Abstract
Wnt proteins are conserved signalling molecules that have an essential role in regulating diverse processes during embryogenesis and adult tissue homoeostasis. Wnts are post-translationally modified by palmitoylation, which is essential for Wnt secretion and function. Intriguingly, the crystal structure of XWnt8 in complex with the extracellular domain of the Frizzled 8 cysteine-rich domain (Fzd8-CRD) revealed that Wnts use the fatty acid as a ‘hotspot’ residue to engage its receptor, which is a unique mode of receptor-ligand recognition. In addition, there are several lines of evidence suggesting that Wnts engage several signalling modulators and alternative receptors by means of fatty acids as a critical contact residue. In the present article, we review our current understanding of Wnt acylation and its functional role in Wnt signalling regulation.
Keywords: Wnt, Frizzled, sFRP, WIF, acylation
Introduction
Wnt signalling is a conserved signalling pathway, regulating a broad range of processes during embryonic development and adult tissue homeostasis [1–4]. Aberrant Wnt signalling is linked to many types of cancer and degenerative diseases. Secreted Wnt ligand family members bind to an array of cell-surface receptors, stimulating distinct signalling cascades, including the canonical β-catenin-dependent pathway, the planar cell polarity pathway, and the Wnt/Ca2+ pathway. The canonical pathway is the most extensively studied Wnt signalling pathway, and is initiated by Wnt binding to the family of Fzd receptors and Lrp5/6 co-receptors. This binding results in the stabilization of β-catenin, which functions as a co-activator of the transcription factors, T-cell factor/lymphoid enhancer factor (TCF/LEF), for regulation of Wnt target gene expression. Fzds, along with the alternative receptors Ror1/2 (receptor tyrosine kinase-like orphan receptor) and Ryk, have been implicated in transducing non-canonical Wnt signalling cascades upon Wnt engagement, and secreted modulators including sFRPs (secreted Frizzled-related protein), WIF-1 (Wnt inhibitory factor), dickkopf (DKK) and sclerostin (SOST) fine tune signalling activation. Wnts are post-translationally palmitoylated by Porcupine, a membrane-bound-O-acyltransferase localized to the endoplasmic reticulum (ER), and it has become increasingly apparent that Wnt acylation is essential for Wnt secretion and function. Current work focuses on delineating the Wnt lipid-mediated interactions with receptors, antagonists and core components of the Wnt secretion pathway, and their functional implications, which we expect will open up new possibilities to therapeutically target Wnt signalling. In this review, we summarize our current understanding of Wnt acylation and highlight various aspects of the functional role of the Wnt lipid in signalling activation, focusing on questions at a molecular level.
Insights into Wnt acylation and the Wnt/Fzd interaction from crystal structure
Wnt proteins undergo extensive post-translational modifications, most importantly, glycosylation and acylation. Whereas N-linked glycosylation appears to primarily enhance stability and/or folding, acylation is essential for Wnt secretion and activity. The initial identification of Wnt acylation provides an explanation for the observed poor solubility and membrane association of Wnts [5]. To date, acylation of two conserved residues have been reported: Cys77 in Wnt3a was found to be acylated by palmitate through a thioester linkage, and Ser209 was reported to be lipidated by an ester-linked palmitoleic acid [5,6]. Whereas mutating Cys77 impairs activity without significantly affecting secretion of Wnt3a, a Wnt3a Ser209Ala mutant is retained in the ER, and has decreased activity, prompting the hypothesis of differential functional roles of the two lipids [5,6]. However, the recent crystal structures of XWnt8 bound to the Fzd8-CRD revealed that only the conserved serine is acylated, and the conserved cysteine is engaged in a disulfide bond with a second, strictly conserved cysteine. Therefore, Cys77 and the corresponding residues thereof cannot serve as an acylation site, supporting the notion that Wnts are only mono-acylated [7].
