Abstract
Background
The objective of this analysis was to evaluate the frequency, distribution of potential etiologies, and survival rates of maternal cardiopulmonary arrest during the hospitalization for delivery in the United States.
Methods
By using data from the Nationwide Inpatient Sample during the years 1998 through 2011, the authors obtained weighted estimates of the number of U.S. hospitalizations for delivery complicated by maternal cardiac arrest. Clinical and demographic risk factors, potential etiologies, and outcomes were identified and compared in women with and without cardiac arrest. The authors tested for temporal trends in the occurrence and survival associated with maternal arrest.
Results
Cardiac arrest complicated 1 in 12,000 or 8.5 per 100,000 hospitalizations for delivery (99% CI, 7.7 to 9.3 per 100,000). The most common potential etiologies of arrest included hemorrhage, heart failure, amniotic fluid embolism, and sepsis. Among patients with cardiac arrest, 58.9% of patients (99% CI, 54.8 to 63.0%) survived to hospital discharge.
Conclusions
Approximately 1 in 12,000 hospitalizations for delivery is complicated by cardiac arrest, most frequently due to hemorrhage, heart failure, amniotic fluid embolism, or sepsis. Survival depends on the underlying etiology of arrest.
Cardiopulmonary arrest is a rare complication during pregnancy and the postpartum period. A number of unique interventions must be considered during resuscitation of the pregnant arrest victim, including the use of left uterine displacement, prompt and often challenging airway management, and an emergent operative delivery of the fetus. The International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science states that “research in the area of maternal resuscitation is lacking, and most of the science is extrapolated from nonpregnant women, manikin studies, or case reports.”1 The committee members called for “epidemiological studies… to document the incidence of cardiac arrest in pregnancy as there is a perception that it is increasing because of increased numbers of women with congenital heart conditions who are now having children.”1
The frequency of maternal cardiopulmonary arrest in the United States is unknown, and there are limited population- level data to inform guideline development or systems preparations for these rare emergencies. The objective of this analysis was to determine the frequency, distribution of potential etiologies, and survival rates of maternal cardiopulmonary arrest during delivery hospitalizations in the United States to provide insights into potentially avoidable maternal morbidity and mortality.
Materials and Methods
Data for the study were derived from the Nationwide Inpatient Sample (NIS), which is the largest all-payer discharge database available in the United States. The database is maintained as part of the Healthcare Cost and Utilization Project sponsored by the Agency for Healthcare Research and Quality.
The NIS is a stratified sample of approximately 20% of all annual U.S. hospital admissions and is designed to represent all admissions to non-Federal hospitals. To create a representative sample, hospitals are selected for inclusion in the NIS based on five characteristics: the number of beds, teaching status, urban/rural location, region (divided into Northeast, South, Midwest, and West), and ownership. Each year, approximately 1,000 hospitals are selected for inclusion with information from all admissions from those hospitals incorporated into the database. The NIS contains sample weights for each of these hospitals which can be applied to obtain national estimates. Additional information on the methodology used to create the NIS can be found on the Healthcare Cost and Utilization Project Web site.* For each patient, the NIS includes age, race, primary expected payer, and up to 15 diagnostic and 15 procedural codes defined in the International classification of Diseases, Ninth Revision, Clinical Modification.†
Inclusion and Exclusion Criteria
We identified all admissions for delivery in the NIS between 1998 and 2011 using a validated coding algorithm of diagnosis and procedure codes, as defined by Kuklina et al.2 Because Diagnosis-Related Group codes changed during the study period, these were not used for selection. Hospitalizations were included if they had a diagnosis code indicating delivery or a procedure code related to delivery (e.g., forceps, breech extraction, vacuum extraction, version and extraction, manually assisted deliveries, episiotomy, hysterotomy, or cesarean delivery). Hospitalizations were excluded if they had a diagnosis code indicating abnormal products of conception (i.e., hydatidiform mole or ectopic pregnancy) or a procedure code indicating abortion.
Outcomes
The primary outcome measure was the population-based incidence of maternal cardiac arrest during hospitalization for delivery in the United States between 1998 and 2011. Secondary outcome measures included: (1) survival to hospital discharge; (2) the association between maternal cardiac arrest and demographic and socioeconomic characteristics, and medical and obstetric diagnoses and procedures; and (3) the association between maternal cardiac arrest and the annual hospital delivery volume. Annual hospital delivery volume was calculated for each hospital by summing all deliveries taking place at the hospital and dividing by the number of years the hospital was included in the study.
Among those hospitalized for delivery, cardiac arrest was defined based on International Classification of Diseases, Ninth Revision, Clinical Modification diagnostic and procedure codes including: 99.60 (cardiopulmonary resuscitation, not otherwise specified), 99.63 (closed-chest cardiac massage), and 37.91 (open-chest cardiac massage) and diagnosis codes including 427.5 (cardiac arrest) and 427.41 (ventricular fibrillation). Cases coded as 668.1 (cardiac complications after anesthesia or sedation in labor and delivery) and 669.4 (other complications of obstetrical procedures) were not included in the outcome measure, because these codes encompass a spectrum of outcomes and are not specific to cardiac arrest.
We examined the distribution of maternal demographic factors (i.e., age, race), data potentially representing socioeconomic status (i.e., primary payer), and coexisting medical conditions and obstetric conditions and procedures in patients with and without cardiac arrest (i.e., malignancy, pulmonary hypertension, ischemic heart disease, congenital heart disease, cardiac valvular disease, pre-existing hypertension, liver disease of pregnancy, systemic lupus erythematosus, drug abuse or dependence, asthma, chronic renal disease, sickle cell disease, and diabetes mellitus, stillbirth, severe preeclampsia, placenta previa, chorioamnionitis, multiple gestation, and cesarean delivery). Sociodemographic variables including age, race, and primary expected payer were recorded directly from the NIS dataset. Medical and obstetrical conditions and obstetrical procedures were defined with the use of previously reported coding algorithms (appendix 1).2,3 By using appropriate International Classification of Diseases, Ninth Revision, Clinical Modification diagnostic codes (appendix 2), we also identified conditions that were the likely proximate etiologies of maternal cardiac arrest: postpartum and antepartum hemorrhage, heart failure or cardiomyopathy, amniotic fluid embolism, sepsis, anesthesia complications, aspiration pneumonitis, venous thrombo embolism, puerperal cerebrovascular disorder, trauma, pulmonary edema, acute myocardial infarction, magnesium toxicity, status asthmaticus, and anaphylaxis. Conditions that affected fewer than 10 women with cardiopulmonary arrest were not considered for further analysis, in compliance with the Healthcare Cost and Utilization Project data use agreement and to ensure patient privacy.
