Abstract
BACKGROUND
Many scoring systems have been developed for the purpose of estimating of mortality and outcomes in intracerebral hemorrhage (ICH). However, the utility of the World Federation of Neurosurgical Society (WFNS) classification, which is routinely used in patients with subarachnoid hemorrhage, has never been specifically assessed in ICH.
METHODS
A retrospective review of the records of consecutive ICH patients admitted over a 2-year period was carried out. Collected data included ICH size, location, intraventricular hemorrhage, age, admission Glasgow Coma Scale scores, and outcomes on discharge. Linear regression was performed to confirm correlations of the WFNS scale and the ICH score separately with good outcome, poor outcome, and in-hospital mortality. Receiver–operator characteristic (ROC) curve was employed to plot WFNS and ICH scores each in relation to in-hospital mortality and poor outcome. Accuracy was estimated by calculating the area under the curves (AUC).
RESULTS
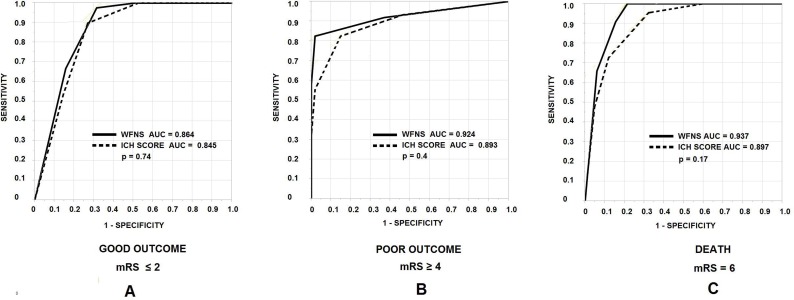
In this study, 128 patients were included. The overall mortality rate was 34.4%. Linear regression showed appropriate fit for both the ICH Score and the WFNS in relation to poor outcome and mortality. The ROC curves for the scales in relation to in-hospital death produced an AUC estimate 0.93 for WFNS and 0.92 for the ICH Score (p = 0.81). For poor outcome, the AUC values were 0.91 and 0.90 for the WFNS and the ICH Score, respectively (p = 0.9). For good outcome, the AUC for WFNS was 0.86 and for the ICH score, 0.85 (p = 0.74).
CONCLUSION
The WFNS classification is as accurate as the ICH score in predicting discharge outcomes and in-hospital mortality. It is a simple clinical scale that can be used to predict outcomes in both ICH and subarachnoid hemorrhage patients.
Keywords: intracerebral hemorrhage, outcomes, mortality, scales
INTRODUCTION
Intracerebral hemorrhage (ICH) is a devastating condition with high morbidity and mortality [1]. Before 2001, a prognostic scale for ICH, similar to the Hunt and Hess classification used in subarachnoid hemorrhage (SAH), did not exist. In 2001, the ICH score was introduced and later validated as a tool for predicting 30-day mortality in patients with ICH [2]. Since then, several modified versions of the ICH score with varying predictive capacities have emerged [3]. The ICH score utilizes a combination of features that are individually associated with high mortality. However, of the five components that comprise this scale, three are radiological. The only clinical piece is the Glasgow Coma Scale (GCS). GCS is also the main component of the World Federation of Neurosurgical Societies (WFNS) classification. In patients with SAH, WFNS classification is used to measure clinical severity on presentation. WFNS classification was developed in 1988 and started as a scale that was largely based upon consensus among experts [4]. It has been shown to have good discriminatory ability for prognostication of SAH [5]. Although in practice it is usually combined with an imaging scale (such as the Fisher Grade), WFNS scale itself quantifies severity on the basis of clinical presentation, but not on radiological. The utility of WFNS has never been specifically examined in patients with ICH. The aim of this study was to determine whether or not WFNS scale can be used to predict in-hospital mortality and discharge in patients with ICH.
METHODS
An Institutional Review Board approval was obtained for this study. A retrospective review of the medical records of consecutive patients with nontraumatic ICH treated at the Ohio State University Medical Center between January 2011 and December 2013 was conducted. We used the International Classification of Diseases, Ninth Revision, diagnosis code for ICH (431) to compile a list of patients for our analysis.
