SUMMARY
Changes in cellular behavior that cause epithelial cells to lose adhesiveness, acquire a motile, invasive phenotype and metastasize to secondary sites are complex and poorly understood. Molecules that normally function to integrate adhesive spatial information with cytoskeleton dynamics and membrane trafficking likely serve important functions in cellular transformation. One such complex is the Exocyst, which is essential for targeted delivery of membrane and secretory proteins to specific plasma membrane sites to maintain epithelial cell polarity. Upon loss of cadherin-mediated adhesion in Dunning R3327-5′A prostate tumor cells, Exocyst localization shifts from lateral membranes to tips of protrusive membrane extensions. Here, it co-localizes and co-purifies with focal complex proteins that regulate membrane trafficking and cytoskeleton dynamics. These sites are the preferred destination of post-Golgi transport vesicles ferrying biosynthetic cargo, such as α5-integrin, which mediates adhesion of cells to the substratum, a process essential to cell motility. Interference with Exocyst activity impairs integrin delivery to plasma membrane and inhibits tumor cell motility and matrix invasiveness. Localization of Exocyst, and by extension targeting of Exocyst-dependent cargo, is dependent on Ral GTPases, which control association between Sec5 and paxillin. Overexpression of Ral-uncoupled Sec5 mutants inhibited Exocyst interaction with paxillin in 5′A cells, as did RNAi-mediated reduction of either RalA or RalB. Reduction of neither GTPase significantly altered steady state levels of assembled Exocyst in these cells, but did change the observed localization of Exocyst proteins.
INTRODUCTION
Prostate cancer is the most commonly diagnosed cancer and is the second leading cause of cancer mortality in American males. This is a complex disease to which many genetic and environmental factors contribute. As with all carcinomas, prostate cancer results from mutations in oncogenes and tumor suppressor genes that cause sequential changes in cellular behavior. Of these changes, those causing a loss of epithelial cellular adhesion, acquisition of an invasive, motile phenotype and ability to metastasize to secondary sites are the most devastating, but also the least understood at the molecular level.
To gain a better understanding of the molecular details underlying the complex series of events leading to advanced prostate cancer, a potentially rewarding approach is to focus on proteins that function in normal epithelial cells to maintain strong intercellular adhesion and promote normal cell polarity. In this regard, E-cadherin is involved in calcium-dependent cell-cell adhesion and establishment of epithelial polarity (Yeaman et al., 1999). Loss of E-cadherin function is associated with increased cellular invasiveness and dedifferentiation of many carcinomas (Takeichi, 1993), and aggressive prostate tumors of both rat and human origin exhibit decreased E-cadherin expression (Bussemakers et al., 1992; Ross et al., 1994). In addition, E-cadherin-mediated cell-cell adhesion is important for recruitment and assembly of proteins involved in tethering post-Golgi transport vesicles to sites of membrane growth and remodeling during cell polarization (“targeting patches”) (Grindstaff et al., 1998; Yeaman et al., 1999). One important component of targeting patches is the Exocyst, a hetero-octameric protein complex first identified in budding yeast but later found to be ubiquitously expressed in eukaryotes (TerBush et al., 1996; Wang and Hsu, 2006). In budding yeast, its localization corresponds to sites of vesicle docking and fusion throughout the cell cycle (Finger and Novick, 1998; TerBush and Novick, 1995). In epithelial cells, Exocyst redistributes from cytosol to plasma membrane sites of cell-cell contact upon initiation of cadherin-mediated adhesion, and serves to ensure efficient delivery of post-Golgi transport vesicles to these sites (Grindstaff et al., 1998; Yeaman et al., 2004).
To function in tethering secretory vesicles to sites of exocytosis, Exocyst must contact both cargo-laden transport vesicles and target sites on the plasma membrane (Guo et al., 1999). Studies in yeast have shown that Exocyst holocomplex assembly involves association between plasma membrane-bound subunits that mark sites of exocytosis and vesicle-associated subunits (Boyd et al., 2004; Guo et al., 1999). Similarly, cell fractionation studies indicate that the mammalian Exocyst may be present as distinct hemicomplexes on vesicle and plasma membranes, and that holocomplex assembly mediates the tethering event (Moskalenko et al., 2003). Exocyst localization, assembly and function is regulated by at least four small GTPases representing members of the Rab, Rho, Arf and Ral subfamilies (Guo et al., 1999; Moskalenko et al., 2002; Novick and Guo, 2002; Prigent et al., 2003). Two of these, Arf6 and Ral, are associated with cell invasiveness and metastasis (Hashimoto et al., 2004; Tchevkina et al., 2005). RalA and RalB are closely related GTPases (~82% identical) that interact with two different Exocyst subunits (Sec5 and Exo84) in vitro (Jin et al., 2005; Moskalenko et al., 2003). Recent evidence suggests that each GTPase regulates different Exocyst activities in vivo (Chien et al., 2006; Lim et al., 2005; Rosse et al., 2006; Shipitsin and Feig, 2004). Ral GTPases may be activated by a family of guanine nucleotide exchange factors (RalGEFs), four of which (RalGDS, RGL1, RGL2 and Rgr) are downstream effectors of Ras (Hofer et al., 1994; Spaargaren and Bischoff, 1994). In recent years it has become increasingly clear that RalGEFs, and by extension Ral GTPases, mediate many of the prometastatic functions of oncogenic Ras mutants (Bodemann and White, 2008; Camonis and White, 2005; Feig, 2003). Although the Exocyst has been implicated in some of these activities, the exact function of this complex in tumorigenesis is far from complete.
Because polarization of epithelial cells is dependent, in part, on adhesive spatial cues directing recruitment and assembly of Exocyst to sites of membrane growth, it is of interest to determine the fate and function of this essential protein complex in cells that have lost the ability to undergo cadherin-mediated intercellular adhesion. In this study, Exocyst assembly and activity is examined in metastatic prostate tumor cells. The guiding hypothesis is that following loss of E-cadherin expression in prostatic tumor cells, Exocyst responds to distinct spatial cues, assumes a novel localization within protrusive cell extensions, and functions to direct the targeted delivery of membrane components required during invasive cell motility.
RESULTS
Loss of E-cadherin expression drives Exocyst recruitment to protrusive cell extensions in metastatic prostate tumor cells
Expression, assembly status and localization of each Exocyst subunit was examined in two clonal cell lines derived from the Dunning rat prostate tumor model (Luo et al., 1997). R3327-5′B cells express several cadherins, including E-cadherin, form monolayers in culture, are poorly invasive in vitro and are non-metastatic in vivo. In contrast, R3327-5′A cells do not express detectable levels of cadherins, exhibit a fibroblast-like morphology and have enhanced invasive and metastatic potential compared with 5′B cells. In vitro, 5′A cells are highly motile, migrating by extending long, slender cellular processes. During ECM invasion, these cells assemble circular dorsal ruffles (invadopodia) through which matrix metalloproteinases are secreted.
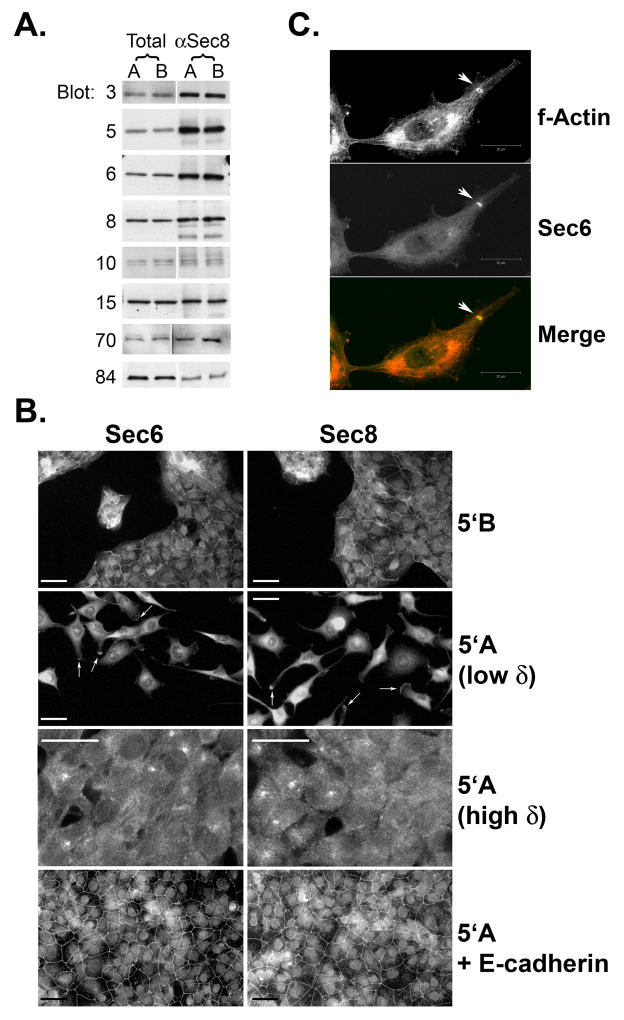
Quantitative immunoblot analysis shows that 5′A and 5′B cells express almost identical levels of each Exocyst subunit (Fig. 1A). Furthermore, quantitative immunoprecipitation with anti-Sec8 antibodies recovered equivalent amounts of each Exocyst subunit from 5′A and 5′B cells, indicating that the status of Exocyst holocomplex assembly is similar in metastatic and non-metastatic prostate tumor cells (Fig. 1A).
Figure 1. Exocyst expression, assembly and localization in non-metastatic and metastatic prostate tumor cells.
(A) Dunning rat R3327-5′A (“A”) and R3327–5′B (“B”) cells were extracted in 1% Triton X-100. Extracts were subjected to immunoprecipitation with antibodies to Sec8. Presence of Sec3, Sec5, Sec6, Sec8, Sec10, Sec15, Exo70 and Exo84 in equivalent amounts of whole cell extracts (“total”) and precipitated immune complexes (“αSec8”) was assessed by SDS-PAGE followed by immunoblotting with specific antibodies. (B) Sub-confluent cultures of R3327–5′B, R3327-5′A or R3327-5′A cells stably expressing human E-cadherin were cultured on type I collagen-coated coverslips, and then processed for immunofluorescent staining with antibodies to Sec6 or Sec8, as described in Materials and Methods. Samples were viewed with a Nikon Microphot-FX microscope (63X objective) and epifluorescent digital images were obtained using a Kodak DCS 760 digital camera. Arrows point to accumulations of Exocyst proteins in protrusive extensions of R3327-5′A cells. (C) Sub-confluent cultures of R3327-5′A cells were cultured on Matrigel-coated coverslips, and then processed for immunofluorescent staining with phalloidin (to label f-actin) and antibodies to Sec6. Arrowhead points to an accumulation of Exocyst within an actin-rich invadopodium. Scale bar = 20 μm.
Localization of endogenous Sec6 and Sec8 (Fig. 1B), as well as other Exocyst subunits (Sec3, Sec5, Sec10, Sec15, Exo70 and Exo84; data not shown), was examined in 5′A and 5′B cells by immunofluorescence microscopy. This analysis revealed striking differences in subcellular localization of Exocyst complexes in the two cell types. In non-invasive 5′B cells, Sec6 and Sec8 were associated with E-cadherin-based adherens junctions along lateral plasma membranes between adjacent cells. No labeling with Sec6/8 antibodies was observed at free, non-contacting membranes of cells. This distribution is similar to that reported previously for kidney epithelial cells (Grindstaff et al., 1998; Yeaman et al., 2004). By contrast, in 5′A cells Sec6 and Sec8 were observed to accumulate in a juxtanuclear region and also within specific sites at the cell periphery, showing especially high concentrations at distal tips of protrusive cell extensions (Fig. 1B, arrows). Tip staining was not observed in all cells. Consistent with previous findings that Exocyst recruitment to intercellular junctions is dependent on cadherin-mediated cell-cell adhesion (Yeaman et al., 2004), no immunostaining of Sec6 or Sec8 was ever observed at sites of cell-cell contact in 5′A cultures, even when cells were seeded at high density (Fig. 1B). In addition, Exocyst labeling was occasionally observed in invadopodia when 5′A cells were seeded on Matrigel-coated coverslips (Fig. 1C, arrowhead). Interestingly, when invadopodia localization was evident in a cell, Exocyst accumulation within pseudopod tips appeared to be reduced or absent.
