Abstract
In addition to motor and/or vocal tics, many individuals with tourette syndrome (TS) or chronic tic disorder (CTD) report frequent, uncomfortable sensory phenomena that immediately precede the tics. To date, examination of these premonitory sensations or urges has been limited by inconsistent assessment tools. In this manuscript, we examined the psychometric properties of a 9-item self-report measure, the Premonitory Urge to Tic Scale (PUTS) and examined the characteristics and correlates of the premonitory urge to tic in a clinical sample of 122 older adolescents and adults with TS or CTD. The PUTS demonstrated adequate internal consistency, temporal stability, and concurrent validity. Premonitory urges were endorsed by the majority of individuals. Most individuals reported some relief from the urges after completing a tic and being able to stop their tics even if only temporarily. Degree of premonitory urges was not significantly correlated with age, and we did not observe any gender differences. Degree of premonitory urges was significantly correlated with estimated IQ and tic severity, but not severity of comorbid obsessive compulsive disorder or attention deficit hyperactivity disorder. It was also not related to concomitant medication status. These findings represent another step forward in our understanding of the premonitory sensations associated with TS and CTD.
Keywords: tourette syndrome, chronic tic disorder, premonitory urge, older adolescents, adults
Tourette syndrome (TS) and chronic tic disorder (CTD) are characterized by sudden, rapid, recurrent motor and/or vocal tics (American Psychiatric Association, 2000). In addition to these observable symptoms, many individuals with TS or CTD also experience frequent, uncomfortable sensory phenomena that immediately precede the tics (for review Scahill, Leckman, & Marek, 1995). These sensory phenomena have been variously described as an itch, pressure, tension, urge, or ache that is temporarily relieved by the completion of the tic. Although first documented in the medical literature in the early 20th century (Patrick, 1905), it is only in the past 20–30 years that the scientific community has begun to systematically assess the nature and frequency of such premonitory sensations in the TS and CTD population.
In contrast to early reports (e.g., Shapiro & Shapiro, 1988), recent evidence suggests that premonitory sensations are experienced by the vast majority of individuals with TS or CTD (e.g., Cohen & Leckman, 1992; Kwak, Dat Vuong, & Jankovic, 2003; Leckman, Walker, & Cohen, 1993). The sensations are also very salient, as many individuals with TS describe the premonitory sensations as more distressing than the tics themselves (Cohen & Leckman, 1992).
Premonitory sensations or urges to tic are also thought to play a central role in the maintenance of tics. Current behavioral models posit that tics are negatively reinforced every time they rid the individual of the discomfort associated with the premonitory urge (Woods et al., 2008). Within this framework, the movements and vocalizations are understood as actions that the individual performs to alleviate the discomfort associated with the premonitory sensations. This conceptualization fits with many patients’ understanding of their illness. For example, Kwak et al. (2003) observed that 71% of individuals with TS believed their tics would no longer occur if the premonitory sensations were eliminated. Similarly, Lang (1991) found that two-thirds of individuals with tics described all of their tics as voluntary responses to involuntary urges. Another 25% of individuals reported that this was true for at least some of their tics. Clearly, a comprehensive understanding of TS and CTD must include examination of the associated premonitory phenomena.
To date, research examining premonitory sensations has primarily employed unpublished interviews and self-report measures. Methods have not been consistently replicated across studies, making cross-sample comparisons difficult. Woods, Piacentini, Himle, and Chang (2005), however, recently developed and published a brief, self-report scale, the Premonitory Urge for Tics Scale (PUTS), for assessing the presence and intensity of premonitory urges to tic in a dimensional manner.
In the first psychometric examination of the PUTS, Woods et al. (2005) administered the measure to 42 youths with TS or CTD ages 8 to 16. The original PUTS consisted of 10 items. Item 10 did not correlate well with the rest of the scale, however, and Woods et al. excluded it from the computation of the total score and further analyses. Using the 9-item PUTS, the authors observed interesting developmental differences in the psychometrics of the measure as well as the relationship between premonitory urges, tic severity, and comorbid psychiatric symptoms. For individuals 11–16 years of age, the measure proved to be internally consistent, temporally stable, and demonstrated good convergent validity with a measure of tic severity. PUTS total score was also significantly positively correlated with comorbid psychiatric symptoms including: symptoms of withdrawal, somatic complaints, anxiety/depression, social problems, aggressive behavior, and symptoms of obsessive compulsive disorder (OCD). For individuals 10 years of age or younger, however, the internal consistency of the measure was not acceptable, temporal stability was lower, and it did not demonstrate concurrent validity with a measure of tic severity. Similarly, PUTS total score was not significantly correlated with comorbid psychiatric symptoms. These findings are consistent with prior reports suggesting that awareness of the premonitory urge to tic does not typically develop until approximately 10 years of age (Leckman et al., 1993). These results would also suggest, in combination with the typical developmental course of TS characterized by a symptomatic peak in early adolescence and decline into adulthood (Leckman, Zhang, & Vitrale, 1998) that, although there may be considerable overlap, we cannot assume that the findings from children will hold true for older adolescents and adults. One very recent paper (Crossley, Seri, Stern, Robertson, & Cavanna, 2013) examined the psychometrics of the 10-item PUTS in an older adolescent and adult sample. However, given Woods et al.’s (2005) recommendation that the PUTS be used as a 9-item measure, data on the reliability and validity of the 9-item PUTS in an older adolescent and adult sample are lacking.