Appendix 1.
ICD-9 CM Definitions for Maternal Conditions
| ICD-9 CM Definition | |
|---|---|
| Malignancy | 140.x–208.x |
| Pulmonary hypertension | 416.0, 416.8, 416.9 |
| Chronic ischemic heart disease | 412.x–414.x |
| Congenital heart disease | 745.0x–747.4x, 648.5x |
| Valvular heart disease | 394.x–397.x, 424.x |
| Pre-existing hypertension | 401.x–405.x, 642.0x–642.2x, 642.7x |
| Liver disorders in pregnancy | 647.x |
| Systemic lupus erythematosus | 710.0x |
| Drug abuse or dependance | 304.x–305.0x, 305.2x–305.9x, 648.3x |
| Asthma | 493.x |
| Chronic renal disease | 581.x–583.x, 585.x, 587.x, 646.2x |
| Sickle cell disease | 282.4x, 282.6x |
| Diabetes mellitus | 250.x, 648.0x, 648.8x |
| Stillbirth | 656.4x, V27.1, V27.3, V27.4, V27.6, V27.7 |
| Severe preeclampsia/eclampsia | 642.5x–642.6x |
| Cesarean delivery | 74.0x–74.2x, 74.4, 74.99 |
| Placenta previa | 641.0x, 641.1x |
| Chorioamnionitis | 658.4x |
| Multiple gestation | V27.2–V27.8, 651.x |
ICD-9 CM = International Classification of Diseases, Ninth Revision, Clinical Modification.
Appendix 2.
Maternal Conditions with the Potential to Cause Cardiac Arrest
| ICD-9 CM Definition | |
|---|---|
| Postpartum hemorrhage | 665.2x, 665.3x, 666.x |
| Antepartum hemorrhage | 641.1x–641.9x, 665.0x, 665.1x |
| Heart failure | 415.0x, 425.x, 428.x, 429.83, 674.5x |
| Amniotic fluid embolism | 673.1x |
| Sepsis | 038.x, 670.x, 995.91, 995.92 |
| Severe anesthesia complications | 668.0–668.2x, 995.86 |
| Aspiration pneumonitis | 507.0, 668.0x, 997.3 |
| Venous thromboembolism | 415.1x, 673.2x |
| Eclampsia | 642.6x |
| Puerperal cerebrovascular disorders | 325, 346.6x, 430.x–432.x, 433.01, 433.11, 433.21, 433.31, 433.81, 433.91, 434.01, 434.11, 434.91, 436.x, 437.1, 437.2, 437.6, 671.5x, 674.0x, 997.02 |
| Trauma | 800.x–904.x |
| Pulmonary edema | 428.1x, 518.4x |
| Acute myocardial infarction | 410.x, 429.5x–429.7x |
| Magnesium toxicity | 275.2 |
| Status asthmaticus | 493.01, 493.11, 493.21, 493.91 |
| Anaphylaxis | 995.0x, 999.4x |
| Aortic dissection or rupture | 441.0x–441.6x |
| Local anesthetic toxicity | 968.5x |
ICD-9 CM = International Classification of Diseases, Ninth Revision, Clinical Modification.
Statistical Analysis
We obtained estimates of the number of U.S. delivery hospitalizations complicated by maternal arrest and by each of the risk factors and potential etiologies of interest by weighting the patient-level discharge data in the NIS files using the svyset and discwt prefix commands in Stata Statistical Software: Release 12.1 (StataCorp LP, College Station, TX). For all analyses, the criterion used for statistical significance was P value less than 0.01, to ensure an appropriate level of confidence given the large size of the dataset. Statistical testing took into account the weighted data and the stratified sampling design of the NIS. Data were complete except for race (n = 13,351,842 [23.5%]), survival (n = 11,061 [0.02%]), age (n = 30,835 [0.1%]), and primary payer (n = 127,369 [0.2%]). Patients with missing race were recorded as “missing” and were retained in the analyses. Patients with missing age or primary payer were excluded from the multivariable analyses. Patients with missing data on survival were excluded from analyses of survival to hospital discharge.
To identify maternal sociodemographic characteristics, medical conditions, and obstetric conditions and procedures that are independently associated with maternal cardiopulmonary arrest, we constructed a multivariable logistic regression model forcing all relevant covariates into the model simultaneously. For this modeling, age was categorized as younger than 20, 20 to 34, 35 to 39, and 40 yr or older, in accordance with the categories established by the U.S. Centers for Disease Control and Prevention.4 Additional variables included in the model included race/ethnicity, primary payer, maternal medical conditions, and obstetric complications and procedures (as listed in appendix 1). Resulting odds ratios and 99% CIs for the covariates are reported. This analysis was repeated excluding from the model cases with cesarean delivery or stillbirth, conditions which are subject to reverse causation (i.e., maternal arrest may result in emergent cesarean delivery and/or fetal demise).
The frequency of conditions with the potential to cause cardiac arrest (appendix 2) was evaluated among the population of women diagnosed with cardiac arrest to identify the most common potential etiologies of cardiac arrest during the hospitalization for delivery. The frequency of arrest among women diagnosed with each condition was examined to identify the likelihood of arrest for each of these conditions. Because a small number of cardiac arrest cases associated with several conditions could result in unreliable estimates, a relative standard error (standard error/weighted estimate) for each estimate was calculated. Estimates may not be reliable if the corresponding relative standard error exceeds 0.30.
