Abstract
BACKGROUND
Imatinib mesylate given orally at a daily dose of 400 mg is the standard of care as initial therapy for patients with chronic myeloid leukemia (CML) in chronic phase (CML-CP). Treatment guidelines propose dose escalation based on clinical assessments of disease response.
METHODS
Response and survival were analyzed in a cohort of patients (n = 106) with newly diagnosed CML-CP who were enrolled on the International Randomized Study of Interferon and STI571 (IRIS) trial, who began treatment with imatinib at a dose of 400 mg daily, and who subsequently underwent dose escalation to either 600 mg or 800 mg daily. Reasons for dose escalation were evaluated retrospectively based on 2 sets of criteria: the IRIS protocol-defined criteria (n = 39 patients) and the European LeukemiaNet (ELN) recommendations (n = 48 patients).
RESULTS
Among all 106 patients who underwent dose escalation, the rates of freedom from progression to accelerated phase or blast phase and overall survival were 89% and 84% at 3 years after dose increase, respectively. A cytogenetic response was obtained in 42% of patients who had their dose escalated based on protocol criteria and in 38% of patients who had their dose escalated according to the ELN recommendations.
CONCLUSIONS
The results from this retrospective analysis supported imatinib dose escalation as an appropriate initial option for patients with CML-CP who were experiencing suboptimal cytogenetic response or resistance.
Keywords: chronic myeloid leukemia in chronic phase, imatinib, dose escalation, suboptimal response
Imatinib mesylate (Glivec, Gleevec; Novartis Pharmaceuticals Corporation, Basel, Switzerland; East Hanover, NJ) is a selective BCR-ABL tyrosine kinase inhibitor. An imatinib dose of 400 mg daily is the standard of care for patients with newly diagnosed chronic myeloid leukemia (CML) in chronic phase (CML-CP). Treatment with imatinib in the first-line setting is associated with an overall survival rate of 88% after 6 years of therapy.1,2 Dose escalation of imatinib to a daily dose of 600 mg or 800 mg has demonstrated a benefit in patients who have a slow or inadequate response and in the setting of disease progression.3 To our knowledge, the experimental arm of the International Randomized Study of Interferon (IFN) and STI571 (IRIS) trial is the largest controlled population of patients with CML-CP in which the rate of response and its durability and/or survival with imatinib treatment can be estimated.1,4
The IRIS trial, which was initiated in 2000, was a multicenter, international, open-label, phase 3 study in which eligible patients with CML-CP were randomized to receive therapy either with imatinib or with IFN-α plus cytarabine. It allowed for a step-wise imatinib dose escalation, first to 600 mg and then to 800 mg, when certain response criteria were not met while the patient was receiving standard dose imatinib, when a major cytogenetic response (MCyR) was lost, or when disease progression occurred. Stepwise dose escalation was recommended because the tolerability of higher doses of imatinib was not well known during the initial portion of the study. The success of imatinib in CML-CP has changed the treatment practices for patients with CML based on expectations of improved response and survival rates.
Recommendations for imatinib dose escalation have been published by the European LeukemiaNet (ELN).5 The ELN recommends imatinib dose escalation to 600 mg or 800 mg daily at certain times of clinical assessments of CML disease response; these assessments correlate with similar or lower disease burden compared with IRIS protocol criteria. The ELN criteria recommend dose escalation of imatinib in case of failure or suboptimal response (Table 1). In the current report, we describe the outcome of patients with newly diagnosed CML-CP who were enrolled on IRIS, who began treatment with imatinib at a dose of 400 mg daily, and who subsequently underwent dose escalation.
Table 1.
Response Criteria From the European LeukemiaNet Recommendations*
| Time, mo | Failure† | Suboptimal Response‡ |
|---|---|---|
| 3 | No HR (stable disease or disease progression) | <CHR |
| 6 | <CHR, no cytogenetic response (Phþ >95%) | <PCyR (Phe >35%) |
| 12 | <PCyR (Ph+ >35%) | <CCyR |
| 18 | <CCyR | <MMR |
| Anytime | Loss of CHR | Additional chromosome abnormalities in Ph + cells |
| Loss of CCyR | Loss of MMR | |
| Mutation | Mutation |
HR indicates hematologic response; CHR, complete hematologic response; PCyR, partial cytogenetic response; Phe, Philadelphia chromosome positive; CCyR, complete cytogenetic response; MMR, major molecular response;.
Excluding criteria related to molecular response, because these were not measured in all patients.
To be interpreted as ‘current dose no longer appropriate.’
To be interpreted as long-term outcome likely to be less favorable.’
MATERIALS AND METHODS
Study Design
Patients enrolled on the IRIS study and randomized to initial treatment with imatinib 400 mg daily were included in this analysis if they underwent dose escalation on or before January 31, 2007 (ie, within 72 months after randomization). The IRIS protocol study design and methods have been previously described.1,4 Two separate analyses were conducted among patients who received imatinib at a dose of ≥ 600 mg per day at least once, 1) including patients retrospectively classified to have their imatinib dose increased according to protocol-defined criteria and 2) including patients retrospectively classified to have their imatinib dose increased according to the ELN recommendations. 5 The objective of the analysis was to evaluate the effect of dose escalation on patient outcomes. It is noteworthy that some criteria specified in the ELN recommendations were not collected routinely in the IRIS trial because of the evolving nature of monitoring criteria during the period of the study. Because ELN guidelines are the most current, these criteria were used in a separate analysis from IRIS criteria to ensure clinical relevance. No attempt to compare ELN and IRIS dose-escalation guidelines was attempted.
The IRIS protocol allowed dose escalation of imatinib to 600 mg and then, 1 month later, to 800 mg for the following reasons: 1) failure to achieve complete hematologic response (CHR) by 3 months, 2) failure to achieve at least a minor CyR (36%-65% Philadelphia chromosome [Ph]-positive metaphases) by 12 months, or 3) loss of an MCyR (≤ 35% Ph-positive metaphases) at any time. Disease progression (development of accelerated phase [AP] or blastic phase CML, loss of CHR or MCyR, or increasing white blood cell [WBC] count) also was considered to be ‘according to IRIS protocol criteria,’ because the label allows for a dose increase for this reason.4 Investigators were not required to contact the sponsor for approval for dose escalations for other reasons not specified by the protocol (eg, loss of a complete CyR [CCyR]; 0% Ph-positive metaphases). A review of safety data through the 42-month time point (July 2004) was included in the analysis for patients who received at least 28 days of cumulative high-dose therapy and had their initial first dose increase by January 31, 2004.