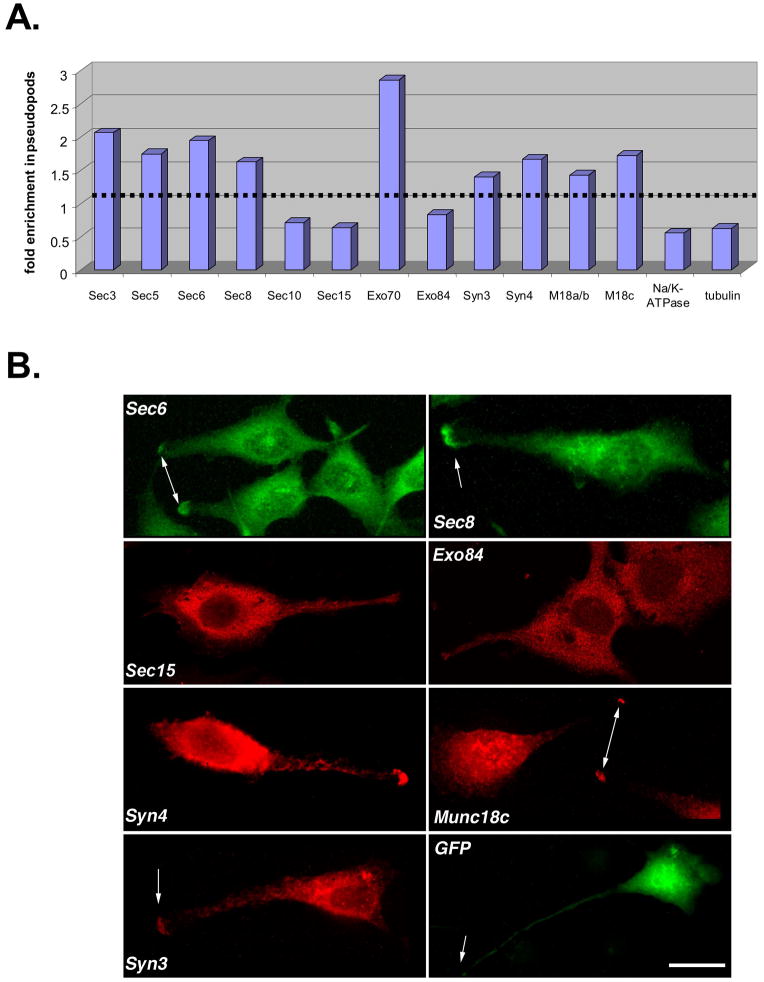
Exocyst enrichment within pseudopods was quantified using a filter assay. This is based on the finding that motile cells, when seeded on porous filter supports (3 μm pores), will attempt to migrate towards a source of chemoattractant placed in the lower chamber, but are prevented from doing so because their nuclei are too large to pass through the pores in the filter (Cho and Klemke, 2002). Hence, only protrusive cellular extensions at the leading edge of cells extend through the filter, and these can be isolated from the remainder of the cell by removing cell bodies from the top of the filter with a cotton swab. The relative enrichment of each Exocyst subunit within pseudopods was quantified and two distribution patterns were observed (Fig. 2A). Some subunits (Sec3, Sec5, Sec6, Sec8 and Exo70) were roughly 1.5–2.7-fold more concentrated within the protrusions than they were elsewhere in the cell. In contrast, other subunits (Sec10, Sec15 and Exo84) were not enriched in pseudopods. That these latter subunits were not concentrated in protrusive cell extensions was supported by immunofluorescence microscopy (Fig. 2B).
Figure 2. Exocyst subunits and SNARE proteins are enriched within protrusive cell extensions.
(A) Dunning rat R3327-5′A prostatic tumor cells were seeded on 75 mm Transwell filters (3.0 μm pore size) and medium containing 10% FBS was added basolaterally to stimulate pseudopod extension, as described in Materials and Methods. An enriched pseudopod fraction was obtained by removing cell bodies from the top of filters with a cotton swab. Indicated proteins were identified by immunoblotting with specific antibodies, and protein levels were quantified using a Molecular Dynamics Typhoon phosphorimager. To determine the fold enrichment of each protein within pseudopods, values were normalized to protein levels present in an equivalent amount of whole cell extract. (B) Distribution of endogenous Sec6, Sec8, Sec15, Exo84, Syntaxin3, Syntaxin4, Munc18c and exogenous GFP in 5′A cells. Sub-confluent cultures of R3327-5′A cells were cultured on type I collagen, and then processed for immunofluorescent staining with indicated antibodies, as described in Materials and Methods. Bound antibodies were detected with appropriate FITC or Texas Red-conjugated secondary antibodies, and epifluorescence images were obtained. Arrows point to accumulations of membrane trafficking components at the tips of pseudopods. Note that accumulations of Sec15, Exo84 and GFP were never observed within pseudopods. Scale bar = 20 μm.
If the Exocyst functions to tether post-Golgi transport vesicles to sites of membrane fusion at distal tips of pseudopods and invadopodia, then it would be anticipated that other proteins that mediate membrane fusion would also accumulate at these sites. Therefore, the localization of two plasma membrane t-SNAREs (syntaxins-3 and –4) and proteins that regulate their activities (Munc18b and Munc18c) were also examined in 5′A cells. Like the Exocyst, each of these proteins was found to localize to both a juxtanuclear region and pseudopod tips (Fig. 2B). The appearance of tip accumulation was not merely a consequence of tips being thicker than other regions of the cell periphery, because cytosolic green fluorescent protein (GFP) did not accumulate at these sites when expressed in cells (Fig. 2B). Quantification of pseudopod accumulation revealed that both t-SNAREs and their cognate Munc18 proteins were approximately 1.5-fold more concentrated within protrusions than they were elsewhere in the cell.
If loss of E-cadherin expression in invasive prostate tumor cells is responsible for redirecting Exocyst assembly to pseudopods, then it should be possible to restore lateral plasma membrane Exocyst localization by re-expressing E-cadherin in these cells. To test this prediction, clonal populations of 5′A cells stably transfected with human E-cadherin cDNA were examined. It was previously reported that ectopic E-cadherin expression was sufficient to repress the elevated invasive and metastatic potential of these cells (Luo et al., 1999). In addition, E-cadherin expression in 5′A cells drove recruitment of Exocyst complexes to intercellular adhesion sites and promoted development of epithelial morphology, similar to that observed in non-metastatic 5′B cells (Fig. 1B). Together, these results highlight an important function for E-cadherin in regulating the sub-cellular localization of the Exocyst in prostate tumor cells, and are consistent with the hypothesis that loss of E-cadherin may contribute to tumorigenesis by facilitating recruitment of Exocyst complexes into pseudopods and invadopodia.
Exocyst is associated with paxillin-containing focal complexes within protrusive cellular extensions at the leading edge of migrating prostate tumor cells
If metastatic prostate tumor cells organize Exocyst assembly within pseudopods in order to target the delivery of cargo to these sites during cell migration, then the Exocyst should be enriched at sites of newly forming cell-substratum adhesions at the leading edge of cells, but not with older cell-substratum contacts, such as focal adhesions at the center or rear of cells. In order to promote directed motility of prostate tumor cells, cultures of 5′A cells were seeded at high density and experimental wounds were introduced by scratching the culture. Behavior of cells at the wound margin was then observed.
Paxillin is a molecular scaffold that organizes signaling proteins responsible for remodeling the plasma membrane and the actin cytoskeleton to orchestrate protrusive activity required for cell motility (Turner et al., 2001). In migrating fibroblasts, paxillin is associated with newly forming focal complexes at the leading edge, as well as with stable focal adhesions at the center and rear of cells. Immunofluorescent labeling studies were performed to determine whether the Exocyst co-localizes with components of paxillin-based signaling complexes. These included paxillin itself (Fig. 3A), the Arf6 GTPase-activating protein GIT1, the SH2/SH3 adaptor protein Nck1/2, and the Rac guanine nucleotide exchange factor β-PIX (Fig. 3B). That each of these proteins was expressed by 5′A cells and that antibodies to each were specific was confirmed by Western blot analysis (Fig. 3C).
Figure 3. Exocyst co-localizes with focal complexes at pseudopod tips of migrating prostate tumor cells.
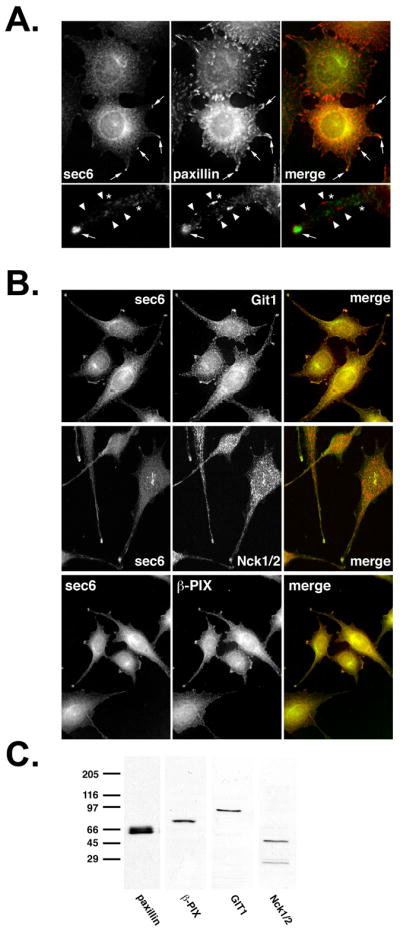
(A) Distribution of endogenous Sec6 and paxillin in R3327-5′A prostatic tumor cells. Cells were seeded on fibronectin-coated glass coverslips for 18 hr, then were fixed with 2% paraformaldehyde, permeabilized with 1% Triton X-100, incubated with mouse anti-Sec6 (mAb 9H5) and rabbit anti-paxillin antibodies, then stained with FITC-conjugated goat anti-mouse and Texas Red-conjugated donkey anti-rabbit IgG. Epifluorescence images were obtained as described in Fig. 1. Arrows point to tips of protrusive pseudopods, within which Sec6 and paxillin appear to co-localize. In lower panels, arrowheads point to structures within pseudopods that stained with anti-paxillin antibodies, but not anti-Sec6 antibodies, and asterixes indicate structures that stained with anti-Sec6 antibodies but not anti-paxillin antibodies. (B) Distribution of endogenous Sec6, Git1, β-PIX and Nck1/2 in 5′A cells. Cells were cultured, fixed and permeabilized as described in Materials and Methods. Sec6 distribution was compared to that of Git1, β-PIX and Nck1/2. Sec6/Git1 and Sec6/β-PIX images were collected by epifluorescence microscopy. Sec6/Nck1/2 images were obtained with a Zeiss confocal laser-scanning microscope (63X objective) using a krypton/argo laser with 488 nm (FITC) and 568 nm (Texas Red) laser lines. (C) Specificity of antibodies. 5′A cells were lysed and approximately 1 μg of protein was loaded per lane onto a 10% SDS-PAGE gel. The proteins were transferred to Immobilon PVDF membranes and incubated with rabbit polyclonal antibodies to paxillin, GIT1 or Nck1/2, or mouse mAb to β-PIX. Blots were then probed with HRP-conjugated secondary antibodies and developed for ECL detection.
