Abstract
Neuroblastoma is the most common extracranial solid tumor in children and, when disseminated, carries a poor prognosis. Even with aggressive combinations of chemotherapy, surgery, autologous bone marrow transplant and radiation, long-term survival remains at 30% and new therapies are needed. Recently, a patient with neuroblastoma who acquired Chagas disease was treated with nifurtimox with subsequent reduction in tumor size. The effect of nifurtimox on the neuroblastoma cell lines CHLA-90, LA1-55n, LA-N2, SMS-KCNR, and SY5Y was examined. Nifurtimox decreased cell viability in a concentration-dependent manner. Cell morphology, TUNEL assay, and caspase-3 activation indicate that cell death was primarily due to apoptosis. Nifurtimox also suppressed basal and TrkB-mediated Akt phosphorylation, and the cytotoxicity of nifurtimox was attenuated by a tyrosine hydroxylase inhibitor (alpha methyl tyrosine). Nifurtimox killed catecholaminergic, but not cholinergic, autonomic neurons in culture. In vivo xenograft models showed inhibition of tumor growth with a histologic decrease in proliferation and increase in apoptosis. These results suggest that nifurtimox induces cell death in neuroblastoma. Therefore, further studies are warranted to develop nifurtimox as a promising new treatment for neuroblastoma.
Keywords: Neuroblastoma, apoptosis, reactive oxygen species, catecholamine, nitrofuran
Introduction
Neuroblastoma is the most common pediatric extracranial solid tumor. Whereas the prognosis for infants with neuroblastoma is generally good, currently only 30% of children diagnosed after 12-15 months of age survive despite aggressive multimodal therapies(1). The children diagnosed over 1 year often have neuroblastoma characterized by n-myc amplification and expression the TrkB receptor which confers chemoresistance (1-3). Current front-line treatments, which include intensive multiagent chemotherapy, surgery, radiation and autologous bone marrow transplant, are often unsuccessful and leave the patients weak and unable to undergo further intensive treatment. Therefore, well tolerated, targeted new treatments are needed.
We previously reported a patient with Stage IV neuroblastoma whose tumor appeared to respond to nifurtimox, a drug used to treat Chagas disease, while undergoing conventional salvage chemotherapy with topotecan and cyclophosphamide (4). Chagas disease is a parasitic disease endemic to South America caused by Trypanosoma cruzi. The mechanism of action of this drug in Chagas disease involves the reduction of nifurtimox to nitro anion free radicals as well as hydrogen peroxide and superoxide free radicals which leads to cell death of the parasite (Maya et al, 2002). This patient acquired Chagas disease from a blood transfusion, and following treatment with nifurtimox her previously refractory neuroblastoma regressed. This observation led us to investigate the effect of nifurtimox on neuroblastoma cells in culture and in xenograft models in order to evaluate its therapeutic potential.
Materials and Methods
Reagents
Nifurtimox (synthesized in the laboratory of Laurent Brard, Brown University, Providence, RI) was dissolved in dimethyl sulfoxide (DMSO) as a 20mg/ml stock and stored in aliquots at -20° C. zVAD-fmk (Calbiochem La Jolla CA) was dissolved in DMSO at a concentration of 10mM and stored at -20° C. Brain derived neurotrophic factor (BDNF) was dissolved in sterile water (100μg/ml) and stored at -80° C (Santa Cruz Biotechnology, Santa Cruz, CA).
Cell Culture and Treatment
The human neuroblastoma cell lines CHLA-90 (Children’s Oncology Group), LA1-55n, LA-N2 (gift from Dr. Ross), SY5Y (ATCC), SMS-KCNR (gifts from Dr. Maris, Children’s Hospital of Philadelphia, Philadelphia, PA) are all neuroblastoma cell lines derived from aggressive childhood tumors, and were maintained in RPMI 1640 media supplemented with 10% (v/v) fetal bovine serum, 100units/ml penicillin and 100μg/ml streptomycin at 37°C in a 5% CO2, humidified incubator. Cells were grown in 48- or 6-well plates or 100mm dishes to 75% confluency. Cells used for evaluating AKT phosphorylation were serum deprived (RPMI 1640 with 0.1% BSA) overnight before treatment. Cells were treated with Nifurtimox (0, 10, 20 μg/ml) for 14 hours for caspase activation studies, or for 2 hours followed by stimulation for 10 minutes with either 100μg/ml BDNF (Santa Cruz Biotechnology, CA) or serum-containing media for analysis of AKT phosphorylation.