Endpoints
The primary study endpoints were event-free survival (EFS) (also referred to as progression-free survival [PFS] in previous publications1) and overall survival. Events were the first occurrence of any of the following: death from any cause, progression to AP or blastic phase (AP-BP) of CML, or loss of an MCyR. A CyR was determined by evaluating at least 20 metaphases per bone marrow sample and was categorized as a CCyR or a partial CyR (PCyR) (1%-35% Ph-positive metaphases). Overall survival was calculated by including deaths from any cause, regardless of bone marrow transplantation (BMT). Patients who discontinued the study were followed for survival.
Statistical Methods
Two analyses were conducted in patients who received imatinib at a dose ≥ 600 mg daily: 1) according to the guidelines for dose escalation provided in the IRIS protocol and 2) a retrospective review of the IRIS database classifying the reason for dose escalation according to the ELN recommendations. Because the criteria for dose escalations evolved in clinical practice after the initiation of the IRIS trial, reasons for dose escalations for all patients were recategorized according to the recommendations released by the ELN (Table 1). The identified reason for dose escalation was checked by a medical review, because it was not required to specify the reason in the case report forms.
A review of safety up to the 42-month time point (July 2004) for patients who received escalated doses of imatinib also was conducted. Only limited collection of safety data within the IRIS trial occurred after July 2004. To allow sufficient time for any adverse events to emerge, patients had to remain on at least 28 days of cumulative higher dose therapy to be included in this analysis; 67 patients of the total 106 qualified for this safety analysis. The frequency of occurrence of grade 3 or 4 events before the dose increase (n = 551; including patients who may have increased later during the trial) was compared with the frequency of events with onset after the first dose increase to at least 600 mg (n = 67).
RESULTS
Of 553 patients who initially were randomized to receive imatinib, 106 patients (19%) had imatinib dose escalation of 600 to 800 mg daily by January 31, 2007. Thirty-five of those patients (33%) who underwent dose escalation had an initial imatinib dose increase to 600 mg daily followed by a second increase to 800 mg daily within 2 months. These patients remained on imatinib 800 mg daily for a median duration of 14 months. Thirteen patients (12%) had dose escalation to 600 mg followed by further escalation to 800 mg more than 2 months later. Dose escalation of imatinib to only 600 mg daily was reported in 47 patients (44%), and initial dose escalation directly to 800 mg daily was reported in 11 patients (10%). Thus, a total of 59 patients received 800 mg of imatinib in the trial.
In the first analysis, ‘dose escalation according to IRIS protocol’ criteria were met for 39 patients. A subset of patients whose disease had progressed was included in this group, because the prescribing information for imatinib allowed for dose escalation for this reason. Thirty-four patients met both ELN and an IRIS protocol criteria for dose escalation; 5 patients met criteria only according to IRIS protocol, and 14 patients met criteria only according to the ELN recommendations (Table 2). The decision to perform imatinib dose escalation for 58 patients on the IRIS protocol was made at the discretion of the treating physician, and the precise reason could not be classified within 1 of these 2 aforementioned groups. Specific details regarding these 58 patients are limited to progression and survival data, because the original case report forms were not designed to capture information relevant to this retrospective analysis.
Table 2.
Reasons for Dose Escalation of Imatinib Among Patients Randomized to Receive Imatinib in the International Randomized Study of Interferon and STI571 Study
| IRIS Protocol Criteria (n539)* | ELN Recommendations (n548)* | ||
|---|---|---|---|
| Reason | No. (%) | Reason | No. (%) |
| No CHR at 3 mo | 7 (18) | 3 mo: Failure or suboptimal response (no HR or CHR) | 7 (15) |
| No MinCyR at 12mo | 8 (21) | 6 mo: Failure (no CHR, no CyR) | 1 (2) |
| Loss of MCyR | 18 (46) | 12 mo: Suboptimal response (no CCyR) | 4 (8) |
| Progression† | 6 (15) | 12 mo: Failure (no MCyR) | 11 (23) |
| 18 mo: Failure (no CCyR) | 10 (21) | ||
| Failure at anytime (confirmed loss of CHR or loss of CCyR) | 15 (31) | ||
IRIS indicates the International Randomized Study of Interferon and STI571 trial; ELN, European LeukemiaNet; CHR, complete hematologic response; HR, hematologic response; MinCyR, minimal cytogenetic response; CyR, cytogenetic response; MCyR, major cytogenetic response; CCyR, complete cytogenetic response.
Includes 34 patients who met both ELN and IRIS protocol criteria for dose escalation,
A subset of patients with disease progression was included in the “per IRIS Protocol Criteria” analysis, because the prescribing information for imatinib allows for dose escalation for this reason.
The median dose intensity was 400 mg daily (range, 251–400 mg daily) before dose escalation. The median time to dose escalation was 22 months for the entire cohort; for patients who underwent dose escalation according to IRIS protocol criteria, it was 14.4. months; and, for patients who underwent dose escalation according to the ELN recommendations, it was 15.6 months. The median time on imatinib after the initial dose increase was 19.4 months for 106 patients with a median dose intensity of 604 mg daily (range, 294–800 mg daily) after dose escalation.
The most common reason for dose escalation among the 39 patients whose dose was increased according to IRIS protocol criteria was loss of MCyR (18 of 39 patients; 46%), followed by lack of minor CyR at 12 months (8 of 39 patients; 21%), and lack of CHR at 3 months (7 of 39 patients; 18%) (Table 2). The most common reasons for dose escalation among the 48 patients whose dose was increased according to the ELN recommendations were failure at any time (15 of 48 patients; 31%), failure at 12 months (11 of 48 patients; 23%), and failure at 18 months (10 of 48 patients; 21%).
