Abstract
Context
High density lipoprotein (HDL) particles perform numerous vascular-protective functions. Animal studies demonstrate that exposure to fine or ultrafine particulate matter (PM) can promote HDL dysfunction. However, the impact of PM on humans remains unknown.
Objective
We aimed to determine the effect of exposure to coarse concentrated ambient particles (CAP) on several metrics of HDL function.
Methods
Thirty-two adults (25.9 ± 6.6 years) were exposed to coarse CAP [76.2 ± 51.5 µg·m−3] in a rural location and filtered air (FA) for 2-hours in a randomized double-blind crossover study. Venous blood collected 2 and 20 hours post-exposures was measured for HDL-mediated efflux of [3H]-cholesterol from cells and 20 hours post-exposures for HDL anti-oxidant capacity by the HDL oxidation index (HOI) and paraoxonase activity. The changes [median (first, third quartiles)] between exposures among 29 subjects with available results were compared by matched Wilcoxon tests.
Results
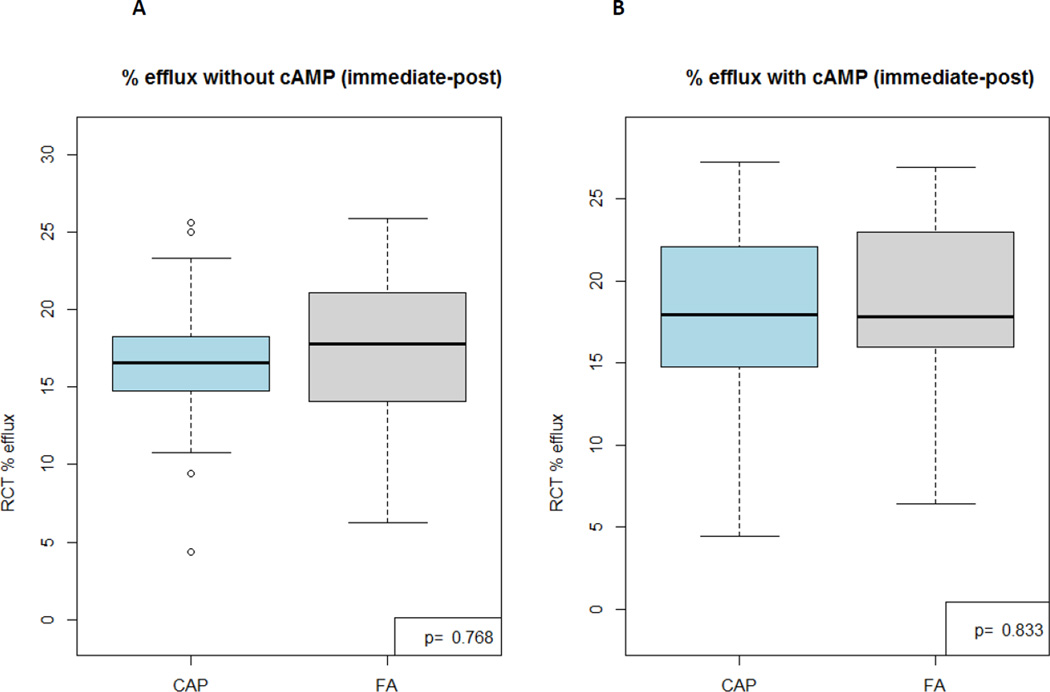
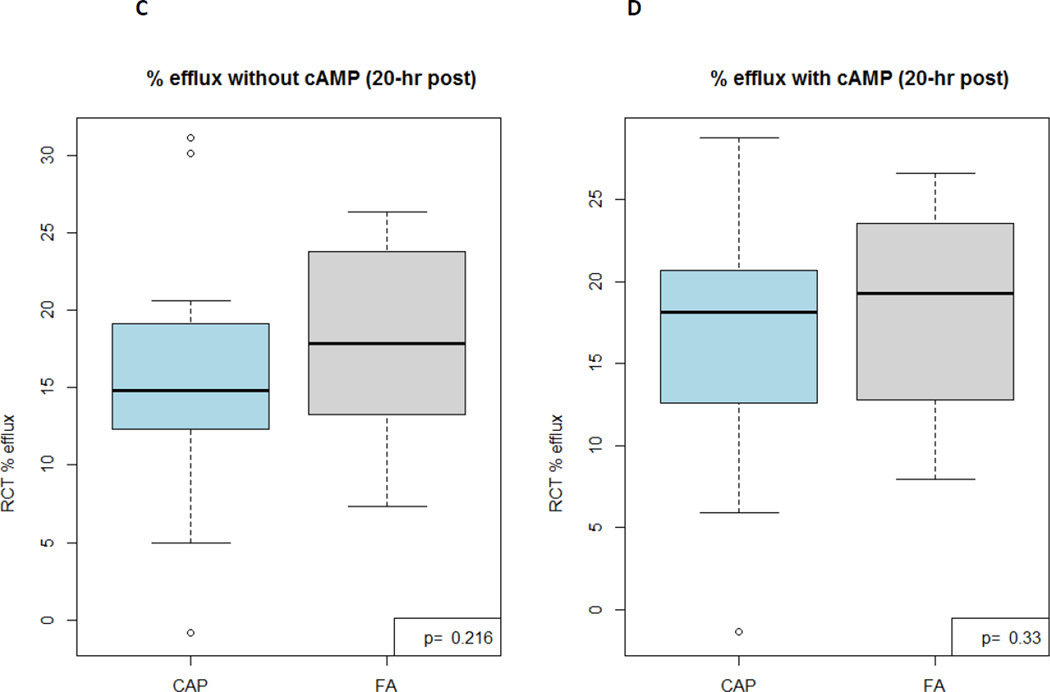
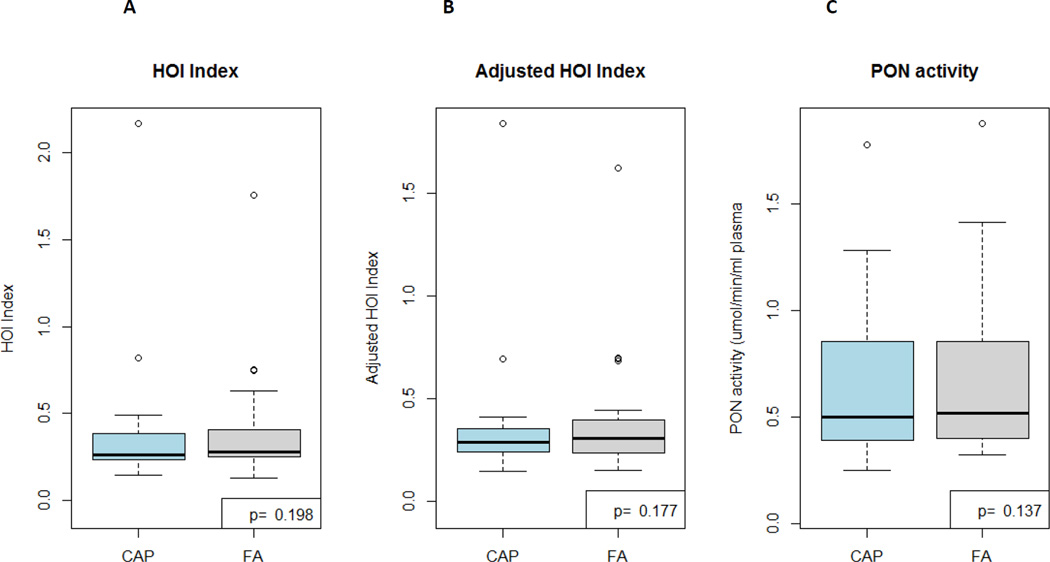
HDL-mediated cholesterol efflux capacity did not differ between exposures at either time point [16.60% (15.17, 19.19) 2-hours post-CAP versus 17.56% (13.43, 20.98) post-FA, p=0.768 and 14.90% (12.47, 19.15) 20-hours post-CAP versus 17.75% (13.22, 23.95) post-FA, p=0.216]. HOI [0.26 (0.24, 0.35) versus 0.28 (0.25, 0.40), p=0.198] and paraoxonase activity [0.54 (0.39, 0.82) versus 0.60 µmol·min−1·ml plasma−1 (0.40, 0.85), p=0.137] did not differ 20-hours post-CAP versus FA, respectively.
Conclusions
Brief inhalation of coarse PM from a rural location did not acutely impair several facets of HDL functionality. Whether coarse PM derived from urban sites, fine particles, or longer-term PM exposures can promote HDL dysfunction warrant future investigations.