Cell Viability Assay
Cell viability was measured with a soluble tetrazolium dye, (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) (MTS) or with Calcein AM. Both methods quantify the amount of vital dye transported into live cells over a fixed period of time. For the MTS Assay, cell viability was measured using the CellTiter 96 AQ One Solution Cell Proliferation Assay kit (Promega, Madison WI). Cells were cultured in 48 well plates (50,000 per well) for 24 hours and then treated with increasing concentrations of nifurtimox (0, 1, 10, and 20 μg/ml) for 24, 48, 72 and 96 hours. Vehicle-treated cells were used as control (0.1% DMSO). At the end of the incubation period, MTS reagent (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium)1 in fresh media was added to each well to a final concentration of 0.5mg/ml and incubated for 4 hours. Absorbance was measured at 490nm using a microplate reader (Multiskan RC, Fisher Scientific Pittsburgh, PA). For the Calcein AM assay, cells were cultured in 48 well plates for 24 hours and then treated with nifurtimox for 48 hours. Vehicle-treated cells were used as controls (0.1% DMSO). After incubation, media was removed and fresh media containing μg/ml Calcein AM (Invitrogen) was added and cells were incubated at 37°C for an additional 30 minutes. Fluorescence was measured at 520em/485ex using a BMG Fluostar microplate reader.
Terminal deoxynucleotidyltransferase mediated dUTP nick end labeling (TUNEL) Asssay
SMS-KCNR cells were grown to 60% confluency in 48 well plates and incubated with increasing concentrations of nifurtimox for 96 hours. Vehicle-treated cells were used as control. Following treatment, morphological features were initially observed with light microscopy using a Nikon Eclipse TS100 fitted with a Fuji digital camera. The cells were washed with PBS, fixed with 4% paraformaldehyde, permeabilized with Triton X-100 at room temperature, labeled with fluorescein-12-dUTP using terminal deoxynucleotidyl transferase and counterstained with propidium iodide (5 μg/ml, Sigma Chemical Co., St Louis). Negative controls (without terminal transferase) were included in each experiment. Apoptosis was detected by fluorescence microscopy on a Nikon Eclipse TE2000-E fitted with a cooled CCD camera).
Western Blot Analysis
Cells cultured in 6-well plates were grown to 75% confluency and collected by scraping and re-suspended in 200μl of E Buffer (10mM Tris pH7.6, 50mM NaCl, 5mM EDTA, 50mM NaF, 0.1mM NaVO4, 1% Triton, 10μg/ml Aprotinin, 10μg/ml Leupeptin, ABSF) and incubated on ice for 20 minutes to lyse the cells. Cell lysates were sonicated for 10 seconds and centrifuged at 14,000 rpm for 20 minutes at 4°C. Protein concentration was determined with BioRad protein assay (BioRad, Hercules, CA). Cell lysates were electrophoresed on a 12%SDS-PAGE and blotted onto Polyvinylidene fluoride (PVDF) membrane, pore size 0.45μm (Millipore). The blots were blocked with 5% non-fat dry milk (BioRad, Hercules, CA) in PBST for 1 hour. The blots were probed with rabbit-derived antibody to caspase 3 and phosphorylated and total Akt, and murine-derived antibody to ERK (Cell Signaling Technology, Beverly MA). Protein bands were visualized using horseradish peroxidaseconjugated secondary antibodies (Amersham-Pharmacia Biotech, Piscataway, NJ) followed by enhanced chemiluminescence (Upstate, Waltham, MA) and photographed using a BioRad Gel Document System GDS 8000 (BioRad, Hercules CA). Blots were stripped and reprobed with beta-actin antibody as an internal loading control (Sigma Chemical Company, St. Louis, MO).
Inhibitor studies
SMS-KCNR cells were pretreated with 50μM Z-VAD-FMK (R&D Systems), a pancaspase inhibitor, for 90 minutes and then treated with nifurtimox (10 μg/ml) for 96 hours. Cell viability was measured by MTS assay as above. SMS-KCNR and SY5Y cells were treated with the tyrosine hydroxylase inhibitor alpha methyl tyrosine (AMPT; Sigma Chemical Company, St. Louis, MO) for 24 hours and then treated with nifurtimox (10 μg/ml) in the presence of inhibitor for 5 days. Cell viability was measured by Calcein AM assay as above.
Preparation of primary autonomic neuron cultures
Catecholaminergic paravertebral sympathetic neurons from E13 chick embryos and cholinergic parasympathetic neurons from ciliary ganglia of E8 chick embryos were isolated and cultured as described previously (Nishi 1996). Ganglia were incubated for 20 minutes at 37 °C. in 0.1% trypsin, dissociated by trituration, and plated onto 24-well plates coated with poly-D-lysine (Sigma Chemical Company, St. Louis, MO) and laminin (purified from EHS tumors in the laboratory of Rae Nishi). The neurons were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) consisting of 10% (v/v) heat inactivated horse serum (Gibco-BRL), 2% (v/v) fetal calf serum (Hyclone), 20 ug/ml penicillin, and 20 units/ml streptomycin; sympathetic neuron medium also included 1 μg/ml 7S NGF (Alomone, Jerusalem Israel) and parasympathetic neuron medium included 10 ng/ml recombinant chicken ciliary neurotrophic factor (chCNTF; produced from a bacterial expression system in the Nishi Lab) . Neurons were treated with vehicle alone or nifurtimox diluted in plating medium one day after plating.
