Amsterdam: Academic Press. [Google Scholar]
- Swanson L. W. (2007). Quest for the basic plan of nervous system circuitry. Brain Res. Rev. 55, 356–372. 10.1016/j.brainresrev.2006.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson L. W., Bota M. (2010). Foundational model of structural connectivity in the nervous system with a schema for wiring diagrams, connectome, and basic plan architecture. Proc. Natl. Acad. Sci. U.S.A. 107, 20610–20617. 10.1073/pnas.1015128107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson L. W., Cowan W. M. (1976). Autoradiographic studies of the development and connections of the septal area in the rat, in The Septal Nuclei: Advances in Behavioral Biology, Vol. 20, ed DeFrance J. F. (New York, NY Springer US; ), 37–64. 10.1007/978-1-4684-3084-4_2 [DOI] [Google Scholar]
- Swanson L. W., Cowan W. M. (1977). Autoradiographic study of organization of efferent connections of hippocampal formation in rat. J. Comp. Neurol. 172, 49–84. 10.1002/cne.901720104 [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Petrovich G. D. (1998). What is the amygdala? Trends Neurosci. 21, 323–331. 10.1016/S0166-2236(98)01265-X [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Sanchez-Watts G., Watts A. G. (2005). Comparison of melanin-concentrating hormone and hypocretin/orexin mRNA expression patterns in a new parceling scheme of the lateral hypothalamic zone. Neurosci. Lett. 387, 80–84. 10.1016/j.neulet.2005.06.066 [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Sharpe L. G. (1973). Centrally induced drinking - comparison of angiotensin-Ii-sensitive and carbachol-sensitive sites in rats. Am. J. Physiol. 225, 566–573. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Sharpe L. G., Griffin D. (1973). Drinking to intracerebral angiotensin-Ii and Carbachol - Dose-response relationships and ionic involvement. Physiol. Behav. 10, 595–600. 10.1016/0031-9384(73)90227-8 [DOI] [PubMed] [Google Scholar]
- Thellier D., Moos F., Richard P., Stoeckel M. E. (1994a). Evidence for connections between a discrete hypothalamic dorsochiasmatic area and the supraoptic and paraventricular nuclei. Brain Res. Bull. 34, 261–274. 10.1016/0361-9230(94)90063-9 [DOI] [PubMed] [Google Scholar]
- Thellier D., Moos F., Richard P., Stoeckel M. E. (1994b). Evidence for reciprocal connections between the dorsochiasmatic area and the hypothalamo neurohypophyseal system and some related extrahypothalamic structures. Brain Res. Bull. 35, 311–322. 10.1016/0361-9230(94)90107-4 [DOI] [PubMed] [Google Scholar]
- Thompson C. L., Pathak S. D., Jeromin A., Ng L. L., MacPherson C. R., Mortrud M. T., et al. (2008). Genomic anatomy of the hippocampus. Neuron 60, 1010–1021. 10.1016/j.neuron.2008.12.008 [DOI] [PubMed] [Google Scholar]
- Thompson R. H., Canteras N. S., Swanson L. W. (1996). Organization of projections from the dorsomedial nucleus of the hypothalamus: a PHA-L study in the rat. J. Comp. Neurol. 376, 143–173. [DOI] [PubMed] [Google Scholar]
- Thompson R. H., Swanson L. W. (1998). Organization of inputs to the dorsomedial nucleus of the hypothalamus: a reexamination with Fluorogold and PHAL in the rat. Brain Res. Brain Res. Rev. 27, 89–118. 10.1016/S0165-0173(98)00010-1 [DOI] [PubMed] [Google Scholar]
- Thompson R. H., Swanson L. W. (2003). Structural characterization of a hypothalamic visceromotor pattern generator network. Brain Res. Brain Res. Rev. 41, 153–202. 10.1016/S0165-0173(02)00232-1 [DOI] [PubMed] [Google Scholar]
- Thompson R. H., Swanson L. W. (2010). Hypothesis-driven structural connectivity analysis supports network over hierarchical model of brain architecture. Proc. Natl. Acad. Sci. U.S.A. 107, 15235–15239. 10.1073/pnas.1009112107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson A. M., West D. C., Hahn J., Deuchars J. (1996). Single axon IPSPs elicited in pyramidal cells by three classes of interneurones in slices of rat neocortex. J. Physiol. 496(Pt 1), 81–102. 10.1113/jphysiol.1996.sp021667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth M., Fuzesi T., Halasz J., Tulogdi A., Haller J. (2010). Neural inputs of the hypothalamic “aggression area” in the rat. Behav. Brain Res. 215, 7–20. 10.1016/j.bbr.2010.05.050 [DOI] [PubMed] [Google Scholar]
- Trojanowski J. Q., Gonatas J. O., Gonatas N. K. (1981). Conjugates of horseradish peroxidase (HRP) with cholera toxin and wheat germ agglutinin are superior to free HRP as orthogradely transported markers. Brain Res. 223, 381–385. 10.1016/0006-8993(81)91151-3 [DOI] [PubMed] [Google Scholar]
- Tweedle C. D., Smithson K. G., Hatton G. I. (1989). Neurosecretory endings in the rat neurohypophysis are en passant. Exp. Neurol. 106, 20–26. 10.1016/0014-4886(89)90140-4 [DOI] [PubMed] [Google Scholar]