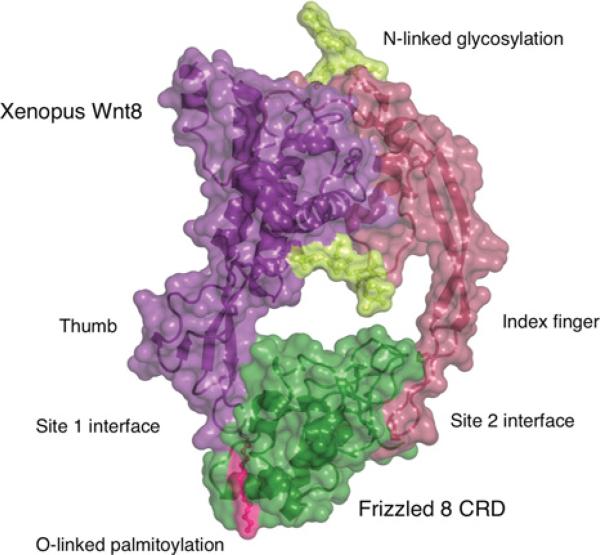
The crystal structure of the XWnt8/Fzd8–CRD complex was determined at 3.25Å resolution and showed that Wnts adopt a novel two-domain structural architecture (Figure 1) [7,8]. The N-terminal domain comprises approximately two-thirds of Wnt, and folds into a seven α-helical globular domain in which the core adopts a saposin-like domain fold. Two inter-helical insertions form a short β-sheet that is stabilized by four disulfide bonds that we commonly refer to as the thumb, with the acylated Ser187 (corresponding to Ser209 in Wnt3a) positioned at the tip of the thumb. Although the electron density accounting for the fatty acid was clearly visible, the resolution of the structure was not sufficient to independently determine the identity of the lipid. Therefore, based on the structure, it was assigned as palmitoleic acid according the modification previously identified using mass spectrometry [6]. Recently, an elegant study revealed that Porcupine strongly prefers palmitoleic acid, but can also catalyze the linkage of fatty acids with an aliphatic chain of 13 to 16 carbons [9]. Notably, in the observed conformation, the aliphatic chain projects away from the thumb without making further contact with the XWnt8 polypeptide chain, and is presented in a highly accessible manner. The C-terminal domain of XWnt8 comprises approximately 100 amino acids, and resembles the cysteine-knot growth factor fold, containing a long, twisted β-hairpin which is stabilized by an extensive network of disulfide bonds, commonly referred to as the index finger.
Figure 1. Overall structure of Xenopus Wnt8 in complex with mouse Fzd8-CRD.
N- and C-terminal domains of XWnt8 are colored in purple and red, respectively, Wnt O-linked palmitoylation in pink, and N-linked glycosylation in yellow. XWnt8 contacts the Fzd8-CRD (shown in green) through two independent binding sites located at the tips of the ‘thumb’ and ‘index finger’.
Fzds are key transducers of Wnt signalling [10]. In mammals, there are 10 Fzds and 19 Wnts that interact with high degree of promiscuity. However, specific Wnt/Fzd pairs, regulating distinct biological functions, are still poorly defined. Fzds belong to the family of seven-pass transmembrane receptors with an extracellular CRD, which is a small, primarily α-helical domain of approximately 120 amino acids that serves as a high affinity Wnt binding domain [11]. It remains to be shown whether the transmembrane domain encompasses an additional low affinity binding site required for Fzd activation.