Clinical benefit of administration of a higher dose was assessed by reviewing clinical milestones that were achieved by 12 months after the increase had occurred. Details regarding the benefits observed after imatinib dose escalation according to either IRIS protocol criteria or the ELN recommendations are shown in Tables 3 and 4, respectively. Within 12 months after dose escalation, 17 of 33 patients (52%) who had a dose escalation of imatinib according to IRIS protocol criteria had achieved their clinical milestone (Table 3). The majority of patients without CHR at 3 months (6 of 7 patients; 86%) had achieved the milestone of CHR. At 12 months after dose escalation, 2 of 8 patients without at least a minor CyR had achieved an MCyR; by 24 months after dose escalation, 4 of these 8 patients (50%) had achieved an MCyR. Of 18 patients with loss of MCyR, 9 patients (50%) had achieved the milestone by 12.5 months, and further improvement in response to CCyR was observed in 3 of 18 patients by 30 months. Overall, 86% of these patients achieved or regained the hematologic response within 12 months of dose escalation, and 42% achieved or regained a CyR.
Table 3.
Clinical Milestones Achieved Within 12 Months After Imatinib Dose Escalation According to the International Randomized Study of Interferon and STI571 Protocol Criteria (N = 39)*
| Reason for Dose Escalation |
No. | Clinical Response by 12 Months |
No. | Best Cytogenetic Response and Time to That Response |
|---|---|---|---|---|
| No CHR at 3 mo | 7 | CHR | 6 | Two of 7 patients (29%) achieved an MCyR by 12-mo follow-up and improved further to CCyR by 33 mo |
| No minor CyR at 12 mo | 8 | MCyR | 2 | Four of 8 patients (50%) achieved an MCyR by 24-mo follow-up and improved further to CCyR by 48 mo |
| Loss of MCyR | 18 | MCyR | 9 | Three of 18 patients (17%) achieved a CCyR by 30 mo |
| Total patients who failed to achieve or lost a clinical response | 33 | Any response | 17 (52%) | |
| Progression | 6 | Clinical improvement with dose escalation† | 4 | Four of 6 patients achieved normalized WBCs; 4 of 4 patients with EMD at baseline had no sign of EMD after treatment; and 1 patient with >20% blasts reverted to CML-AP |
CHR indicates complete hematologic response; MCyR, major cytogenetic response; CCyR, complete cytogenetic response; WBCs, white blood cells; EMD, extramedullary disease; CML-AP, chronic myeloid leukemia in accelerated-phase.
Twelve months ±14 days.
Greater than 1 clinical symptom per patient.
Table 4.
Clinical Milestones Achieved by 12 Months After Dose Escalations According to European LeukemiaNet Recommendations (N = 48)*
| Reason for Dose Escalation | No. | Clinical Response by 12 Months |
No. | Best CyR and Time to That Response |
|---|---|---|---|---|
| At 3 mo: Failure or suboptimal response (no HR or <CHR) | 7 | CHR | 6 | Two of 6 patients had a CCyR at 8 mo and 33 mo |
| At 6 mo: Failure (no CHR, no CyR) | 1 | CHR or MCyR | 0 | |
| At 12 mo: Suboptimal response (no CCyR) | 4 | CCyR | 0 | One of 4 patients had an MCyR at 12.5 mo; 2 of 4 patients had a CCyR at 14 mo and 15.5 mo |
| At 12 mo: Failure (no MCyR) | 11 | MCyR | 6 | Five of 11 responses occurred 2.5–11 mo after dose escalation; 1 of 11 patients had an MCyR at 21 mo; 1 of 11 patients had a CCyR at 24 mo |
| At 18 mo: Failure (no CCyR) | 10 | CCyR | 2 | Two of 10 responses occurred at 3 mo and 9 mo after dose escalation; 1 of 10 patients had a CCyR at 29 mo |
| Failure at anytime (confirmed loss of CHR, or loss associated with progression to AP/BC, or loss of CCyR) | 15 | CHR, MCyR, or CCyR | 0, 3, 4 | Seven of 15 CyR responses occurred between 2 mo and 10 mo after dose escalation; 2 of 15 patients had an additional CCyR at 15 mo and 21 mo |
| Total | 48 | Improved response | 21 (44%) |
CyR indicates cytogenetic response; HR, hematologic response; CHR, complete hematologic response; CCyR, complete cytogenetic response; MCyR, major cytogenetic response; AP, accelerated phase; BC, blast crisis. *Twelve months ±14 days.
The clinical response milestones were achieved within 12 months in 21 of 48 patients (44%) who had a dose escalation of imatinib according to the ELN recommendations (Table 4). The milestone of CHR was achieved within 12 months in 6 of 7 patients who had failure or suboptimal response at 3 months. Six of 11 patients with failure at 12 months achieved an MCyR within 12 months after a dose increase, 2 of 10 patients with failure at 18 months experienced a CCyR within the 12 months after dose increase, and 7 of 15 patients with failure at any time achieved at least a MCyR within 1 year after dose increase. With continued follow-up, several additional patients achieved an MCyR and a CCyR. None of the patients with failure at 6 months (n = 1) or suboptimal response at 12 months (n = 4) achieved their clinical milestone at 12 months. At later follow-up, an MCyR was reported in 1 patient who had a suboptimal response at 12 months; this patient had achieved only a minor CyR before dose escalation. Two other patients who had suboptimal responses at 12 months achieved a CCyR in subsequent follow-up; these patients had obtained an MCyR before dose escalation. In summary, 67% of patients who had their dose escalated based on the ELN recommendations achieved or regained a hematologic response within 12 months of dose escalation, and 38% achieved or regained a CyR.