To evaluate temporal trends in maternal cardiac arrest and survival, we determined the frequency of arrest in each year of the study period, as well as the in-hospital survival for those patients who arrested. We tested for temporal trend in the occurrence of maternal arrest and in survival using univariate logistic regression, modeling year of delivery as a continuous independent variable. We next used multivariable logistic regression models to assess whether temporal trends in cardiac arrest were independent of secular trends in maternal sociodemographics characteristics, medical conditions, and obstetric conditions and procedures (appendix 1), and whether trends in survival were independent of trends in the proximate etiologies of arrest.
We then determined the frequency of arrest and survival after arrest stratified by annual hospital delivery volume. Annual delivery volume for each hospital was calculated, by year, using unique hospital-identifying codes. We stratified annual delivery volume into four categories, 1,000 or less, 1,001 to 2,000, 2,001 to 4,000, and greater than 4,000, corresponding to low, low–moderate, moderate–high, and high delivery volume centers. Differences between the hospital categories in terms of arrest and survival were evaluated using Pearson chi-square test. The effect of hospital delivery volume on the risk of arrest was then estimated using a multivariable logistic regression model after adjustment for demographic characteristics and comorbidities/obstetric conditions. Likewise, the effect of hospital volume on the risk of survival after arrest was evaluated using a multivariable logistic regression model after adjusting for proximate etiologies of arrest.
Results
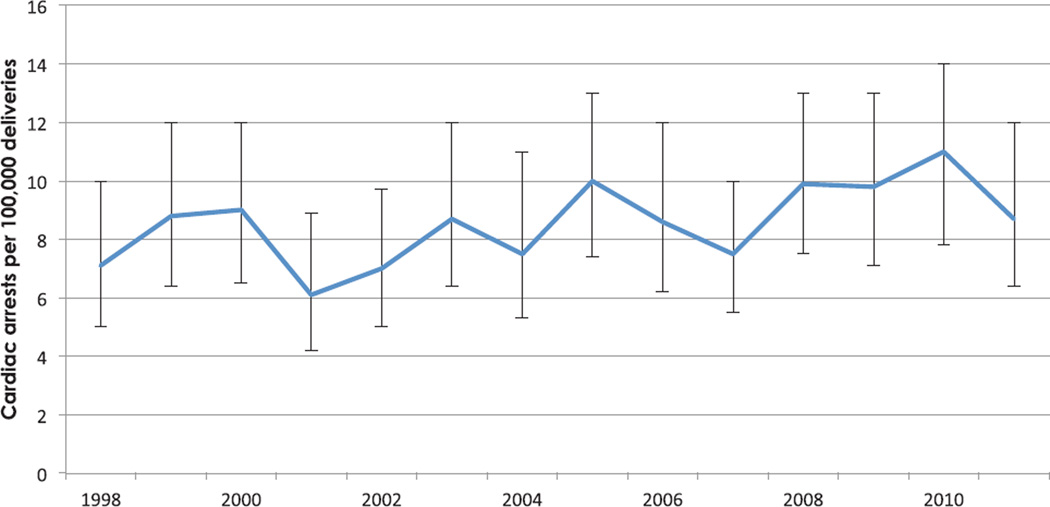
On the basis of weighted analysis, among 56,900,512 hospitalizations for delivery between 1998 and 2011, there were 4,843 cases of cardiopulmonary arrest, corresponding to an event rate of 8.5 arrests per 100,000 hospitalizations for delivery (99% CI, 7.7 to 9.3 per 100,000). This frequency did not change over time (P = 0.017) (fig. 1). After adjustment for changes in patient demographic and clinical conditions, any trend in the risk of arrest over time did not reach the threshold for statistical significance (adjusted odds of arrest per year increase, 0.97; 99% CI, 0.94 to 1.01; P = 0.04).
Fig. 1.
The frequency per 100,000 deliveries of maternal cardiac arrest by year, the Nationwide Inpatient Sample 1998–2011. Error bars denote the 99% CI.
Women who suffer cardiac arrest are more likely to be aged 35 yr or older, more likely to be black (i.e., non- Hispanic black by U.S. Centers for Disease Control and Prevention race/ethnicity coding), and more likely to receive care funded by Medicaid as opposed to private insurance (table 1). The maternal medical conditions most strongly associated with cardiopulmonary arrest include pulmonary hypertension, malignancy, cardiovascular disease (i.e., ischemic heart disease, congenital heart disease, cardiac valvular disease, and pre-existing hypertension), liver disease, and systemic lupus erythematosus. The obstetric conditions most strongly associated with cardiac arrest include stillbirth, cesarean delivery, severe preeclampsia/eclampsia, and placenta previa. After excluding cesarean delivery and stillbirth from the multivariable model to predict cardiac arrest, the effect size estimates for severe preeclampsia/eclampsia and placenta previa increased, but all other estimates remained stable (data not shown).
Table 1.