Quantification of neuronal number using phase microscopy
The total number of sympathetic and parasympathetic neurons in 10 different fields of view was counted at 200× using phase contrast optics on a Nikon Eclipse TE200 microscope. Neurons were counted if their cell bodies appeared phase-bright with neurites the length of at least two cell body diameters.
Measurement of reactive oxygen species
Production of reactive oxygen species (ROS) was determined using dichlorodihydrofluorescein (DCF). Approximately 8 × 105 neuroblastoma cells (SMS-KCNR, SY5Y, CHLA-90, LAN2) were plated into 25ml culture flasks in complete media (RPMI 10%FBS, glutamine, penicillin, streptomycin) overnight. The next day media was changed to RPMI without phenol red and with glutamine. Nifurtimox was added to a final concentration of 10 or 20ug/ml using minimal lighting. DMSO was added to the control cells. Cells were incubated in the dark at 37 degrees for 30 minutes. Carboxylated DCF (Invitrogen, Carlsbad, CA) (10mM in DMSO and stored as a single use aliquot) was added to each foil protected flask to a final concentration of 20uM. Cells were incubated in the dark at 37 °C for 1 hour and centrifuged. Cells were rinsed with PBS plus 1%FBS, then re-suspended in PBS 1%FBS: Accumax (Phoenix FLOW, San Diego, CA) was added and incubated for 5 minutes at 37 °C. Cells were centrifuged and re-suspended in 500ul of PBS with 1% FBS and 0.2% sodium azide and fluorescence was measured by flow cytometry.
In Vivo Mouse Xenograft
Six week old female nude mice (nu/nu Phox) (Charles River Laboratories, Wilmington, MA) were used. For xenografting, 107 SMS-KCNR cells suspended in 0.2ml of Matrigel on ice were injected subcutaneously into the left flank of 6 mice per group using a 27G needle. Tumor growth was observed 10-14 days following inoculation in >90% of animals. At this time animals were fed either control 5g pellets or 5g pellets containing 150mg/kg of mouse body weight of Nifurtimox (daily dose) (Bio-Serv, Frenchtown, NJ). Tumor measurements were followed using the ellipsoid formula (length × width × height × 0.52). Treatment was continued until the largest tumor exceeded 3.0cm3 at which time all the mice were euthanized. Tumors were collected and weighed. These studies were approved by the Animal Care and Use Committee at the University of Vermont. Three separate experiments with 16 mice each (8 mice/group) were performed. Tumor sections were fixed in 10% neutral buffered formalin and processed for paraffin-embedded sections.
5μ sections were deparaffinized and either stained with hematoxylin and eosin or stained with Ki67 (Sp6; Lab Vision, Fremont, CA) to determine the MIB-1 index (number of positive tumor cell nuclei/total tumor cell nuclei) or cleaved-caspase-3 antibody (Cell Signaling, MA). Mitotic and apoptotic indices were determined by counting the number of mitotic figures or pyknotic cells/mm2.
Statistical Analysis
Basic descriptive data are presented as means standard deviations or standard errors. Inverse linear regression models were used to estimate IC50 values. Analysis of variance approaches were used for analyses that involved multiple groups. If statistical significance was observed, then multiple comparisons using the Fisher’s Least Significant Difference method was employed. Student t-tests were utilized when only two groups were involved and variances were similar while unequal variance t-tests were used when the latter assumption was not justified. A randomized block design analysis of variance was employed to compare tumor weights under Nifurtimox and control conditions for replicated experiments. All statistical tests used a 5% significance level.
Results
Nifurtimox induces apoptosis of neuroblastoma cells in culture
We tested the effect of nifurtimox on growth of CHLA-90, LA1-55n, LA-N2, SMS-KCNR, and SY5Y neuroblastoma cells in culture. As shown in Figure 1, nifurtimox inhibits the growth of all five cell lines in a concentration dependent manner. After 2 days of exposure to 20μg/ml of nifurtimox, the number of cells is decreased to 5.9% of vehicle-treated controls in CHLA-90 (p<0.001), 31.3% in LA1-55n (p<0.001), 32.9% in LA-N2 (p<0.001), 3.6% in SMS-KCNR (p<0.001), and 1.8% in SY5Y (p<0.001). The concentration required to reach 50% inhibition of growth (IC50) for these cell lines was estimated to be 12.18 μg/ml for CHLA-90, 13.62 μg/ml for LA1-55n, 17.17 μg/ml for LA-N2, 10.98 μg/ml for SMS-KCNR and 9.15 μg/ml for SY5Y. In contrast, cytotoxic effects are not observed on normal epithelial cells in culture exposed to nifurtimox (4).
Figure 1. Nifurtimox decreases neuroblastoma cell viability.
