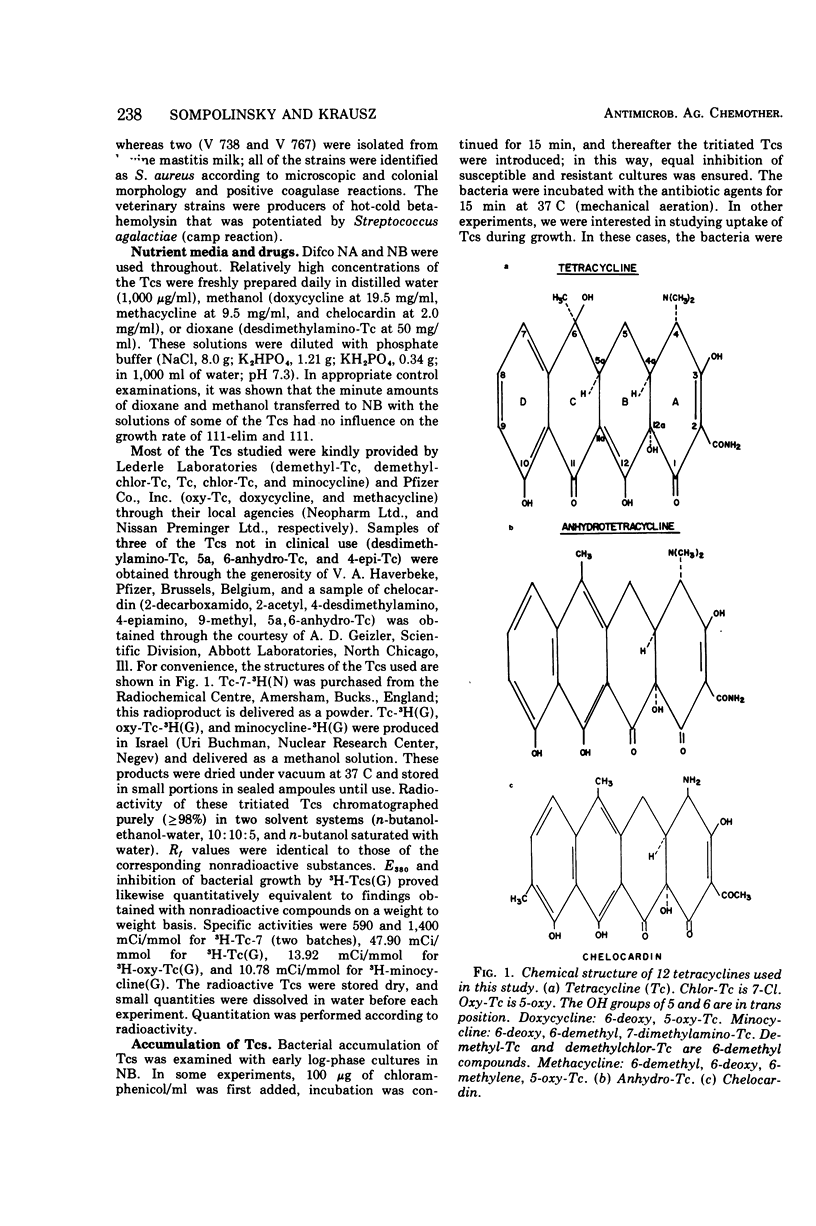
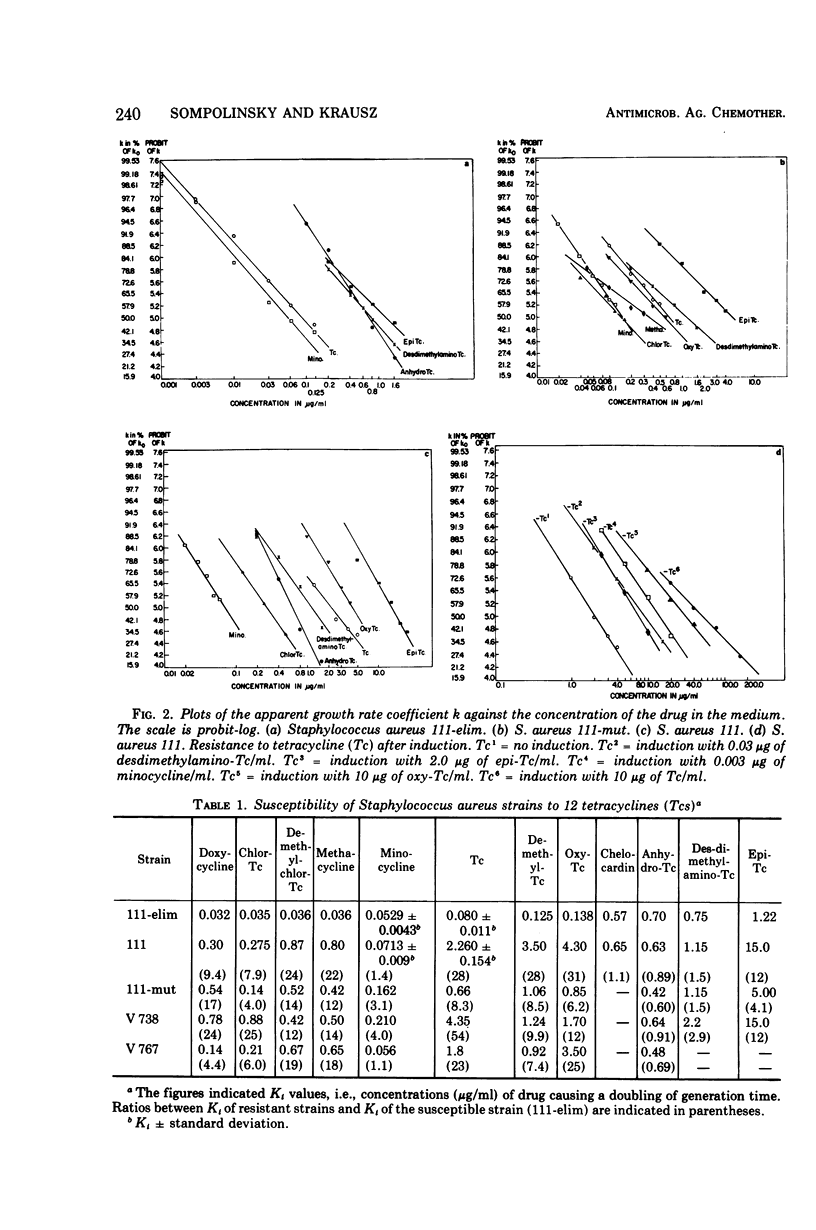
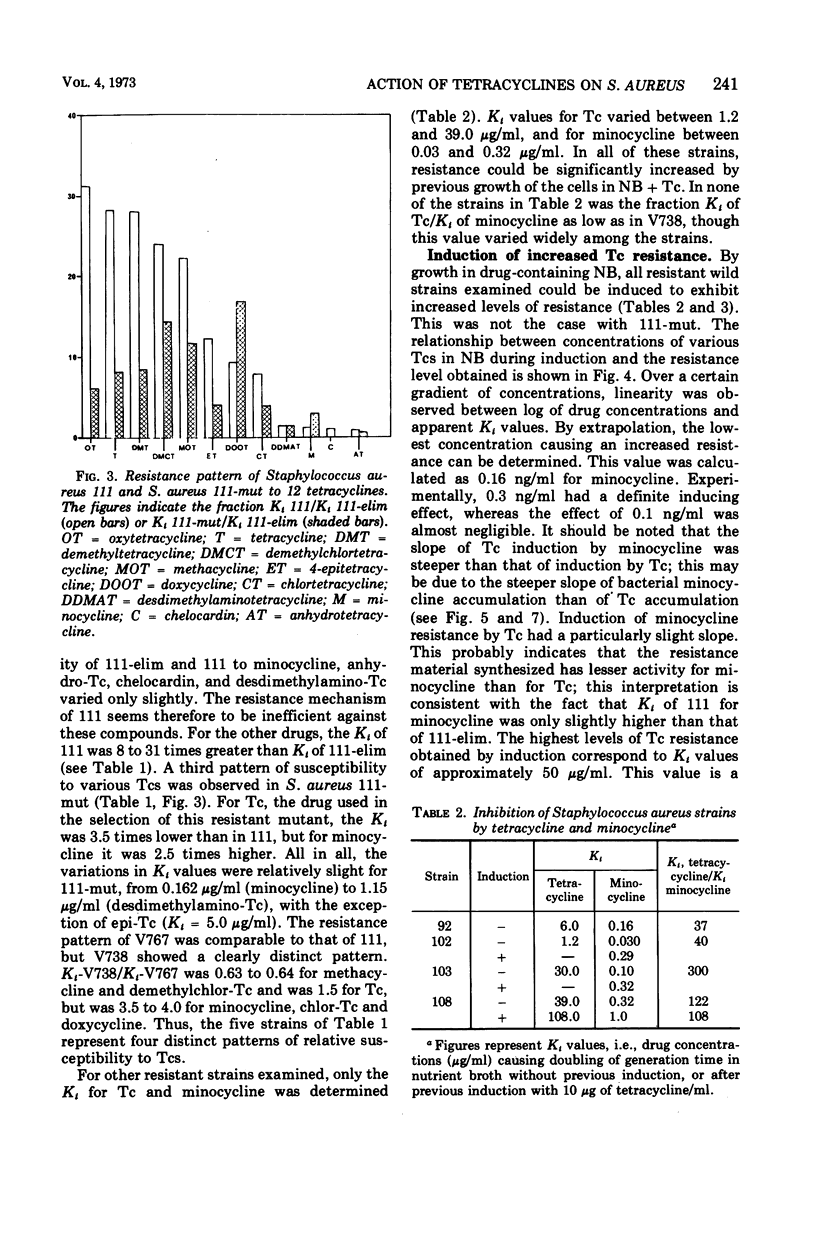
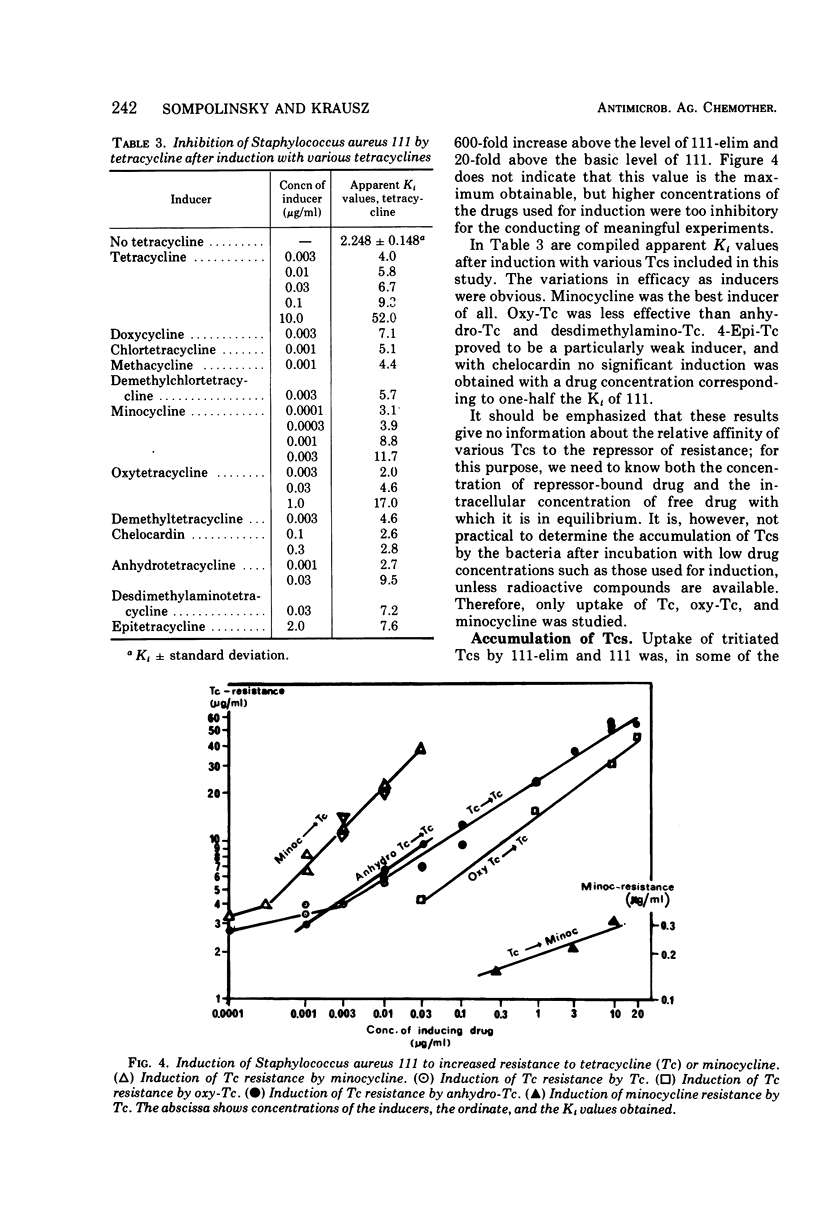
Abstract
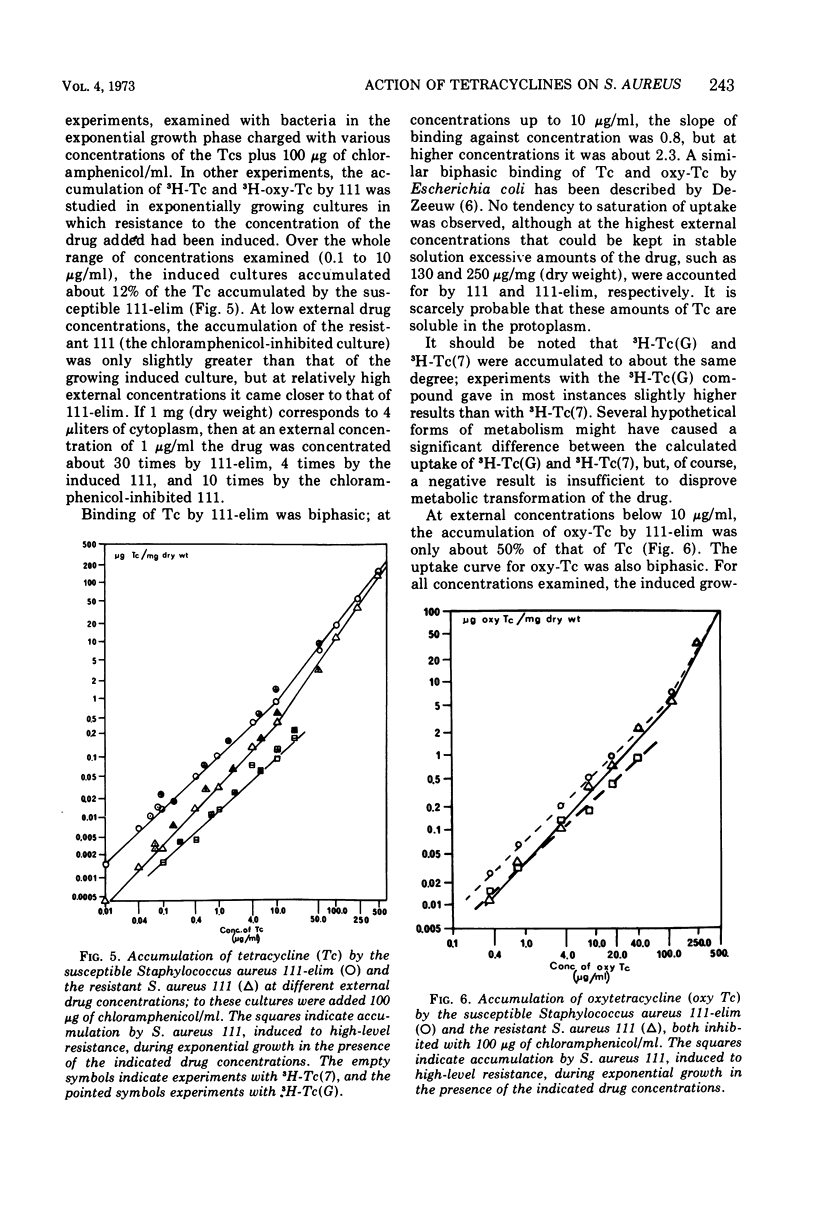
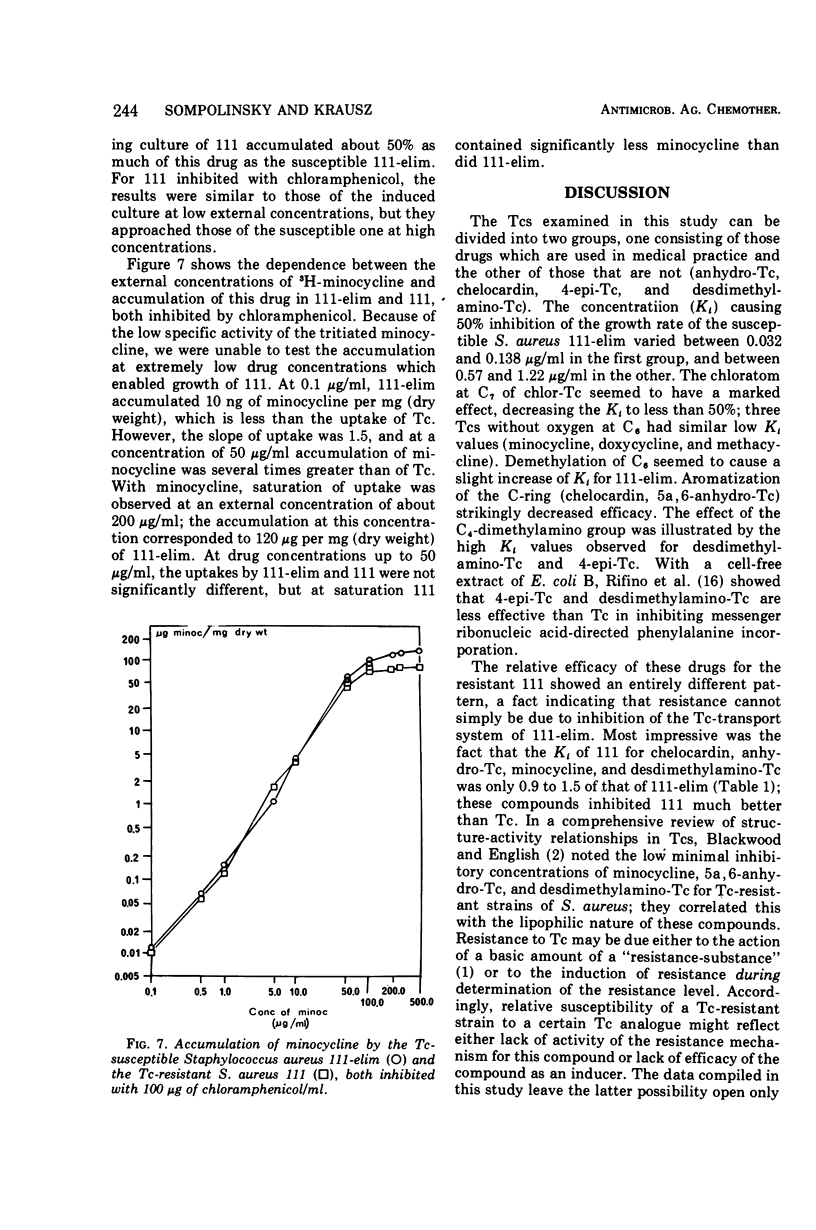
The relative growth inhibition caused by 12 tetracyclines in a susceptible strain of Staphylococcus aureus (111-elim) and in the same strain carrying a resistance-plasmid (111) showed entirely different patterns. For four of the tetracyclines (minocycline, anhydrotetracycline, chelocardin, and desdimethylaminotetracycline), the strain with the tetracycline plasmid (111) had virtually the same tolerance as the susceptible strain (111-elim). A resistant mutant of strain 111-elim showed a third pattern of relative growth inhibition, and another distinct pattern was observed in a veterinary wild strain of S. aureus. Of the 12 tetracyclines, 11 were effective inducers