The Association between Sociodemographic Characteristics, Maternal Medical Conditions, and Obstetric Conditions and Procedures and Maternal Cardiac Arrest, the Nationwide Inpatient Sample 1998–2011
| Arrest, N (%) | No Arrest, N (%) | aOR* (99% CI) | |
|---|---|---|---|
| Overall | |||
| Age group, yr | |||
| <20 | 327 (6.8) | 6,021,192 (10.6) | 0.8 (0.5–1.0) |
| 20–34 | 3,191 (65.9) | 42,762,659 (75.2) | Ref |
| 35–39 | 965 (19.9) | 6,597,067 (11.6) | 1.6 (1.3–2.0)† |
| ≥40 | 359 (7.4) | 1,483,916 (2.6) | 2.0 (1.5–2.8) |
| Race/ethnicity | |||
| White | 1,500 (31.0) | 23,264,619 (40.9) | Ref |
| Black | 1,191 (24.6) | 5,914,624 (10.4) | 2.3 (1.8–3.0) |
| Hispanic | 879 (18.1) | 9,957,035 (17.5) | 1.3 (1.0–1.7) |
| Asian/Pacific Islander | 200 (4.1) | 2,042,190 (3.6) | 1.4 (0.9–2.2) |
| Other | 195 (4.0) | 2,366,238 (4.2) | 1.2 (0.7–1.9) |
| Unknown | 878 (18.1) | 13,350,964 (23.5) | 1.1 (0.8–1.4) |
| Primary payer | |||
| Medicare | 83 (1.7) | 280,194 (0.5) | 2.1 (0.8–5.1) |
| Medicaid | 2,209 (45.7) | 22,495,861 (39.6) | 1.3 (1.1–1.6) |
| Private insurance | 2,221 (45.9) | 30,509,870 (53.7) | Ref |
| Self-pay | 195 (4.0) | 1,937,426 (3.4) | 1.5 (0.9–2.2) |
| No charge | 18 (0.4) | 131,388 (0.2) | 1.7 (0.7–3.9) |
| Other | 112 (2.3) | 1,413,565 (2.5) | 1.2 (0.6–2.1) |
| Maternal medical conditions | |||
| Pulmonary hypertension | 92 (1.9) | 7,904 (0) | 13.3 (6.0–29.6) |
| Malignancy† | 38 (0.8) | 21,579 (0) | 12.5 (4.7–33.0) |
| Ischemic heart disease† | 30 (0.6) | 6,751 (0) | 7.6 (2.1–27.5) |
| Liver disease† | 53 (1.1) | 54,158 (0.1) | 5.5 (2.3–13.1) |
| Congenital heart disease† | 48 (1.0) | 42,600 (0.1) | 4.2 (1.6–11.0) |
| Systemic lupus erythematosus† | 60 (1.2) | 49,803 (0.1) | 4.1 (1.8–9.8) |
| Cardiac valvular disease | 179 (3.7) | 343,650 (0.6) | 3.8 (2.2–6.3) |
| Pre-existing hypertension | 457 (9.4) | 863,155 (1.5) | 2.7 (1.9–3.7) |
| Chronic renal disease | 83 (1.7) | 110,236 (0.2) | 2.6 (1.2–5.5) |
| Sickle cell disease† | 38 (0.8) | 68,711 (0.1) | 2.6 (1.0–6.4) |
| Drug abuse/dependance | 153 (3.2) | 651,875 (1.1) | 1.8 (1.1–2.9) |
| Asthma | 258 (5.3) | 1,281,158 (2.3) | 1.7 (1.1–2.4) |
| Diabetes mellitus | 495 (10.2) | 3,154,125 (5.5) | 1.0 (0.8–1.4) |
| Maternal obstetrical conditions/procedures | |||
| Stillbirth | 386 (8.0) | 361,976 (0.6) | 12.9 (9.4–17.7) |
| Cesarean delivery | 3,758 (77.6) | 16,546,570 (29.1) | 6.7 (5.4–8.3) |
| Severe preeclampsia/eclampsia | 701 (14.5) | 682,730 (1.2) | 6.5 (5.0–8.3) |
| Placenta previa | 257 (5.3) | 296,571 (0.5) | 4.4 (2.9–6.5) |
| Chorioamnionitis | 159 (3.3) | 1,013,164 (1.8) | 1.3 (0.8–2.0) |
| Multiple gestation | 184 (3.8) | 986,535 (1.7) | 0.8 (0.5–1.3) |
Bold font denotes a statistically significant result.
On the basis of weighted analysis, age was missing for 30,835 individuals (0.05%) and primary payer was missing for 127,369 (0.2%). Patients with missing values for either of these variables were excluded from the logistic regression model (n = 158,164, 0.3%).
Estimates with a relative standard error (i.e., standard error/weighted estimate) >0.30 may not be reliable.
aOR = adjusted odds ratio; N = number; Ref = referent category.
The potential etiologies of arrest are listed in table 2. Hemorrhagic conditions including antepartum hemorrhage and postpartum hemorrhage contributed to 38.1% of all cases. Heart failure, amniotic fluid embolism, sepsis, anesthesia complications, aspiration pneumonitis, venous thromboembolism, and eclampsia each contributed to between 6 and 14% of cases. Amniotic fluid embolism was the diagnosis most frequently complicated by cardiac arrest, followed by acute myocardial infarction and venous thromboembolism (table 2). Even though postpartum and antepartum hemorrhage were the leading potential etiologies of cardiac arrest during the hospitalization for delivery, fewer than 1 in 1,000 women who experienced antepartum or postpartum hemorrhage also experienced cardiac arrest.
Table 2.
Distribution of Maternal Cardiac Arrests (n = 4,843), the Nationwide Inpatient Sample 1998–2011
| Potential Proximate Etiology of Maternal Cardiac Arrest, N (%) |
Cause-specific Cardiac Arrest Frequency per 1,000 Women with Each Condition |
Survival to Hospital Discharge,* N (%) |
|
|---|---|---|---|
| Postpartum hemorrhage | 1,349 (27.9) | 0.8 | 739 (55.1) |
| Antepartum hemorrhage | 813 (16.8) | 0.9 | 433 (53.2) |
| Heart failure | 645 (13.3) | 15.6 | 458 (71.1) |
| Amniotic fluid embolism | 645 (13.3) | 252.7 | 337 (52.5) |
| Sepsis | 544 (11.2) | 2.1 | 256 (46.9) |
| Anesthesia complication | 379 (7.8) | 29.5 | 310 (81.9) |
| Aspiration pneumonitis | 346 (7.1) | 20.3 | 287 (82.9) |
| Venous thrombo embolism | 346 (7.1) | 43.9 | 144 (41.5) |
| Eclampsia | 296 (6.1) | 6.2 | 226 (76.5) |
| Puerperal cerebrovascular disorder | 212 (4.4) | 13.6 | 85 (40.0) |
| Trauma | 125 (2.6) | 3.9 | 29 (23.3) |
| Pulmonary edema | 118 (2.4) | 11.2 | 83 (70.9) |
| Acute myocardial infarction | 150 (3.1) | 89.8 | 85 (56.3) |
| Magnesium toxicity | 66 (1.4) | 5.2 | 57 (85.9) |
| Status asthmaticus† | 54 (1.1) | 12.6 | 29 (53.7) |
| Anaphylaxis† | 15 (0.3) | 10.8 | 15 (100) |
| Aortic dissection/rupture† | 14 (0.3) | 31.0 | 0 |
Numbers of arrests from local anesthetic toxicity cannot be reported due to restrictions on reporting small cell sizes.