In the crystal structure, XWnt8 engages the Fzd8-CRD through two separated binding sites located at the tips of the thumb (site 1) and index finger (site 2), resembling a hand that grabs its target with an extended thumb and index finger (Figure 1) [7]. Intriguingly, the palmitoleic acid binds in an elongated hydrophobic groove, generating extensive hydrophobic and van der Waals interactions. Though additional protein–protein interactions between XWnt8 and Fzd8-CRD further strengthen the site 1 interface, the Wnt lipid is clearly a “hotspot” residue that contributes considerably to the total binding energy, and is essential for Fzd binding. The high degree of conservation of hydrophobic residues forming the lipid-binding groove in other Fzd-CRDs implies a conserved lipid-mediated recognition mode that imparts a range of Wnt/Fzd affinities. The site 2 interaction is mediated by an 11-amino acid cyclic peptide located at the tip of the index finger binding to an Fzd-specific shallow cavity. The notion of Wnt/Fzd cross-reactivity rather than mono-specificity is supported by the observation that interactions are largely made between chemically conserved amino acids. However, binding studies clearly show that Wnts and Fzds are not broadly degenerate, and both interfaces are important for Wnt/Fzd specificity and affinity.
It remains an open question whether Wnts adopt an alternative conformation in the free, receptor-unbound state in which the lipid moiety is protected by the XWnt8 polypeptide chain from an aqueous environment [7]. However, the requirement of detergent to maintain the solubility and activity of recombinant Wnts argues for a scenario in which the lipid remains exposed. Upon secretion, Wnts localize to the cell membrane or exosomes, or bind to Wnt receptors, Wnt antagonists, carrier proteins like Swim and lipoproteins through direct contact with the Wnt lipid. However, the hypothesis of a closed, lipid-shielded conformation was fueled by the crystal structure of the N-terminal domain of WntD [12]. WntD is an exception within the Wnt family; it is a non-lipidated, Drosophila-specific Wnt homologue with poorly defined function. It neither binds to any Drosophila Fzd-CRDs, nor is it capable of activating the β-catenin-dependent Wnt pathway. The acylated Ser187 is replaced by a glutamine in WntD, precluding acylation. The crystal structure of WntD confirmed the non-lipidated nature and revealed an alternative folded-back and hydrogen-bond-stabilized conformation of the tip of the thumb. Though the rest of the structure superimposes well with XWnt8, prompting the hypothesis that this conformation could represent the conformation of the thumb in the closed state.
Implication of Wnt acylation in Wnt/Ror2 interaction
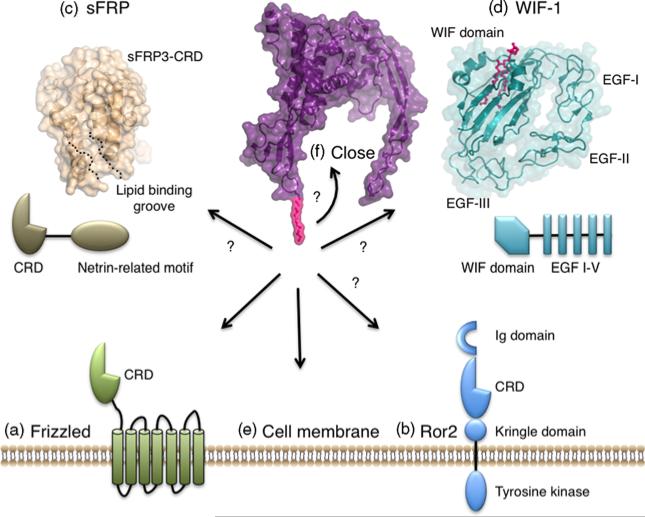
In addition to the classical Wnt receptors Fzds and Lrp5/6, the receptor tyrosine kinase, Ror2, functions as an alternative receptor for Wnts [13–15]. It plays an essential role during embryogenesis of various organs by primarily regulating cell polarity and migration. Ror2 consists of an immunoglobulin-like domain, an Fzd-like CRD domain and a Kringle domain within the extracellular domain, and a tyrosine kinase domain followed by Pro-rich and Ser/Thr-rich regions within the intracellular domain (Figure 2). Ror2 has been shown to bind through its CRD to several Wnt subtypes; Wnt5a, however, is considered the signalling-competent Ror2 ligand. Several, to some extent controversial, signalling mechanisms of Ror2 have been reported, and much remains to be understood regarding receptor activation and signal transduction. Wnt5a binding to Ror2 was shown to induce receptor homodimerization, tyrosine or Ser/Thr phosphorylation through receptor and non-receptor kinases, leading to inhibition of β-catenin signalling and/or activation of alternative signalling pathways [16–20]. In addition, Wnt5a was shown to form a signalling complex with Fzd and Ror2, and Ror2 may function as a decoy receptor to sequester various Wnt ligands [21–24].