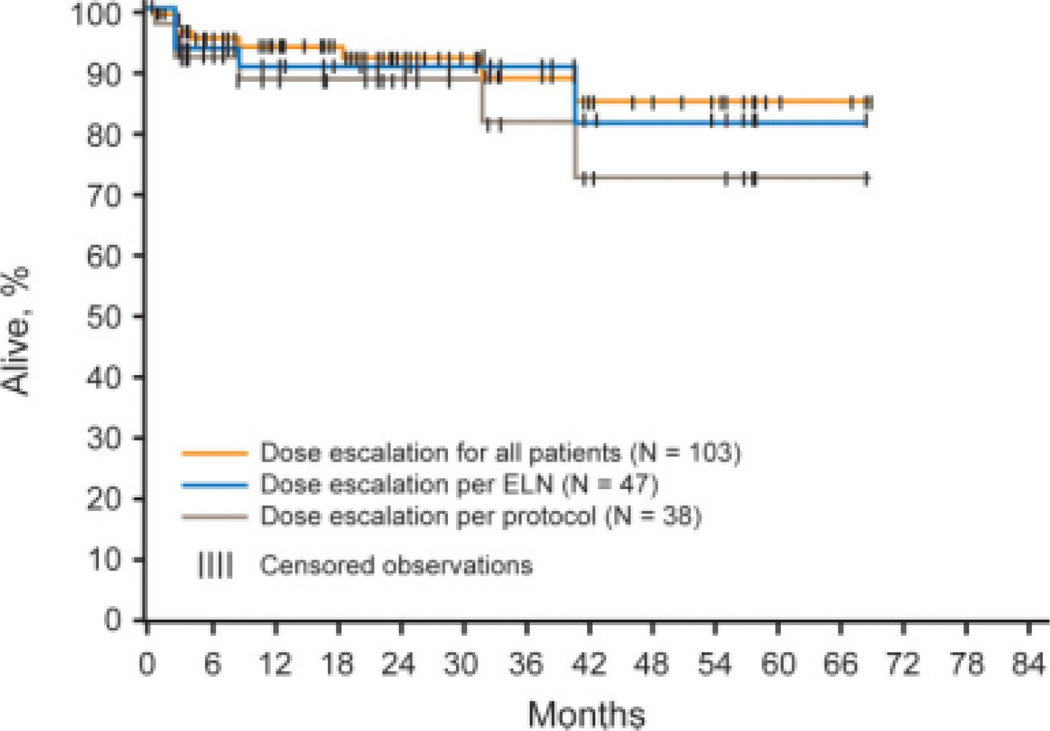
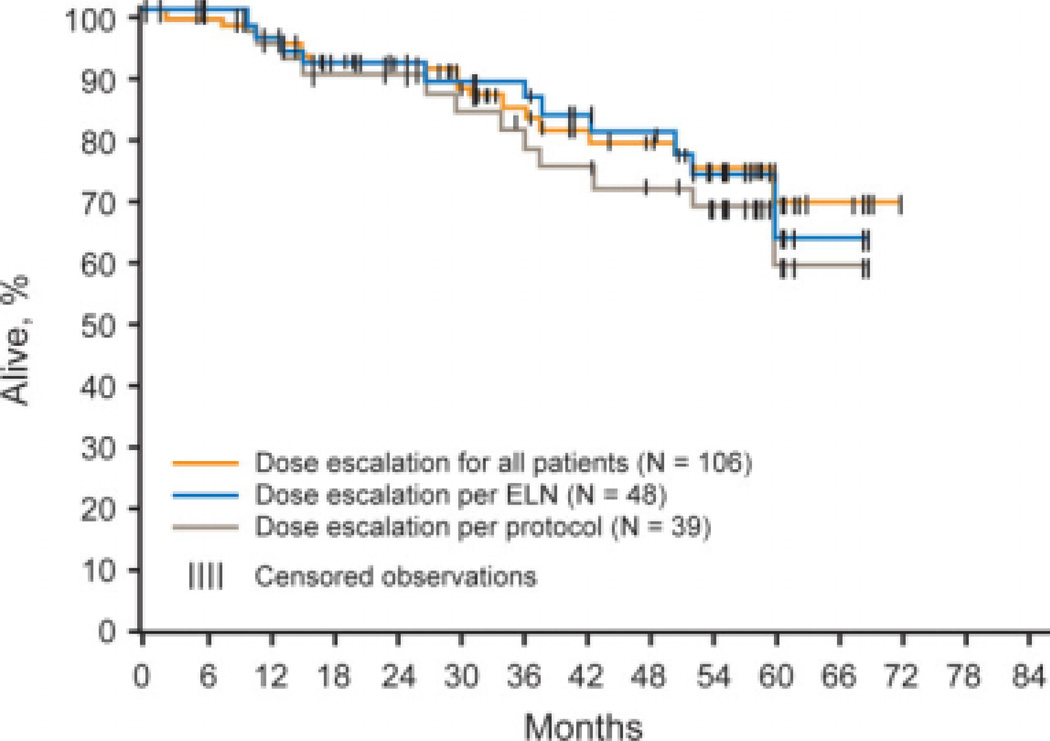
For the total population of 106 patients who had dose escalation of imatinib while on the IRIS trial, the estimated PFS rates at 12 months and 36 months after the dose escalation were 94% and 89%, respectively (Fig. 1). The estimated overall survival rates in the total group at 12 months and 36 months were 96% and 84%, respectively (Fig. 2). The PFS rates at 12 months and 36 months in the patients who had dose escalation of imatinib according to IRIS protocol criteria were 88% and 81%, respectively; and the overall survival rates at the same time points were 95% and 81%, respectively. The PFS rate among patients who had dose escalation of imatinib according to the ELN recommendations was 90% at both 12 months and 36 months; and the overall survival rates at the same time points were 96% and 89%, respectively. The 6 patients who had dose escalation of imatinib at the time of disease progression had a 2-year survival rate of 83%.
FIGURE 1.
Progression-free survival (PFS) (accelerated phase, or blast phase, or death related to chronic myeloid leukemia) for pooled cohorts of dose-escalated patients according to the International Randomized Study of Interferon and STI571 (IRIS) protocol, the European LeukemiaNet (ELN) recommendations, and for the entire patient population. Three patients were not included in the PFS analysis because they had disease progression before dose escalation.
FIGURE 2.
Overall survival for pooled cohorts of dose-escalated patients according to the International Randomized Study of Interferon and STI571 (IRIS) protocol, the European LeukemiaNet (ELN) recommendations, and for the entire patient population.