Survival is missing for 0.2% of those with cardiopulmonary arrest.
Estimates with a relative standard error (i.e., standard error/weighted estimate) >0.30 may not be reliable.
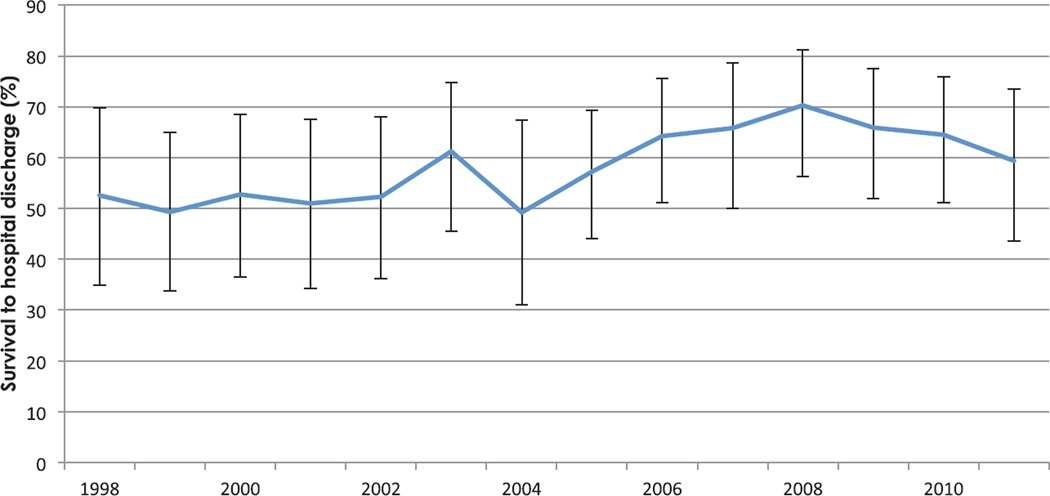
Overall, 59.0% of women (99% CI, 54.9 to 63.1%) who suffered cardiac arrest survived to hospital discharge. Survival, which varied substantially by potential etiology, was lowest for aortic dissection or rupture and trauma and highest for aspiration pneumonitis and medication-related complications including anaphylaxis, local anesthetic toxicity, magnesium toxicity, and anesthesia-related complications (table 2). Survival improved during the time period studied (P = 0.001) (fig. 2). After adjustment for changes in the potential etiologies in arrest, the temporal trend toward improved survival persisted (odds of survival per year increase, 1.07; 99% CI, 1.03 to 1.12).
Fig. 2.
The frequency of survival to hospital discharge in delivery-associated cardiac arrest by year, the Nationwide Inpatient Sample 1998–2011. Error bars denote the 99% CI.
Of those women with cardiac arrest, 74.0% had one of the coexisting diagnoses considered as a potential etiology of arrest. There was no difference in survival between those with (58.1%) and those without (61.2%) a coexisting diagnostic code with the potential to cause the cardiac arrest (P = 0.06).
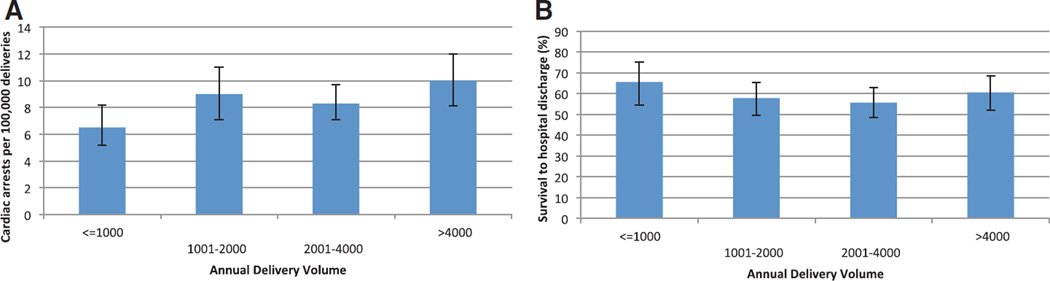
Frequency of cardiopulmonary arrest varied by annual hospital delivery volume (P = 0.0036) and was more frequent in facilities with more than 1,000 deliveries per year when compared with smaller volume facilities (fig. 3A); these differences were no longer significant after adjustment for differences in maternal demographic characteristics, comorbidities, and obstetric conditions. Survival did not vary based on delivery volume (fig. 3B) (P = 0.25), even after adjusting for the distribution of potential etiologies of arrest.
Fig. 3.
By annual hospital delivery volume, the (A) frequency per 100,000 deliveries of maternal cardiac arrest and (B) frequency of survival to hospital discharge from maternal cardiac arrest, the Nationwide Inpatient Sample 1998–2011. Error bars denote the 99% CI.
Discussion
By using a nationally representative sample of admission for delivery in the United States during a 14-yr period, we found that approximately 1 in 12,000 hospitalizations is complicated by maternal cardiac arrest. Although hemorrhage accounts for the largest fraction of maternal arrests, amniotic fluid embolism has the strongest disease-specific association with cardiac arrest. Survival to hospital discharge is highly dependent on the arrest etiology and is lowest for aortic dissection or rupture and trauma and highest for aspiration pneumonitis and medication-related complications including anaphylaxis, local anesthetic toxicity, magnesium toxicity, and anesthesia-related complications. Whereas the frequency of arrest has been fairly stable over the past decade, survival has improved. This study provides population-based data that extend the findings of case reports and series and can serve as the basis for programmatic changes in the preparation and management for maternal cardiac arrest.