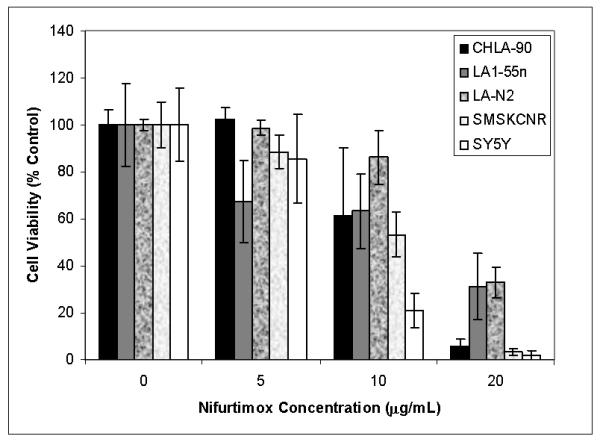
CHLA90, LA1-55n, LA-N2, SMSKCNR,and SY5Y neuroblastoma cells were incubated with increasing nifurtimox concentrations in 48 well plates for 2 days. Cell viability was quantified using Calcein AM and expressed as percent of vehicle control.
To determine whether nifurtimox treatment causes cell death by apoptosis, TUNEL assays to detect DNA fragmentation and assays for activated caspase-3, an initiator of the apoptotic cascade (5) were performed. When SMS-KCNR cells were exposed to nifurtimox (0-20μg/ml) for 96 hours, there was a concentration-dependent increase in the number of TUNEL-labeled nuclei (Figure 2A). Along with DNA fragmentation, confirmation of apoptosis is seen with caspase activation, as indicated by the concentration-dependent activation of caspase-3 in SMS-KCNR, SY5Y, CHLA-90, and LAN2 cells exposed to nifurtimox for 14 hrs; this caspase activation was not observed in vehicle treated controls (Figure 2B).
Figure 2. Nifurtimox induces apoptosis of neuroblastoma cells.
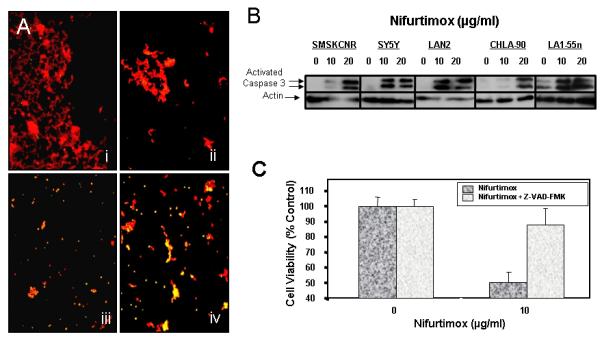
(A) SMS-KCNR cells were cultured and incubated 0,1.0, 10 and 20 μg/ml nifurtimox for 96, then fixed and stained for TUNEL (green). Cells were counterstained with propidium iodide (red) to label DNA. Photographs were taken using epifluorescence with a 10× objective. i: Vehicle control, ii: 1 μg/ml, iii: 10 μg/ml, and iv: 20 μg/ml. Note the increase in number of apoptotic nuclei (yellow spots) with increasing nifurtimox dose. (B) Cells grown for 24 hrs in the indicated concentration of nifurtimox were assayed for activated caspase 3 by western blot. Increased activation of caspase 3 was observed with increasing concentrations of nifurtimox. (C) SMS-KCNR Cells were pretreated with 50μM Z-VAD-FMK for 90 minutes before treatment with nifurtimox (10 μg/ml) and Z-VAD-FMK for 96 hours. Cell viability was measured by MTS assay. Controls without Z-VAD had decreased cell number, but Z-VAD rescued cells from dying.
To confirm that nifurtimox-mediated apoptosis was the result of caspase activation, SMS-KCNR cells were pretreated with 50μM Z-VAD-FMK, a pancaspase inhibitor that irreversibly binds to the catalytic sites of caspases 1 through 9 (6), for 90 minutes before and during treatment with nifurtimox. Whereas exposure to 10 μgm/ml of Nifurtimox for 96 hours decreased the viability of SMS-KCNR cells by 50%, the viability of Z-VAD-FMK pretreated cultures was comparable to vehicle-treated controls, p=0.002 (Figure 2C). Therefore, inhibiting the activity of caspases prevents nifurtimox-induced cytotoxicity.
Nifurtimox suppresses Akt phosphorylation
BDNF binds to the TrkB receptor on neuroblastoma cells via an autocrine loop, leading to phosphorylation of Akt (which plays a vital role in cell survival) and so ultimately causing chemoresistance (3, 7). Thus, we tested whether nifurtimox could interfere with TrkB signaling. In the absence of nifurtimox, stimulation of SMS-KCNR cells with BDNF resulted in phosphorylation of Akt (Figure 3A, Lane 1). Addition of nifurtimox (10 and 20 μg/ml) inhibited Akt phosphorylation as indicated by decreased band intensities (Figure 3A, Lanes 3 and 4), although total Akt protein levels were not altered (Figure 3A). Similarly, nifurtimox completely inhibited serum stimulated Akt phosphorylation at levels as low as 1 μg/ml (Figure 3B).