Of 106 patients who had dose increases, 42 patients (40%) remained on study treatment at the date of data cutoff (January 31, 2007), including 31 patients (29%) who were receiving doses ≥600 mg (Table 5). Seven patients (7%) crossed over to the IFN-a plus cytarabine arm because of loss of MCyR in 1 patient (1%), loss of CHR in 4 patients (4%), and increasing WBC count in 2 patients (2%). The following were reasons for discontinuation in 57 patients who had a dose escalation but did not remain on study treatment: unsatisfactory therapeutic effect (36 patients; 34%), protocol violations (4 patients; 4%), and adverse events and abnormal procedures (2 patients each; 2%). Five patients (5%) withdrew consent, and 2 patients (2%) discontinued because of administrative problems or loss of follow-up. For 6 patients (6%) ‘no longer requires study drug (BMT)’ was given as the reason, indicating that these patients underwent transplantation. Patients who discontinued imatinib because of an unsatisfactory therapeutic effect after dose escalation were on imatinib study treatment altogether for a median of 47 months (range, 8.3–72.9 months). Not all patients who discontinued imatinib because of an ‘unsatisfactory therapeutic effect’ had documented progression of disease or loss of MCyR. Patients with lack of CyR or fluctuating Phpositivity without loss of MCyR were included in the ‘unsatisfactory therapeutic effect’ category.
Table 5.
Reasons for Imatinib Discontinuation Documented for Patients Who Had Dose Escalations in the International Randomized Study of Interferon and STI571 Study (N = 106)
| Variable | No. (%) |
|---|---|
| Remained on imatinib at data cutoff | 42 (40) |
| Patients receiving ≥600 mg/d dose at cutoff | 31 (29) |
| Discontinued imatinib* | 64 (60) |
| Unsatisfactory effect† | 36 (34) |
| Protocol violation | 4 (4) |
| Adverse event(s) | 2 (2) |
| Abnormal procedure | 2 (2) |
| Withdrawal of consent | 5 (5) |
| Administrative problems/lost to follow-up | 2 (2) |
| BMT (no longer required study drug) | 6 (6) |
| Crossed over to IFN-α plus cytarabine | 7 (7) |
BMT indicates bone marrow transplantation; IFN-α, interferon alpha.
Discontinued on or before January 31, 2007.
Patients who discontinued because of unsatisfactory therapeutic effect were on imatinib study treatment for a median of 47 months (range, 8.3– 72.9 months).
On the basis of a data cutoff date of July 31, 2004, 67 patients who underwent imatinib dose escalation for a cumulative duration of at least 28 days qualified for the safety analysis. Relative to treatment with the standard daily imatinib dose of 400 mg, an increased frequency of grade 3 or 4 adverse events was observed after dose escalation to ≥600 mg daily for superficial edema (0.9% before vs 1.5% after increase), headache (0.4% vs 1.5%, respectively), abdominal pain (3.1% vs 4.5%, respectively), hemorrhage (1.3% vs 3.0%, respectively), pyrexia (0.7% vs 1.5%, respectively), anemia (3.1% vs 7.5%, respectively), and thrombocytopenia (8.2% vs 10.4%, respectively) (Table 6).
Table 6.
Grade 3 or 4 Adverse Events That Increased in Frequency After Imatinib Dose Escalation to >600 mg for 28 Days (July 31, 2004 Cutoff)
| AEs | Percentage of Patients (No.) | ||
|---|---|---|---|
| All Patients During Study, N5551 |
Before Increase to ≥600 mg Daily, N5551 |
After Increase to ≥600 mg Daily, N567/After Increase to ≥800 mg Daily, N540 |
|
| Nonhematologic AEs | |||
| Fluid retention | 2.2 | 2 | 1.5 |
| Other fluid retention | 1.1 | 1.1 | 1.1 |
| Superficial edema | 1.1 | 0.9 | 1.5 |
| Headache | 0.5 | 0.4 | 1.5 |
| Abdominal pain | 3.6 | 3.1 | 4.5 |
| Hemorrhage | 1.6 | 1.3 | 3 |
| GI hemorrhage | 0.5 | 0.2 | 3 |
| Pyrexia | 0.9 | 0.7 | 1.5 |
| Grade 3 or 4 hematologic AEs | |||
| Anemia | 4 (22) | 3.1 (17) | 7.5 (5)/7.5(3) |
| Thrombocytopenia | 9.3 (51) | 8.2 (45) | 10.4 (7)/10 (4) |
AEs indicates adverse events; GI, gastrointestinal.
DISCUSSION
Dose escalation of imatinib in patients with newly diagnosed CML-CP in the IRIS study was effective in patients who had disease that was not responding optimally to imatinib 400 mg daily. Approximately 20% of patients had dose increases from the standard imatinib dose of 400 mg daily to 600 or 800 mg daily. Of the patients who had dose escalation based on IRIS protocol criteria, 86% achieved or regained their hematologic response within 12 months of dose escalation, and 42% achieved or regained a CyR. Of the patients who had dose escalation according to the ELN recommendations, 67% achieved or regained a hematologic response within 12 months of dose escalation, and 38% achieved or regained a CyR. Three years after the dose increase, the overall rate of freedom from progression to AP and BP was 89%, and the 3-year survival rate was 84%. Most patients attained a clinical benefit within the first 12 months after dose increase.
Previous studies regarding the benefit of dose escalation of imatinib after IFN failure reached different conclusions. Some studies highlighted the durability of responses gained with imatinib dose escalation,6 whereas others suggested these responses may be brief.7,8 In a study from the University of Texas M. D. Anderson Cancer Center, 74 patients had dose escalation for unsatisfactory response. Among 51 patients who had dose escalation for cytogenetic recurrence or refractoriness, 32 patients (63%) improved their CyR to a CCyR (n = 15), a PCyR (n = 12), or a minor CyR (n = 5); these responses were sustained in 14 patients (27%). Responses were more modest and less durable in patients who were treated for hematologic recurrence.6 In a recent update of 103 patients from that same study, 49% of patients who had imatinib dose escalation for cytogenetic resistance or recurrence regained a CCyR; their estimated 2-year event-free survival rate was 85%.9 This update of the IRIS data also highlights the benefit of imatinib dose escalation in achieving better and durable responses, primarily in patients who were experiencing a suboptimal CyR or cytogenetic resistance/recurrence.