of higher tetracycline resistance in S. aureus 111, but no correlation was found between the efficacy of the tetracyclines as inducers and as inhibitors of growth of 111-elim or 111. At external drug concentrations causing doubling of the generation time (Ki), 111-elim accumulated tetracycline, oxytetracycline, and minocycline to a degree corresponding to several thousand molecules per coccus. At a fixed external drug concentration, 111 accumulated less tetracycline and oxytetracycline than 111-elim, whereas comparison at their respective Ki values showed accumulation to be significantly higher for 111 than for 111-elim. The accumulation of tetracyclines is assumed to involve both surface sorption and active membrane transport. Resistance is probably due to decreased accumulation of the drugs, and a hypothesis explaining the mechanism of resistance is offered.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avtalion R. R., Ziegler-Schlomowitz R., Pearl M., Wojdani A., Sompolinsky D. Depressed resistance to tetracycline in Staphylococcus aureus. Microbios. 1971 Mar;3(10):165–180. [PubMed] [Google Scholar]
- Connamacher R. H., Mandel H. G., Hahn F. E. Adaptation of populations of Bacillus cereus to tetracycline. Mol Pharmacol. 1967 Nov;3(6):586–594. [PubMed] [Google Scholar]
- Connamacher R. H., Mandel H. G. Studies on the intracellular localization of tetracycline in bacteria. Biochim Biophys Acta. 1968 Sep 24;166(2):475–486. doi: 10.1016/0005-2787(68)90235-9. [DOI] [PubMed] [Google Scholar]
- Craven G. R., Gavin R., Fanning T. The transfer RNA binding site of the 30 S ribosome and the site of tetracycline inhibition. Cold Spring Harb Symp Quant Biol. 1969;34:129–137. doi: 10.1101/sqb.1969.034.01.019. [DOI] [PubMed] [Google Scholar]