Figure 3. Nifurtimox suppresses Akt Phosphorylation.
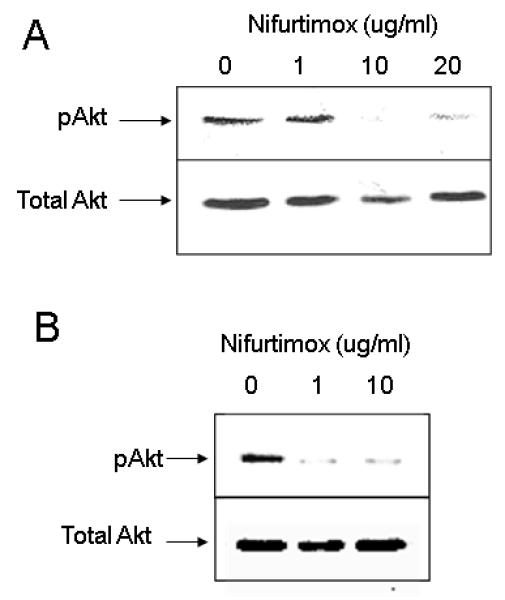
(A) BDNF mediated TrkB-Akt signaling: SMS-KCNR cells were serum deprived for 18 hours, treated with 0, 1.0, 10 and 20μg/ml Nifurtimox for 90 minutes and then stimulated with BDNF (100μg/ml) for 10 minutes. Cells were lysed and analyzed by western blot analysis using phospho-Akt antibodies (upper panel). The blots were stripped and reporbed with antibodies specific for total Akt protein (lower panel). (B) AKT phosphorylation: SMS-KCNR cells were treated with Nifurtimox for 90 minutes in the presence of serum, cells were lysed and analyzed by western blot analysis using antibodies specific for phospho-Akt (upper panel) and total Akt (lower panel) sequentially as above.
Nifurtimox cytotoxicity is enhanced by catecholamines
To test whether nifurtimox interacts with catecholamines to produce cytotoxicity, we pre-treated neuroblastoma cell lines SMS-KCNR and SY5Y with the tyrosine hydroxylase inhibitor alpha-methyl-p-tyrosine (AMPT) at a concentration of AMPT known to suppress catecholamine content (Specter, Sjoerdsma et al 1964). Both SMS-KCNR and SY5Y cell lines showed approximately 40% increase in cell viability in the presence of AMPT for 5 days, p<0.001 (Figure 4A). This attenuation of cytotoxicity is presumably due to the decreased catecholamine content caused by AMPT.
Figure 4. Nifurtimox is toxic to catecholaminergic, but not cholinergic, cells.
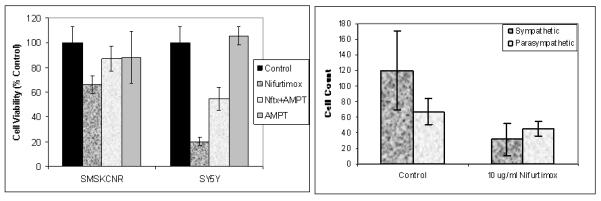
(A) Inhibition of catecholamine synthesis with AMPT reverses the toxic effect of nifurtimox on SMSKCNR and SY5Y neuroblastoma cells in culture. Cells were pretreated with or without AMPT for 1 hr, then 20 μg/ml of nifurtimox was added and cell viability assessed using Calcein AM. (B) Catecholaminergic sympathetic neurons and cholinergic parasympathetic neurons were treated with vehicle (DMSO) containing medium, or medium containing 10 μg/ml nifurtimox. After 48 hours of treatment, a significant decrease in the number of nifurtimox-treated catecholaminergic neurons was observed (one-way ANOVA, p =0.016). In contrast to the decrease in catecholaminergic neurons, the number of cholinergic neurons were not significantly different after treatment with nifurtimox. Results represent the mean +/- SEM from triplicate samples.
To further examine the role catecholamines play in nifurtimox-induced cytotoxicity, we treated lumbar sympathetic neurons and parasympathetic ciliary ganglion neurons in cell culture with nifurtimox. Many (but not all) of the sympathetic neurons synthesize and store catecholamines(8). The number of healthy sympathetic neurons decreased 75% compared with DMSO vehicle-treated controls after incubation with 10 μg/ml nifurtimox for 24 hrs, p=0.016 (Figure 4B). In contrast, all of the parasympathetic neurons are cholinergic and do not synthesize catecholamines (9) (and the number of cholinergic neurons did not change even after 48 hours of treatment (Figure 4B). Nifurtimox-treated catecholaminergic neurons, but not cholinergic neurons, appeared degenerative. Furthermore, both Schwann cells and fibroblasts were also present in both types of neuronal cultures and were unaffected by nifurtimox. Thus, cells that contain catecholamines are more sensitive to the cytotoxic effects of nifurtimox, and since neurons are postmitotic, this shows that the nifurtimox does not only act by interfering with proliferation.