Reasons for resistance or refractoriness to imatinib therapy include, but are not limited to, over-expression of BCR-ABL, gene amplification, transporter genes (either decreased expression of the octomer-binding protein gene OCT1 or increased expression of the multidrug resistance gene MDR1), and BCR-ABL kinase domain mutations. Several of these mechanisms may cause relative resistance to imatinib, which could be overcome with dose escalation.10,11 However, some mutations may render the disease completely resistant or significantly resistant to imatinib (eg, T315I mutations; or mutations for which the 50% inhibitory concentration of imatinib is increased significantly). For these patients, a change to the second-generation tyrosine kinase inhibitors may be more beneficial than escalating the dose of imatinib.12,13
The results of this study should be interpreted in the context of the retrospective nature of the analysis and the changing criteria for suboptimal response or resistance to imatinib over the time course of the trial. Within this context, the analysis provides reasonable evidence for the benefit of imatinib dose escalation in patients with CML-CP who were experiencing a suboptimal cytogenetic response or cytogenetic relapse.
In summary, this retrospective analysis indicates that imatinib dose escalation is an effective initial strategy for patients with CML-CP who do not achieve the expected response to standard therapy. Prospective studies currently are underway to explore the role of high-dose imatinib further in the CML treatment paradigm.
Acknowledgments
Conflict of Interest Disclosures
Supported by Novartis (clinicaltrials.gov no. NCT00006343).
Dr. Kantarjian has received research grants from Novartis Pharmaceuticals Corporation and Bristol-Myers Squibb Company.
Dr. Larson has received research grants from Novartis Pharmaceuticals Corporation and Bristol Myers Squibb Company.
Dr. Guilhot has acted as a consultant and has received research support from Bristol-Myers Squibb Company and Novartis Pharmaceuticals Corporation.
Dr. O’Brien has acted as a consultant and received research support from Novartis Pharmaceuticals Corporation and Bristol-Myers Squibb Company.
Drs. Mone, Rudoltz, and Krahnke are employees of Novartis Pharmaceuticals Corporation.
Dr. Cortes has acted as a consultant and received research support from Novartis Pharmaceuticals Corporation.
Dr. Druker has acted as a consultant for the following: Ambit Biosciences, ARIAD Pharmaceuticals, Avalon Pharmaceuticals, Bioexpertise, Bristol-Myers Squibb Company, Calistoga Pharmaceuticals, Cylene Pharmaceuticals, Nodality, Portola, Roche, SGX Pharmaceuticals, Sequenom, Sofinnova Ventures, and Upstate Biotechnology. He is a stockholder in Breakthrough Therapeutics and MolecularMD and has received grant support from Novartis Pharmaceuticals Corporation and Bristol-Myers Squibb Company. He also has received royalties from the Dana-Farber Cancer Institute and is a patent owner of “Mutated ABL Kinase Domains.” Oregon Health and Science University (OHSU) and Dr. Druker have a financial interest in MolecularMD. The technology used in this research has been licensed to MolecularMD. The potential conflict of interest has been reviewed and managed by the OHSU Conflict of Interest in Research Committee and the Integrity Program Oversight Council. Dr. Druker currently is the prinicipal investigator on several Novartis and Bristol-Myers Squibb clinical trials. His institution has contracts with these companies to pay for patient costs, nurse and data manager salaries, and institutional overhead. Dr. Druker does not derive a salary nor does his laboratory receive funds from these contracts.
Appendix
Study Management Committee: Oregon Health and Science University Cancer Institute Research and Patient Care, Portland, Ore (B. J. Druker); University Hospital Poitier, Poitier, France (F. Guilhot); University of Chicago, Chicago, Ill (R. A. Larson); and University of Newcastle-upon-Tyne, Newcastle-upon-Tyne, UK (S. G. O’Brien).
Independent Data Monitoring Board: Rambam Medical Center, Haifa, Israel (J. Rowe); Wayne State University, Barbara Ann Karmanos Cancer Institute, Detroit, Mich (C. A. Schiffer); and International Drug Development Institute, Brussels, Belgium (M. Buyse).
Protocol Working Group: Policlinico San Orsola-Malpighi, Bologna, Italy (M. Baccarani); Hospital Clinical, Barcelona (F. Cervantes); Erasmus Medical Center, Rotterdam, the Netherlands (J. Cornelissen); Johannes Gutenberg Universitat, Mainz, Germany (T. Fischer); Universitat Heidelberg, Mannheim, Germany (A. Hochaus); Hanson Institute Centre for Cancer, Adelaide, Australia (T. Hughes); Medical University of Vienna, Vienna, Austria (K. Lechner); Aarhus Amtsygehus, Aarhus, Denmark (J. L. Nielsen); CHU de Bordeaux, Pessac, France (J. Reiffers); Hopital Saint Louis, Paris (P. Rousselot); San Luigi Gonzaga Hospital, Turin, Italy (G. Saglio); Vancouver Hospital, Vancouver, British Columbia, Canada (J. Shepherd); Akademiska Sjukhuset, Uppsala, Sweden (B. Simonsson); University Hospital, Basel, Switzerland (A. Gratwohl); Imperial College, London, UK (J. M. Goldman); University of Michigan Health System, Ann Arbor, Mich (M. Talpaz); Mater Misericordiae Public Hospital, Brisbane, Australia (K. Taylor); and University Hospital Gathuisberg, Leuven, Belgium (G. Verhoef).