Paxillin, GIT1, Nck1/2 and β-PIX were all enriched at pseudopods tips, similar to the Exocyst (Fig. 3). Each of these proteins was also present at juxtanuclear sites. Close inspection of cells by confocal microscopy revealed that Sec6 and paxillin were co-localized at distal tips of pseudopods at the leading edge of migrating cells at the wound margin (Fig. 3A, lower panels, arrows), but that behind these sites, more proximal to cell bodies, the two proteins had distinct distributions (Fig. 3A, lower panels, arrowheads and asterixes). Importantly, paxillin was also enriched within adhesion sites elsewhere in the cell, but Sec6 was not enriched at these sites. Paxillin-associated signaling proteins were, like Sec6, enriched within pseudopods tips but did not appear to accumulate within other paxillin-positive structures (Fig. 3B). Therefore, these results indicate that Exocyst is enriched at sites containing newly forming cell-substrate focal complexes at the leading edge of migrating cells, but is absent from older contact sites elsewhere in cells.
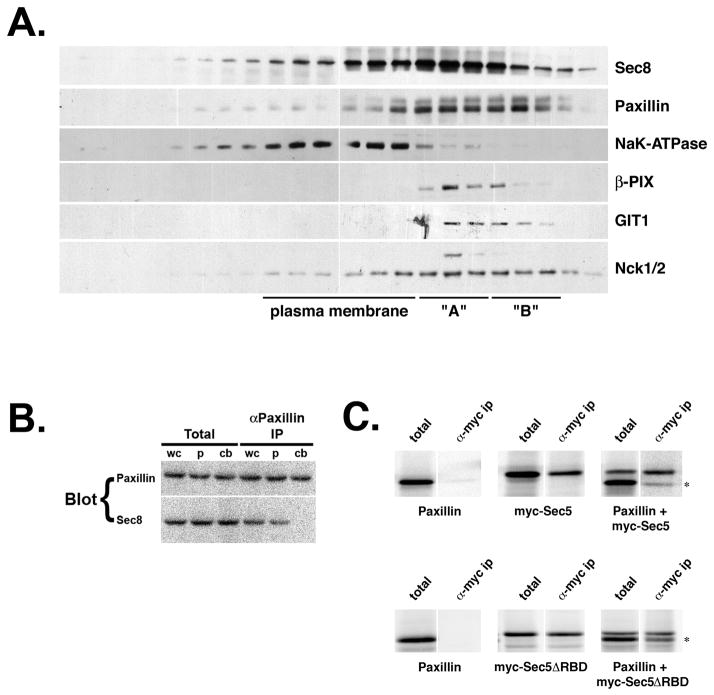
Paxillin, β-PIX, GIT1 and Nck1/2 co-fractionate with Sec8 in density gradients of post-nuclear supernatant obtained from mechanically homogenized 5′A prostate tumor cells (Fig. 4A). Analysis of gradient fractions by western blotting with specific antibodies reveals a peak of Sec8 in fractions corresponding to a density (δ) of ~1.15 – 1.17 g/ml (Fig. 4A, peak “A”). Thus, Sec8 is associated with membranes that have a higher density than the bulk of plasma membrane, as defined by the presence of sodium-potassium ATPase (Fig. 4A, NaK-ATPase, peak δ ~1.10 – 1.13 g/ml). A fraction of paxillin was recovered in the Sec8-containing peak, although a second peak of paxillin was recovered in higher density fractions that did not contain a peak of Sec8 (Fig. 4A, peak “B”). Importantly, β-PIX and GIT1 were primarily recovered within the Sec8-containing fractions, and not with the higher density paxillin-containing fractions. The SH2/SH3 adaptor Nck1/2 was found to co-fractionate with both paxillin-containing peaks, but a slightly larger (~47 kDa) protein that was detected by anti-Nck1/2 antibodies was present exclusively in fractions that contained Sec8. This band appears to represent a phosphorylated form of Nck1/2, because treatment of fractions with alkaline phosphatase caused it to disappear (data not shown). These results indicate that Sec8 co-fractionates in density gradients with membranes containing paxillin and associated proteins involved in regulating vesicle trafficking and cytoskeleton dynamics.
Figure 4. Exocyst is associated with paxillin-containing complexes within pseudopods of migrating prostate tumor cells.
(A) Fractionation of R3327-5′A cells in iodixanol gradients. 5′A cells were homogenized in a ball bearing cell cracker. Post-nuclear supernatant was fractionated by isopycnic centrifugation through five-step iodixanol gradients, as described in Materials and Methods. Fractions (0.5 ml) were collected and densities determined with a refractometer. Presence of Sec8, paxillin, NaK-ATPase α subunit, β-PIX, GIT1 and Nck1/2 in gradient fractions was assayed by SDS-PAGE followed by immunoblotting with specific antibodies. Protein levels were quantified using a Molecular Dynamics Phosphorimager. Fractions corresponding to peak levels of NaK-ATPase, Sec8 and paxillin are labeled “plasma membrane”, “A” and “B”, respectively. (B) Co-immunoprecipitation of Sec8 with paxillin from isolated 5′A pseudopods. 5′A cells were cultured on 75 mm Transwell filters (3 μm pores), and induced to extend pseudopods, as described for Fig. 2. Whole cells (“wc”), isolated pseudopods (“p”) or cell bodies (“cb”) were isolated in CSK buffer after rubbing either the top or bottom of filters with a cotton swab, as appropriate. Extracts (“total”) and precipitated immune complexes (“αPaxillin IP”), normalized to total protein content, were assessed by SDS-PAGE followed by immunoblotting with specific antibodies to paxillin or Sec8. Note that Sec8 co-precipitates with paxillin immune complexes, but only from pseudopods and not cell bodies. (C) Sec5 binds paxillin in vitro. Plasmids encoding paxillin and/or myc-Sec5 or Sec5 lacking its Ral-binding domain (myc-Sec5ΔRBD) were used to prime coupled transcription and translation reactions in the presence of [35S]methionine/cysteine. Aliquots of translation products were assessed directly (“total”) or after immunoprecipitation with anti-myc antibodies (“α-myc ip”). Bands representing paxillin that co-immunoprecipitated with Myc-tagged Sec5 are indicated (asterixes).
Results of density gradient fractionation suggest that fractions containing the peak of Sec8 (Fig. 4A, peak “A”) may contain pseudopod tip complexes, whereas fractions containing the peak of paxillin (Fig. 4A, peak “B”) may contain older focal adhesion complexes that do not contain Exocyst proteins. To confirm the prediction that Exocyst associates preferentially with focal complexes at the leading edge of migrating cells, paxillin-containing protein complexes were immunoprecipitated from cell bodies or pseudopods isolated from 5′A cells induced to undergo frustrated chemotaxis, as described for Fig. 2. This analysis confirmed that Exocyst complexes were only associated with paxillin-containing complexes isolated from pseudopods, and not with more abundant paxillin complexes associated with other cellular structures (Fig. 4B).
To determine whether the Exocyst directly binds paxillin, and to identify which subunit is responsible for this interaction, each individual Exocyst subunit was expressed together with paxillin in coupled in vitro transcription/translation reactions. This analysis revealed a direct interaction between Sec5 and paxillin (Fig. 4C). None of the other Exocyst subunits associated with paxillin in this assay (data not shown). This interaction was independent of the amino terminal Ral-binding domain of Sec5, indicating that paxillin binds a different part of Sec5 than that involved in binding Ral GTPases (Fig. 4C).
Biosynthetic cargo is delivered to Exocyst-containing pseudopods
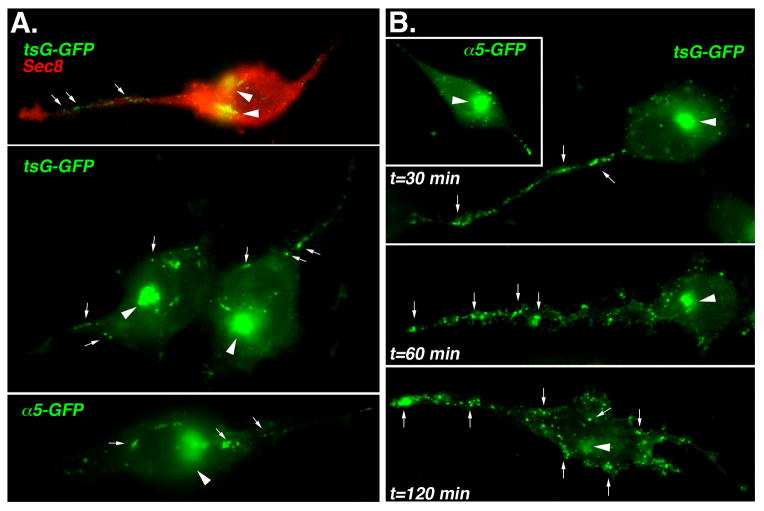
Morphological transport assays were performed to determine whether exocytic cargo is preferentially targeted to Exocyst-enriched sites in pseudopods. For these studies, cells were transfected with plasmids encoding GFP-tagged forms of either α5-integrin (α5-GFP) or a temperature-sensitive mutant of the vesicular stomatitis virus (VSV) G protein (tsG-GFP). The latter protein is a convenient marker of exocytosis because its transport through the secretory pathway can be manipulated by shifting the temperature at which cells are cultured. Following transfection, cells were incubated at 39.5 °C for 4 hours to accumulate tsG-GFP in the endoplasmic reticulum, then shifted to 19 °C for two hours to allow the protein to accumulate within the trans-Golgi network (TGN). Finally, cells were shifted to 32 °C for various lengths of time to permit protein trafficking from the TGN to the plasma membrane. In cells fixed after incubation at 32°C for 30 minutes, much of the GFP-tagged cargo remained in perinuclear compartments (Fig. 5A, arrowheads). In addition, post-Golgi transport intermediates containing tsG-GFP or α5-GFP were observed in the cytoplasm at some distance from the perinuclear vicinity, and these appeared to be enriched in pseudopods compared with other parts of the cell (Fig. 5A, arrows).
Figure 5. Polarized trafficking of biosynthetic cargo to pseudopods in prostate tumor cells.
R3327-5′A cells were transfected with plasmids encoding GFP-VSVG (tsG-GFP) or GFP α5-integrin (α5-GFP) and morphological transport assays were performed as described in Materials and Methods. Following accumulation of cargo proteins in the TGN, cultures were shifted to 32 °C either in the absence (A) or presence (B) of 0.5% tannic acid for various lengths of time to facilitate the trafficking of accumulated GFP-fusion proteins from the TGN to the plasma membrane. Following 30 min at 32 °C (A) or indicated times (B), cells were fixed with 2 % paraformaldehyde, permeabilized and stained with anti-Sec8 mAb, which was detected using a Texas Red-conjugated secondary antibody. Epifluorescence images were obtained as described in Fig. 1. Arrowheads point to TGN and arrows point to examples of post-TGN transport intermediates. Note that more post-TGN transport vesicles appear to be delivered to pseudopods than to other parts of the cell, even when the TGN is located on the opposite side of the nucleus from the pseudopod. In tannic acid pre-fixed samples (B), pseudopods accumulate transport vesicles, but with prolonged incubation vesicles also begin to accumulate within the cell body.