Nifurtimox increases reactive oxygen species in neuroblastoma cells
SMS-KCNR, SY5Y, CHLA-90, LAN2 cells were treated with nifurtimox, incubated with carboxyl-DCF, fluorescence was then measured by flow cytometry. Oxidation of DCF by reactive oxygen species (ROS) causes increased fluorescence. With increasing doses of nifurtimox there was an increase in fluorescence, indicating greater levels of inctracellular ROS (Figure 5).
Figure 5. Nifurtimox induces formation of reactive oxygen species in neuroblastoma cells.
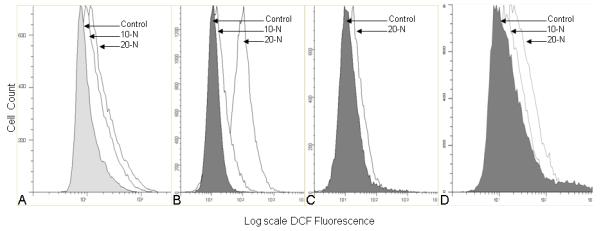
Cells were grown in T25 flasks and incubated in nifurtimox (10-N=10μg/ml, 20-N=20μg/ml) for 30 minutes prior to treatment with carboxylated DCF. Cells were collected and analyzed by flow cytometry. A dose-dependent increase in production of ROS in response to nifurtimox was seen (fold increase in DCF reported at 20μg/ml). (A) SMS KCNR; 1.5 fold increase, (B) SY5Y; 10.5 fold increase, (C) CHLA-90; 2 fold increase, (D) LAN2; 2 fold increase.
Nifurtimox inhibited tumor growth in vivo in mice xenografts
In addition to its effects in vitro, nifurtimox was also able to reduce the growth of neuroblastoma tumors in a xenograft model. Nude mice (nu/nu) were injected in the left flank with SMS-KCNR cells and allowed to form palpable tumors for 10-14 days. Then mice were fed 5g food pellets daily with or without 150mg/kg nifurtimox. After 28 days of treatment (when control mice reached maximum tumor size of 3.0cm3) the mice were sacrificed. There were no signs of drug related toxicity. Tumors were excised and weighed. Treatment with nifurtimox resulted in inhibition of tumor growth. The average tumor weight in control mice was 1.14 grams compared to an average weight of 0.3 grams in mice receiving nifurtimox (p=0.03) (Figure 6A). Combining these results with three additional replicated experiments of nifurtimox and control conditions in a randomized block design analysis of variance confirmed the significance of the tumor weight differences (F(1,3) = 31.90, p = 0.011) (Supplemental Table 1). Immunohistochemical staining showed that tumors treated with nifurtimox had decreased cell proliferation as evidenced by decrease in Ki67 positive staining (75.3% versus 93.2%, p<0.001) and decrease in mitotic index (33.5 versus 50.2, p=0.012) as well as an increase in apoptosis as evidenced by an increase in the pyknotic index (63.7 versus 39.5, p=0.022). (Figure 6B-C)
Figure 6. Nifurtimox induces apoptotic cell death and inhibits tumor growth in vivo in a neuroblastoma mouse xenograft.
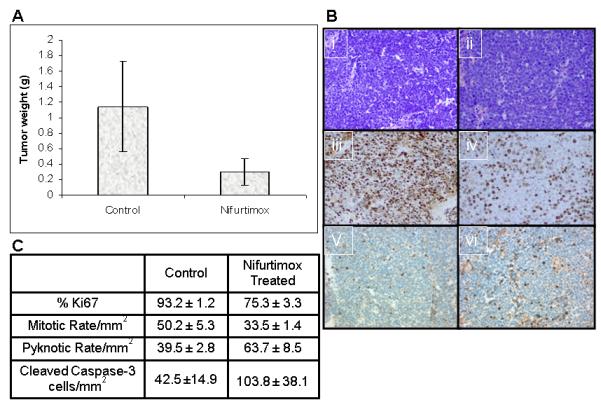
(A) Nude mice injected SMSKCNR were fed food pellets with or without 150 μg/kg nifurtimox per day. After 28 days of treatment mice were sacrificed and tumors were harvested and weighed. There was a significant decrease in the mean tumor size of mice treated with nifurtimox (p=0.03). (B) Tumors were stained with H&E (i=control, ii=nifurtimox treated) and stained with Ki67 (iii=control, iv=nifurtimox treated) and cleaved caspase-3 (v=control, vi=nifurtimox treated). (C) Immunohistochemical stained sections were counted for %Ki67 positive, the mitotic rate, the pyknotic rate and cleaved caspase-3 from each tumor. A significant decrease in cell proliferation by Ki67 (p<0.001) and mitotic rate (p=0.012) as well as an increase in apoptosis by pyknotic rate (p=0.022) and cleaved caspase-3 (p=0.0022) was seen.