Keywords: Air pollution, atherosclerosis, high density lipoprotein
Introduction
Fine particulate matter (PM) < 2.5 µm (PM2.5) air pollution is a leading cause of global morbidity and mortality, with a substantial portion of the deaths attributable to cardiovascular (CV) causes. Supporting these associations, numerous studies have shown that PM2.5 exposures are capable of eliciting a variety of adverse biological responses which could potentially play a role in triggering acute CV events (Brook et al. 2010).
On the other hand, whether coarse PM (2.5–10 µm in aerodynamic diameter; PM10-2.5) is also associated with adverse CV health effects is less clear given the mixed results from epidemiological observations (Brunekreef et al. 2005; Chang et al. 2011; Peng et al. 2008; Puett et al. 2009; Zanobetti et al. 2009). In addition to its larger size, coarse PM differs from PM2.5 in several regards including sources and components. PM10-2.5 itself is a heterogeneous mixture of particles typically generated from mechanical activities (e.g., crushing, suspension of ground materials), rather than from the combustion of fossil fuels as with PM2.5. The sources of coarse PM range from farming, roadway dust, to construction activities. Its major constituents (metals, crustal material such as silicon, calcium, and bio-aerosols including endotoxins) also differ according to nearby activities and landscape features (Brunekreef et al. 2005). In light of the uncertainty regarding the CV health effects of coarse PM, we have recently undertaken a series of experiments investigating the potential biological effects of acute exposures.