Upon docking and fusion with the plasma membrane, it is no longer possible to observe accumulation of GFP-labeled transport vesicles in the cytoplasm. It could be argued, therefore, that post-Golgi transport intermediates only appear to be targeted to pseudopods because they have farther to travel than do intermediates that dock and fuse within the cell body. Therefore, terminal docking and fusion events were impaired by application of the non-permeable fixative tannic acid to cells in order to more definitively resolve whether exocytosis was polarized towards pseudopods. Tannic acid crosslinks proteins at the plasma membrane, thereby “freezing” the vesicle fusion machinery (Polishchuk et al., 2004). However, cytoplasmic events, including post-Golgi vesicle budding and transport to the cell periphery proceed unimpeded in the presence of tannic acid for at least one hour. Under these conditions, a clear accumulation of both tsG-GFP and α5-GFP-containing transport intermediates was observed within pseudopods (Fig. 5B). After prolonged incubation at 32 °C, vesicles began to accumulate within the cell body, indicating either that pseudopods had exceeded their capacity for vesicles or that transport machinery was failing due to extended exposure to tannic acid. Nevertheless, these results indicate that exocytosis is polarized towards Exocyst-enriched tips of pseudopods in 5′A prostate tumor cells.
Exocyst assembly within pseudopods is required for exocytosis and invasive cell motility
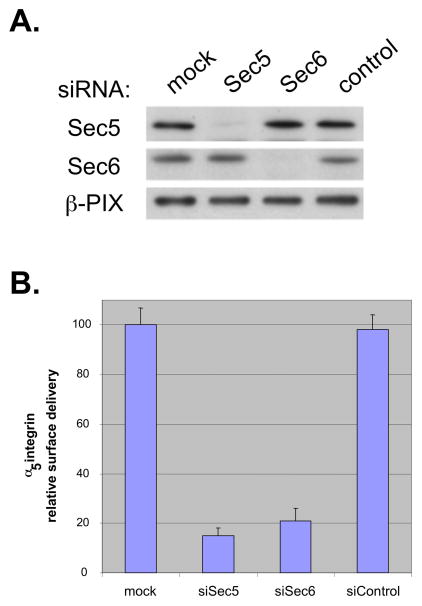
To determine whether the Exocyst is required for biosynthetic protein trafficking in metastatic prostate tumor cells, the efficiency of delivery of newly synthesized α5-integrin to the surface of 5′A cells that had normal or reduced levels of Sec5 or Sec6 was compared. Cells were transfected with siRNAs specific for Sec5 or Sec6 or a control siRNA. 24 hr later, cells were transfected with plasmid encoding α5-GFP, then returned to the incubator for another 24 hr. On the day of the experiment (48 hr after siRNA transfection), one set of cultures was lysed and analyzed for expression of Sec5, Sec6 or a loading control (β-PIX) by Western blotting (Fig. 6A), and another set was used to quantify α5-GFP trafficking (Fig. 6B). Triplicate wells containing either control or Exocyst knock-down cells were subjected to pulse-chase and surface biotinylation analysis to quantify the amount of newly synthesized α5-GFP that arrived at the surface during a one hour delivery period. This revealed that the efficiency of delivery of newly synthesized α5-GFP to the plasma membrane of 5′A cells was significantly reduced following RNAi-mediated reduction of Sec5 or Sec6 expression. The amount of α5-integrin that was synthesized in control and experimental cultures was similar (data not shown), but the fraction that was inserted into the plasma membrane of cells with reduced Sec5 or Sec6 levels was reduced to approximately 18 – 22 % of the levels observed in mock-transfected cells or in cells transfected with control siRNA. Therefore, Exocyst function is required for trafficking of newly synthesized α5-integrin from the TGN to the plasma membrane of prostate tumor cells.
Figure 6. Exocyst is required for exocytosis of newly synthesized α5-integrin in prostate tumor cells.
(A) RNAi mediated reduction of Sec5 and Sec6 expression. R3327-5′A were transfected with either nothing (“mock”) or with siRNAs targeting Sec5, Sec6 or a control non-targeting siRNA (“control”), as described in Materials & Methods. 60 hr post-transfection, cells were lysed and lysates were analyzed by SDS-PAGE and immunoblotting for Sec5, Sec6 and β-PIX. Protein levels were quantified using a Molecular Dynamics Typhoon phosphorimager. (B) Metabolic pulse-chase and surface biotinylation analysis of α5 integrin trafficking in prostate tumor cells. Delivery of newly synthesized α5-GFP to the surface of R3327-5′A cells was assessed as described in Materials and Methods. Experiments were performed twice, each time with triplicate wells of cells. Relative surface delivery was defined as the mean signal obtained from three replicate biotinylated α5-GFP bands, normalized to the mean of the total α5-GFP recovered in the initial immunoprecipitates.
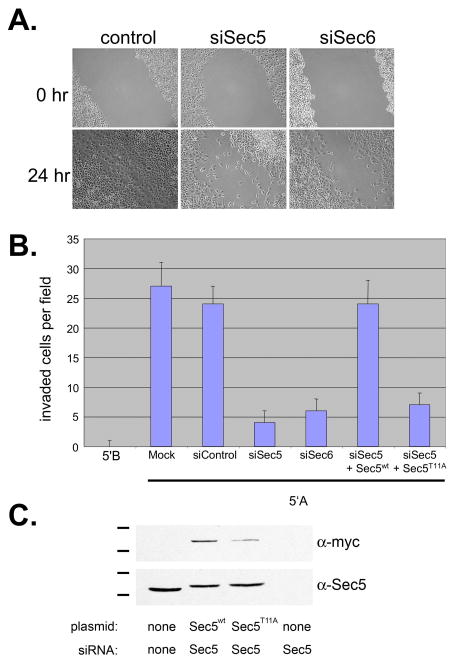
Because Exocyst complexes are enriched within pseudopods and invadopodia and are required for exocytosis of α5-GFP-laden transport vesicles, it is reasonable to expect that Exocyst assembly within pseudopods and invadopodia is required for invasive cell motility. Wound healing experiments were performed initially, to determine whether Exocyst assembly is required for directed motility of 5′A prostate tumor cells. Following RNAi-mediated reduction of either Sec5 or Sec6, cells were significantly impaired in their ability to close wounds relative to control cells (Fig. 7A). 24 hr after wounding monolayers, control monolayers had completely healed, whereas wounded monolayers of cells with reduced Sec5 or Sec6 levels had closed wounds to only ~ 35–40% of the controls. Scattered individual cells were observed within the wounds in these cultures, but coordinated migration of the population was impaired following Sec5 or Sec6 knockdown.
Figure 7. Ral-coupled Exocyst activity is required for invasive motility of prostate tumor cells.
(A) Wound healing assay. Confluent monolayers of R3327-5′A cells, transfected with siRNAs specific for Sec5, Sec6 or a control non-targeting siRNA, were experimentally wounded by scratching, and analysis was performed as described in Materials & Methods. (B) Matrigel invasion assay. Non-metastatic R3327-5′B cells, or metastatic R3327-5′A cells transfected with indicated siRNAs and rescue constructs, were seeded on Matrigel-coated Transwell filters. Invasion assays were performed as described in Materials & Methods. (C) Sec5 expression analysis. 5′A prostate tumor cells transfected with indicated siRNAs and/or rescue constructs, were extracted and analyzed by SDS-PAGE and immunoblotting with antibodies to Sec5 (endogenous and ectopic proteins detected) or c-myc (ectopic Sec5 detected).
A hallmark of metastatic tumor cells is their ability to degrade basal laminae and subjacent connective tissue, enter the bloodstream and migrate to distant sites in the body. To determine whether Exocyst function is required for the invasive motility of 5′A prostate tumor cells, the ability of cells to migrate across Matrigel-coated filters was assayed (Fig. 7B). Non-metastatic 5′B prostate tumor cells were not able to degrade Matrigel and so remained on the upper surface of filters during invasion assays. In contrast, a significant number of metastatic 5′A cells were able to degrade Matrigel and migrate across filters during a 24 hr incubation (Fig. 7B). In 5′A cells transfected with Sec5 or Sec6 siRNAs, but not with control siRNA, invasiveness was impaired and cells remained in the upper chamber, similar to non-metastatic 5′B cells (Fig. 7B). Co-transfection of cells with Sec5 siRNA and a plasmid encoding myc-tagged Sec5 harboring a silent mutation to render the mRNA resistant to RNAi-mediated silencing restored Sec5 expression (Fig. 7C) and rescued ECM invasiveness to levels comparable to control 5′A cells (Fig. 7B).
Ral GTPases regulate Exocyst association with paxillin-containing focal complexes and polarized growth of protrusive cellular extensions
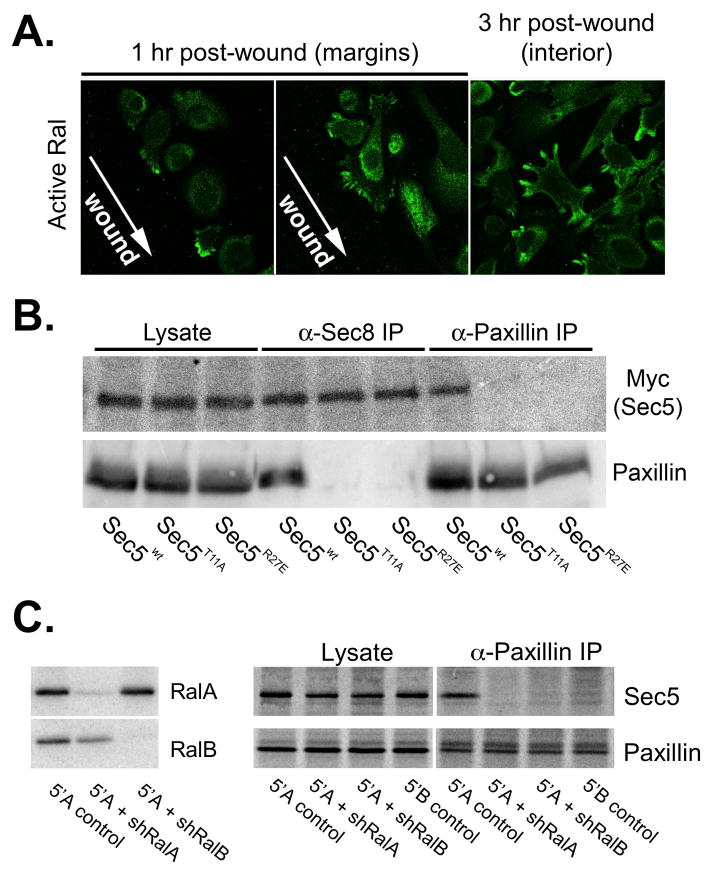
Ral GTPases directly bind Sec5 and Exo84 and somehow regulate Exocyst function, either by controlling holocomplex assembly or local accumulation at sites of membrane remodeling (Camonis and White, 2005). Because Sec5 is required for polarized trafficking and invasive motility of metastatic prostate tumor cells, and in vitro binding studies suggest that Sec5 mediates Exocyst association with paxillin, we sought to determine whether Ral binding to Sec5 is required for invasive motility, and whether this involves modulating either Exocyst assembly or association with paxillin. Similar to findings in other cell types (Takaya et al., 2004), active Ral GTPases were found to be enriched within protrusive cellular extensions at the leading edge of motile prostate tumor cells and, to a lesser extent, in a perinuclear compartment (Fig. 8A). Thus, Ral GTPases are positioned to regulate Exocyst activites at two sites in these cells.
Figure 8. Ral GTPases are required for Exocyst association with paxillin in cells.