Accordingly, the present manuscript has two aims: 1) to examine the psychometric properties of the 9-item PUTS in a clinical sample of older adolescents and adults with diagnosed TS or CTD; and 2) to examine the characteristics and correlates of the premonitory urge to tic, as measured by the 9-item PUTS, in a clinical sample of older adolescents and adults with diagnosed TS or CTD. More specifically, we aim to describe the nature, prevalence, and degree of the premonitory urge to tic, as well as the relationship between the degree of premonitory urges to tic and gender, age, an estimate of IQ, tic severity, and concomitant medication status. Additionally, because OCD and attention deficit hyperactivity disorder (ADHD) are the most common comorbid conditions for individuals with TS and CTD (Zohar, Apter, King, Pauls, Leckman, & Cohen, 1999), we sought to explore the relationship between the degree of premonitory urges to tic and comorbid OCD and ADHD symptom severity.
We hypothesized that, similar to Woods et al.’s (2005) finding in youths, the PUTS would demonstrate good internal consistency, temporal stability, and concurrent validity in an older adolescent and adult sample. Also consistent with previous findings (Crossley et al., 2013; Woods et al., 2005) we hypothesized that premonitory urges would be endorsed by the majority of individuals and that feelings of being “wound up” or having an “energy in my body that needs to get out” would be the most commonly endorsed premonitory sensations. Consistent with the behavioral model of tics, we predicted that most individuals would report some relief from the urges to tic after completing the tic and would report being able to stop the tics, even if only for a short time. Because our sample was limited to older adolescents and adults, who have surpassed the age at which awareness of the premonitory urge typically develops (Leckman et al., 1993), we did not necessarily expect a correlation between age and premonitory urges. We did, however, hypothesize that total scores on the PUTS would be equal to or higher than those observed by Woods et al. (2005) in a youths ages 11–16. Given the lack of an observed association between premonitory urges and IQ in children (Woods et al., 2005), we also hypothesized that the degree of premonitory urges to tic would not be correlated with an estimate of IQ.
Further predictions regarding the characteristics and correlates of the premonitory urge to tic are more difficult to render. Prior work has produced mixed findings which may be partially related to differences in methodology (e.g., comparing individuals with premonitory urges to those without premonitory urges versus examining relationships in a continuous manner) and differences in methods of measurement. Leckman et al. (1993) observed a gender difference such that men were more likely to report premonitory urges than women. Two more recent reports (Sutherland Owens et al. 2011; Crossley et al., 2013), however, failed to observe any gender differences. As mentioned above, Woods et al. (2005) found a relationship between degree of premonitory urges and tic severity as well as symptoms of OCD, but not problems with attention. Similarly, Kurlan, Lichter, and Hewitt (1989) observed that all of the individuals in their sample who reported premonitory sensations also had comorbid obsessive-compulsive symptoms. In contrast, Leckman et al. (1993) found no relationship between the premonitory urge to tic and tic severity or obsessive-compulsive symptom severity. Cohen and Leckman (1992) also found no difference in tic severity between individuals with or without premonitory sensations. Additionally, Sutherland Owens et al. (2011) did not observe any relationship between premonitory urge and tic severity or comorbid OCD symptoms. Crossley et al. (2013) did not observe a correlation between medication use and the premonitory urge to tic. However, it has not yet been examined whether individuals not taking any medication differ from those taking medication differ in the degree of reported premonitory urges.
Consequently, we chose to conduct exploratory analyses examining the relationship between the premonitory urge to tic and gender, tic severity, severity of comorbid OCD and ADHD symptoms, and medication status. Because the PUTS is a dimensional measure, it may allow us to examine the relationship between the premonitory urge to tic and various demographic and symptomatic variables in a continuous manner, thereby providing some clarity regarding the previously mixed findings.
Method
Procedure
Data were collected as part of a multi-site randomized controlled trial comparing a Comprehensive Behavioral Intervention for Tics (CBIT) to Psychoeducation and Supportive Therapy (PST) for individuals age 16 or older with TS or CTD (see Wilhelm et al., 2012, for full description). In brief, 122 individuals were randomly assigned to receive 8 sessions of CBIT (n = 63) or PST (n = 59) over 10 weeks. Participants were enrolled at Massachusetts General Hospital/Harvard Medical School, Yale University, and The University of Texas Health Science Center at San Antonio. All study procedures were approved by the Institutional Review Boards at each site. Participants provided written consent (or assent and parental consent in the case of participants under 18 years of age) prior to the initiation of any study procedures. Clinician-rated and self-report measures were collected at screening, baseline, mid-treatment, post-treatment, and follow-up. For the purposes of this manuscript, we report screening and baseline data only. Clinician-rated measures were administered by individuals with a master’s degree or higher in a mental health care field. Raters received extensive training and were required to demonstrate reliability on 3 video-recorded assessments prior to the initiation of the study (see also Wilhelm et al., 2012 for a full description of rater supervision, monitoring, and reliability assessments).