Numerous pathways have been elucidated whereby PM could plausibly trigger acute CV events (Brook et al. 2010). Recent evidence also supports the contention that long-term exposures can even promote the development of chronic CV disease-states such as atherosclerosis (Adar et al 2013). One emerging biological pathway potentially responsible for a portion of both these acute and chronic health effects is air pollutant-mediated high-density lipoprotein (HDL) dysfunction. In addition to being the central mediator of reverse cholesterol transport (RCT), HDL plays key roles in vascular homeostasis (e.g., anti-inflammatory, anti-oxidant activities) which together serve as powerful inhibitors of the atherosclerotic process and also help to prevent plaque instability/vulnerability (Bandeali et al 2012; Eren et al. 2012; Rosenson et al. 2011; Soran et al 2009). Beyond the association between low HDL-cholesterol levels, dysfunctional HDL particles per se (in particular impaired cholesterol efflux capacity and anti-oxidant actions) are of clinical importance as they have been shown to be independent predictors of CV events and/or the presence of underlying atherosclerosis (Khera et al 2011; Li et al 2013B; Patel et al 2011). In the context of air pollution, recent animal experiments have also demonstrated that exposure to fine or ultrafine PM (<0.1 µm) over a few weeks are capable of impairing several aspects of HDL functionality including anti-inflammatory capacity, anti-oxidant capacity and paraoxonase activity (Araujo et al 2008; Li et al 2013A; Yin et al. 2013).
However, no study has evaluated the effect of ambient PM air pollutants (of any size fraction or duration of exposure) on HDL function in humans. While disease-states that induce HDL dysfunction (e.g., diabetes) as well as the ensuing sequelae (e.g., atherosclerotic progression) are most commonly thought to be chronic processes, it is becoming increasingly clear that even acute pro-inflammatory/oxidative insults (e.g., infections) can impair HDL function within hours-to-days (G et al 2011; Eren et al. 2012; Patel et al 2011; Smith 2010). This abrupt genesis of dysfunctional HDL particles (e.g., shifting from an anti- to a pro-oxidant phenotype) could plausibly play a role in the acute instigation of plaque instability and thus the attendant CV events (Eren et al. 2012). As such, we aimed to explore if acute exposure to coarse concentrated ambient particle (CAP) in a rural location is capable of altering clinically-relevant facets of HDL function – the ability of HDL to facilitate the efflux of cholesterol from cells, antioxidant capacity (HDL oxidant index [HOI]) and paraoxonase activity (Eren et al 2012; Khera et al 2011; Patel et al 2011; Rosenson et al. 2011). In this paper we report the changes in HDL function (pre-defined secondary outcomes) induced by coarse PM from a recently completed controlled exposure study.
Methods
The Institutional Review Board of the University of Michigan approved this study. During a screening visit subjects signed an informed consent document and had blood labs drawn (fasting lipids, glucose) and a history and physical exam performed. Inclusion criteria included nonsmoking adults (no active smoking within the past year per self-report during the screening visit and living in nonsmoking households) who were from 18–50 years of age without any established CV disease or traditional CV risk factors. To be enrolled subjects were required to have screening blood pressures < 140/90 mm Hg and fasting glucose levels < 126 mg·dL−1. Subjects were excluded if they were taking medications (e.g., statins) or over-the-counter pills (e.g., anti-oxidants) that might alter study outcomes.
Enrolled subjects entered into a randomized double-blind crossover study (from May 2011 to June 2012) comparing the health effects of 2-hour-long exposures to coarse CAP versus filtered air (FA). Subjects came to the research facility on each day having fasted for > 8 hours with randomized exposures occurring from 10 am to noon. Approximately 12.5 ml peripheral venous blood was drawn into sodium heparin tubes 20 hours post-exposures for later analyses of HOI and paraoxonase. Approximately 4 ml of venous blood was drawn into EDTA tubes both 2 and 20 hours post-exposures for later analyses of HDL-mediated cholesterol efflux assays. The plasma was separated from blood within 2 hours after collection and immediately stored in a −80 degree Celsius (°C) freezer for later batched analyses. There was a 1–3 week washout period between exposures.
Exposure Facility and Air Pollution Measurements
Dexter Michigan was selected as the location for coarse PM exposures as it is highly influenced by rural sources (http://www.epa.gov/castnet/javaweb/site_pages/ANA115.html). This site is >10 km from freeways and >60 km west of the Detroit metropolitan area. Coarse CAP was generated by a 2-stage virtual impactor system (Demokritou et al. 2002; Moffet et al. 2004) which concentrates ambient coarse PM (predominantly from 2.5–10 µm) without substantively altering the composition or chemistry. The AIRCARE mobile air research laboratory has been described in detail elsewhere (Harkema et al. 2004). Randomized, blinded exposures were delivered to subjects who were resting seated within an air-tight chamber via a facemask with an air flow rate between 25–28 liters·minute−1. Gaseous pollutants (e.g., ozone) remained at or below ambient levels for both exposures. To generate FA a high efficiency PM filter was inserted at the inlet of the concentrator (i.e., “upstream” to the series of virtual impactors). Coarse CAP mass levels were monitored during exposures downstream to the concentrator with a DataRAM monitor (Thermo Scientific, Waltham, MA). Outdoor and within chamber temperatures (maintained at approximately 24 degrees Celsius) were also monitored. Filter samples were collected immediately upstream of the exposure chamber on 47-mm Teflon filters (Pall, Ann Arbor, MI) at a flow rate of 6 L/min. The samples were analyzed gravimetrically for particle mass using a microbalance (MT-5 Mettler Toledo, Columbus, OH) in a temperature/humidity-controlled clean laboratory as described in the Federal Reference Method (USEPA. 1997. Reference method for the determination of fine particulate matter as PM2.5 in the atmosphere. EPA 40 CFR Pat 50. Washington DC).