(A) Localization of active Ral following wounding of prostatic tumor cells. Cells were transfected with X-Press-tagged Exo84 Ral-binding domain and monolayers were wounded by scratching. Active Ral GTPase localization was determined by immunofluorescence staining with anti-X-Press antibodies at indicated time points. (B) Co-immunoprecipitation of Exocyst and paxillin is dependent on Ral-binding capability of Sec5. Cells were transfected with plasmids encoding myc-tagged Sec5 (wild type (wt) or Ral-uncoupled mutants (T11A or R27E)). Cells were extracted in 1% Triton X-100 and extracts were subjected to immunoprecipitation with antibodies to Sec8 or paxillin. Presence ectopic myc-Sec5 and paxillin in equivalent amounts of whole cell extracts (“lysate”) and precipitated immune complexes was assessed by SDS-PAGE followed by immunoblotting with specific antibodies. (C) RNAi mediated reduction of RalA and RalB expression. R3327-5′A were infected with recombinant lentiviruses coding shRNAs specific for RalA or RalB. Stable clones of cells were selected in puromycin and assessed for RalA and RalB expression by immunoblotting with specific antibodies. (D) R3327-5′B, R3327–5′A or R3327–5′A cells expressing shRNAs targeting RalA or RalB were extracted in 1% Triton X-100. Extracts were subjected to immunoprecipitation with antibody to paxillin. Presence Sec5 and paxillin in equivalent amounts of whole cell extracts (“lysate”) and precipitated immune complexes (“α-paxillin IP”) was assessed by SDS-PAGE followed by immunoblotting with specific antibodies.
Ral binding to Sec5 is required for invasive motility of aggressive prostate tumor cells. Expression of a Sec5 variant harboring a point mutation that disrupts binding to Ral (Sec5T11A) (Fukai et al., 2003) failed to rescue matrix invasiveness following siRNA-mediated reduction of endogenous Sec5 (Fig. 7B & 7C). Neither Sec5T11A nor Sec5R27E, a second Ral-uncoupled variant, bound paxillin when expressed in 5′A cells, although both proteins were incorporated into Exocyst complexes that could be recovered by immunoprecipitation with anti-Sec8 antibodies (Fig. 8B). It is unlikely that either mutation disrupts paxillin binding directly, as the Ral-binding domain of Sec5 was dispensable for paxillin binding in vitro (Fig. 4C). However, incorporation of Ral-uncoupled ectopic Sec5 subunits into endogenous Exocyst complexes blocked the association between paxillin and Exocyst in 5′A prostate tumor cells, as shown by the failure to co-immunoprecipitate paxillin with Sec8 in cells expressing either Sec5T11A or Sec5R27E (Fig. 8B).
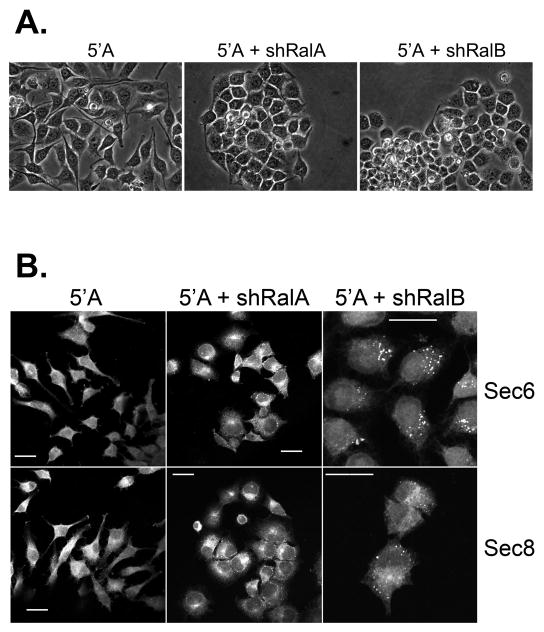
To gain further insight into the mechanism by which Ral GTPases regulate Exocyst-dependent activities in metastatic prostate tumor cells, and to identify which Ral GTPase (RalA or RalB) is required for this regulation, loss-of-function studies were performed. Lentiviral expression of specific shRNAs efficiently reduced expression of either RalA or RalB in 5′A cells (Fig. 8C). Surprisingly, RNAi-mediated knockdown of either GTPase inhibited association of Exocyst complexes with paxillin (Fig. 8C). Examination of cells revealed that reduction of either RalA or RalB impaired the ability of cells to extend long, slender pseudopods associated with invasive motility (Fig. 9A). Cells with reduced expression of either GTPase were observed to grow as cohesive colonies, and only rarely were cells witnessed to detach from and migrate away from these colonies. These results indicate that both RalA and RalB contribute to altered cellular morphogenesis and motility in prostatic tumor cells, and that targeting of Exocyst complexes to paxillin-containing focal complexes requires inputs from both GTPases.
Figure 9. Ral GTPases are required for localization of Exocyst to protrusive cell extensions.
(A) Morphology of R3327–5′A or R3327–5′A cells expressing shRNAs targeting RalA or RalB. Note that RNAi mediated reduction of either RalA or RalB suppressed polarized growth of protrusive cell extensions, and cells tended to grow as more tightly compacted colonies than control R3327–5′A prostate tumor cells. (B) Localization of Sec6 and Sec8 in prostate tumor cells is altered following reduction of RalA or RalB expression. R3327–5′A cells or R3327–5′A cells expressing shRNAs targeting RalA or RalB were fixed and labeled with antibodies against Sec6 or Sec8. Note that Exocyst proteins are concentrated in perinuclear compartments, similar to those observed in parental 5′A cells, when RalA expression is reduced. In contrast, these Exocyst subunits accumulate in large cytoplasmic vesicles in cells when RalB expression is reduced.
RNAi-mediated knockdown of either GTPase did not significantly reduce the amount of assembled Exocyst in 5′A prostate tumor cells, as determined by co-immunoprecipation of three subunits (Sec5, Sec15 and Exo84) with Sec8 (Fig. S1). However, reduction of Ral expression altered the subcellular distribution of Exocyst complexes in distinct ways. In cells with reduced expression of RalA, Sec6 and Sec8 were primarily present within a perinuclear compartment, whereas in cells with reduced expression of RalB these proteins accumulated in large cytoplasmic vesicles (Fig. 9B). Therefore, Ral GTPases appear to regulate subcellular localization, rather than assembly of Exocyst complexes in 5′A prostate tumor cells.
DISCUSSION
Mechanisms that regulate assembly and recruitment of the Exocyst to sites of active membrane remodeling are complex and poorly understood. Recent work in both budding yeast and mammalian cells has highlighted an essential role for protein-lipid interactions involving Sec3 and Exo70 subunits with phosphatidylinositol 4,5-bisphosphate (He et al., 2007; Liu et al., 2007; Zhang et al., 2008). While such interactions appear to be essential for overall membrane binding, they are not sufficient to account for the highly localized enrichment of Exocyst complexes often observed in polarized cells. For example, in renal epithelial cells the Exocyst is concentrated within a relatively narrow region of the lateral plasma membrane, just below the tight junction. Exocyst accumulation at this site requires components of both calcium-dependent (E-cadherin) and calcium-independent (nectin) cell adhesion systems (Yeaman et al., 2004). The importance of E-cadherin-mediated cell-cell adhesion in regulating membrane recruitment and assembly of Exocyst on lateral plasma membranes is supported by the current study, because ectopic expression of E-cadherin in metastatic prostate tumor cells was sufficient to restore assembly of intercellular junctions and return Exocyst complexes to these sites. These molecular events are associated with suppression of invasive motility in vitro and metastasis in vivo.
Although mechanisms that sort Exocyst complexes into cadherin-based intercellular junctions are incompletely understood, it is likely that interactions with multidomain scaffolding proteins, such as SAP97 and CASK, which assemble on lateral plasma membranes of epithelial cells, participate in this process. Interactions between PDZ domains on these or related proteins and a C-terminal PDZ target on Sec8 have been shown in neurons (Sans et al., 2003), adipocytes (Inoue et al., 2006) and oligodendrocytes (Anitei et al., 2006), but their significance in sorting Exocyst components in epithelial cells has not been established.
In cells lacking cadherin-mediated adhesion, distinct spatial cues likely mediate recruitment and assembly of Exocyst complexes at sites of membrane remodeling. In the metastatic prostate cancer cells examined here, Exocyst components were enriched within protrusive cellular extensions at the leading edge of motile cells, and were occasionally observed in invadopodia. Paxillin likely represents one important spatial determinant for Exocyst localization in these cells. Exocyst components co-localized, co-fractionated and co-immunoprecipitated with paxillin from leading edge complexes. Although molecular specifics of this interaction not yet known, it is noteworthy that only the Sec5 subunit was observed to interact with paxillin in vitro. This in vitro interaction did not require Ral GTPases nor did it involve the Ral-binding domain of Sec5. However, Ral binding to Sec5 was necessary to facilitate Exocyst binding to paxillin in cells. This conclusion is based on findings that overexpression of Ral-uncoupled Sec5 mutants inhibited Exocyst interaction with paxillin in 5′A cells, as did RNAi-mediated reduction of either RalA or RalB. Importantly, neither reduction of RalA nor RalB significantly altered the steady state level of assembled Exocyst complex in these cells, but the localization of Exocyst proteins was somewhat different when either GTPase was absent. In the absence of RalA, Sec6 and Sec8 accumulated in a perinuclear compartment, whereas depletion of RalB resulted in accumulations of Sec6 and Sec8 in large vesiculated structures in the cytoplasm. Therefore, Ral GTPases appear to function in spatial regulation of Exocyst function, rather than Exocyst assembly per se, during tumor cell motility. Moreover, RalA and RalB may regulate different aspects of Exocyst function in the perinuclear compartment and plasma membrane.
A previous study showed that Ral was selectively activated at the leading edge of motile epithelial cells (Takaya et al., 2004). We also observed activation of Ral at the leading edge of motile prostate tumor cells. Therefore, it seems plausible that Ral facilitates Sec5-paxillin interactions specifically at the leading edge of migrating cells and that this contributes to Exocyst enrichment and polarized exocytosis at these sites. Interestingly, in non-invasive 5′B cells, which express both E-cadherin and paxillin, Exocyst is found associated with lateral membrane cell-cell contacts and is not associated with paxillin. This implies that a hierarchy of spatial cues and protein-protein interactions controls the spatial distribution of Exocyst complexes within these cells.
Several reports have highlighted distinct functions for RalA and RalB in tumorigenic transformation (Bodemann and White, 2008; Camonis and White, 2005). There appear to be cell type specific activities associated with each GTPase, even within the relatively narrow biological context of cell migration. Loss-of-function analyses have shown that RalB, but not RalA, is limiting for migration of normal rat kidney cells in wound healing assays (Rosse et al., 2006), as well as human bladder and prostate cancer cells in transwell migration assays (Oxford et al., 2005). Interestingly, in both of these studies reduction of RalA expression reversed the inhibitory effect of RalB knockdown, suggesting that RalA and RalB might serve antagonistic functions in cell migration. In contrast to these reports, other studies have highlighted a role for RalA in chemotactic migration of C2C12 skeletal myoblasts (Suzuki et al., 2000), and human bladder cancer cell lines (Gildea et al., 2002), and in signaling to the Exocyst during neurite branching (Lalli and Hall, 2005). The data presented here implicate both RalA and RalB in signaling Exocyst recruitment to paxillin-containing complexes during polarized growth of protrusive cellular extensions. Thus, our data are consistent with previous studies in human pancreatic carcinoma cells, which showed that both RalA and RalB are required for invasive motility (Lim et al., 2006).