Participants
The 122 individuals (78 male; 44 female; 98 White, non-Hispanic; 17 White, Hispanic; 1 Black; 5 Asian/Pacific Islander; 1 other) who completed the baseline assessment ranged in age from 16 to 69 (mean age = 31.6, SD = 13.7 years). Of those, 103 met DSM-IV-TR (American Psychiatric Association, 2000) diagnostic criteria for TS; 19 (15.6%) met DSM-IV-TR diagnostic criteria for CTD (18 motor tics only; 1 vocal tic only); 19 (15.6%) met diagnostic criteria for OCD; and 34 (27.9%) met diagnostic criteria for ADHD. Seventy-one individuals (58.2%) were unmedicated; 8 (6.6%) were on a stable dose of tic medication only; 23 (18.9%) were on a stable dose of tic medication and other psychiatric medication; and 20 (16.4%) were on a stable dose of other psychiatric medication only at the time of enrollment.
Measures
Diagnostic assessment
Our procedures for diagnostic assessment were modeled on those developed by the Tourette Syndrome Association International Consortium for Genetics (TSAICG). At study entry raters administered The Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P; First, Spitzer, Gibbon, & Williams, 2002), a semi-structured clinical interview, to establish DSM-IV diagnoses. Because the SCID does not include a module for the diagnosis of tic disorders, raters also administered a semi-structured interview developed for this purpose by the TSAIG (TSAICG, 2007). Similarly, the SCID does not include a module for the diagnosis of ADHD. In this case, we adopted the diagnostic procedures commonly used by researchers of adulthood ADHD (e.g., Safren, Sprich, Mimiaga, Surman, Knouse, Groves, & Otto, 2010) and administered the ADHD module of the Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (K-SADS-PL; Kaufman, Birmaher, Brent, Rao, Flynn, Moreci, Williamson, & Ryan, 1997) to determine this diagnosis. Questions were phrased in the past tense and raters asked the participants if similar concerns were currently present.
Premonitory Urge for Tics Scale (PUTS; Woods et al., 2005)
The PUTS is a 9-item2 self-report questionnaire measuring premonitory sensations in individuals with tics. Each item is scored from 1 (not at all true) to 4 (very much true). The total score is computed by summing the 9 items. Thus, total scores range from 9 to 36, and higher scores represent greater premonitory urges. The PUTS has demonstrated good internal consistency, test-retest reliability, and construct validity among youths between 11 and 16 years of age (Woods et al., 2005). The present manuscript reports the first psychometric data on the 9-item PUTS in an older adolescent and adult sample.
Yale Global Tic Severity Scale (YGTSS; Leckman, Riddle, Hardin, & Ort, 1989)
The YGTSS is a clinician-rated measure of tic severity. Raters assess motor and phonic tics separately in five domains: number, frequency, intensity, complexity, and interference. Each domain is rated on a scale from 0 to 5, with 5 indicating greater severity. Total scores for motor and phonic tics can therefore range from 0 to 25. The Total Tic Severity Score is the sum of the motor and phonic tic scores and can range from 0 to 50. Additionally, raters provide an impairment score from 0 to 50 (with higher scores indicating greater impairment) reflecting the individual’s overall impaired functioning due to his or her tics. The YGTSS total score combines the total tic severity and impairment scores, and ranges from 0 to 100. The YGTSS has demonstrated good internal consistency and inter-rater reliability as well as excellent convergent and divergent validity (Leckman et al., 1989). In the present sample, the measure demonstrated adequate internal consistency (α = .78).
Yale-Brown Obsessive Compulsive Scale (YBOCS; Goodman, Price, Rasmussen, & Mazure, 1989a, 1989b)
The YBOCS is a 10-item clinician-rated measure of OCD symptom severity. Raters assess obsessions and compulsions separately in five domains: time spent, interference, distress, resistance, and control. Each domain is rated on a scale from 0 to 4. Thus, total scores range from 0 to 40, with higher scores indicating greater symptom severity. The YBOCS has demonstrated good inter-rater reliability and internal consistency as well as convergent and discriminant validity (Goodman et al., 1989a, 1989b). In the present sample, the measure demonstrated excellent internal consistency (α = .94).
Attention Deficit Hyperactivity Disorder Rating Scale (ADHD-RS; DuPaul, Power, Anastopoulos, & Reid, 1998)
The ADHD-RS is a 18-item self-report measure derived from the DSM-IV ADHD criteria. Each item is rated from 0 (never or rarely) to 3 (very often). Thus, total scores range from 0 to 54 with higher scores representing greater symptom severity. The scale was originally developed and validated in a pediatric population, but it has been previously used in adult ADHD populations (e.g., Spencer, Wilens, Biederman, & Faraone, 1995; Wilens et al., 1996). In the present sample, the measure demonstrated excellent internal consistency (α = .93).
Wechsler Test of Adult Reading (WTAR; The Psychological Corporation, 2001)
The WTAR is a 50-item measure that provides an estimate of overall IQ. Subjects read a list of 50 words aloud, and for each word that they pronounce correctly they earn one point. The WTAR has excellent internal consistency, test-retest reliability, and convergent validity. In the U.S. Standardization Sample, correlations between WTAR scores and full-scale IQ, as measured by the Wechsler Adult Intelligence Scale-III (WAIS-III) ranged from .63 to .80 for individuals 16–69 years of age. Correlations between WTAR score and verbal IQ and performance IQ ranged from .66 to .80 and from .45 to .72, respectively (The Psychological Corporation, 2001).