HDL isolation and cholesterol efflux methods
Stored plasma samples were adjusted to d=1.21 g/mL with anhydrous potassium bromide powder and centrifuged (Sorvall WX-90, TFT-80.2 rotor, 429,600 g, 8 hours, 4°C). The top 200 µL from the tubes was removed and 0.45 µm filtered into chromatography vials. Next, the obtained total lipoprotein fraction was resolved on Superose 6 100/300 GL column (GE Healthcare) using fast protein liquid chromatography (Bio-Rad DuoFlow equipped with C96 autosampler with cooling). Lipoproteins were eluted with an isocratic buffer containing 10 mM phosphate buffer, 154 mM NaCl, and 0.02% sodium azide, pH 7.4, at a flow rate of 0.5 mL/min. Fractions containing HDL were collected by automatic fraction collector at 4°C, yielding albumin-free HDL at concentrations of 0.7–2.5 mg/ml. The presence and purity of HDL was confirmed by 15% PAGE electrophoresis using ApoA-I (Sigma) as a reference.
HDL-mediated cholesterol efflux experiments were performed as described previously (Li et al. 2013B), with minor modifications. RAW cells (mouse macrophages) were obtained from The American Type Culture Collection and cultured in Roswell Park Memorial Institute (RPMI) medium supplemented with 5% fetal bovine serum (FBS). Cells were plated at a density of 4×105 cells per well in 24-well culture plates. Cells were allowed to attach overnight after which medium was replaced with in 250 µL of Dulbecoo’s Modified Eagle Medium (DMEM) containing 0.2% BSA, 1 µCi of H3-cholesterol with or without 0.3 mM 8-chloroadenosine 3′,5′-cyclic-monophosphate (cAMP) (Sigma). Cells then were allowed to load with H3-cholesterol for a total of 8 hours. The cells were washed three times and equilibrated for one hour in DMEM. HDL- mediated cholesterol efflux was then determined by incubating the test HDL with H3-cholesterol labeled cells for 14 hours at 37°C. Radioactivity in the medium supernatants and total cell radioactivity were measured on scintillation counter.
HDL-mediated cholesterol efflux capacity was calculated as: % Efflux = 100 × [Disintegrations per minute (DPM) of Efflux Medium] / ([DPM of Efflux Medium + Cell DPM]); non-specific efflux (without HDL) data were subtracted from % Efflux for each sample. In the case of cAMP-stimulated cells, adenosine-5'-triphosphate-binding cassette transporter (ABC)-A1 and ABCG1-mediated efflux were calculated using following equations: a) %Efflux(ABCA1+ABCG1) with cAMP = %Efflux(+cAMP); b) %Efflux(ABCA1) = %Efflux(+cAMP) — %Efflux(without cAMP)]. Thus, cAMP-stimulated efflux represents total of ABCA1+ABCB1-mediated cholesterol efflux while efflux without cAMP stimulation represents predominately ABCG1-mediated efflux.
HDL anti-oxidant capacity and paraoxonase activity methods
HDL anti-oxidant capacity was assessed by a fluorescence assay that evaluated the ability of HDL to inhibit LDL oxidation, determined by dichlorofluorescein (DCF) fluorescence. HDL cholesterol was isolated from thawed plasma samples using the dextran-sulphate precipitation method. Briefly, 50 µl of plasma was incubated with 10 µl of LipiDirect Magnetic HDL cholesterol precipitating reagent (Reference Diagnostics, Inc, Bedford, MA) for 10 minutes at room temperature. The mixture was then centrifuged at 12000 rpm for 5 minutes at 4°C and the HDL containing supernatant was collected. HDL cholesterol concentration was determined by a colorimetric assay (Thermo Scientific, Middletown, VA). The HDL supernatant was used in the assay to evaluate the ability of HDL to inhibit LDL-induced oxidation of dihydrodichlorofluorescein (DCFH) into the fluorescent DCF as previously described (Yin et al 2013). The DCFH reagent was prepared by incubating dichlorofluorescein diacetate (DCF-DA) (Sigma-Aldrich, St. Louis, MO) at 2 mg/ml with 0.1 M NaOH. DCF-DA was hydrolyzed to DCFH and the mixture was neutralized with 10× PBS. Equal concentrations of HDL and LDL cholesterol (50 µg /ml) were mixed with Tris-HCl buffer (pH 7.4) up to a total volume of 100 µl and added to a black well flat bottom microtiter plate in triplicate. The plate was incubated at 37°C for 1 hour. Next, 20 µl of the DCFH reagent was added to each well and the plate was incubated at 37°C for 3 hours. DCF fluorescence intensity was determined using a plate reader (SynergyMx, BioTek, Vermont) at an excitation wavelength of 485 nm and emission wavelength of 530 nm. HDL anti-oxidant capacity was expressed as a HDL oxidant index (HOI), determined by the ratio of DCF fluorescence in the presence and absence of HDL cholesterol. An index < 1.0 denotes protective anti-oxidant HDL whereas an index > 1.0 indicates pro-oxidant HDL. The samples were assayed in triplicates, and the mean intra-assay coefficient of variation for all samples was 9.0%. To control for assay variability across different plates, a normal plasma HDL sample from a healthy individual was included on each plate (control sample). The inter-plate and inter-day variability calculated using this control sample was 5.7% and 15.7%, respectively. The HOI values of test samples obtained in each plate were adjusted according to the variability exhibited by the control sample on each plate to generate adjusted HOI values.