How might Ral activation and Exocyst recruitment to pseudopod tip complexes contribute to cell polarization during invasive motility? First, the vesicle tethering function of the Exocyst is likely to be required for delivery of specific components as well as bulk membrane from the trans-Golgi network (TGN), recycling endosomes or other internal stores to the growing protrusive cell extension. Results presented here show that Exocyst function is required for efficient exocytosis of newly synthesized α5-integrin. This is consistent with results of TIRF microscopy, which revealed that fusion of vesicles carrying newly synthesized LDL receptor, an Exocyst-dependent cargo molecule (Grindstaff et al., 1998), occurs predominantly at the leading edge of migrating NRK cells (Schmoranzer et al., 2003). In addition, directed motility of Swiss 3T3 fibroblasts was impaired by expression of dominant-negative protein kinase D mutant (Prigozhina and Waterman-Storer, 2004), which has been shown to impair post-TGN membrane trafficking and Exocyst recruitment to the plasma membrane (Liljedahl et al., 2001; Yeaman et al., 2001).
It is also likely that other Exocyst-specific functions, distinct from a principle role in vesicle tethering, contribute to its activity in mediating invasive motility of tumor cells. One such function is to coordinate cytoskeleton remodeling with vesicle trafficking. In one of the earliest papers describing a connection between Ral and the Exocyst, it was suggested that Sec5 mediates Ral-induced filopodia formation via a mechanism that is distinct from Exocyst-dependent membrane trafficking processes (Sugihara et al., 2002). Overexpression of Exo70 has been reported to promote filopodia formation in NRK cells (Wang et al., 2004), and a similar phenotype has been seen in R3327-5′A cells (data not shown). In addition, Exo70 was recently shown to interact with the Arp2/3 complex and to regulate its activity within nascent lamellipodia of migrating NRK cells (Zuo et al., 2006). Given that the overall dimensions of a fully assembled octameric Exocyst holocomplex exceeds 13 nm × 30 nm (Hsu et al., 1998), this provides a large interface to accommodate numerous protein-protein interactions that might serve to integrate Exocyst-dependent vesicle tethering with other processes involved in cellular morphogenesis. Considering the pace with which novel Exocyst-interacting partners are being identified, this seems likely to be the case.
MATERIALS AND METHODS
Reagents
Mouse monoclonal antibodies against Sec6 (9H5 and 10C3) and Sec8 (2E9, 2E12, 5C3, 8F12 and 10C2) have been described previously (Hsu et al., 1996; Kee et al., 1997). Mouse monoclonal antibodies against Sec5 and Exo84 were generously provided by Dr. Richard Scheller (Genentech). Rabbit polyclonal antibodies against Sec3, Sec5, Sec10, Sec15, Exo70 and Exo84 were generated by Covance and have been described previously (Yeaman, 2003). Rabbit polyclonal antibodies against the conserved cytoplasmic domain of mouse E-cadherin (E2), α-catenin, and the α subunit of the sodium, potassium ATPase (NaKα) have been described previously (Marrs et al., 1993). Rabbit anti-Munc18c was generously provided by Dr. David James (Garvan Institute, Australia). Rabbit polyclonal antibodies to RalB (BD Transduction Labs), paxillin, GIT1 and Nck1/2 (Santa Cruz Biotechnology, Inc.), and syntaxin3 (AbCam), and mouse mAbs to β-tubulin (Sigma), β-PIX, Munc18a/b, RalA and syntaxin4 (BD Transduction Labs) were obtained from commercial sources. FITC-goat anti-mouse, Texas Red-donkey anti-mouse and Texas Red-donkey anti-rabbit IgG were purchased from Jackson Immunoresearch Labs (West Grove, PA). Mouse anti-c-Myc agarose affinity gel was from Sigma (St. Louis, MO).
Plasmid encoding temperature-sensitive mutant viral glycoprotein VSVG-tsO45 fused to GFP (ts-G-GFP) was generously provided by Drs. Suzie Scales (Genentech) (Scales et al., 1997). Plasmids encoding α5-integrin or paxillin fused to GFP (α5-GFP, paxillin-GFP) were kindly provided by Dr. Alan F. Horwitz (University of Virginia) (Laukaitis et al., 2001). Myc-tagged rSec5 was expressed from the pCMV-myc vector (Clontech). Ral-uncoupled mutants (rSec5T11A and rSec5R27E) were generated by site-directed mutagenesis with the Quick-Change kit (Strategene) and expressed from the pCMV-myc vector (Clontech). X-Press-tagged rExo84 Ral-binding domain was expressed from the pcDNA3.1/HisC vector (Invitrogen). For in vitro transcription/translation studies, the coding sequence of paxillin was subcloned into the pcDNA3.1 vector, which contains a T7 RNA polymerase promoter. Myc-tagged rSec5 was expressed from the pGBKT7 plasmid (Clontech). A truncated rSec5 construct, deleted of its amino terminal 120 amino acid Ral-binding domain (Myc-rSec5ΔRBD), was expressed from the pcDNA3.1/HisC vector.
Synthetic siRNAs targeting Sec5 or Sec6 and standard negative control non-targeting siRNAs were ordered from Dharmacon. Sec5 and Sec6 targets were designed using the following sense sequences previously shown to be effective for rat Exocyst proteins (Rosse et al., 2006): CGGCAGAAUGGAUGUCUGC (Sec5-1), GGUCGGAAAGACAAGGCAGAU (Sec5-2), and CUGGAGGCAGAGCAUCAACAC (Sec6). Plasmids coding shRNAs specific for RalA (CGCUGCAAUUAGAGACAACUA; clone TRCN0000004865) or RalB (GAGUUUGUAGAAGACUAUGAA; clone TRCN0000072957) in the pLKO.1 vector were purchased from Open Biosystems (Huntsville, AL).
Cell Culture Methodology
Dunning rat prostate tumor cell lines (R3327-5′A and R3327-5′B) were maintained in high glucose Dulbecco’s modified Eagle’s media (DMEM, Life Technologies) supplemented with 10% fetal bovine serum (FBS), penicillin, streptomycin, and kanamycin. Plasmids were transfected using Lipofectamine2000 reagent (Life Technologies) according to the manufacturer’s instructions. siRNAs were transfected at 160 nM using Oligofectamine (Life Technologies) according to the manufacturer’s instructions. Lentivirus production in 293FT packaging cells followed established protocols (The RNAi Consortium). Stable knockdown of RalA and RalB was achieved by lentiviral infection of R3327-5′A cells and selection in 5 μg/ml puromycin.
Immunofluorescent Staining
Cells were fixed in 4% paraformaldehyde for 30 min, then permeabilized by incubation at 0°C for 10 min with 1% Triton X-100 in buffer containing 10 mM Pipes, pH 6.8, 50 mM NaCl, 300 mM sucrose, 3 mM MgCl2 and protease inhibitors (1 mM pefabloc, and 10 μg/ml each of aprotinin, antipain, leupeptin, pepstatin A) (CSK buffer). Antibodies were diluted in blocking buffer (Ringer’s saline (154 mM NaCl, 1.8 mM Ca2+, 7.2 mM KCl, 10 mM Hepes, pH 7.4) containing 0.2 % BSA, 0.5% normal goat serum and 0.5% normal donkey serum) and applied to cells for 2 h at 4°C. After five washes in blocking buffer, FITC and Texas Red conjugated secondary antibodies, diluted 1:200, were applied for 1 h at 4°C. Coverslips and filters were washed five times and mounted in VectaShield (Vector Laboratories, Burlingame, CA). Samples were viewed with either a Nikon Microphot-FX microscope (63X or 100X objectives) or a Zeiss 510 confocal laser scanning microscope (63X objective) using krypton/argon laser with 488 nm (FITC, GFP) and 543 nm (Texas Red) laser lines, as noted in figure legends. Digital images of data collected from the Nikon Microphot-FX microscope were obtained with a Kodak DCS 760 digital camera.
Morphological assay for polarized trafficking of α5-GFP and ts-G-GFP
R3327-5′A cells were transfected with plasmids encoding either α5-GFP or ts-G-GFP. To accumulate ts-G-GFP in the TGN, cells were suspended with trypsin-EDTA 24 hr after transfection, re-plated on collagen-coated coverslips and incubated at 40°C for 5 hr to accumulate ts-G-GFP in the endoplasmic reticulum. Cultures were shifted to 32°C for 7.5 min to facilitate protein folding, and subsequently transferred to 19°C for 2 hr to accumulate protein in the TGN. To accumulate α5-GFP in the TGN, cells were suspended with trypsin-EDTA 24 hr after transfection, re-plated on collagen-coated coverslips and incubated at 37°C for 1 hr then transferred to 19°C for 4 hr. Trafficking of protein from the TGN to plasma membrane was initiated by shifting cultures from 19°C to 32°C. In some cases, cultures were treated with 0.5 % (w/v) tannic acid prior to and during the final 32°C incubation period to inhibit vesicle fusion with the plasma membrane (Polishchuk et al., 2004). At indicated time points, cultures were fixed and labeled with antibodies to Sec8 followed by Texas Red anti-mouse secondary antibody.
Gel Electrophoresis and Immunoblotting
Protein samples were incubated in SDS-PAGE sample buffer for 10 min at 65°C before separation in 7.5%, 10% or 12.5% SDS polyacrylamide gels. Proteins were electrophoretically transferred from gels to Immobilon PVDF membrane (Millipore Corp., Bedford, MA). Blots were blocked in Blotto (5% nonfat dry milk, 0.1% sodium azide in 150 mM NaCl, 10 mM TrisHCl, pH 7.5 [TBS]) overnight at 4°C. Primary antibodies were incubated with blots at room temperature for 1 h. After five washes, 10 min each, in TBS containing 0.1% Tween-20, the blots were incubated with 125I-labeled goat anti-mouse or goat anti-rabbit secondary antibody (Amersham) for 1 h at room temperature. Blots were washed as above and exposed to phosphorimager screens. The amount of labeled antibody bound to the blots was determined directly using a Phosphorimager (Typhoon, Molecular Dynamics, Sunnyvale, CA) and ImageQuant software (version 1.2, Molecular Dynamics, Sunnyvale, CA).
Cell Fractionation in Iodixanol Gradients
Cells were homogenized in isotonic sucrose buffer (0.25 M sucrose in 20 mM HEPES-KOH, pH 7.2, 90 mM KOAc, 2 mM Mg(OAc)2, and protease inhibitors) by repeated passage through a ball bearing homogenizer (Varian Physics, Stanford University). Separation of different membrane compartments was achieved by centrifugation in five-step 10–15–20–25–30% (wt/vol) iodixanol gradients. Briefly, post-nuclear supernatant was mixed with Opti-Prep (60% (wt/vol) iodixanol, Nycomed, Oslo, Norway) and homogenization buffer to generate a solution containing 30% iodixanol. This was overlayed in centrifuge tubes with equal volumes of 25%, 20%, 15%, and 10% iodixanol, and samples were centrifuged at 350,000 × g for 3 h at 4°C in a Beckman NVT65 rotor. Fractions (0.5 ml) were collected, refractive indices were read, and proteins were separated by SDS-PAGE. Proteins were transferred from gels to Immobilon P membranes for immunoblotting as described above.
Isolation of pseudopods
Pseudopod isolation was performed similar to methods described previously (Cho and Klemke, 2002). R3327-5′A cells were incubated for 24 hr in serum-free DMEM containing 0.5 % (w/v) BSA and antibiotics. Cells were suspended and seeded at a density of 1.5 × 107 cells/filter on Transwell filters (# 3420, 75 mm diameter, 3.0 μm pore size). Following attachment at 37°C for 2 hr in serum-free medium, complete medium containing 10% FBS was applied to the basal chamber to stimulate chemotaxis. Cultures were returned to 37°C and incubated for 4 hr to allow pseudopod growth. Cell bodies were manually removed from the tops of filters by gentle scrubbing with a cotton swab, leaving the detached pseudopods adherent to the bottoms of filters. For analysis of total cellular protein, cell bodies were not removed. Lastly, intact cells or isolated pseudopods were solubilized in extraction buffer (CSK with protease inhibitors) and protein content was quantified using a micro BCA assay (Pierce).