Discussion
Neuroblastoma is a leading cause of cancer death in children. Current therapies for children with disseminated disease are associated with severe toxicity, and lead to cure in only a minority of cases. Thus, the development of new therapies—especially ones with more favorable toxicity profiles—would represent a significant improvement in the treatment of this disease.
Nifurtimox has been used around the world for 30 years for the treatment of Chagas disease and is well tolerated in children. The most common side effects found in adults are nausea, vomiting, abdominal pain, memory loss, sleep disturbances, seizures, psychosis, tremors, muscle weakness, paresthesias and polyneuritis. In a study of 67 children using nifurtimox for Chagas disease, no patients were excluded from the study due to adverse drug effects (10). A previously reported neuroblastoma patient who received nifurtimox for Chagas disease also did not experience any significant side effects from nifurtimox (4). It was the clinical response in this patient that led to the studies reported here.
Exposure of five different neuroblastoma cell lines to nifurtimox led to significant levels of apoptosis as demonstrated by the dose-dependent presence of DNA fragmentation and activated caspase-3; inhibition of caspase with Z-VAD-FMK abrogated the effect of nifurtimox. Similarly, nude mice with neuroblastoma xenografts showed a decrease in tumor growth when exposed to nifurtimox, with histologic changes consistent with apoptosis.
Nifurtimox is a nitroheterocyclic compound, which contains a nitro group that is essential for its efficacy. The nitro group can be reduced to the nitro anion radical in cell free systems by interacting with cytochrome P-450 reductase and xanthine oxidase, but also with catecholamines(11-14). Nitro anions can then reduce oxygen to the superoxide anion radical and hydrogen peroxide. When used to treat Chagas disease, it is the nitro anion free radicals and oxyradicals that appear to kill the parasite Trypanosoma cruzi(15, 16).
Exposure of neuroblastoma cells to nifurtimox did indeed lead to an increase in intracellular reactive oxygen species as measured by the DCF assay (Figure 5). The largest increase in DCF is seen in the SY5Y cell line (10.5 fold increase), this cell line is also the most sensitive in cell viability assays. The presence of catecholamines in these cells may play an important role in this effect and lead to some degree of specificity. Whereas nifurtimox led to degeneration of normal sympathetic neurons in culture, neither non-neural cells nor parasympathetic nerve cells were affected by nifurtimox. Furthermore, inhibition of dopamine synthesis by the tyrosine hydroxylase inhibitor AMPT attenuated the cytotoxicity of nifurtimox for neuroblastoma cells. In addition to the formation of the nitro anion radical, catecholamines and nifurtimox may interact to generate semiquinone free radicals that exacerbate damage of functionally important biomolecules, which would also contribute to apoptosis (12). It is interesting in this regard that the interaction between nifurtimox and catecholamines is thought to be the underlying cause of the neurological side effects resulting from treatment of patients with Chagas disease (12).
Suppression of basal and TrkB-mediated Akt phosphorylation by nifurtimox may also contribute to the cytotoxicity of nifurtimox for neuroblastoma cells. TrkB is a receptor tyrosine kinase with a high affinity for neurotrophins, and is expressed almost exclusively in biologically unfavorable neuroblastomas (2, 3). Binding of brain-derived neurotrophic factor to TrkB leads to the activation of the Ras/MAPK and PI3K/Akt signaling pathways. In neuroblastoma, the BDNF-TrkB-Akt pathway has been shown to be critical for cell survival and to protect cells from DNA damaging therapeutic agents, thus contributing to the development of chemoresistance (3, 17, 18). Both basal levels of Akt phorphorylation as well as BDNF-induced increases in Akt phosphorylation were inhibited by nifurtimox. Whether this effect might enhance the efficacy of conventional cytotoxic agents, either in neuroblastoma or in other tumors, remains to be answered.
Nifurtimox is cytotoxic both in vitro and in vivo and it lacks many of the toxicities associated with conventional antineoplastic drugs. The enhanced generation of free radicals in catecholamine-containing cells should result in some degree of targeted effect, and its effect on inhibiting anti-apoptotic pathways may produce synergy with other cytotoxic agents. These features make it an attractive candidate for further clinical investigation.
Supplementary Material
Supplemental Figure 1. Combination of results of in vivo mice experiments with three additional replicated experiments of nifurtimox and control conditions in a randomized block design analysis of variance confirmed the significance of the tumor weight differences (F(1,3) = 31.90, p = 0.011)).
Acknowledgments
Financial support: Training Grant from the Department of Pediatrics, Division of Hematology/Oncology Brown University, Vermont Cancer Center, Lake Champlain Cancer Research Organization, Children’s Miracle Network, and the Penelope and Sam Fund for Neuroblastoma Research to Dr. Saulnier Sholler. NS25767 to Dr. Nishi). Brown University Seed Grant, a NICHD, K12 HD043447 BIRCWH Scholar Grant to Dr. Brard.