The following investigators participated in the International Randomized Study of Interferon and STI571 (IRIS) trial: Australia: Royal Brisbane Hospital, Herston (S. Durant); Monash Medical Center, Melbourne (A. Schwarer); Sir Charles Gardner Hospital, Perth (D. Joske); Australia Leukemia and Lymphoma Group, Melbourne (J. Seymour); Royal Melbourne Hospital, Parkville (A. Grigg); St. Vincent′s Hospital, Darlinghurst (D. Ma); Royal North Shore Hospital, St. Leonards (C. Arthur); Westmead Hospital, Westmead (K. Bradstock); and Royal Prince Alfred Hospital, Sydney (D. Joshua). Belgium: A. Z. Sint-Jan, Brugge (A. Louwagi)e; Institut Jules Bordet, Brussels (P. Martiat); and Cliniques Universitaries, Yvoir (A. Bosly). Canada: McGill University, Montreal, Quebec (C. Shistok); Princess Margaret Hospital, Toronto, Ontario (J. Lipton); Queen Elizabeth II Health Sciences Center, Halifax, Nova Scotia (D. Forrest); McMaster University Medical Centre, West Hamilton, Ontario (I. Walker); Universite de Montreal, Montreal, Quebec (D-C. Roy); Cancer Care Manitoba, Winnepeg, Manitoba (M. Rubinger); Ottawa Hospital Regional Cancer Centre, Ottawa, Ontario (I. Bence-Bruckler); University of Calgary and Tom Baker Cancer Center, Calgary, Alberta (D. Stewart); London Regional Cancer Centre, London, Ontario (M. Kovacs); and Cross Cancer Center, Edmonton, Alberta (A-R. Turner). Denmark: Kobenhavns Amts Sygehus I Gentofte, Hellerup (H. Birgens); and Danish University of Pharmaceutical Sciences and University of Southern Denmark, Copenhagen (O. Bjerrum). France: Hopital Claude Huriez, Lille (T. Facon); Hotel Dieu Hospital, Nantes (J-L. Harousseau); Henri Mondor Hospital, Creteil (M. Tulliez); Centre Hospitalier Universitaire (CHU) Braboi, Vandoueuvreles Nancy (A. Guerci); Institut Paoli-Calmettes, Marseille (D. Blaise); Hopital Civil, Strasbourg (F. Maloisel); and CHU la Miletrie, Poitiers (M. Michallet). Germany: University of Regensburg, Regensburg (R. Andreesen); Krankenhaus Muenchen-Schwabig, Munich (C. Nerl); University Hospital Rostock, Rostock (M. Freund); Heinrich-Heine-Universitaet Duesseldorf, Duesseldorf (N. Gattermann); University Hospital Carl Gustav Carus, Dresden (G. Ehninger); University Hospital, Leipzig (M. Deininger); Johann Wolfgang Goethe University Hospital, Frankfurt (O. Ottmann); University Hospital Rechts der Isar, Munich (C. Peschel); University of Heidelberg, Heidelberg (S. Fruehauf); Philipps-Universitaet. Marburg (A. Neubauer); Humboldt-Universitaet, Berlin (P. le Coutre); and Robert-Bosch-Hospital, Stuttgart (W. Aulitzky). Italy: University Hospital, Udine (R. Fanin); San Orsola Hospital, Bologna (G. Rosti); Universita La Sapienza, Rome (F. Mandelli); Instituto di Ricovero e Cura a Carattre Scientifico Policlinico San Matteo, Pavia (M. Lazzarino); Niguarda Ca′Granda Hospital, Milan (E. Morra); Azienda Osperdaliera e Cliniche Univerditarie San Martino, Largo R. Benzi, Genoa (A. Carella); University of Pisa, Pisa (M. Petrini); Azienda Ospedaliera Bianchi-Malcrino-Morelli, Reggio Calabria (F. Nobile); University of Bari, Policlinico, Bari (V. Liso); Cardelli Hospital, Naples (F. Ferrara); University of Parma, Parma (V. Rizzoli); Ospedal Civile, Pescara (G. Fiortoni); and Institute of Hematology and Medical Oncology Seragnoli, Bologna (G. Martinelli). Netherlands: Vrje Universiteit Academic Medical Center, Amsterdam (G. Ossenkoppele). New Zealand: University of Auckland, Auckland (P. Browett). Norway: Medisinsk Avedling, Rikshospitalet, Oslo (T. Gedde-Dahl); Ulleval Sykehus, Oslo (J-M. Tangen); and Hvidovre Hospital, Betalende (I. Dahl). Spain: Hospital Clinic, Villarroel, Barcelona (J. Odrizoala); University of Barcelona, Barcelona (J. C. Hernandez Boulda); Hospital Universitarui de la Princesa, Madrid (J. L. Steegman); Hospital Universitario de Salamanca, Salamanca (C. Canizo); San Carlos Clinical Hospital, Madrid (J. Diaz); Institut Catala d′Oncologia, Barcelona (A. Grenena); and Hospital Lluis Alcanyis, Cta Xativa-Silla (M. N. Fernandez). Sweden: Karolinska Hospital, Stockholm (L. Stenke); Huddinge Sjukhus, Huddinge (C. Paul); Medicinkliniken Universitetssjukhuset, Obrebro (M. Bjoreman); Regionsjukhuset, Linkoping (C. Malm); Sahlgrenska Hospital, Goteborg (H. Wadenvik); Endokinsekt/Medklin Universitetssjukhuset, Lund (P-G. Nilsson); and Universitetssjukhuset Malmo University Hospital, Malmo (I. Turesson). Switzerland: Kantonsspital, St. Gallen (U. Hess); and University of Bern, Bern (M. Solenthaler). UK: University of Nottingham and Nottingham City Hospital, Liverpool (R. E. Clark); Cambridge Institute for Medical Research, Cambridge (A. R. Green); Glasgow Royal Infirmary, Glasgow (T. L. Holyoake); Manchester Royal Infirmary, Manchester (G. S. Lucas); Leeds General Infirmary, Leeds (G. Smith); Queen Elizabeth Hospital, Edgbaston, Birmingham (D. W. Milligan); Derriford Hospital, Plymouth (S. J. Rule); and University Hospital of Wales, Cardiff (A. K. Burnett). US: Walt Disney Memorial Cancer Institute, Orlando, Fla (R. Moroose); Roswell Park Cancer Center, Buffalo, NY (M. Wetzler); Gibbs Cancer Center, Spartanburg, SC (J. Bearden); Ohio State University School of Medicine, Columbus Ohio (S. Cataland); University of New Mexico Health Sciences Center, Albuquerque, NM (I. Rabinowitz); University of Maryland Cancer Center, Baltimore, Md (B. Meisenberg); Montgomery Cancer Center, Montgomery, Ala (K. Thompson); State University of New York Upstate Medical Center, Syracuse, NY (S. Graziano); University of Alabama at Birmingham, Birmingham, Ala (P. Emanuel); Hematology and Oncology Inc., Dayton, Ohio (H. Gross); Billings Oncology Associates, Billings, Mont (P. Cobb); City of Hope National Medical Center, Duarte, Calif (R. Bhatia); Cancer Center of Kansas, Wichita, Kan (S. Dakhil); Alta Bates Comprehensive Cancer Center, Berkeley, Calif (D. Irwin); Cancer Research Center