Although these data verify that maternal cardiopulmonary arrest is a rare complication, they do not confirm the perception that the rate of arrests per year is increasing. Similarly, the Scottish Confidential Audit of Severe Maternal Morbidity found a stable rate of cardiac arrest between 2003 and 2011 in their population-based data.5–7 Notably, cardiac arrest seems to be more common during hospitalization for delivery in the United States than in Scotland, where 20 cardiac arrests were reported between 2003 and 2011,5 corresponding to an event rate of 3.9 per 100,000 live births (95% CI, 2.2 to 5.6).‡ The Scottish surveillance system relies on active reporting by designated midwife coordinators for each delivery unit, whereas the current study relied on administrative codes for cardiac arrest. Although the frequency of cardiac arrest may be higher in the United States, it is also possible that the threshold for submitting hospital charges is lower than the threshold for completing a nonremunerated clinical report to a central governmental organization.
The American Heart Association8 draws from reports of maternal mortality4,6,7 in describing the differential diagnosis for the cause of maternal cardiac arrest. Results from the current analysis suggest that the etiologies of maternal cardiac arrest during admission for delivery are similar, but not identical, to the etiologies that underlie maternal mortality. Cardiomyopathy and cardiovascular disease combined constitute the leading cause of maternal death in the United States (24% of all deaths) and the United Kingdom (20.3%), resulting in cause-specific mortality ratios of 3.5 and 2.3 per 100,000 pregnancies, respectively.4,6,7,9 The prominence of cardiovascular disease in these reports likely reflects the requirement for maternal mortality surveillance data to capture all deaths that take place during, and up to 1 full year after, pregnancy. Conversely, hemorrhage is the leading potential etiology of maternal cardiac arrest during admission for delivery, accounting for 38.1% of these events, followed by heart failure and acute myocardial infarction, which together account for 15.2% of all events. Although a comprehensive list of differential diagnoses should be considered when caring for an obstetric cardiac arrest victim, the most likely etiologies of cardiac arrest during the hospitalization for delivery seem to be hemorrhage, heart failure, amniotic fluid embolism, and sepsis.
Risk factors for maternal cardiopulmonary arrest seem to reflect the distribution of arrest etiologies. For example, placenta previa is a known cause of obstetric hemorrhage, is associated with placenta accreta, and is a plausible risk factor for hemorrhagic arrest. Similarly, it is conceivable that women of advanced maternal age would be at increased risk of arrest given their increased risk of preeclampsia and cardiac disease. Even after adjusting for maternal age, medical, and obstetric conditions, cardiopulmonary arrest is more common in minority women when compared with white women and among women with Medicaid insurance when compared those with private insurance. Although these associations parallel relationships demonstrated with maternal death,4 the genetic, behavioral, environmental, and health system factors that underlie these health disparities are unknown.10
An expanding body of evidence suggests that the population-level risks for serious complications of pregnancy have increased. Since 1998, the U.S. childbearing population has become older,11 and individual women are more likely to enter pregnancy with a chronic health condition, such as chronic hypertension12 or cardiac disease.13–15 Severe maternal morbidity during the hospitalization for delivery has increased particularly postpartum hemorrhage, acute renal failure, shock, acute myocardial infarction, respiratory distress syndrome, and maternal sepsis.16–18 Despite the increasing presence of these maternal comorbidities and complications, it is notable that the event rate of maternal cardiopulmonary arrest remained stable.
Survival to hospital discharge after arrest was approximately 60% in our study, and improved slightly during the 14 yr of this study. This rate exceeds those previously reported after in-hospital cardiac arrest in an obstetric population in Belgium (17%),19 in-hospital cardiac arrest in a nonobstetric population in the United States (21.2%),20 perioperative cardiac arrest for nonobstetric surgery in the United States (31.7%),21 and in a population of pregnant arrest victims described in a systematic review of published case reports (54%).22 This high survival rate may reflect the relative health and fitness of the childbearing population, or the inclusion of postpartum cardiac arrest events. The trend toward improved survival persisted after adjusting for changes in the potential etiologies of arrest, possibly due to clinically important improvements in both clinical care and the appropriateness of the selected birthing setting based on the presence of maternal comorbid conditions.3 Recent evidence from the American Heart Association Get With the Guidelines initiative confirms that adherence to resuscitation guidelines improves outcomes among nonobstetric in-hospital patients with cardiac arrest.23 However, cardiopulmonary arrest in the maternal population involves complexities and distractions that likely reduce the overall quality of resuscitation.24–26 Thus, it is difficult to assume that recent policy documents, which describe implementation strategies to improve performance and adherence to guidelines for teams providing maternal cardiopulmonary resuscitation, are responsible for this finding.27
Although cardiac arrest was most common in the largest delivery centers, events were observed even in centers with fewer than 1,000 deliveries per year. The event frequency is sufficiently rare that individual providers may encounter only one maternal cardiac arrest in a lifetime. This introduces significant challenges to the acquisition and maintenance of competency in resuscitation protocols and skills. Emergency manuals, checklists, simulation or team training, or a combination of interventions may help to ensure that individuals and teams are prepared to deliver effective and timely maternal cardiopulmonary resuscitation.28–30
This analysis is limited by the cross-sectional design and the use of administrative data. With cross-sectional analyses, it is not possible to determine the temporal relationship between events, specifically between delivery and cardiac arrest; event frequencies and survival rates for antepartum, intrapartum, and postpartum cardiac arrest may differ from the overall values reported in this analysis. The etiologies reported in table 2 reflect coexisting diagnoses that were identified among those women with a cardiac arrest, and that have the capacity to cause cardiac arrest. The relevant clinical records are not available to confirm the extent to which these diagnoses caused the arrest. Although cesarean delivery demonstrated at least a five-fold association with cardiac arrest when compared with vaginal delivery, this association may reflect perimortem cesarean delivery as an intervention to treat maternal cardiac arrest, rather than as a primary cause of arrest. Alternatively, cesarean delivery may be the mode of delivery chosen for women with high-risk conditions, such as preeclampsia and abnormal placentation, and may therefore be a marker for women with impending cardiac arrest.31 Certain diagnoses, such as placenta accreta, is not represented in the International Classification of Diseases, Ninth Revision, Clinical Modification coding system, and others, such as obesity, are not reliably coded in administrative records. Finally, survival to hospital discharge does not capture clinically important endpoints, such as neurologically intact survival or neonatal outcomes. Despite these limitations, administrative data provide the best existing source of data for studying this rare yet vitally important problem. The NIS is the largest representative dataset available in the United States, and the inclusion of more than a decade of data yields sufficient power to analyze an event as rare as maternal cardiopulmonary arrest.