Figure 2. Demonstrated and putative Wnt-lipid-mediated interactions.
Interactions of the Wnt palmitoylation with a) the CRD of Frizzled; b) the CRD of Ror2; c) the CRD of sFRP; d) the WIF domain of WIF-1; e) the cell membrane and f) the Wnt core to form a closed conformation are discussed. The crystal structure of sFRP3-CRD (wheat) revealed a hydrophobic Fzd-like lipid-binding groove, and the crystal structure of the WIF domain and EGF-like domains I–III of human WIF-1 (cyan) with bound phospholipid (pink) are shown.
Given that Wnts are mono-lipidated, this raises the question of whether two Wnts are required for Ror2 homodimerization and Ror2/Fzd cross-linking, or whether Ror2 uses an alternative interface for Wnt engagement. It should be noted that sequence alignments and molecular modelling of the Ror2-CRD do not reveal a conspicuous, Fzd-like hydrophobic binding groove, and it remains unclear whether the Wnt lipid serves as a ‘hotspot’ residue for Ror2 binding. Quantitative binding measurements and/or structural characterization of complexes involving Wnts and Ror2 could clarify the molecular basis of the Wnt/Ror2 interaction, the role of the Wnt lipid, binding versus signalling specificity and composition and stoichiometry of Wnt5a– Ror2 signalling complexes.
Modulation of Wnts through sFRPs and WIF
Wnt signalling is modulated by a number of secreted antagonists, including sFRPs and WIF-1, which bind directly to Wnts to inhibit the formation of a Wnt–receptor signalling complex [3]. Mammals encode five members of the sFRP family, which are widely expressed during embryogenesis and adult tissue homoeostasis, and each comprises an N-terminal Fzd-like CRD domain, followed by a netrin-related motif (Figure 2). The crystal structure of the mouse sFRP3-CRD revealed a lipid-binding groove analogous to Fzd8-CRD, and we can expect that sFRPs interacts with Wnt in a manner comparable to Fzd using the Wnt lipid as the crucial ‘hotspot’ residue, thereby sequestering Wnts from Fzd [11]. Though sFRP-CRD is sufficient for Wnt binding and inhibition [25], a direct interaction between Wnt and the netrin-like domain has been demonstrated [26,27], raising the question of the molecular mechanism of how sFRPs potently compete with Fzds for Wnt binding: is antagonism based on a high affinity form of the CRD, additional contacts mediated by the netrin-like motif leading to enhanced affinity or higher local concentration of sFRPs compared with Fzd?
In addition to their inhibitory role, sFRPs and soluble Fzd-CRD were shown to enhance β-catenin-dependent Wnt signalling in a concentration- and context-dependent manner [26,28–30]. Several potential mechanisms of this biphasic signalling modulation have been suggested. Interestingly, sFRP and soluble Fz-CRDs were shown to enhance the diffusion and solubility of Wnts, likely by direct engagement of the Wnt lipid, with resultant enhancing of Wnt signalling in more distal cells [31,32]. This finding suggests that the involvement of a solubility-regulating post-translational modification that is utilized by receptors and antagonists as a ‘hotspot’ contact accounts for at least a part of the dual antagonistic–agonistic activity. However, it will be critical to perform a comprehensive quantitative characterization of the pairwise Wnt/sFRP and Fzd binding affinities in order to understand the full spectrum of Wnt inhibition by different sFRPs. In addition, it will need to be shown if this activity is specific to sFRPs or common to soluble proteins that directly engage the Wnt lipid.