Immunoprecipitation
Whole cells, pseudopods or cell bodies were extracted in CSK buffer for 30 min at 4°C. Extracts were precleared with 5 μl of nonimmune serum and 50 μl Staphylococcus aureus cells (Pansorbin; Calbiochem Novabiochem, La Jolla, CA) for 1 hr at 4°C. For Sec8 immunoprecipitation, mAbs 2E12, 5C3 and 10C2 were covalently cross-linked to Protein A Sepharose beads (Pharmacia LKB Nuclear, Gaithersburg, MD) with dimethyl pimelimidate (DMP), and 20 μl of immunoadsorbant was used per immunoprecipitation. Immunoprecipitation of paxillin was performed with a specific rabbit polyclonal antibody (5 μg per sample) pre-bound to Protein A Sepharose beads. Immunoadsorbants were incubated with pre-cleared cell extracts for 2 h at 4°C, then washed under stringent conditions and prepared for SDS-PAGE as described previously (Yeaman et al., 2004).
In Vitro Transcription/Translation Analysis
Plasmids encoding individual myc-tagged Exocyst subunits (in pGBKT7 vectors) were added singly or together with plasmid encoding paxillin (in pcDNA 3.1) to TNT Quick Coupled Transcription/Translation Systems (Promega Corp.). Reactions were incubated in the presence of [35S]methionine/cysteine (Perkin-Elmer), according to manufacturer’s instructions. Aliquots of total translated product or anti-myc immunoprecipitated material were analyzed by SDS-PAGE and autoradiography.
Metabolic Pulse-Chase and Surface Biotinylation Analysis
R3327-5′A cells were transfected with siRNAs specific for Sec5 or Sec6 or a control siRNA. 24 hr later, cells were transfected with plasmid encoding α5-GFP and returned to the incubator. The following morning, cells were suspended with trypsin and split into four identical replicate cultures and returned to the incubator for 12 – 18 hr. On the day of the experiment (~60 hr after siRNA transfection), one set of cultures was lysed and analyzed for expression of Sec5, Sec6 or a loading control (β-PIX) by Western blotting, and the remaining cultures were used to quantify α5-GFP trafficking. Triplicate wells containing either control or Exocyst knock-down cells were incubated in methionine/cysteine-free culture medium to starve them for 30 min, then pulse-labeled for 30 min in the same medium supplemented with 1 mCi/ml [35S]methionine/cysteine. Following the labeling period, cultures were washed three times in chase medium (complete DMEM supplemented with 5-fold excess methionine and cysteine), and returned to the incubator for one hour in the same medium. At the end of the chase incubation, cultures were placed on ice, washed with ice-cold Ringer’s saline, and labeled with Sulfo-NHS-SS-Biotin (Pierce). Following extensive washes in quenching buffer (TBS containing 50 mM NH4Cl and 0.5 % BSA), cells were lysed and pre-cleared as described above, and α5-GFP was immunoprecipitated with anti-GFP antibodies. The biotinylated (eg surface-exposed) cohort of α5-GFP was subsequently recovered from total immunoprecipitates by avidin precipitation. Samples were analyzed by SDS-PAGE and bands were quantified with a phosphorimager following fluorographic enhancement of gels with Amplify solution (Amersham). Relative surface delivery of α5-GFP was defined as the mean signal obtained from three replicate biotinylated α5-GFP bands, normalized to the mean of the total α5-GFP recovered in initial immunoprecipitates.
Wound-healing assays
R3327-5′A cells were transfected with siRNAs specific for Sec5, Sec6 or a control siRNA using Oligofectamine. 72 hr post-transfection, confluent cell monolayers were experimentally wounded by scratching with a pipette tip, rinsed with Ringer’s saline, and fed with fresh medium containing 10% serum. Analysis of wounds was performed by photographing several areas of each wound at multiple time points over a 48 hr period using a Nikon TE300 microscope equipped with a Nikon Coopix 5000 camera. Areas of wound closure were quantified using Image-Pro Plus software (Media Cybernetics, Silver Spring, MD).
Matrigel Invasion Assay
5′B or 5′A cells were transfected without or with Sec5, Sec6 or control siRNAs and cultured for 60 hr. Cells were suspended in serum-free DMEM containing 0.5 % (w/v) BSA and antibiotics and seeded at a density of 5×104 cells/filter on Transwell filters (# 3422, 6.5 mm diameter, 8.0 μm pore size) coated with 20 μg of Matrigel. Following attachment of cells at 37°C for 2 hr in serum-free medium, complete medium containing 10% FBS was applied to the basal chamber to stimulate migration. Cultures were returned to 37°C and incubated for 24 hr to allow invasion to occur. At the end of the invasion period, cells remaining on the upper surface of filters were manually removed by gentle scrubbing with a cotton swab, and cells that had invaded through the Matrigel-coated filters were fixed with 100% methanol, stained with DAPI, and counted manually. To control for variability in plating efficiency between samples, replicate filters were treated identically to those described above, except that cells remaining on the upper surface were not removed at the end of the invasion period. Cells were fixed with methanol, labeled with DAPI and the mean cell density of each culture was calculated by manually counting cells in multiple random fields of each filter.
Supplementary Material
R3327–5′A cells or R3327–5′A cells expressing shRNAs targeting RalA or RalB were extracted in 1% Triton X-100. Extracts were subjected to immunoprecipitation with antibodies to Sec8. Presence Sec8, Sec5, Sec15 and Exo84 in equivalent amounts of whole cell extracts (“total”) and precipitated immune complexes (“α-Sec8 IP”) was assessed by SDS-PAGE. Indicated proteins were identified by immunoblotting with specific antibodies, and protein levels were quantified using a Molecular Dynamics Typhoon phosphorimager. Band intensities for each protein pair were calculated and normalized to levels present parental 5′A cells, which is defined as “1”.
Acknowledgments
This work was supported by grants from the National Institutes of Health (GM067002) and the U.S. Department of Defense (DAMD 17-03-1-0187). Brian Leper, Melinda Schwarz and Hsiang Wen are gratefully acknowledged for their technical assistance in generating and characterizing reagents and performing these studies.
References
- Anitei M, Ifrim M, Ewart MA, Cowan AE, Carson JH, Bansal R, Pfeiffer SE. A role for Sec8 in oligodendrocyte morphological differentiation. J Cell Sci. 2006;119:807–18. doi: 10.1242/jcs.02785. [DOI] [PubMed] [Google Scholar]
- Bodemann BO, White MA. Ral GTPases and cancer: linchpin support of the tumorigenic platform. Nat Rev Cancer. 2008;8:133–40. doi: 10.1038/nrc2296. [DOI] [PubMed] [Google Scholar]
- Boyd C, Hughes T, Pypaert M, Novick P. Vesicles carry most exocyst subunits to exocytic sites marked by the remaining two subunits, Sec3p and Exo70p. J Cell Biol. 2004;167:889–901. doi: 10.1083/jcb.200408124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussemakers MJ, van Moorselaar RJ, Giroldi LA, Ichikawa T, Isaacs JT, Takeichi M, Debruyne FM, Schalken JA. Decreased expression of E-cadherin in the progression of rat prostatic cancer. Cancer Res. 1992;52:2916–22. [PubMed] [Google Scholar]
- Camonis JH, White MA. Ral GTPases: corrupting the exocyst in cancer cells. Trends Cell Biol. 2005;15:327–32. doi: 10.1016/j.tcb.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Chien Y, Kim S, Bumeister R, Loo YM, Kwon SW, Johnson CL, Balakireva MG, Romeo Y, Kopelovich L, Gale M, Jr, et al. RalB GTPase-mediated activation of the IkappaB family kinase TBK1 couples innate immune signaling to tumor cell survival. Cell. 2006;127:157–70. doi: 10.1016/j.cell.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Cho SY, Klemke RL. Purification of pseudopodia from polarized cells reveals redistribution and activation of Rac through assembly of a CAS/Crk scaffold. J Cell Biol. 2002;156:725–36. doi: 10.1083/jcb.200111032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig LA. Ral-GTPases: approaching their 15 minutes of fame. Trends Cell Biol. 2003;13:419–25. doi: 10.1016/s0962-8924(03)00152-1. [DOI] [PubMed] [Google Scholar]
- Finger FP, Novick P. Spatial regulation of exocytosis: lessons from yeast. J Cell Biol. 1998;142:609–12. doi: 10.1083/jcb.142.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukai S, Matern HT, Jagath JR, Scheller RH, Brunger AT. Structural basis of the interaction between RalA and Sec5, a subunit of the sec6/8 complex. Embo J. 2003;22:3267–78. doi: 10.1093/emboj/cdg329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gildea JJ, Harding MA, Seraj MJ, Gulding KM, Theodorescu D. The Role of Ral A in Epidermal Growth Factor Receptor-regulated Cell Motility. Cancer Res. 2002;62:982–5. [PubMed] [Google Scholar]
- Grindstaff KK, Yeaman C, Anandasabapathy N, Hsu SC, Rodriguez-Boulan E, Scheller RH, Nelson WJ. Sec6/8 complex is recruited to cell-cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in epithelial cells. Cell. 1998;93:731–40. doi: 10.1016/s0092-8674(00)81435-x. [DOI] [PubMed] [Google Scholar]
- Guo W, Roth D, Walch-Solimena C, Novick P. The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. Embo J. 1999;18:1071–80. doi: 10.1093/emboj/18.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto S, Onodera Y, Hashimoto A, Tanaka M, Hamaguchi M, Yamada A, Sabe H. Requirement for Arf6 in breast cancer invasive activities. Proc Natl Acad Sci U S A. 2004;101:6647–52. doi: 10.1073/pnas.0401753101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Xi F, Zhang X, Zhang J, Guo W. Exo70 interacts with phospholipids and mediates the targeting of the exocyst to the plasma membrane. Embo J. 2007;26:4053–65. doi: 10.1038/sj.emboj.7601834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer F, Fields S, Schneider C, Martin GS. Activated Ras interacts with the Ral guanine nucleotide dissociation stimulator. Proc Natl Acad Sci U S A. 1994;91:11089–93. doi: 10.1073/pnas.91.23.11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SC, Hazuka CD, Roth R, Foletti DL, Heuser J, Scheller RH. Subunit composition, protein interactions, and structures of the mammalian brain sec6/8 complex and septin filaments. Neuron. 1998;20:1111–22. doi: 10.1016/s0896-6273(00)80493-6. [DOI] [PubMed] [Google Scholar]
- Hsu SC, Ting AE, Hazuka CD, Davanger S, Kenny JW, Kee Y, Scheller RH. The mammalian brain rsec6/8 complex. Neuron. 1996;17:1209–19. doi: 10.1016/s0896-6273(00)80251-2. [DOI] [PubMed] [Google Scholar]