In conclusion, maternal cardiopulmonary arrest is a rare event for which resuscitation requires complex coordination between various specialties. Approximately 1 in 12,000 hospitalizations for delivery is complicated by cardiac arrest, most frequently associated with hemorrhage, heart failure, amniotic fluid embolism, or sepsis. Survival depends on the likely etiology of arrest, but seems to be improving over time. The epidemiology of maternal cardiac arrest described in this report may be used to guide the further development of training courses and systems preparations to manage these life-threatening emergencies.
What We Already Know about This Topic
Cardiac arrest is rare during pregnancy and the postpartum period, but whether its incidence is changing and whether resuscitation is effective are largely unknown
What This Article Tells Us That Is New
In a review from the Nationwide Inpatient Sample of over 50 million hospitalizations for delivery from 1998 to 2011, cardiac arrest occurred in 8.5 per 100,000 hospitalizations, with no change in the rate over this time period
Nearly 60% of women survived to discharge, with an increase in survival over this time period
Acknowledgments
Support was provided solely from institutional and/or departmental sources.
Footnotes
HCUP Nationwide Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP). Rockville, MD, Agency for Healthcare Research and Quality, 1998–2007. Available at: http://www.hcup-us.ahrq.gov/. Accessed September 27, 2013.
Center for Disease Control and Prevention. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), 2011. Available at: http://www.cdc.gov/nchs/icd/icd9cm.htm. Accessed September 27, 2013.
Vital Events Reference Tables 2011, Section 3: Births. General Register Office for Scotland. Available at: http:// www.gro-scotland.gov.uk/statistics/theme/vital-events/general/ref-tables/2011/section-3-births.html. Accessed July 30, 2011.
Competing Interests
The authors declare no competing interests.
References
- 1.Morrison LJ, Deakin CD, Morley PT, Callaway CW, Kerber RE, Kronick SL, Lavonas EJ, Link MS, Neumar RW, Otto CW, Parr M, Shuster M, Sunde K, Peberdy MA, Tang W, Hoek TL, Böttiger BW, Drajer S, Lim SH, Nolan JP. Advanced Life Support Chapter Collaborators: Part 8: Advanced life support: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2010;122(16 suppl 2):S345–S421. doi: 10.1161/CIRCULATIONAHA.110.971051. [DOI] [PubMed] [Google Scholar]
- 2.Kuklina EV, Whiteman MK, Hillis SD, Jamieson DJ, Meikle SF, Posner SF, Marchbanks PA. An enhanced method for identifying obstetric deliveries: Implications for estimating maternal morbidity. Matern Child Health J. 2008;12:469–477. doi: 10.1007/s10995-007-0256-6. [DOI] [PubMed] [Google Scholar]
- 3.Mhyre JM, Bateman BT, Leffert LR. Influence of patient comorbidities on the risk of near-miss maternal morbidity or mortality. Anesthesiology. 2011;115:963–972. doi: 10.1097/ALN.0b013e318233042d. [DOI] [PubMed] [Google Scholar]
- 4.Berg CJ, Callaghan WM, Syverson C, Henderson Z. Pregnancy-related mortality in the United States, 1998 to 2005. Obstet Gynecol. 2010;116:1302–1309. doi: 10.1097/AOG.0b013e3181fdfb11. [DOI] [PubMed] [Google Scholar]
- 5.Lennox C, Marr L. Scottish Confidential Audit of Severe Maternal Morbidity 9th Annual Report. Edinburgh, Scotland, Healthcare Improvement Scotland. 2013:1–62. [Google Scholar]
- 6.Cantwell R, Clutton-Brock T, Cooper G, Dawson A, Drife J, Garrod D, Harper A, Hulbert D, Lucas S, McClure J, Millward-Sadler H, Neilson J, Nelson-Piercy C, Norman J, O’Herlihy C, Oates M, Shakespeare J, de Swiet M, Williamson C, Beale V, Knight M, Lennox C, Miller A, Parmar D, Rogers J, Springett A. Saving Mothers’ Lives: Reviewing maternal deaths to make motherhood safer: 2006–2008. The Eighth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG. 2011;118(suppl 1):1–203. doi: 10.1111/j.1471-0528.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- 7.Lewis G. The Seventh Report on Confidential Enquiries into Maternal Deaths in the United Kingdom. London, United Kingdom: CEMACH; 2007. The Confidential Enquiry into Maternal and Child Health (CEMACH). Saving Mother’s Lives: Reviewing Maternal Deaths to Make Motherhood Safer—2003–2005; pp. 1–267. [Google Scholar]
- 8.Vanden Hoek TL, Morrison LJ, Shuster M, Donnino M, Sinz E, Lavonas EJ, Jeejeebhoy FM, Gabrielli A. Part 12: Cardiac arrest in special situations: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 suppl 3):S829–S861. doi: 10.1161/CIRCULATIONAHA.110.971069. [DOI] [PubMed] [Google Scholar]
- 9.Mhyre JM. Maternal mortality. Curr Opin Anaesthesiol. 2012;25:277–285. doi: 10.1097/ACO.0b013e3283530580. [DOI] [PubMed] [Google Scholar]
- 10.Bryant AS, Worjoloh A, Caughey AB, Washington AE. Racial/ethnic disparities in obstetric outcomes and care: Prevalence and determinants. Am J Obstet Gynecol. 2010;202:335–343. doi: 10.1016/j.ajog.2009.10.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin JA, Hamilton BE, Ventura SJ, Osterman MJ, Matthews T. Births: Final data for 2011. National Vital Statistics Reports. 2013;62:1–90. [PubMed] [Google Scholar]