Results
Preliminary Analyses
As previously discussed, Woods et al. (2005) found that the 10th item of the PUTS did not correlate well with the rest of the items and therefore recommended excluding it from the scale. To confirm that the same approach would be appropriate with our sample, we correlated each item with the 10-item total score and every other item. Item 10 was significantly correlated with the total score, r = .39, p < .001. However, it demonstrated the weakest correlation with the total score relative to the other nine items. Moreover, it was significantly correlated with only two of the other nine items. In contrast, each of the other nine items was significantly correlated with at least six of the other nine items. Therefore, in keeping with Woods et al. (2005), we excluded item 10 from the measure. We have only included item 10 in our presentation of item-by-item results. Table 1 presents the correlation between each item and the 9-item total score.
Table 1.
Item mean, standard deviation, and correlation with 9-item total score
| Item | Mean | SD | Correlation with total score |
|---|---|---|---|
| 1. Right before I do a tic, I feel like my insides are itchy. | 1.66 | .93 | .42* |
| 2. Right before I do a tic, I feel pressure inside my brain or body. | 2.09 | 1.11 | .64* |
| 3. Right before I do a tic, I feel “wound up” or tense inside. | 2.53 | 1.03 | .78* |
| 4. Right before I do a tic, I feel like something is not “just right.” | 2.48 | 1.07 | .76* |
| 5. Right before I do a tic, I feel like something isn’t complete. | 2.20 | 1.08 | .65* |
| 6. Right before I do a tic, I feel like there is energy in my body that needs to get out. | 2.75 | 1.06 | .69* |
| 7. I have these feelings almost all the time before I do a tic. | 2.76 | 1.01 | .75* |
| 8. These feelings happen for every tic I have. | 2.30 | 1.01 | .56* |
| 9. After I do the tic, the itchiness, energy, pressure, tense feelings, or feelings that something isn’t “just right” or complete go away, at least for a little while. | 2.76 | 1.05 | .61* |
| 10. I am able to stop my tics, even if only for a short period of time. | 2.81 | 1.01 | N/A-not included in total |
Significant at the .01 level.
Psychometric findings
Internal Consistency and Temporal Stability
To examine the internal consistency of the PUTS, we computed Cronbach’s alpha. The PUTS demonstrated good internal consistency, α = .83 (Groth-Marnat, 2003). To examine the temporal stability of the PUTS, we correlated total PUTS score at screening with total PUTS score at baseline, which occurred within 2 weeks of the screening visit. The PUTS demonstrated adequate temporal stability, r = .79, p < .001.
Concurrent Validity
To test concurrent validity, we examined the relationship between degree of premonitory urges and the dimensions of tic severity as measured by the YGTSS. Total PUTS score was moderately positively correlated with tic severity, as measured by the total tic score of the YGTSS, r =.32, p < 0.001. We also observed small to medium positive correlations between total PUTS score and number of tics, r = .29, p = .001, frequency of tics, r = .20, p = .03, complexity of tics, r = .25, p = .006, and tic-related interference, r = .32, p < .001. Total PUTS score was not significantly correlated with tic intensity, r = .16, p = .08. Taken together, these results suggest that those individuals with greater premonitory urges also experience more severe tics. However, the modest size of the correlations (all between .16 and .32) and the differential relationships between PUTS and the dimensions of the YGTSS would suggest that the PUTS is measuring a feature of the disorder that is separable from tic severity.
Summary data
Over 90% of individuals endorsed premonitory urges to some extent (n = 114, 93.4%). Eight individuals (6.6%) obtained a total score of 9, indicating the complete absence of premonitory urges. Total PUTS scores ranged from 9 to 33 with a mean score of 21.5 (SD = 6.1).
Phenomenology of the urge
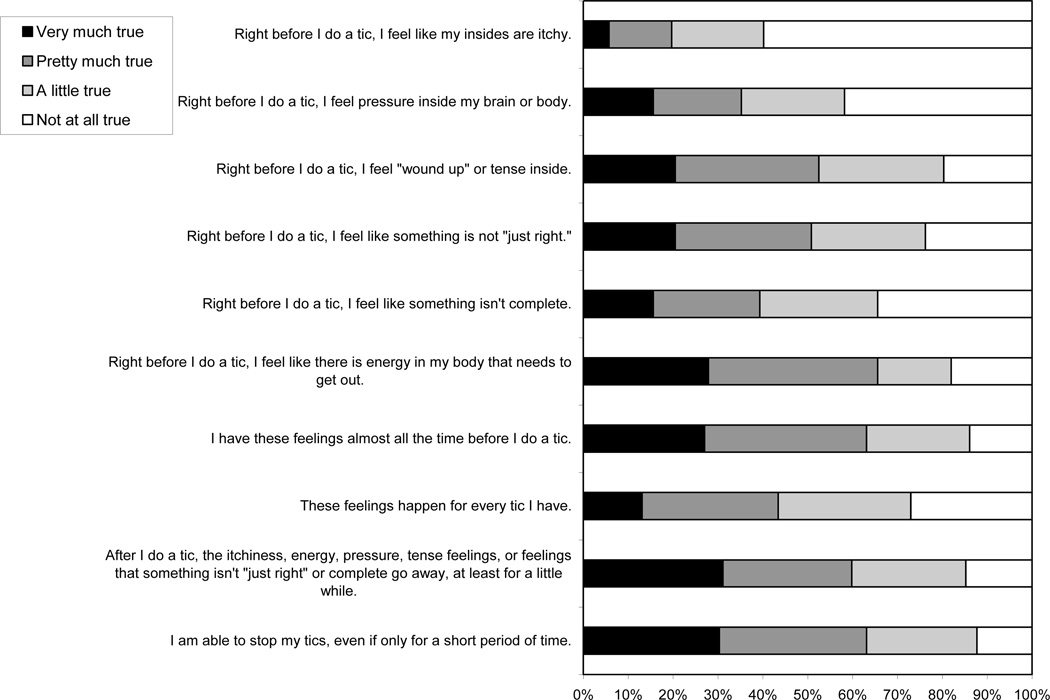
The means and standard deviations for items 1–10 are listed in Table 1. Also see Figure 1 for depiction of item-by-item response frequencies. In the following summary, we describe the percentage of individuals who endorsed each item to some degree (i.e., provided an item rating of 2 [“a little true”] or higher).