- Veening J. G., Coolen L. M. (1998). Neural activation following sexual behavior in the male and female rat brain. Behav. Brain Res. 92, 181–193. 10.1016/S0166-4328(97)00190-3 [DOI] [PubMed] [Google Scholar]
- Veening J. G., Coolen L. M. (2014). Neural mechanisms of sexual behavior in the male rat: emphasis on ejaculation-related circuits. Pharmacol. Biochem. Behav. 121, 170–183. 10.1016/j.pbb.2013.12.017 [DOI] [PubMed] [Google Scholar]
- Veening J. G., Swanson L. W., Cowan W. M., Nieuwenhuys R., Geeraedts L. M. (1982). The medial forebrain bundle of the rat. II. An autoradiographic study of the topography of the major descending and ascending components. J. Comp. Neurol. 206, 82–108. 10.1002/cne.902060107 [DOI] [PubMed] [Google Scholar]
- Veronneau-Longueville F., Rampin O., Freund-Mercier M. J., Tang Y., Calas A., Marson L., et al. (1999). Oxytocinergic innervation of autonomic nuclei controlling penile erection in the rat. Neuroscience 93, 1437–1447. 10.1016/S0306-4522(99)00262-6 [DOI] [PubMed] [Google Scholar]
- Wang M. E., Fraize N. P., Yin L., Yuan R. K., Petsagourakis D., Wann E. G., et al. (2013). Differential roles of the dorsal and ventral hippocampus in predator odor contextual fear conditioning. Hippocampus 23, 451–466. 10.1002/hipo.22105 [DOI] [PubMed] [Google Scholar]
- Watts A. G., Sanchez-Watts G. (2007). Rapid and preferential activation of Fos protein in hypocretin/orexin neurons following the reversal of dehydration-anorexia. J. Comp. Neurol. 502, 768–782. 10.1002/cne.21316 [DOI] [PubMed] [Google Scholar]
- Weiland N. G., Orikasa C., Hayashi S., McEwen B. S. (1997). Distribution and hormone regulation of estrogen receptor immunoreactive cells in the hippocampus of male and female rats. J. Comp. Neurol. 388, 603–612. [DOI] [PubMed] [Google Scholar]
- Wiegand S. J., Terasawa E. (1982). Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Neuroendocrinology 34, 395–404. 10.1159/000123335 [DOI] [PubMed] [Google Scholar]
- Witkin J. W., Paden C. M., Silverman A. J. (1982). The luteinizing hormone-releasing hormone (LHRH) systems in the rat brain. Neuroendocrinology 35, 429–438. 10.1159/000123419 [DOI] [PubMed] [Google Scholar]
- Witter M. P. (2006). Connections of the subiculum of the rat: topography in relation to columnar and laminar organization. Behav. Brain Res. 174, 251–264. 10.1016/j.bbr.2006.06.022 [DOI] [PubMed] [Google Scholar]
- Woolley C. S., Weiland N. G., McEwen B. S., Schwartzkroin P. A. (1997). Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density. J. Neurosci. 17, 1848–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wotjak C. T., Kubota M., Liebsch G., Montkowski A., Holsboer F., Neumann I., et al. (1996). Release of vasopressin within the rat paraventricular nucleus in response to emotional stress: a novel mechanism of regulating adrenocorticotropic hormone secretion? J. Neurosci. 16, 7725–7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouterlood F. G., Groenewegen H. J. (1985). Neuroanatomical tracing by use of Phaseolus vulgaris-leucoagglutinin (PHA-L): electron microscopy of PHA-L-filled neuronal somata, dendrites, axons and axon terminals. Brain Res. 326, 188–191. 10.1016/0006-8993(85)91402-7 [DOI] [PubMed] [Google Scholar]
- Wray S., Hoffman G. (1986). A developmental study of the quantitative distribution of LHRH neurons within the central nervous system of postnatal male and female rats. J. Comp. Neurol. 252, 522–531. 10.1002/cne.902520408 [DOI] [PubMed] [Google Scholar]
- Zaborszky L. S., Wouterlood F. G., Lanciego J. L., Heimer L. (2006). Neuroanatomical Tract-Tracing 3: Molecules, Neurons, and Systems. New York, NY: Springer. [Google Scholar]
- Zahm D. S., Parsley K. P., Schwartz Z. M., Cheng A. Y. (2013). On lateral septum-like characteristics of outputs from the accumbal hedonic “hotspot” of Pecina and Berridge with commentary on the transitional nature of basal forebrain “boundaries”. J. Comp. Neurol. 521, 50–68. 10.1002/cne.23157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Hernandez V. S. (2013). Synaptic innervation to rat hippocampus by vasopressin-immuno-positive fibres from the hypothalamic supraoptic and paraventricular nuclei. Neuroscience 228, 139–162. 10.1016/j.neuroscience.2012.10.010 [DOI] [PubMed] [Google Scholar]
- Ziegler D. R., Cullinan W. E., Herman J. P. (2002). Distribution of vesicular glutamate transporter mRNA in rat hypothalamus. J. Comp. Neurol. 448, 217–229. 10.1002/cne.10257 [DOI] [PubMed] [Google Scholar]
- Zingg B., Hintiryan H., Gou L., Song M. Y., Bay M., Bienkowski M. S., et al. (2014). Neural networks of the mouse neocortex. Cell 156, 1096–1111. 10.1016/j.cell.2014.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]