- De Zeeuw J. R. Accumulation of tetracyclines by Escherichia coli. J Bacteriol. 1968 Feb;95(2):498–506. doi: 10.1128/jb.95.2.498-506.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan J. B., Morse M. L. Carbohydrate transport in Staphylococcus aureus. II. Characterization of the defect of a pleiotropic transport mutant. Biochim Biophys Acta. 1965 Sep 27;109(1):172–183. doi: 10.1016/0926-6585(65)90101-9. [DOI] [PubMed] [Google Scholar]
- FRANKLIN T. J., GODFREY A. RESISTANCE OF ESCHERICHIA COLI TO TETRACYCLINES. Biochem J. 1965 Jan;94:54–60. doi: 10.1042/bj0940054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett E. R., Miller G. H., Brown M. R. Kinetics and mechanisms of action of antibiotics on microorganisms. V. Chloramphenicol and tetracycline affected Escherichia coli generation rates. J Pharm Sci. 1966 Jun;55(6):593–600. doi: 10.1002/jps.2600550613. [DOI] [PubMed] [Google Scholar]
- Inoue M., Hashimoto H., Mitsuhashi S. Mechanism of tetracycline resistance in Staphylococcus aureus. I. Inducible resistance to tetracycline. J Antibiot (Tokyo) 1970 Feb;23(2):68–74. doi: 10.7164/antibiotics.23.68. [DOI] [PubMed] [Google Scholar]
- Izaki K., Kiuchi K., Arima K. Specificity and mechanism of tetracycline resistance in a multiple drug resistant strain of Escherichia coli. J Bacteriol. 1966 Feb;91(2):628–633. doi: 10.1128/jb.91.2.628-633.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskin A. I., May Chan W. Inhibition by tetracyclines of polyuridylic acid directed phenylalanine incorporation in Escherichia coli cell-free systems. Biochem Biophys Res Commun. 1964;14:137–142. doi: 10.1016/0006-291x(64)90243-8. [DOI] [PubMed] [Google Scholar]
- OKAMOTO S., MIZUNO D. MECHANISM OF CHLORAMPHENICOL AND TETRACYCLINE RESISTANCE IN ESCHERICHIA COLI. J Gen Microbiol. 1964 Apr;35:125–133. doi: 10.1099/00221287-35-1-125. [DOI] [PubMed] [Google Scholar]
- Reeve E. C. Genetic analysis of some mutations causing resistance to tetracycline in Escherichia coli K12. Genet Res. 1968 Jun;11(3):303–309. doi: 10.1017/s0016672300011484. [DOI] [PubMed] [Google Scholar]
- Rifino C. B., Bousquet W. F., Knevel A. M., Belcastro P., Martin A. N. Effect of certain tetracycline analogs on phenylalanine-14C incorporation by Escherichia coli B cell-free extracts. J Pharm Sci. 1968 Feb;57(2):351–352. doi: 10.1002/jps.2600570232. [DOI] [PubMed] [Google Scholar]
- Sompolinsky D., Krawitz T., Zaidenzaig Y., Abramova N. Inducible resistance to tetracycline in Staphylococcus aureus. J Gen Microbiol. 1970 Aug;62(3):341–349. doi: 10.1099/00221287-62-3-341. [DOI] [PubMed] [Google Scholar]
- Sompolinsky D., Zaidenzaig Y., Ziegler-Schlomowitz R., Abramova N. Mechanism of tetracycline resistance in Staphylococcus aureus. J Gen Microbiol. 1970 Aug;62(3):351–362. doi: 10.1099/00221287-62-3-351. [DOI] [PubMed] [Google Scholar]