Paraoxonase-1 (PON-1) is an important HDL-associated anti-oxidant enzyme. Its activity was performed using 5 µl of plasma in the assay mixture consisting of paraoxon substrate, 2 mM CaCl2, 2.0 M NaCl in 100 mM tris–HCl buffer (pH 8.5) as previously described (Yin et al 2013). The hydrolysis of paraoxon substrate (diethyl-p-nitrophenyl phosphate) to p-nitrophenol by PON-1 was quantified by spectrophotometry. The assay was performed at room temperature and the kinetics of p-nitrophenol formation was determined by recording absorbance at 405 nm every 15 seconds for 4 minutes. The enzyme activity was expressed as µmol p-nitrophenol formed per minute for every 1 mL of plasma.
Statistical Methods
Summary statistics were computed for continuous measures as mean ± standard deviation (SD) or median (interquartile range, IQR) if the distribution was skewed. The matched pairs of HDL function measurements after CAP versus FA exposures were compared using the matched Wilcoxon tests among the 29 subjects with all results completed. The associations between HDL function metrics were analyzed using linear mixed models. All analyses were performed using the statistical software package R (version 2.14.1).
Results
Subjects (n=32) who completed the study were healthy, as per the inclusion criteria (Table 1). PM10-2.5 levels were higher during coarse CAP compared to FA exposures (Table 2). There were no differences between exposures in ambient or intra-chamber temperatures (results not shown). There were no significant differences detected in cholesterol efflux capacity metrics to HDL from subjects exposed 2 hours or 20 hours following CAP versus FA exposures (Table 3; Figure 1). There was a non-significant trend for decreased PON activity (p=0.137) 20 hours following CAP as hypothesized but no differences in the HOI indices (Table 4; Figure 2). Except for the 2 metrics of cholesterol efflux, the correlations between differing HDL functional assays were not significant (Table 5).
Table 1.
Subject Characteristics (n=32; 16 female subjects)
| Variable | Mean | SD | Minimum | Maximum |
|---|---|---|---|---|
| Age (years) | 25.9 | 6.6 | 18.0 | 46.0 |
| Weight (kg) | 78.4 | 16.3 | 55.9 | 111.4 |
| Height (m) | 1.7 | 0.1 | 1.6 | 2.0 |
| Body mass index (kg·m−2) | 26.3 | 5.7 | 18.3 | 43.5 |
| Fasting glucose (mg·dL−1)* | 86.9 | 6.9 | 70.0 | 103.0 |
| Total cholesterol (mg·dL−1) | 163.9 | 31.4 | 104.0 | 244.0 |
| HDL-C (mg·dL−1) | 55.4 | 15.9 | 25.0 | 91.0 |
| LDL-C (mg·dL−1) | 88.8 | 26.1 | 49.0 | 135.0 |
| Triglycerides (mg·dL−1) | 106.0 | 80.9 | 40.0 | 401.0 |
HDL-c, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SD, standard deviation
Missing 1 subject data point
Table 2.
PM10-2.5 concentrations during exposures (n=32)
| Exposure | Mean | SD | Minimum | Maximum |
|---|---|---|---|---|
| Filtered air | 10.1 | 7.1 | 2.6 | 27.4 |
| Coarse CAP | 76.2* | 51.5 | 10.3 | 246.5 |
CAP, concentrated ambient particles; SD, standard deviation
PM10-2.5 concentrations are in µg·m−3 and determined by Teflon filter-based gravimetric mass measurements for the 2-hour period of exposures. Mass levels below the detection limit (6.8 µg·m3) were recorded at this value for analyses (n=15, filtered air exposures only)
P values <0.01 for differences of mean or median levels between exposure types
Table 3.
HDL reverse cholesterol transport activity (n=29)
| Outcome | Coarse CAP Exposure | Filtered Air Exposure | ||
|---|---|---|---|---|
| immediate-post | 20-hr post | immediate-post | 20-hr post | |
| HDL efflux capacity without cAMP (%)* | 16.60 (15.17, 19.19) | 14.90 (12.47, 19.15) | 17.56 (13.43, 20.98) | 17.75 (13.22, 23.95) |
| HDL efflux capacity with cAMP (%)* | 18.19 (14.75, 22.11) | 17.61 (13.22, 20.67) | 17.71 (15.10, 22.12) | 19.81 (13.32, 22.10) |
Results are median (first, third quartile)
cAMP, 0.3 mM 8-chloroadenosine 3′,5′-cyclic-monophosphate
HDL efflux with cAMP (added stimulus) assessed ABCA1 and ABCG1-mediated RCT activity together, while results without cAMP predominantly assessed ABCG1-mediated RCT activity alone.
Figure 1. HDL-mediated cholesterol efflux activity results 2-hours (A, B [with cAMP added]) and 20-hours (C, D [with cAMP added]) post CAP versus FA exposures (n=29).
CAP, coarse concentrated ambient particles; FA, filtered air; cAMP, 0.3 mM 8-chloroadenosine 3′,5′-cyclic-monophosphate
These experiments evaluated the ability of subjects’ HDL particles to cause efflux of H3-cholesterol from mouse macrophages.
P values are calculated by paired Wilcoxon Signed Rank tests
Table 4.