- 12.Bateman BT, Bansil P, Hernandez-Diaz S, Mhyre JM, Callaghan WM, Kuklina EV. Prevalence, trends, and outcomes of chronic hypertension: A nationwide sample of delivery admissions. Am J Obstet Gynecol. 2012;206:134.e1–134.e8. doi: 10.1016/j.ajog.2011.10.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuklina E, Callaghan W. Chronic heart disease and severe obstetric morbidity among hospitalisations for pregnancy in the USA: 1995–2006. BJOG. 2011;118:345–352. doi: 10.1111/j.1471-0528.2010.02743.x. [DOI] [PubMed] [Google Scholar]
- 14.Leary PJ, Leary SE, Stout KK, Schwartz SM, Easterling TR. Maternal, perinatal, and postneonatal outcomes in women with chronic heart disease in Washington State. Obstet Gynecol. 2012;120:1283–1290. doi: 10.1097/aog.0b013e3182733d56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karamlou T, Diggs BS, McCrindle BW, Welke KF. A growing problem: Maternal death and peripartum complications are higher in women with grown-up congenital heart disease. Ann Thorac Surg. 2011;92:2193–2198. doi: 10.1016/j.athoracsur.2011.05.088. discussion 2198–9. [DOI] [PubMed] [Google Scholar]
- 16.Callaghan WM, Kuklina EV, Berg CJ. Trends in postpartum hemorrhage: United States: 1994–2006. Am J Obstet Gynecol. 2010;202:353.e1–353.e6. doi: 10.1016/j.ajog.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol. 2012;120:1029–1036. doi: 10.1097/aog.0b013e31826d60c5. [DOI] [PubMed] [Google Scholar]
- 18.Wanderer JP, Leffert LR, Mhyre JM, Kuklina EV, Callaghan WM, Bateman BT. Epidemiology of obstetric-related ICU admissions in Maryland: 1999–2008. Crit Care Med. 2013;41:1844–1852. doi: 10.1097/CCM.0b013e31828a3e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dijkman A, Huisman CM, Smit M, Schutte JM, Zwart JJ, van Roosmalen JJ, Oepkes D. Cardiac arrest in pregnancy: Increasing use of perimortem caesarean section due to emergency skills training? BJOG. 2010;117:282–287. doi: 10.1111/j.1471-0528.2009.02461.x. [DOI] [PubMed] [Google Scholar]
- 20.Chan PS, Berg RA, Spertus JA, Schwamm LH, Bhatt DL, Fonarow GC, Heidenreich PA, Nallamothu BK, Tang F, Merchant RM. AHA GWTG-Resuscitation Investigators: Risk-standardizing survival for in-hospital cardiac arrest to facilitate hospital comparisons. J Am Coll Cardiol. 2013;62:601–609. doi: 10.1016/j.jacc.2013.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krishna Ramachandran S, Mhyre J, Kheterpal S, Christensen RE, Tallman K, Morris M, Chan PS. American Heart Association’s Get With The Guidelines-Resuscitation Investigators: Predictors of survival from perioperative cardiopulmonary arrests: A retrospective analysis of 2,524 events from the get with the guidelines-resuscitation registry. Anesthesiology. 2013;119:1322–1339. doi: 10.1097/ALN.0b013e318289bafe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Einav S, Kaufman N, Sela HY. Maternal cardiac arrest and perimortem caesarean delivery: Evidence or expert-based? Resuscitation. 2012;83:1191–1200. doi: 10.1016/j.resuscitation.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Ornato JP, Peberdy MA, Reid RD, Feeser VR, Dhindsa HS. NRCPR Investigators: Impact of resuscitation system errors on survival from in-hospital cardiac arrest. Resuscitation. 2012;83:63–69. doi: 10.1016/j.resuscitation.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Lipman SS, Wong JY, Arafeh J, Cohen SE, Carvalho B. Transport decreases the quality of cardiopulmonary resuscitation during simulated maternal cardiac arrest. Anesth Analg. 2013;116:162–167. doi: 10.1213/ANE.0b013e31826dd889. [DOI] [PubMed] [Google Scholar]
- 25.Cohen SE, Andes LC, Carvalho B. Assessment of knowledge regarding cardiopulmonary resuscitation of pregnant women. Int J Obstet Anesth. 2008;17:20–25. doi: 10.1016/j.ijoa.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Einav S, Matot I, Berkenstadt H, Bromiker R, Weiniger CF. A survey of labour ward clinicians’ knowledge of maternal cardiac arrest and resuscitation. Int J Obstet Anesth. 2008;17:238–242. doi: 10.1016/j.ijoa.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 27.Hui D, Morrison LJ, Windrim R, Lausman AY, Hawryluck L, Dorian P, Lapinsky SE, Halpern SH, Campbell DM, Hawkins P, Wax RS, Carvalho JC, Dainty KN, Maxwell C, Jeejeebhoy FM. The American Heart Association 2010 guidelines for the management of cardiac arrest in pregnancy: Consensus recommendations on implementation strategies. J Obstet Gynaecol Can. 2011;33:858–863. doi: 10.1016/S1701-2163(16)34991-X. [DOI] [PubMed] [Google Scholar]
- 28.Lipman SS, Daniels KI, Arafeh J, Halamek LP. The case for OBLS: A simulation-based obstetric life support program. Semin Perinatol. 2011;35:74–79. doi: 10.1053/j.semperi.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Lipman SS, Carvalho B, Cohen SE, Druzin ML, Daniels K. Response times for emergency cesarean delivery: Use of simulation drills to assess and improve obstetric team performance. J Perinatol. 2013;33:259–263. doi: 10.1038/jp.2012.98. [DOI] [PubMed] [Google Scholar]
- 30.Arriaga AF, Bader AM, Wong JM, Lipsitz SR, Berry WR, Ziewacz JE, Hepner DL, Boorman DJ, Pozner CN, Smink DS, Gawande AA. Simulation-based trial of surgical-crisis checklists. N Engl J Med. 2013;368:246–253. doi: 10.1056/NEJMsa1204720. [DOI] [PubMed] [Google Scholar]
- 31.Clark SL, Belfort MA, Dildy GA, Herbst MA, Meyers JA, Hankins GD. Maternal death in the 21st century: Causes, prevention, and relationship to cesarean delivery. Am J Obstet Gynecol. 2008;199:36 e1–36 e5. doi: 10.1016/j.ajog.2008.03.007. [DOI] [PubMed] [Google Scholar]