In contrast to sFRP, the Wnt antagonist WIF-1 does not contain an Fzd-like CRD, but comprises an N-terminal WIF domain followed by five epidermal growth factor (EGF)-like domains (Figure 2) [33]. Though the WIF domain is sufficient for Wnt binding and inhibition of Wnt signalling and cross-reacts with several Wnts including Wnt3a, 4, 5a, 7a and 11 with high affinity, the EGF-like domains were recently shown to enhance binding and activity [34–36]. However, the molecular mechanism of WIF-1-mediated Wnt inhibition still remains unclear.
The NMR and crystal structures of the human WIF-1 domain revealed a β-sandwich fold, with three short helices lining one side [36,37]. Intriguingly, alkyl-chain-containing detergent molecules and phosphatidylcholines, which were derived from the recombinant protein expression and purification process, were bound to a hydrophobic cavity within the core and hydrophobic surfaces residues, respectively, prompting the hypothesis that the WIF domain could engage Wnt proteins through binding of the aliphatic chain. In addition, WIF-1 enhances the solubility of Wnts in in vitro conditions, thus arguing in favor of a Wnt lipid-mediated interaction. However, this hypothesis needs further validation from the crystal structure of the WIF-1 in complex with Wnt.
Implication of Wnt acylation in drug development
Owing to the central role of Wnt signalling in the regeneration of a multitude of adult tissues, and the development and progression of many types of cancers, strategies to therapeutically target Wnt signalling for use in regenerative medicine and oncology are increasingly being investigated. This includes, but is not limited to, the chemical inhibition of Porcupine function leading to the blockade of Wnt acylation and secretion [38–40], chemical stabilization of the downstream effector, Axin [38,41], resulting in enhanced β-catenin degradation and the use of soluble decoy receptors [42] to antagonize receptor activation. However, broadly inhibiting Wnt signalling that simultaneously antagonizes Wnt-dependent cellular activities, such as the regeneration of the intestinal epithelium and bone, has raised significant safety concerns. Therefore, approaches to enhance specificity are being investigated. This is most successfully exemplified with romozozumab, a humanized monoclonal antibody that targets sclerostin – a Wnt antagonist expressed in bone and cartilage tissue – to increase bone mass, and which is currently being tested in clinical trails for the treatment of post-menopausal osteoporosis [43,44]. The Wnt–Fzd interaction presents an excellent opportunity to specifically target this pathway. In addition, identification of the lipid as a ‘hotspot’ residue provides a framework to more effectively target the interaction. Interestingly, the binding site of vantictumab, a monoclonal Fzd antibody, which is currently being tested in clinical trails for the inhibition of a range of cancers, was mapped to bind in close proximity to the lipid-binding groove [45]. However, although vantictumab was originally selected for Fzd7-CRD, it cross-reacts with five out of ten human Fzds. Through the visualization of the Wnt–Fzd interaction, and the continuing enhancement of our understanding of the molecular mechanism of Wnt signalling regulation at the cell membrane, we will be able to pursue more rational approaches to the design of Wnt antagonists and agonists with enhanced and tailored specificities.
Concluding remarks
Wnt proteins are important regulators of many diverse processes during embryogenesis and adult tissue homoeostasis. Wnt activity is transduced by an array of cell-surface receptors and fine-tuned by a large number of secreted modulators. Much remains to be understood about the interactions of Wnts with receptors and modulators that ultimately determine specificity of Wnt activity. In particular, understanding the molecular basis of the interactions, the molecular determinants for binding specificity and the relative binding affinities will be essential to understand receptor specificity and discrimination, differential signalling activity and the specificity of Wnt inhibition. Considerable progress has been made in the isolation and characterization of Wnts; it is an exciting time in the progression of our understanding of Wnt protein biochemistry.
Acknowledgments
Funding
We acknowledge support from the US National Institutes of Health [grant number RO1 GM097015]; the Stinehart/Reed Foundation; the Howard Hughes Medical Institute to K.C.G.; and the Jane Coffin Childs Memorial Fund to C.Y.J.