Figure 1.
Item-by-item response frequencies.
Items 1–6 assess the quality of the premonitory urge. As predicted, “an energy in my body that needs to get out” and an inner feeling of being “wound up” or tense were the most commonly endorsed sensations, with over 80% of individuals endorsing these items. Feelings of something being not “just right” or incomplete were endorsed by 76.2% and 65.6% of individuals, respectively. Feelings of “pressure inside my brain or body” were endorsed by 58.2% of individuals. Only 40.2% of individuals endorsed inner sensations of itchiness.
Items 7 and 8 assess the frequency of the premonitory urge to tic. Approximately 86% of individuals endorsed item 7 (I have these feelings almost all the time before I do a tic). Nearly three out of four (73.0%) participants endorsed item 8 (These feelings happen for every tic I have).
Item 9 (After I do a tic, the itchiness, energy, pressure, tense feelings, or feelings that something isn’t “just right” or complete go away, at least for a little while) assesses the presumed functional relationship between the tic and the urges to tic. Consistent with the behavioral model of tics, it was endorsed by over 85% of individuals.
Item 10 (I am able to stop my tics, even if only for a short period of time) assesses the ability to suppress tics rather than the premonitory urge. Consistent with our understanding of tics as semi-voluntary behaviors, 87.7% of individuals reported being able to stop their tics even if only for a short period of time.
Correlates of the degree of premonitory urges
To examine factors potentially associated with degree of premonitory urges, we conducted a t-test comparing the total PUTS score for males to that of females, and conducted correlations between total PUTS score and the following variables: age, estimated IQ, tic severity, OCD symptom severity, and ADHD symptom severity. To examine the effect of medication status on the premonitory urge to tic, we conducted a between-groups ANOVA comparing those individuals taking no medication, tic medication only, tic and other psychiatric medication, or other psychiatric medication only.
Males and females did not significantly differ in the degree of premonitory urges, t (120) = .32, p = .75 (male M = 21.4; female M = 21.8). Total PUTS score was not significantly correlated with age, r = .10, p = .29. We did observe a small to medium positive correlation between total PUTS score and estimated full-scale IQ, as measured by the WTAR, , r = .21, p =.02, such that greater premonitory urges were associated with higher estimated IQ.
As described above, total PUTS score was moderately positively correlated with tic severity, as measured by the total tic score of the YGTSS, r = 0.32, p < 0.001, suggesting that those individuals with greater premonitory urges also experience more severe tics. To examine whether degree of premonitory urges differentially correlated with motor or vocal tic severity, we repeated the above correlations for the motor tic and phonic tic subscale scores separately. A different pattern of results emerged. Total PUTS score was not significantly correlated with overall motor tic severity. Moreover, of the five motor tic subscales, we observed only one significant correlation, a small to medium positive relationship between total PUTS score and motor tic complexity, r = .19, p = .04. In contrast, total PUTS score was moderately positively correlated with overall phonic tic severity, r = .30, p = .001, and weakly to moderately positively correlated with all five phonic tic subscales (number: r = .30, p = .01; frequency: r = .24, p = .01; intensity: r = .21, p = .02, complexity: r = .19, p = .04; interference: r = .34, p < .001). Taken together, these results suggest the relationship between premonitory urge severity and overall tic severity may be primarily accounted for by the relationship between premonitory urge severity and phonic tic severity.
Overall OCD symptom severity, as measured by the YBOCS total score, was not significantly correlated with the total PUTS score, r = .11, p = .25. Because of previous work suggesting a relationship between “just right” perceptions (tapped by items 4 & 5) and symptoms of OCD among individuals with TS (Leckman, Walker, Goodman, Paul, & Cohen, 1994), we decided to examine whether subjects with comorbid OCD endorsed these items more strongly than those without comorbid OCD. To do this, we conducted two independent sample t-tests comparing individuals with (n = 19) and without (n = 103) current comorbid OCD on the mean score of items 4 (Right before I do a tic, I feel like something is not “just right”) and 5 (Right before I do a tic, I feel like something isn’t complete). Individuals with comorbid OCD did more strongly endorse item 4 (not “just right”), t (120) = 2.37, p = .02. Item 5 (feelings of incompleteness) was also more strongly endorsed by those with comorbid OCD, although not significantly so t (120) = 1.89, p = .06.