References
- 1.Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11(8):1466–77. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 2.Brodeur GM, Maris JM, Yamashiro DJ, et al. Biology and genetics of human neuroblastomas. J Pediatr Hematol Oncol. 1997;19(2):93–101. doi: 10.1097/00043426-199703000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Ho R, Eggert A, Hishiki T, et al. Resistance to chemotherapy mediated by TrkB in neuroblastomas. Cancer Res. 2002;62(22):6462–6. [PubMed] [Google Scholar]
- 4.Sholler GL Saulnier, Kalkunte S, Greenlaw C, et al. Antitumor activity of nifurtimox observed in a patient with neuroblastoma. J Pediatr Hematol Oncol. 2006;28(10):693–5. doi: 10.1097/01.mph.0000212994.56812.f2. [DOI] [PubMed] [Google Scholar]
- 5.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281(5381):1312–6. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 6.Puppo M, Pastorino S, Melillo G, et al. Induction of apoptosis by flavopiridol in human neuroblastoma cells is enhanced under hypoxia and associated with N-myc proto-oncogene down-regulation. Clin Cancer Res. 2004;10(24):8704–19. doi: 10.1158/1078-0432.CCR-03-0422. [DOI] [PubMed] [Google Scholar]
- 7.Jaboin J, Kim CJ, Kaplan DR, et al. Brain-derived neurotrophic factor activation of TrkB protects neuroblastoma cells from chemotherapy-induced apoptosis via phosphatidylinositol 3′-kinase pathway. Cancer Res. 2002;62(22):6756–63. [PubMed] [Google Scholar]
- 8.Rohrer H, Ernsberger U. The differentiation of the neurotransmitter phenotypes in chick sympathetic neurons. Advances in Pharmacology. 1998;42:891–5. doi: 10.1016/s1054-3589(08)60890-x. [DOI] [PubMed] [Google Scholar]
- 9.Nishi R, Berg DK. Survival and development of ciliary ganglion neurons grown alone in cell culture. Nature. 1979;277(5693):232–4. doi: 10.1038/277232a0. [DOI] [PubMed] [Google Scholar]
- 10.Solari A, Ortiz S, Soto A, et al. Treatment of Trypanosoma cruzi-infected children with nifurtimox: a 3 year follow-up by PCR. The Journal of Antimicrobial Chemotherapy. 2001;48(4):515–9. doi: 10.1093/jac/48.4.515. [DOI] [PubMed] [Google Scholar]
- 11.Dubin M, Grinblat L, Villamil SH Fernandez, et al. Nitrofuran inhibition of microsomal lipid peroxidation. FEBS letters. 1987;220(1):197–200. doi: 10.1016/0014-5793(87)80902-x. [DOI] [PubMed] [Google Scholar]
- 12.Rao DN, Mason RP. Generation of nitro radical anions of some 5-nitrofurans, 2- and 5-nitroimidazoles by norepinephrine, dopamine, and serotonin. A possible mechanism for neurotoxicity caused by nitroheterocyclic drugs. The Journal of Biol Chem. 1987;262(24):11731–6. [PubMed] [Google Scholar]
- 13.Paulino-Blumenfeld M, Hansz M, Hikichi N, et al. Electronic properties and free radical production by nitrofuran compounds. Free Radical Res Comm. 1992;16(4):207–15. doi: 10.3109/10715769209049174. [DOI] [PubMed] [Google Scholar]
- 14.Letelier ME, Izquierdo P, Godoy L, et al. Liver microsomal biotransformation of nitro-aryl drugs: mechanism for potential oxidative stress induction. J Appl Toxicol. 2004;24(6):519–25. doi: 10.1002/jat.999. [DOI] [PubMed] [Google Scholar]
- 15.Docampo R, Stoppani AO. Generation of superoxide anion and hydrogen peroxide induced by nifurtimox in Trypanosoma cruzi. Archives of Biochemistry and Biophysics. 1979;197(1):317–21. doi: 10.1016/0003-9861(79)90251-0. [DOI] [PubMed] [Google Scholar]
- 16.Moreno SN, Mason RP, Docampo R. Reduction of nifurtimox and nitrofurantoin to free radical metabolites by rat liver mitochondria. Evidence of an outer membrane-located nitroreductase. The Journal of Biol Chem. 1984;259(10):6298–305. [PubMed] [Google Scholar]
- 17.Middlemas DS, Kihl BK, Zhou J, Zhu X. Brain-derived neurotrophic factor promotes survival and chemoprotection of human neuroblastoma cells. The Journal of Biol Chem. 1999;274(23):16451–60. doi: 10.1074/jbc.274.23.16451. [DOI] [PubMed] [Google Scholar]
- 18.Middlemas DS, Kihl BK, Moody NM. Brain derived neurotrophic factor protects human neuroblastoma cells from DNA damaging agents. J Neurooncol. 1999;45(1):27–36. doi: 10.1023/a:1006342423175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Combination of results of in vivo mice experiments with three additional replicated experiments of nifurtimox and control conditions in a randomized block design analysis of variance confirmed the significance of the tumor weight differences (F(1,3) = 31.90, p = 0.011)).