of Hawaii, Honolulu, Hawaii (B. Issell); University of Nebraska Medical Center, Omaha, Neb (S. Pavletic); Columbus Community Clinical Oncology Program, Columbus, Ohio (P. Kuebler); Michigan State University Hematology/Oncology, Lansing, Mich (E. Layhe); Brown University School of Medicine, Providence, RI (P. Butra); Loyola University Medical Center, Shreveport, La (J. Glass); Duke University Medical Center, Durham, NC (J. Moore); University of Vermont, Burlington, Vt (B. Grant); University of Tennessee, Memphis, Tenn (H. Neill); University of Louisville, Louisville, Ky (R. Herzig); Sarah Cannon Cancer Center, Nashville, Tenn (H. Burris); University of Minnesota, Minneapolis, Minn (B. Petersen); Cleveland Clinic Foundation, Cleveland, Ohio (M. Kalaycio); Fred Hutchinson Cancer Research Center, Seattle, Wash (D. Stirewalt); University of Utah, Salt Lake City, Utah (W. Samlowski); Memorial Sloan-Kettering Cancer Center, New York, NY (E. Berman); University of North Carolina School of Medicine, Charlotte, NC (S. Limentani); Atlanta Cancer Center, Atlanta, Ga (T. Seay); University of North Carolina School of Medicine, Chapel Hill, NC (T. Shea); Indiana Blood and Marrow Institute, Beech Grove, Ind (L. Akard); San Juan Regional Cancer Center, Farmington, NM (G. Smith); University of Massachusetts Memorial Medical Center, Worcester, Mass (P. Becker); Washington University School of Medicine, St. Louis, Mo (S. Devine); Veterans Affairs Medical Center, Milwaukee, Wis (R. Hart); Louisiana State University Medical Center, New Orleans, La (R. Veith); Decatur Memorial Hospital, Decatur, Ill (J. Wade); Rocky Mountain Cancer Centers, Denver, Colo (M. Brunvad); Oncology-Hematology Group of South Florida, Miami, Fla (L. Kalman); Memphis Cancer Center, Memphis, Tenn (D. Strickland); Henry Ford Hospital, Detroit, Mich (M. Shurafa); University of California, San Diego, Medical Center, La Jolla, Calif (A. Bashey); Western Pennsylvania Cancer Institute, Pittsburgh, Pa (R. Shadduck); Tulane Cancer Center, New Orleans, La (H. Safah); Southbay Oncology Hematology Partners, Campbell, Calif (M. Rubenstein); University of Texas Southwest Medical Center, Dallas, Tex (R. Collins); Cancer Care Associates, Tulsa, Okla (A. Keller); Robert H. Lurie Comprehensive Cancer Center, Chicago, Ill (M. Tallman); Northern New Jersey Cancer Center, Hackensack, NJ (A. Pecora); University of Pittsburgh Medical Center, Hillman Cancer Center, Pittsburgh, Pa (M. Agha); Texas Oncology, Dallas, Tex (H. Homes); and New Mexico Oncology Hematology Consultants Albuquerque, NM (R. Guidice).
References
- 1.Druker BJ, Guilhot F, O’Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2007;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 2.Hochhaus A, Druker BJ, Larson RA, et al. IRIS 6-year follow-up: sustained survival and declining annual rate of transformation in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib [abstract] Blood. 2007;110 Abstract 25. [Google Scholar]
- 3.Kantarjian HM, Talpaz M, O’Brien S, et al. Dose escalation of imatinib mesylate can overcome resistance to standard-dose therapy in patients with chronic myelogenous leukemia. Blood. 2003;101:473–475. doi: 10.1182/blood-2002-05-1451. [DOI] [PubMed] [Google Scholar]
- 4.O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low dose cytarabine for newly diagnosed chronic phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 5.Baccarani M, Saglio G, Goldman J, et al. Evolving concepts in the management of chronic myeloid leukemia Recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006;6:1809–1820. doi: 10.1182/blood-2006-02-005686. [DOI] [PubMed] [Google Scholar]
- 6.Kantarjian H, Cortes J, O’Brien S, et al. Long-term survival benefit and improved complete cytogenetic and molecular response rates with imatinib mesylate in Philadelphia chromosome-positive, chronic-phase chronic myeloid leukemia after failure of interferon-α. Blood. 2004;104:1979–1988. doi: 10.1182/blood-2004-02-0711. [DOI] [PubMed] [Google Scholar]
- 7.Zonder J, Pemberton P, Brandt H, Mohamed A, Schiffer C. The effect of dose increase of imatinib mesylate in patients with chronic or accelerated phase chronic myelogenous leukemia with inadequate hematologic or cytogenetic response to initial treatment. Clin Cancer Res. 2003;9:2092–2097. [PubMed] [Google Scholar]
- 8.Martin D, Goldman J, Olavarria E, Apperley J. Transient benefit only from increasing the imatinib dose in CML patients who do not achieve complete cytogenetic remissions on conventional doses. Blood. 2003;102:2702–2703. doi: 10.1182/blood-2003-06-2042. [DOI] [PubMed] [Google Scholar]
- 9.Jabbour E, Kantarjian K, Atallah E, et al. Impact of imatinib mesylate dose escalation on resistance and suboptimal responses to standard-dose therapy in patients (pts) with chronic myeloid leukemia (CML) [abstract] Blood. 2007;110 Abstract 1035. [Google Scholar]
- 10.Shah NP, Tran C, Lee FY, et al. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004;305:399–401. doi: 10.1126/science.1099480. [DOI] [PubMed] [Google Scholar]
- 11.Gorre ME, Mohammed M, Ellwood K, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–880. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- 12.Hochhaus A, Kantarjian H, Baccarani M, et al. Dasatinib induces notable hematologic and cytogenetic responses in chronic phase chronic myeloid leukemia after failure of imatinib therapy. Blood. 2007;109:2303–2309. doi: 10.1182/blood-2006-09-047266. [DOI] [PubMed] [Google Scholar]
- 13.Kantarjian H, Giles F, Gattermann N, et al. Nilotinib (formerly amn107), a highly selective BCR-ABL tyrosine kinase inhibitor, is effective in patients with Philadelphia chromosome-positive chronic myelogenous leukemia in chronic phase following imatinib resistance and intolerance. Blood. 2007;110:3540–3546. doi: 10.1182/blood-2007-03-080689. [DOI] [PubMed] [Google Scholar]