Overall ADHD symptom severity, as measured by the ADHD-RS, was not significantly correlated with the total PUTS score, r = .08, p = .39. Total PUTS score also did not significantly differ based on medication status, F(3, 118) = .75, p = .52.
Discussion
In summary, the PUTS appears to be a reliable measure of the premonitory urge to tic among adults. The estimates of internal consistency and temporal stability were acceptable and comparable to those observed by Woods et al. (2005) in youths 11–16 years of age. It also showed good concurrent validity when compared with the YGTSS, demonstrating that it is tapping a related but distinct feature of the disorder.
Consistent with several previous reports, the premonitory urge to tic was endorsed to some degree by an overwhelming majority (93.4%) of individuals. Consistent with Crossley et al. (2013) and Woods et al. (2005), the present sample most frequently endorsed “energy in my body that needs to get out” and an inner feeling of being “wound up” or tense. Inner sensations of itchiness were the least commonly endorsed sensations. This differs from previous reports that have emphasized an itch-like quality to the urges. These findings could have implications for the language that we use when inquiring about the premonitory urge to tic among our patients. Describing the sensations in an accurate manner could improve patients’ ability to recognize them. We would recommend that clinicians focus their descriptions of the premonitory urge on a feeling of being wound up, tense, or having energy in the body that needs to get out.
The majority of participants reported experiencing some relief from the urge to tic upon completion of a tic, as well as being able to stop their tics even if only for a short time. Taken together, these results provide further support for the functional relationship between the urge to tic and the tic itself that is so central to the behavioral model of tics.
We did not observe a relationship between age and the degree of premonitory urges to tic. The mean score among adults (adult M = 21.5, SD = 6.1) was slightly elevated relative to that observed in youths ages 11–16 by Woods et al. (2005; youth M = 18.6, SD = 4.6). This is not surprising. Previous work suggests that awareness of the premonitory urge to tic develops around 10 years of age (Leckman et al., 1993). It may be that by adulthood awareness of the urge is firmly established and remains relatively stable across the lifespan.
In contrast to Leckman and colleagues’ (1993) observation that males were more likely to report premonitory urges than females, we did not observe any gender differences in the degree of premonitory urges. This is consistent with the more recent findings of Sutherland Owens and colleagues (2011) and Crossley et al. (2013).
The relationship between estimated IQ and the premonitory urge to tic was somewhat surprising. Although it is a modest relationship, it is possible that increased IQ is indicative of greater verbal ability, which enables patients to label and describe the premonitory sensations and their relationship to the tics. We would advise caution in the interpretation of this finding, however, given the brief nature of the IQ assessment. Future work employing a full IQ battery could confirm this preliminary hypothesis.
That premonitory urge severity was related to overall tic severity is not surprising. If tics are semi-voluntary responses to the premonitory urges, as the behavioral model of tics suggests, then more severe urges should be related to more severe tics. However, the unique relationship between the degree of premonitory urges and phonic tic severity was unexpected. The reasons for this are not entirely clear. Because vocal tics are often more noticeable than motor tics, they may be more often associated with negative social consequences. Some researchers have hypothesized that negative social consequences (i.e., social punishment) may intensify the premonitory urges via conditioning (Capriotti, Brandt, Ricketts, Espil, & Woods, 2012). In other words, because the urges come to signal the onset of a tic that is then punished by the environment, the urge itself may become increasingly more aversive over time. Future work would of course be necessary to replicate the relationship between degree of premonitory urges and phonic tic severity and to test this and possibly other alternative explanatory hypotheses. It would also be interesting to directly assess the premonitory urges for different types of tics (motor vs. phonic, simple vs. complex) to determine whether the quality, intensity, or frequency of the urges differs in any systematic manner.
We did not observe the relationship between degree of premonitory urges and overall severity of comorbid OCD symptoms previously observed by Woods et al. (2005). Our data supported a more specific relationship between “just right” premonitory sensations and OCD symptom severity consistent with that described by Leckman et al. (1994). Consistent with Woods et al. (2005), however, degree of premonitory urges was not related to degree of comorbid ADHD symptoms. Consistent with Crossley et al. (2013), it was also not related to medication status.
The present study is not without limitations. A primary limitation is that our sample consisted of a clinical sample of individuals. It is possible that our findings may not generalize to the larger population of individuals with TS or CTD. By restricting our examination to a clinical sample, we may have also limited the range of possible scores and therefore limited our ability to detect relationships with other variables. Additionally, our sample was limited to older adolescents and adults. Although our primary aim was to examine the PUTS and premonitory urges in this age range, future studies examining premonitory urges among individuals of all ages could provide more nuanced information on the developmental course of premonitory urges.