HDL oxidation index and paraoxonase activity results after exposures (n=29)
| Outcome | Coarse CAP Exposure | Filtered Air Exposure |
|---|---|---|
| HOI | 0.26 (0.24, 0.35) | 0.28 (0.25, 0.40) |
| Adjusted HOI* | 0.29 (0.24, 0.35) | 0.30 (0.24, 0.39) |
| Paraoxonase activity (µmol·min−1·ml plasma−1) | 0.54 (0.39, 0.82) | 0.60 (0.40, 0.85) |
Results are median (first, third quartile)
Lab values were collected and analyzed only 20 hours post-exposures.
HOI, HDL oxidation index; CAP, concentrated ambient particles
Figure 2. HDL oxidation index (A), adjusted HDL oxidation index (B), and PON activity 20 hours following CAP versus FA exposures (n=29).
CAP, coarse concentrated ambient particles; FA, filtered air; PON, paraoxonase
HDL anti-oxidant capacity was assessed by a fluorescence assay that evaluated the ability of HDL to inhibit LDL oxidation, determined by dichlorofluorescein (DCF) fluorescence.
PON activity was determined as the hydrolysis of paraoxon substrate (diethyl-p-nitrophenyl phosphate) to p-nitrophenol by PON-1 by spectrophotometry.
P values are calculated by paired Wilcoxon Signed Rank tests
Table 5.
Correlations between metrics of HDL function (n=29)
| HDL efflux capacity with cAMP |
HDL efflux capacity without cAMP |
HOI | Paraoxonase | |
|---|---|---|---|---|
| HDL efflux capacity with cAMP | 0.762 (p<0.001) | −0.197 (p=0.106) | 0.116 (p=0.290) | |
| HDL efflux capacity without cAMP | −0.139 (p=0.334) | 0.062 (p=0.501) | ||
| HOI | −0.065 (p=0.846) |
cAMP, 0.3 mM 8-chloroadenosine 3′,5′-cyclic-monophosphate; HOI, HDL oxidation index
Discussion
Brief exposure to coarse CAP from a rural location did not impair HDL function over a period of 20 hours. Three clinically-relevant markers including the HOI (i.e., the capacity of HDL to prevent LDL oxidation), paraoxonase activity (i.e., an important HDL-associated anti-oxidant enzyme), and HDL-mediated cholesterol efflux capacity metrics were not significantly altered by the inhalation of high levels of coarse PM compared to FA. As such, we did not find evidence in this short-term study to support that HDL dysfunction is likely a pertinent mechanism explaining the heightened CV risk shown to occur following acute elevations in ambient coarse PM levels in some epidemiological studies (Brunekreef et al. 2005; Chang et al. 2011; Peng et al. 2008; Puett et al. 2009; Zanobetti et al. 2009)
While a prior experiment has shown that short-term occupational exposure to welding fumes can cause an acute reduction in HDL cholesterol levels, lipoprotein functional parameters were not directly assessed (Rice et al. 2011). Therefore, this was the first study to explore the effect of any ambient air pollutant on HDL function in humans. Numerous questions thus remain unanswered by this single experiment. Whether coarse PM comprised of differing chemical constituents (e.g., urban particles), fine or ultrafine particles, and/or longer-term exposures to PM (of any size fraction) are capable of impairing HDL function in humans warrant future investigations. Moreover, HDL particles convey a variety of pleiotropic actions normally protecting vascular health and homeostasis (Bandeali et al 2012; Eren et al. 2012; Rosenson et al. 2011; Soran et al 2009). The potential role played by HDL dysfunction in mediating several of the germane air pollution-induced adverse CV health effects (e.g., endothelial dysfunction, atherosclerosis) (Brook et al 2010) should also be explored.
Prior Animal Studies Relating Air Pollutants to HDL Function
There have been 3 published studies regarding the effects of air pollutants on HDL function. All of them were conducted over several weeks of exposures in animals and none of them evaluated coarse PM (Araujo et al 2008; Li et al 2013 (A); Yin et al. 2013). Araujo et al (2008) were the first to show that air pollutants could negatively impact HDL functionality. In this experiment, both fine and ultrafine PM exposures impaired the anti-inflammatory capacity of HDL measured by a cell-based assay. There was indirect evidence to support that this HDL dysfunction may have contributed to the concomitant increase in systemic atherosclerosis. More recent experiments have demonstrated that diesel exhaust and ultrafine PM can both impair HOI and paraoxonase activity, measured by the same (Yin et al. 2013) or similar techniques (Li et al 2013A) as in our current study. However, while diesel exhaust impaired HDL anti-oxidant and anti-inflammatory capacities, it did not induce changes in the HDL-mediated cholesterol efflux capacity (Yin et al, 2013). In these studies, diesel exhaust (Yin et al 2013) and ultrafine PM (Li et al 2013A) led to negative correlations between HOI and PON activity, suggesting that alteration in PON activity could be causally related to the HDL dysfunction. However, in the present study while we did observe a trend for decreased paraoxonase activity 20 hours following CAP versus FA as hypothesized, there were also trends for unexpected (paradoxical) decreased HOI values, which is suggestive of HDL particles with stronger antioxidant capacities. Given the statistical non-significance of both outcomes it is not possible to know if this is a true biological trend or simply chance alone. Future larger studies will be able to address this issue.