Data that were collected included admission age, GCS, ICH volume, the presence of intraventricular hemorrhage (IVH), and ICH location. The GCS scores were primarily extracted from the ambulance records. Hematoma volume was measured on the initial head computed tomography (CT) scan using the ABC/2 method [6]. Outcomes on hospital discharge were documented as scores on the modified Rankin scale (mRS), a stroke outcome scale with scores ranging from 0 (no symptoms at all) to 6 (dead). Also, abstracted was the existence of motor deficit (including aphasia) from the initial examination in order to calculate the WFNS scores. All patients with ICH admitted to the Ohio State University Medical Center are managed in accordance with the American Heart Association/American Stroke Association guidelines [7].
A dichotomy was created separating discharge outcomes into good (mRS ≤ 2) and poor (mRS ≥ 4). Regression analysis was then performed to confirm association of each scale to good and poor outcome, as well as to in-hospital death. Determination coefficients (r2) were calculated to estimate the amount of variance in outcome explained by the scales. Bland–Altman plot was performed to assess the correlation of mRS to WFNS score and mRS to the ICH score. Finally, receiver–operator characteristic (ROC) curve was used to plot WFNS and ICH scores each in relation to in-hospital mortality and poor outcome. We estimated accuracy by calculating the area under the curves (AUC). A p value of ≤ 0.05 was considered significant. Minitab® 17 (Minitab Inc., State College, Pennsylvania) and JMP® (Statistical Discovery / SAS, Cary, North Carolina) software were used for statistical analysis.
RESULTS
A total of 128 patients with an average age of 70.7 ± 13.6 were included in our study. In-hospital mortality rate was 34.4%. Table 1 shows the characteristics of this cohort in more detail.
Table 1. Characteristics of the study cohort. SD = standard deviation, ICH = intracerebral hemorrhage, IVH = intraventricular hemorrhage, mRS = modified Rankin Scale.
| Age | Mean ± SD | 70.7 ± 13.6 |
| Sex | Male | 78(60.9%) |
| Female | 50(39.1%) | |
| Glasgow coma scale score | Median | 13 |
| ICH Volume | Median | 17.4 mL |
| Mean ± SD | 34.4 ± 47.2 mL | |
| ICH Location | Basal ganglia | 45(35.2%) |
| Brainstem | 6(4.7%) | |
| Cerebellum | 12(9.4%) | |
| Lobar | 47(36.7%) | |
| Thalamus | 18(14.1%) | |
| IVH | 55(43%) | |
| Outcome | mRS = 0 | 12(9.4%) |
| mRS = 1 | 12(9.4%) | |
| mRS = 2 | 15(11.7%) | |
| mRS = 3 | 15(11.7%) | |
| mRS = 4 | 17(13.3%) |
In linear regression with in-hospital death as the dependent variable, the r2 for WFNS was 0.51 and 0.49 for the ICH score, suggesting equal approximation of variance between the two scales. For poor outcome, r2 values for the WFNS and the ICH score were 0.55 and 0.47, respectively. Bland–Altman plots showed correlation of 0.80 between WFNS and mRS (p < 0.0001), and 0.74 between the ICH score and mRS (p < 0.0001). The ROC curves for the scales in relation to in-hospital death produced an AUC estimate 0.93 for WFNS and 0.92 for the ICH score (p = 0.81) (Fig. 1). For poor outcome, the AUC values were 0.91 and 0.90 for the WFNS and the ICH score, respectively (p = 0.9). For good outcome, the AUC for WFNS was 0.86 and for the ICH score, 0.85 (p = 0.74).
Figure 1. Receiver–operator characteristic (ROC) curves comparing ICH score (dotted line) and WFNS classification (solid line) with good discharge outcome (A), poor discharge outcome (B), and in-hospital mortality (C). AUC = area under the curve.
DISCUSSION
This study showed that the WFNS classification, which is routinely used in patients presenting with SAH, is also applicable in patients with ICH. Moreover, it is at least as accurate as the ICH score in the prediction of mortality and outcomes. The advantages of the WFNS scale are simplicity and versatility. It can be used to estimate in-hospital mortality and discharge outcomes without incorporation of the radiological data. Specifically, the measurement of ICH volume may be challenging to physicians who are not very familiar with neuroimaging.