The present manuscript represents another step forward in our efforts to understand the premonitory sensations associated with TS and CTD. We hope that standardization in measurement of the premonitory urge to tic will facilitate the advancement of our knowledge regarding this important feature of the disorder. We also hope that a greater understanding of the characteristics and correlates of premonitory urges will inform treatment. Current behavioral treatments for tics (e.g., Habit Reversal Training (HRT), Comprehensive Behavioral Intervention for Tics (CBIT); see Woods et al., 2008) focus heavily on the premonitory urge. Patients are helped to increase their awareness of the premonitory urge to tic and to break the connection between the premonitory urge and the tic by implementing a competing response upon detecting this urge. Therefore, a thorough understanding and assessment of the premonitory urges to tic are essential to the clinician and patient. Moreover, advances in our knowledge regarding the role that premonitory urges play in the etiology and maintenance of tic disorders may help us to further refine our treatment approaches.
Highlights.
The PUTS demonstrated adequate internal consistency, temporal stability, and concurrent validity.
Premonitory urges were endorsed by the majority of individuals with tourette syndrome and chronic tic disorder.
Degree of premonitory urges was significantly associated with estimated IQ and tic severity, but not with age, gender, severity of comorbid obsessive compulsive disorder, attention deficit hyperactivity disorder, or concomitant medication status.
Acknowledgments
This research was supported by grants 5R01MH069877 (Dr. Wilhelm), R01MH069874 (Dr. Scahill), and RO1MH069875 (Dr. Petersen) from the National Institute of Mental Health (NIMH) with subcontracts to Dr. Piacentini and Dr. Woods. Dr. Walkup consulted on this grant. The funding agency played no role in the study design, the collection, analysis or interpretation of data, or in the preparation or review of this manuscript. We would like to acknowledge James Dizura and Haibei Liu for assistance with the study data. Additionally, we would like to thank the CBITS team and all of the patients who participated in this trial.
Abbreviations
- ADHD
attention deficit hyperactivity disorder
- ADHD-RS
Attention Deficit Hyperactivity Disorder Rating Scale
- CBIT
Comprehensive Behavioral Intervention for Tics
- CTD
chronic tic disorder
- IQ
Intelligence Quotient
- DSM-IV-TR
Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision
- K-SADS-PL
Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version
- OCD
obsessive compulsive disorder
- PST
Psychoeducation and Supportive Therapy
- PUTS
Premonitory Urge to Tic Scale
- SCID-I/P
Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition
- TS
tourette tyndrome
- WTAR
Wechsler Test of Adult Reading
- YBOCS
Yale-Brown Obsessive Compulsive Scale
- YGTSS
Yale Global Tic Severity Scale
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Because this study was designed prior to the publication of the initial psychometric data on the PUTS (Woods et al., 2005), participants completed the original 10-item version of the PUTS. We focus our analyses on the first 9 items, but present data regarding item 10 when indicated.
Contributor Information
Hannah E. Reese, Email: hreese@partners.org.
Alan L. Peterson, Email: petersona3@uthscsa.edu.
Douglas W. Woods, Email: dwoods@uwm.edu.
John Piacentini, Email: jpiacentini@mednet.ucla.edu.
Sabine Wilhelm, Email: sabinewilhelmwilhelm@earthlink.net.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed., text rev. Washington DC: Author; 2000. [Google Scholar]
- Capriotti MR, Brandt BC, Ricketts EJ, Espil FM, Woods DW. Comparing the effects of differential reinforcement of other behavior and response-cost contingencies on tics in youth with Tourette syndrome. Journal of Applied Behavior Analysis. 2012;45:251–263. doi: 10.1901/jaba.2012.45-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AJ, Leckman JF. Sensory phenomena associated with Gilles de la Tourette's syndrome. Journal of Clinical Psychiatry. 1992;53(9):319–323. [PubMed] [Google Scholar]
- Crossley E, Seri S, Stern JS, Robertson MM, Cavanna AE. Premonitory urges for tics in adult patients with Tourette syndrome. Brain and Development. doi: 10.1016/j.braindev.2012.12.010. in press. [DOI] [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD Rating Scale—IV: Checklists, norms, and clinical interpretation. New York, NY: Guilford Press; 1998. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York, NY: Biometrics Research; 2002. [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C. The Yale-Brown Obsessive Compulsive Scale: I. Development, use, and reliability. Archives of General Psychiatry. 1989a;46(11):1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. http://dx.doi.org/10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C. The Yale-Brown Obsessive Compulsive Scale: II. Validity. Archives of General Psychiatry. 1989b;46:1012–1016. doi: 10.1001/archpsyc.1989.01810110054008. http://dx.doi.org/10.1001/archpsyc.1989.01810110054008. [DOI] [PubMed] [Google Scholar]