- Inoue M, Chiang SH, Chang L, Chen XW, Saltiel AR. Compartmentalization of the exocyst complex in lipid rafts controls Glut4 vesicle tethering. Mol Biol Cell. 2006;17:2303–11. doi: 10.1091/mbc.E06-01-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R, Junutula JR, Matern HT, Ervin KE, Scheller RH, Brunger AT. Exo84 and Sec5 are competitive regulatory Sec6/8 effectors to the RalA GTPase. Embo J. 2005;24:2064–74. doi: 10.1038/sj.emboj.7600699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee Y, Yoo JS, Hazuka CD, Peterson KE, Hsu SC, Scheller RH. Subunit structure of the mammalian exocyst complex. Proc Natl Acad Sci U S A. 1997;94:14438–43. doi: 10.1073/pnas.94.26.14438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalli G, Hall A. Ral GTPases regulate neurite branching through GAP-43 and the exocyst complex. J Cell Biol. 2005;171:857–69. doi: 10.1083/jcb.200507061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laukaitis CM, Webb DJ, Donais K, Horwitz AF. Differential dynamics of alpha 5 integrin, paxillin, and alpha-actinin during formation and disassembly of adhesions in migrating cells. J Cell Biol. 2001;153:1427–40. doi: 10.1083/jcb.153.7.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljedahl M, Maeda Y, Colanzi A, Ayala I, Van Lint J, Malhotra V. Protein kinase D regulates the fission of cell surface destined transport carriers from the trans-Golgi network. Cell. 2001;104:409–20. doi: 10.1016/s0092-8674(01)00228-8. [DOI] [PubMed] [Google Scholar]
- Lim KH, Baines AT, Fiordalisi JJ, Shipitsin M, Feig LA, Cox AD, Der CJ, Counter CM. Activation of RalA is critical for Ras-induced tumorigenesis of human cells. Cancer Cell. 2005;7:533–45. doi: 10.1016/j.ccr.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Lim KH, O’Hayer K, Adam SJ, Kendall SD, Campbell PM, Der CJ, Counter CM. Divergent roles for RalA and RalB in malignant growth of human pancreatic carcinoma cells. Curr Biol. 2006;16:2385–94. doi: 10.1016/j.cub.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Liu J, Zuo X, Yue P, Guo W. Phosphatidylinositol 4,5-bisphosphate mediates the targeting of the exocyst to the plasma membrane for exocytosis in mammalian cells. Mol Biol Cell. 2007;18:4483–92. doi: 10.1091/mbc.E07-05-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Lubaroff DM, Hendrix MJ. Suppression of prostate cancer invasive potential and matrix metalloproteinase activity by E-cadherin transfection. Cancer Res. 1999;59:3552–6. [PubMed] [Google Scholar]
- Luo J, Sharma N, Seftor EA, De Larco J, Heidger PM, Hendrix MJ, Lubaroff DM. Heterogeneous Expression of Invasive and Metastatic Properties in a Prostate Tumor Model. Pathol Oncol Res. 1997;3:264–271. doi: 10.1007/BF02904285. [DOI] [PubMed] [Google Scholar]
- Marrs JA, Napolitano EW, Murphy-Erdosh C, Mays RW, Reichardt LF, Nelson WJ. Distinguishing roles of the membrane-cytoskeleton and cadherin mediated cell-cell adhesion in generating different Na+, K(+)-ATPase distributions in polarized epithelia. J Cell Biol. 1993;123:149–64. doi: 10.1083/jcb.123.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskalenko S, Henry DO, Rosse C, Mirey G, Camonis JH, White MA. The exocyst is a Ral effector complex. Nat Cell Biol. 2002;4:66–72. doi: 10.1038/ncb728. [DOI] [PubMed] [Google Scholar]
- Moskalenko S, Tong C, Rosse C, Mirey G, Formstecher E, Daviet L, Camonis J, White MA. Ral GTPases regulate exocyst assembly through dual subunit interactions. J Biol Chem. 2003;278:51743–8. doi: 10.1074/jbc.M308702200. [DOI] [PubMed] [Google Scholar]
- Novick P, Guo W. Ras family therapy: Rab, Rho and Ral talk to the exocyst. Trends Cell Biol. 2002;12:247–9. doi: 10.1016/s0962-8924(02)02293-6. [DOI] [PubMed] [Google Scholar]
- Oxford G, Owens CR, Titus BJ, Foreman TL, Herlevsen MC, Smith SC, Theodorescu D. RalA and RalB: antagonistic relatives in cancer cell migration. Cancer Res. 2005;65:7111–20. doi: 10.1158/0008-5472.CAN-04-1957. [DOI] [PubMed] [Google Scholar]
- Polishchuk R, Di Pentima A, Lippincott-Schwartz J. Delivery of raft-associated, GPI-anchored proteins to the apical surface of polarized MDCK cells by a transcytotic pathway. Nat Cell Biol. 2004;6:297–307. doi: 10.1038/ncb1109. [DOI] [PubMed] [Google Scholar]
- Prigent M, Dubois T, Raposo G, Derrien V, Tenza D, Rosse C, Camonis J, Chavrier P. ARF6 controls post-endocytic recycling through its downstream exocyst complex effector. J Cell Biol. 2003;163:1111–21. doi: 10.1083/jcb.200305029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigozhina NL, Waterman-Storer CM. Protein kinase D-mediated anterograde membrane trafficking is required for fibroblast motility. Curr Biol. 2004;14:88–98. doi: 10.1016/j.cub.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Ross JS, Figge HL, Bui HX, del Rosario AD, Fisher HA, Nazeer T, Jennings TA, Ingle R, Kim DN. E-cadherin expression in prostatic carcinoma biopsies: correlation with tumor grade, DNA content, pathologic stage, and clinical outcome. Mod Pathol. 1994;7:835–41. [PubMed] [Google Scholar]
- Rosse C, Hatzoglou A, Parrini MC, White MA, Chavrier P, Camonis J. RalB mobilizes the exocyst to drive cell migration. Mol Cell Biol. 2006;26:727–34. doi: 10.1128/MCB.26.2.727-734.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sans N, Prybylowski K, Petralia RS, Chang K, Wang YX, Racca C, Vicini S, Wenthold RJ. NMDA receptor trafficking through an interaction between PDZ proteins and the exocyst complex. Nat Cell Biol. 2003;5:520–30. doi: 10.1038/ncb990. [DOI] [PubMed] [Google Scholar]
- Scales SJ, Pepperkok R, Kreis TE. Visualization of ER-to-Golgi transport in living cells reveals a sequential mode of action for COPII and COPI. Cell. 1997;90:1137–48. doi: 10.1016/s0092-8674(00)80379-7. [DOI] [PubMed] [Google Scholar]
- Schmoranzer J, Kreitzer G, Simon SM. Migrating fibroblasts perform polarized, microtubule-dependent exocytosis towards the leading edge. J Cell Sci. 2003;116:4513–9. doi: 10.1242/jcs.00748. [DOI] [PubMed] [Google Scholar]
- Shipitsin M, Feig LA. RalA but not RalB enhances polarized delivery of membrane proteins to the basolateral surface of epithelial cells. Mol Cell Biol. 2004;24:5746–56. doi: 10.1128/MCB.24.13.5746-5756.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaargaren M, Bischoff JR. Identification of the guanine nucleotide dissociation stimulator for Ral as a putative effector molecule of R-ras, H-ras, K-ras, and Rap. Proc Natl Acad Sci U S A. 1994;91:12609–13. doi: 10.1073/pnas.91.26.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugihara K, Asano S, Tanaka K, Iwamatsu A, Okawa K, Ohta Y. The exocyst complex binds the small GTPase RalA to mediate filopodia formation. Nat Cell Biol. 2002;4:73–8. doi: 10.1038/ncb720. [DOI] [PubMed] [Google Scholar]
- Suzuki J, Yamazaki Y, Li G, Kaziro Y, Koide H. Involvement of Ras and Ral in chemotactic migration of skeletal myoblasts. Mol Cell Biol. 2000;20:4658–65. doi: 10.1128/mcb.20.13.4658-4665.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaya A, Ohba Y, Kurokawa K, Matsuda M. RalA activation at nascent lamellipodia of epidermal growth factor-stimulated Cos7 cells and migrating Madin-Darby canine kidney cells. Mol Biol Cell. 2004;15:2549–57. doi: 10.1091/mbc.E03-11-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. Cadherins in cancer: implications for invasion and metastasis. Curr Opin Cell Biol. 1993;5:806–11. doi: 10.1016/0955-0674(93)90029-p. [DOI] [PubMed] [Google Scholar]
- Tchevkina E, Agapova L, Dyakova N, Martinjuk A, Komelkov A, Tatosyan A. The small G-protein RalA stimulates metastasis of transformed cells. Oncogene. 2005;24:329–35. doi: 10.1038/sj.onc.1208094. [DOI] [PubMed] [Google Scholar]
- TerBush DR, Maurice T, Roth D, Novick P. The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. Embo J. 1996;15:6483–94. [PMC free article] [PubMed] [Google Scholar]
- TerBush DR, Novick P. Sec6, Sec8, and Sec15 are components of a multisubunit complex which localizes to small bud tips in Saccharomyces cerevisiae. J Cell Biol. 1995;130:299–312. doi: 10.1083/jcb.130.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CE, West KA, Brown MC. Paxillin-ARF GAP signaling and the cytoskeleton. Curr Opin Cell Biol. 2001;13:593–9. doi: 10.1016/s0955-0674(00)00256-8. [DOI] [PubMed] [Google Scholar]
- Wang S, Hsu SC. The molecular mechanisms of the mammalian exocyst complex in exocytosis. Biochem Soc Trans. 2006;34:687–90. doi: 10.1042/BST0340687. [DOI] [PubMed] [Google Scholar]
- Wang S, Liu Y, Adamson CL, Valdez G, Guo W, Hsu SC. The mammalian exocyst, a complex required for exocytosis, inhibits tubulin polymerization. J Biol Chem. 2004;279:35958–66. doi: 10.1074/jbc.M313778200. [DOI] [PubMed] [Google Scholar]
- Yeaman C. Ultracentrifugation-based approaches to study regulation of Sec6/8 (exocyst) complex function during development of epithelial cell polarity. Methods. 2003;30:198–206. doi: 10.1016/s1046-2023(03)00026-4. [DOI] [PubMed] [Google Scholar]
- Yeaman C, Grindstaff KK, Nelson WJ. New perspectives on mechanisms involved in generating epithelial cell polarity. Physiol Rev. 1999;79:73–98. doi: 10.1152/physrev.1999.79.1.73. [DOI] [PubMed] [Google Scholar]
- Yeaman C, Grindstaff KK, Nelson WJ. Mechanism of recruiting Sec6/8 (exocyst) complex to the apical junctional complex during polarization of epithelial cells. J Cell Sci. 2004;117:559–70. doi: 10.1242/jcs.00893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman C, Grindstaff KK, Wright JR, Nelson WJ. Sec6/8 complexes on trans-Golgi network and plasma membrane regulate late stages of exocytosis in mammalian cells. J Cell Biol. 2001;155:593–604. doi: 10.1083/jcb.200107088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Orlando K, He B, Xi F, Zhang J, Zajac A, Guo W. Membrane association and functional regulation of Sec3 by phospholipids and Cdc42. J Cell Biol. 2008;180:145–58. doi: 10.1083/jcb.200704128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X, Zhang J, Zhang Y, Hsu SC, Zhou D, Guo W. Exo70 interacts with the Arp2/3 complex and regulates cell migration. Nat Cell Biol. 2006;8:1383–8. doi: 10.1038/ncb1505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
R3327–5′A cells or R3327–5′A cells expressing shRNAs targeting RalA or RalB were extracted in 1% Triton X-100. Extracts were subjected to immunoprecipitation with antibodies to Sec8. Presence Sec8, Sec5, Sec15 and Exo84 in equivalent amounts of whole cell extracts (“total”) and precipitated immune complexes (“α-Sec8 IP”) was assessed by SDS-PAGE. Indicated proteins were identified by immunoblotting with specific antibodies, and protein levels were quantified using a Molecular Dynamics Typhoon phosphorimager. Band intensities for each protein pair were calculated and normalized to levels present parental 5′A cells, which is defined as “1”.