The original ICH score is quite intuitive and applicable. It has been validated and is possibly the scale used by most practitioners. Since its introduction, significant effort has been placed into modifying the ICH score in order to increase its sensitivity. These modifications primarily consist of incorporating a wide range of clinical features, such as the Graeb Scale (for IVH), pulse pressure, National Institutes of Health Stroke Scale, pre-ICH impairment, hydrocephalus, history of hypertension, serum glucose, and dialysis dependency [3]. These modified scales have their strengths and limitations. However, fitting in a plethora of patient characteristics in an aim to be comprehensive will not necessarily produce the ideal prognostication scale for ICH. Aside from having a reasonably high predicative value, a scale needs to be simple; one that is easy to memorize and apply. The WFNS scale especially allows non-neurologists/non-neurosurgeons to use the same scale for both ICH and SAH, relying principally on clinical examination. Additionally, the WFNS can be used to quantify the clinical severity of ICH.
The strengths of the ICH score and the WFNS in predicting outcomes come from the inclusion of GCS. Indeed, GCS score alone has been shown to be as predicative as various existing ICH-grading scales [8]. It is simple and versatile enough to be used independently, but what is discounted in the GCS is existence of motor deficit, which can have a heavy impact on outcome.
The biggest limitation of our study was that it was based on retrospective data. The retrospective nature of our study also did not allow us to assess long-term outcomes after discharge from the hospital, considering that patients who are severely disabled on discharge may improve and eventually become independent after rehabilitation. We did not have access to all patients’ follow-up record so that we could, similar to the ICH score, determine morality or functional status at 30 days. Also, our sample size was small, yet it was close to the cohort size used in formulating the ICH score (152) [2].
In summary, the WFNS classification is accurate and simple enough to be applied in both SAH and ICH patients. This may be particularly useful for emergency department physicians or medical intensivists. This study primarily shows equivalence of WFNS and the ICH score in predicting mortality and outcomes. Prospective studies may help confirm this finding.
DISCLOSURES
The author has nothing to disclose.
References
- Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344:1450–1460. doi: 10.1056/NEJM200105103441907. Available at: http://www.nejm.org/doi/full/10.1056/NEJM200105103441907. [DOI] [PubMed] [Google Scholar]
- Hemphill JC, 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: A simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32:891–897. doi: 10.1161/01.str.32.4.891. Available at: http://stroke.ahajournals.org/content/32/4/891.full. [DOI] [PubMed] [Google Scholar]
- Hwang BY, Appelboom G, Kellner CP, Carpenter AM, Kellner MA, Gigante PR, Sander Connolly E. Clinical grading scales in intracerebral hemorrhage. Neurocrit Care. 2010;13:141–151. doi: 10.1007/s12028-010-9382-x. Available at: http://link.springer.com/article/10.1007/s12028-010-9382-x. [DOI] [PubMed] [Google Scholar]
- Report of world federation of neurological surgeons committee on a universal subarachnoid hemorrhage grading scale. J Neurosurg. 1988;68:985–986. doi: 10.3171/jns.1988.68.6.0985. [DOI] [PubMed] [Google Scholar]
- van Heuven AW, Dorhout Mees SM, Algra A, Rinkel GJ. Validation of a prognostic subarachnoid hemorrhage grading scale derived directly from the Glasgow coma scale. Stroke. 2008;39:1347–1348. doi: 10.1161/STROKEAHA.107.498345. Available at: http://stroke.ahajournals.org/content/39/4/1347.full. [DOI] [PubMed] [Google Scholar]
- Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, Khoury J. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304–1305. doi: 10.1161/01.str.27.8.1304. Available at: http://stroke.ahajournals.org/content/27/8/1304.full. [DOI] [PubMed] [Google Scholar]
- Morgenstern LB, Hemphill JC, 3rd, Anderson C, Becker K, Broderick JP, Connolly ES, Jr, Greenberg SM, Huang JN, MacDonald RL, Messe SR, Mitchell PH, Selim M, Tamargo RJ. Guidelines for the management of spontaneous intracerebral hemorrhage: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41:2108–2129. doi: 10.1161/STR.0b013e3181ec611b. Available at: http://stroke.ahajournals.org/content/41/9/2108.full.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry-Jones AR, Abid KA, Di Napoli M, Smith CJ, Vail A, Patel HC, King AT, Tyrrell PJ. Accuracy and clinical usefulness of intracerebral hemorrhage grading scores: A direct comparison in a UK population. Stroke. 2013;44:1840–1845. doi: 10.1161/STROKEAHA.113.001009. Available at: http://stroke.ahajournals.org/content/44/7/1840.full.pdf+html. [DOI] [PubMed] [Google Scholar]