The putative mechanisms underlying the air pollution-induced HDL dysfunction in prior animal studies have not been fully elucidated. However, HDL particles can rapidly switch their phenotype into dysfunctional particles in the setting of acute pro-inflammatory or oxidative insults such as infections, illnesses, or acute coronary syndromes (G et al 2011; Eren et al. 2012; Patel et al 2011; Smith 2010). In fact, HDL can become pro-atherosclerotic in these situations over several hours-to-days. Cytokine-related alterations in acute phase proteins secreted from the liver leading to an altered protein cargo within HDL particles and/or myeloperoxidase-induced oxidation of HDL proteins (e.g., apoprotein-A1) after PM inhalation may be responsible. Air pollution is well-known to elicit numerous systemic pro-inflammatory and oxidative responses (Brook et al 2010). In theory, given the close proximity of inhaled pollutant particles that reach the terminal bronchioles/alveoli to HDL (either within the microvasculature or the lung interstitium), there may be direct effects of deposited air pollutant components (e.g., pro-oxidant metals or organic carbon species) upon HDL composition and subsequent function. Alternatively, lung-based cells known to interact with PM (e.g., resident macrophages) may produce oxidants as part of an immune response that subsequently affects the nearby HDL particle. As such, prior research suggests that our hypothesis was plausible that even a 2-hour exposure to coarse CAP could impair one or more aspect of HDL function.
Study Limitations
We speculate that our present findings were negative largely because the time course of exposure was inadequate to elicit the requisite systemic responses that produce dysfunctional HDL. Alternatively, the coarse CAP derived from a rural setting may not have contained the necessary chemical constituents. The PM components are indeed likely important for the genesis of HDL dysfunction as ultrafine has been shown to cause larger changes than fine PM (Araujo et al 2008). Ongoing filter analyses of PM components during these exposures and coarse CAP exposures in a different urban setting will hopefully provide insights into these latter issues.
Unlike ultrafine and fine PM, coarse particles are more commonly deposited in larger extra-thoracic airways (Geiser and Kreyling, 2010). This may have limited the “dose” of coarse PM that actually reached subjects’ bronchioles/alveoli upon inhalation. It may be a prerequisite that particles deposit in smaller airways in order to instigate HDL dysfunction (as mechanistically speculated in the prior section). Nevertheless, it is estimated that between 15–30% of inhaled coarse PM will reach small airways (Geiser and Kreyling, 2010). It is also not known precisely where in the pulmonary tree it is required for particles to reach or interact with lung-based cells in order to promote HDL dysfunction (or any systemic CV actions for that matter) (Brook et al. 2010). Future studies evaluating the potential differences on HDL dysfunction elicited by inhaled PM of varying sizes may help elucidate this issue.
Finally, we did not measure HDL function prior to exposures. The cross-over design of our study assumes that pre-exposure values are the same between study days. This has been the case in prior experiments for other health outcomes (Brook et al 2009). It is possible that unmeasured pre-exposure differences in HDL functionality might have obscured an impact of CAP compared to FA; however, we find this unlikely given the negative results (p-values) and reasonably large sample size for a controlled human exposure study.
Conclusions
In this first study to investigate the effect of air pollution on HDL function in humans, brief inhalation of coarse PM from a rural location did not acutely impair several facets of HDL functionality. Future studies should investigate whether coarse PM of differing characteristics, fine particles, or longer-term exposures can promote HDL dysfunction.
Acknowledgments
This study was funded by grants from the NIH CTSA grant (UL1RR024986), NIEHS ONES RO1 Award ES016959, and from the United States Environmental Protection Agency RD83479701 and R833740.
Footnotes
Declaration of interest statement
The authors report no declarations of interest.
References
- Adar SD, Sheppard L, Vedal S, Polak JF, Samspon PD, Diez Roux AV, Budoff M, Jacobs DR, Barr RG, Watson K, Kaufman JD. Fine particulate air pollution and the progression of carotid intima-media thickness: A prospective cohort study from the Multi-Ethnic Study of Atherosclerosis and Air Pollution. PLOS Med. 2013;10:e1001430. doi: 10.1371/journal.pmed.1001430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo JA, Barajas B, Leinman M, Wang X, Bennett BJ, Gong KW, Navab M, Harkema J, Sioutas C, Lusis AJ, Nel AE. Ambient particulate pollutants in the ultrafine range promote early atherosclerosis and systemic oxidative stress. Circ Res. 2008;102:589–596. doi: 10.1161/CIRCRESAHA.107.164970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandeali S, Farmer J. High-density lipoprotein and atherosclerosis: the role of antioxidant activity. Curr Athero Rep. 2012;14:101–107. doi: 10.1007/s11883-012-0235-2. [DOI] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA, III, Brook JR, Bhatnagar A, Diez-Roux A, Holguin F, Hong Y, Luepker RV, Mittleman M, Peters A, Siscovich D, Smith SC, Jr, Whitsel L, Kaufman JD. Particulate matter air pollution and cardiovascular disease. An update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Brook RD, Urch B, Dvonch JT, Bard RL, Speck M, Keeler G, Morishita M, Kaciroti N, Harkema J, Corey P, Silverman F, Wellenius G, Mittleman MA, Rajagopalan S, Brook JR. Insights into the Mechanisms and Mediators of the Effects of Air Pollution Exposure on Blood Pressure and Vascular Function in Healthy Humans. Hypertension. 2009;54:659–667. doi: 10.1161/HYPERTENSIONAHA.109.130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunekreef B, Forsberg B. Epidemiological evidence on effects of coarse airborne particles on health. Eur Respir J. 2005;26:309–318. doi: 10.1183/09031936.05.00001805. [DOI] [PubMed] [Google Scholar]