Abbreviations
- CRD
cysteine-rich domain
- EDF
epidermal growth factor
- ER
endoplasmic reticulum
- Fzd
Frizzled
- sFRP
secreted Frizzled-related protein
- WIF-1
Wnt inhibitory factor
References
- 1.Willert K, Nusse R. Wnt proteins. Cold Spring Harb. Perspect. Biol. 2012;4:a007864. doi: 10.1101/cshperspect.a007864. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 3.Cruciat CM, Niehrs C. Secreted and transmembrane wnt inhibitors and activators. Cold Spring Harb. Perspect. Biol. 2013;5:a015081. doi: 10.1101/cshperspect.a015081. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat. Rev. Mol. Cell. Biol. 2009;10:468–477. doi: 10.1038/nrm2717. PubMed. [DOI] [PubMed] [Google Scholar]
- 5.Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 6.Takada R, Satomi Y, Kurata T, Ueno N, Norioka S, Kondoh H, Takao T, Takada S. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev. Cell. 2006;11:791–801. doi: 10.1016/j.devcel.2006.10.003. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 7.Janda CY, Waghray D, Levin AM, Thomas C, Garcia KC. Structural basis of Wnt recognition by Frizzled. Science. 2012;337:59–64. doi: 10.1126/science.1222879. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bazan JF, Janda CY, Garcia KC. Structural architecture and functional evolution of Wnts. Dev. Cell. 2012;23:227–232. doi: 10.1016/j.devcel.2012.07.011. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao X, Hannoush RN. Single-cell imaging of Wnt palmitoylation by the acyltransferase porcupine. Nat. Chem. Biol. 2014;10:61–68. doi: 10.1038/nchembio.1392. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 10.Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, Andrew D, Nathans J, Nusse R. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382:225–230. doi: 10.1038/382225a0. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 11.Dann CE, Hsieh JC, Rattner A, Sharma D, Nathans J, Leahy DJ. Insights into Wnt binding and signalling from the structures of two Frizzled cysteine-rich domains. Nature. 2001;412:86–90. doi: 10.1038/35083601. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 12.Chu ML, Ahn VE, Choi HJ, Daniels DL, Nusse R, Weis WI. Structural studies of Wnts and identification of an LRP6 binding site. Structure. 2013;21:1235–1242. doi: 10.1016/j.str.2013.05.006. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green JL, Kuntz SG, Sternberg PW. Ror receptor tyrosine kinases: orphans no more. Trends Cell Biol. 2008;18:536–544. doi: 10.1016/j.tcb.2008.08.006. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green J, Nusse R, van Amerongen R. The role of Ryk and Ror receptor tyrosine kinases in Wnt signal transduction. Cold Spring Harb. Perspect. Biol. 2014;6 doi: 10.1101/cshperspect.a009175. doi: 10.1101/cshperspect.a009175 CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Amerongen R. Alternative Wnt pathways and receptors. Cold Spring Harb. Perspect. Biol. 2012;4 doi: 10.1101/cshperspect.a007914. doi: 10.1101/cshperspect.a007914 CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Rubin B, Bodine PV, Billiard J. Wnt5a induces homodimerization and activation of Ror2 receptor tyrosine kinase. J. Cell. Biochem. 2008;105:497–502. doi: 10.1002/jcb.21848. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 17.Mikels A, Minami Y, Nusse R. Ror2 receptor requires tyrosine kinase activity to mediate Wnt5A signaling. J. Biol. Chem. 2009;284:30167–30176. doi: 10.1074/jbc.M109.041715. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamamoto H, Yoo SK, Nishita M, Kikuchi A, Minami Y. Wnt5a modulates glycogen synthase kinase 3 to induce phosphorylation of receptor tyrosine kinase Ror2. Genes Cells. 2007;12:1215–1223. doi: 10.1111/j.1365-2443.2007.01128.x. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 19.Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, Koshida I, Suzuki K, Yamada G, Schwabe GC, et al. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells. 2003;8:645–654. doi: 10.1046/j.1365-2443.2003.00662.x. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 20.Akbarzadeh S, Wheldon LM, Sweet SM, Talma S, Mardakheh FK, Heath JK. The deleted in brachydactyly B domain of ROR2 is required for receptor activation by recruitment of Src. PloS One. 2008;3:e1873. doi: 10.1371/journal.pone.0001873. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grumolato L, Liu G, Mong P, Mudbhary R, Biswas R, Arroyave R, Vijayakumar S, Economides AN, Aaronson SA. Canonical and noncanonical Wnts use a common mechanism to activate completely unrelated coreceptors. Genes Dev. 2010;24:2517–2530. doi: 10.1101/gad.1957710. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim C, Forrester WC. Functional analysis of the domains of the C elegans Ror receptor tyrosine kinase CAM-1. Dev. Biol. 2003;264:376–390. doi: 10.1016/j.ydbio.2003.09.007. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 23.Billiard J, Way DS, Seestaller-Wehr LM, Moran RA, Mangine A, Bodine PV. The orphan receptor tyrosine kinase Ror2 modulates canonical Wnt signaling in osteoblastic cells. Mol. Endocrinol. 2005;19:90–101. doi: 10.1210/me.2004-0153. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 24.Green JL, Inoue T, Sternberg PW. The C. elegans ROR receptor tyrosine kinase, CAM-1, non-autonomously inhibits the Wnt pathway. Development. 2007;134:4053–4062. doi: 10.1242/dev.005363. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 25.Lin K, Wang S, Julius MA, Kitajewski J, Moos M, Jr, Luyten FP. The cysteine-rich frizzled domain of Frzb-1 is required and sufficient for modulation of Wnt signaling. Proc. Natl. Acad. Sci. U.S.A. 1997;94:11196–11200. doi: 10.1073/pnas.94.21.11196. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uren A, Reichsman F, Anest V, Taylor WG, Muraiso K, Bottaro DP, Cumberledge S, Rubin JS. Secreted frizzled-related protein-1 binds directly to Wingless and is a biphasic modulator of Wnt signaling. J. Biol. Chem. 2000;275:4374–4382. doi: 10.1074/jbc.275.6.4374. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 27.Bhat RA, Stauffer B, Komm BS, Bodine PV. Structure-function analysis of secreted frizzled-related protein-1 for its Wnt antagonist function. J. Cell. Biochem. 2007;102:1519–1528. doi: 10.1002/jcb.21372. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 28.Sagara N, Kirikoshi H, Terasaki H, Yasuhiko Y, Toda G, Shiokawa K, Katoh M. FZD4S, a splicing variant of frizzled-4, encodes a soluble-type positive regulator of the WNT signaling pathway. Biochem. Biophys. Res. Commun. 2001;282:750–756. doi: 10.1006/bbrc.2001.4634. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 29.von Marschall Z, Fisher LW. Secreted Frizzled-related protein-2 (sFRP2) augments canonical Wnt3a-induced signaling. Biochem. Biophys. Res. Commun. 2010;400:299–304. doi: 10.1016/j.bbrc.2010.08.043. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xavier CP, Melikova M, Chuman Y, Uren A, Baljinnyam B, Rubin JS. Secreted Frizzled-related protein potentiation versus inhibition of Wnt3a/beta-catenin signaling. Cell. Signal. 2014;26:94–101. doi: 10.1016/j.cellsig.2013.09.016. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mii Y, Taira M. Secreted Frizzled-related proteins enhance the diffusion of Wnt ligands and expand their signalling range. Development. 2009;136:4083–4088. doi: 10.1242/dev.032524. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 32.Kumar S, Zigman M, Patel TR, Trageser B, Gross JC, Rahm K, Boutros M, Gradl D, Steinbeisser H, Holstein T, et al. Molecular dissection of Wnt3a-Frizzled8 interaction reveals essential and modulatory determinants of Wnt signaling activity. BMC Biol. 2014;12:44. doi: 10.1186/1741-7007-12-44. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsieh JC, Kodjabachian L, Rebbert ML, Rattner A, Smallwood PM, Samos CH, Nusse R, Dawid IB, Nathans J. A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature. 1999;398:431–436. doi: 10.1038/18899. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 34.Banyai L, Kerekes K, Patthy L. Characterization of a Wnt-binding site of the WIF-domain of Wnt inhibitory factor-1. FEBS Lett. 2012;586:3122–3126. doi: 10.1016/j.febslet.2012.07.072. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 35.Surmann-Schmitt C, Widmann N, Dietz U, Saeger B, Eitzinger N, Nakamura Y, Rattel M, Latham R, Hartmann C, von der Mark H, et al. Wif-1 is expressed at cartilage-mesenchyme interfaces and impedes Wnt3a-mediated inhibition of chondrogenesis. J. Cell Sci. 2009;122:3627–3637. doi: 10.1242/jcs.048926. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 36.Malinauskas T, Aricescu AR, Lu W, Siebold C, Jones EY. Modular mechanism of Wnt signaling inhibition by Wnt inhibitory factor 1. Nat. Struct. Mol. Biol. 2011;18:886–893. doi: 10.1038/nsmb.2081. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liepinsh E, Banyai L, Patthy L, Otting G. NMR structure of the WIF domain of the human Wnt-inhibitory factor-1. J. Mol. Biol. 2006;357:942–950. doi: 10.1016/j.jmb.2006.01.047. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 38.Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, Wei S, Hao W, Kilgore J, Williams NS, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat. Chem. Biol. 2009;5:100–107. doi: 10.1038/nchembio.137. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dodge ME, Moon J, Tuladhar R, Lu J, Jacob LS, Zhang LS, Shi H, Wang X, Moro E, Mongera A, et al. Diverse chemical scaffolds support direct inhibition of the membrane-bound O-acyltransferase porcupine. J. Biol. Chem. 2012;287:23246–23254. doi: 10.1074/jbc.M112.372029. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu J, Pan S, Hsieh MH, Ng N, Sun F, Wang T, Kasibhatla S, Schuller AG, Li AG, Cheng D, et al. Targeting Wnt-driven cancer through the inhibition of Porcupine by LGK974. Proc. Natl. Acad. Sci. U.S.A. 2013;110:20224–20229. doi: 10.1073/pnas.1314239110. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner S, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 42.DeAlmeida VI, Miao L, Ernst JA, Koeppen H, Polakis P, Rubinfeld B. The soluble wnt receptor Frizzled8CRD-hFc inhibits the growth of teratocarcinomas in vivo. Cancer Res. 2007;67:5371–5379. doi: 10.1158/0008-5472.CAN-07-0266. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 43.Li X, Ominsky MS, Warmington KS, Morony S, Gong J, Cao J, Gao Y, Shalhoub V, Tipton B, Haldankar R, et al. Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J. Bone Miner. Res. 2009;24:578–588. doi: 10.1359/jbmr.081206. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 44.Ominsky MS, Vlasseros F, Jolette J, Smith SY, Stouch B, Doellgast G, Gong J, Gao Y, Cao J, Graham K, et al. Two doses of sclerostin antibody in cynomolgus monkeys increases bone formation, bone mineral density, and bone strength. J. Bone Miner. Res. 2010;25:948–959. doi: 10.1002/jbmr.14. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 45.Gurney A, Axelrod F, Bond CJ, Cain J, Chartier C, Donigan L, Fischer M, Chaudhari A, Ji M, Kapoun AM, et al. Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc. Natl. Acad. Sci. U.S.A. 2012;109:11717–11722. doi: 10.1073/pnas.1120068109. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]