- Groth-Marnat G. Handbook of Psychological Assessment. New Jersey: John Wiley & Sons, Inc; 2003. [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. http://dx.doi.org/10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kurlan R, Lichter D, Hewitt D. Sensory tics in Tourette's syndrome. Neurology. 1989;39(5):731–734. doi: 10.1212/wnl.39.5.731. http://dx.doi.org/10.1212/WNL.39.5.731. [DOI] [PubMed] [Google Scholar]
- Kwak C, Dat Vuong K, Jankovic J. Premonitory sensory phenomenon in Tourette’s Syndrome. Movement Disorders. 2003;18(12):1530–1533. doi: 10.1002/mds.10618. http://dx.doi.org/10.1002/mds.10618. [DOI] [PubMed] [Google Scholar]
- Lang A. Patient perception of tics and other movement disorders. Neurology. 1991;41(2, Pt 1):223–228. doi: 10.1212/wnl.41.2_part_1.223. http://dx.doi.org/10.1212/WNL.41.2_Part_1.223. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Riddle MA, Hardin MT, Ort SI. The Yale Global Tic Severity Scale: Initial testing of a clinician-rated scale of tic severity. Journal of the American Academy of Child and Adolescent Psychiatry. 1989;28(4):566–573. doi: 10.1097/00004583-198907000-00015. http://dx.doi.org/10.1097/00004583-198907000-00015. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Walker DE, Cohen DJ. Premonitory urges in Tourette's syndrome. American Journal of Psychiatry. 1993;150(1):98–102. doi: 10.1176/ajp.150.1.98. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Walker DE, Goodman WK, Pauls DL, Cohen DJ. “Just Right” perceptions associated with compulsive behavior in Tourette’s Syndrome. American Journal of Psychiatry. 1994;151:675–680. doi: 10.1176/ajp.151.5.675. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Zhang H, Vitale A. Course of tic severity in Tourette’s Syndrome: The first two decades. Pediatrics. 1998;102:234–245. doi: 10.1542/peds.102.1.14. [DOI] [PubMed] [Google Scholar]
- Patrick HT. Convulsive tic. Journal of the American Medical Association. 1905;44:437–442. http://dx.doi.org/10.1001/jama.1905.92500330005001a. [Google Scholar]
- The Psychological Corporation. Wechsler Test of Adult Reading manual. Author: San Antonio, TX; 2001. [Google Scholar]
- Safren SA, Sprich S, Mimiaga MJ, Surman C, Knouse L, Groves M, Otto MW. Cognitive Behavioral Therapy vs Relaxation With Educational Support for Medication-Treated Adults With ADHD and Persistent Symptoms. A Randomized Controlled Trial. JAMA. 2010;304(8):875–880. doi: 10.1001/jama.2010.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scahill LD, Leckman JF, Marek KL. Sensory phenomena in Tourette’s Syndrome. In: Weiner WJ, Lang AE, editors. Behavioral Neurology of Movement Disorders. New York, NY: Raven Press; 1995. pp. 273–280. [PubMed] [Google Scholar]
- Shapiro AK, Shapiro ES. In: Gilles de Tourette Syndrome. Sensory tics. 2nd ed. Shapiro AK, Shapiro ES, Young JG, Feinberg TE, editors. New York: Raven Press; 1988. pp. 356–360. [Google Scholar]
- Spencer T, Wilens T, Biederman J, Faraone SV. A double-blind, crossover comparison of methylphenidate and placebo in adults with childhood-onset attention-deficit hyperactivity disorder. Archives of General Psychiatry. 1995;52(6):434–443. doi: 10.1001/archpsyc.1995.03950180020004. http://dx.doi.org/10.1001/archpsyc.1995.03950180020004. [DOI] [PubMed] [Google Scholar]
- Sutherland Owens AN, Miguel EC, Swerdlow NR. Sensory gating scales and premonitory urges in Tourette Syndrome. The Scientific World Journal. 2011;11:736–741. doi: 10.1100/tsw.2011.57. http://dx.doi.org/1100/tsw.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourette Syndrome Association International Consortium for Genetics (TSAIG) Genome scan for Tourette disorder in affected-sibling-pair and multigenerational families. Am J Hum Genet. 2007;80:265–272. doi: 10.1086/511052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE, Biederman J, Prince J, Spencer TJ, Faraone SV, Warburton R, Geller D. Six-week, double-blind, placebo-controlled study of desipramine for adult attention deficit hyperactivity disorder. American Journal of Psychiatry. 1996;153(9):1147–1153. doi: 10.1176/ajp.153.9.1147. Retrieved from http://ajp.psychiatryonline.org/journal.aspx?journalid=13. [DOI] [PubMed] [Google Scholar]
- Wilhelm SPeterson AL, Piacentini J, Woods DW, Deckersbach T, Sukhodolsky DG, Scahill L. Randomized trial of behavior therapy for adults with Tourette Syndrome. Archives of General Psychiatry. 2012;69(8):795–803. doi: 10.1001/archgenpsychiatry.2011.1528. Retrieved from http://ajp.psychiatryonline.org/journal.aspx?journalid=13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods DW, Piacentini J, Himle MB, Chang S. Premonitory urge for tics scale (PUTS): Initial psychometric results and examination of the premonitory urge phenomenon in youths with tic disorders. Journal of Developmental and Behavioral Pediatrics. 2005;26(6):397–403. doi: 10.1097/00004703-200512000-00001. Retrieved from http://journals.lww.com/jrnldbp/pages/default.aspx. [DOI] [PubMed] [Google Scholar]
- Woods DW, Piacentini JC, Chang S, Deckersbach T, Ginsburg GS, Peterson AL, Scahill LD, Walkup JT, Wilhelm S. Managing Tourette syndrome: A behavioral intervention for children and adults. New York, NY: Oxford University Press; 2008. [Google Scholar]
- Zohar AH, Apter A, King RA, Pauls DL, Leckman JF, Cohen DJ. Epidemiological studies. In: Leckman JF, Cohen DJ, editors. Tourette’s syndrome—tics, obsessions, compulsions: Developmental pathology and clinical care. New York: Wiley; 1999. pp. 177–193. [Google Scholar]