- Chang HH, Peng RD, Dominici F. Estimating the acute health effects of coarse particulate matter accounting for exposure measurement error. Biostat. 2011;12:637–652. doi: 10.1093/biostatistics/kxr002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eren E, Yilmaz N, Aydin O. High density lipoprotein and it’s dysfunction. Open Biochem J. 2012;6:78–93. doi: 10.2174/1874091X01206010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- G HB, Rao VS, Kakkar VV. Friend turns Foe: Transformation of anti-inflammatory HDL to proinflammatory HDL during acute-phase response. Cholesterol. 2011;274629 doi: 10.1155/2011/274629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiser and Kreyling. Deposition and biokinetics of inhaled nanoparticles. Part Fibre Tox. 2010;7:2. doi: 10.1186/1743-8977-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkema JR, Keeler GJ, Wagner JG, Morishita M, Timm E, Hotchkiss J, et al. Health Effects Institute Research Report 120. Boston MA: Health Effects Institute; 2004. Effects of inhaled urban air particulates on normal and hypersecretory airways in rats. [PubMed] [Google Scholar]
- Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri KJ, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Navan M, Pakbin P, Ning Z, Navab K, Hough G, Morgan TE, Finch CE, Araujo JA, Fogelman AM, Sioiutas C, Hsiai T. Ambient ultrafine particles alter lipid metabolism and HDL anti-oxidant capacity in LDLR-null mice. J Lipid Res. 2013 (A);54:1608–1615. doi: 10.1194/jlr.M035014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X-M, Tamg WJW, Mosior MK, Huang Y, Wu Y, Matter W, Gao V, Schmitt D, DiDonato JA, Fisher EA, Smith JD, Hazen SL. Paradoxical Association of Enhanced Cholesterol Efflux With Increased Incident Cardiovascular Risks. Arterioscl Thromb Vasc Biol. 2013 (B);33:1696–1701. doi: 10.1161/ATVBAHA.113.301373. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffet RC, Shields LG, Berntsen J, Devlin RB, Prather KA. Characterization of an ambient coarse particle concentrator used for human exposure studies: aerosol size distributions, chemical composition, and concentration enrichment. Aerosol science and technology. 2004;38:1123–1137. [Google Scholar]
- Patel PJ, Khera AV, Jafri K, Wilensky RL, Rader DJ. The anti-oxidative capacity of high-density lipoprotein is reduced in acute coronary syndrome but not in stable coronary artery disease. J Am Coll Cardiol. 2011;58:2068–2075. doi: 10.1016/j.jacc.2011.08.030. [DOI] [PubMed] [Google Scholar]
- Peng PD, Chang HH, Bell ML, McDermott A, Zeger SL, Samet JM, Dominici F. Coarse particulate matter air pollution and hospital admissions for cardiovascular and respiratory diseases among Medicare patients. JAMA. 2008;299:2172–2179. doi: 10.1001/jama.299.18.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puett RC, Hart JE, Yanosky JD, Paciorek C, Schwartz J, Suh H, Speizer FE, Laden F. Chronic fine and coarse particulate exposure, mortality, and coronary heart disease in the Nurses’ Health Study. Environ Health Perspect. 2009;117:1697–1701. doi: 10.1289/ehp.0900572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice MB, Cavallari J, Fang S, Christiani D. Acute decrease in HDL cholesterol associated with exposure to welding fumes. J Occup Environ Med. 2011;53:17–21. doi: 10.1097/JOM.0b013e3182028d20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenson RS, Brewer HB, Chapman MJ, Fazio S, Hussain MM, Kontush A, Krauss RM, Otvos JD, Remaley AT, Schaefer EJ. HDL measures, particle heterogeneity, proposed nomenclature, and relation to atherosclerotic cardiovascular events. Clin Chem. 2011;57:392–410. doi: 10.1373/clinchem.2010.155333. [DOI] [PubMed] [Google Scholar]
- Smith JD. Myeloperoxidase, inflammation, and dysfunctional high-density lipoprotein. J Clin Lipidol. 2010;4:382–388. doi: 10.1016/j.jacl.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soran H, Younis NN, Charlton-Menys V, Durrington P. Variation in paraoxonase-1 activity and atherosclerosis. Curr Opin Lipidol. 2009;20:265–274. doi: 10.1097/MOL.0b013e32832ec141. [DOI] [PubMed] [Google Scholar]
- Yin F, Lawal A, Pciks J, Fox JR, Larson T, Navab, Fogelman AM, Rosenfeld ME, Araujo JA. Diesel exhaust induces systemic lipid peroxidation and development of dysfunctional pro-oxidant and pro-inflammatory high-density lipoprotein. Arterioscler Thromb Vasc Biol. 2013;33:1153–1161. doi: 10.1161/ATVBAHA.112.300552. [DOI] [PubMed] [Google Scholar]
- Zanobetti A, Schwartz J. The effect of fine and coarse particulate matter air pollution on mortality: A national analysis. Environ Health Perspect. 2009;117:898–903. doi: 10.1289/ehp.0800108. [DOI] [PMC free article] [PubMed] [Google Scholar]