Abstract
Because eosinophils express CD52 antigen, 12 patients with refractory or relapsed hypereosinophilic syndrome were treated with alemtuzumab, an anti-CD52 antibody. Brisk elimination of signs and symptoms of the disease was achieved in almost all patients, with durability of response better in those on maintenance therapy. Rechallenge with alemtuzumab in relapsing patients yields the same efficacy repeatedly. Adverse effects are mostly related to immunosuppression.
Background
Relapsing, refractory patients with idiopathic hypereosinophilic syndrome (I-HES) and chronic eosinophilic leukemia–not otherwise specified (CEL-NOS) do not have many effective, durable therapeutic options. Alemtuzumab, an anti-CD52 antibody, has been reported to be an effective therapy due to inherent expression of CD52 on eosinophils.
Methods
A retrospective chart review of 12 patients treated with alemtuzumab at our center until 2012.
Results
Ten (83%) of 12 patients achieved complete hematologic response (CHR) after a median of 1 week for a median duration of 66 weeks, with the elimination of disease-related symptoms; 2 patients achieved partial hematologic remission hematologic remission (PHR). Patients with CHR who received alemtuzumab maintenance (n = 5) had a significantly longer time to progression than those patients who were only observed (n = 5) (P = .01). Eleven patients relapsed (only one while on maintenance), and 6 were rechallenged with alemtuzumab. Five (83%) achieved second CHR after a median of 3.5 weeks, for a median duration of 123 weeks. Again, those given maintenance (n = 3) had a longer time to progression than those who were only observed (P = .04). Adverse effects were mostly related to infusion reactions and lymphopenia-related viral infections (despite antibiotic prophylaxis). One patient developed Epstein-Barr virus–related lymphoma.
Conclusions
Alemtuzumab is an effective treatment for patients with relapsed, refractory idiopathic hypereosinophilic syndrome and chronic eosinophilic leukemia–not otherwise specified, in terms of both CHR achievement (even after repeated rechallenges) and duration (particularly if provided as a maintenance therapy). Common adverse effects are related to infusion reactions and immunosuppression.
Keywords: Alemtuzumab, Chronic eosinophilic leukemia, Idiopathic hypereosinophilic syndrome, Therapy
Introduction
Eosinophilic bone marrow disorders are a heterogeneous group.1,2 The 2008 World Health Organization classification of hematologic neoplasms has identified 4 subtypes: (i) myeloid and lymphoid neoplasms with eosinophilia and abnormalities of platelet-derived growth factor receptor alpha (PDGFRα), platelet-derived growth factor receptor beta (PDGFRβ), or fibroblast growth factor receptor 1 (FGFR1); (ii) chronic eosinophilic leukemia–not otherwise specified (CEL-NOS); (iii) lymphocytic variant of hypereosinophilic syndrome (L-HES), and (iv) idiopathic hypereosinophilic syndrome (I-HES).3 CEL-NOS is distinguished from I-HES by the presence of clonal cytogenetic abnormality or increased blasts (>2% in the peripheral blood or >5% in bone marrow but <20% in both compartments).3
Molecular abnormalities that contain PDGFRα and PDGFRβ genes are highly sensitive to low-dose imatinib,4–6 whereas immunosuppressive treatments are commonly effective long term for lymphocytic variant of hypereosinophilic syndrome.7 CEL-NOS and I-HES are usually treated in the case of a symptomatic organ involvement, primarily with prednisone but also with hydroxyurea (HU), interferon alfa, and high-dose imatinib; bone marrow transplant (BMT) is the only curative option.8 Affected patients are usually exposed to multiple different therapies over the disease course to counteract disease signs and symptoms.3 The largest published retrospective multicenter clinical analysis of therapy success (188 cases, including patients with I-HES, L-HES, and FIP1L1-PDGFRα) has described complete responses in more than 50% of patients when using traditional treatments. However, the duration of response has not been described.9 For example, although corticosteroids are usually effective, their long-term use is associated with a number of adverse effects. However, unmaintained remissions are rare,10 and corticosteroid-resistant cases respond poorly to traditional therapies.11
A number of new therapeutic agents have been evaluated in I-HES and CEL-NOS, including tyrosine kinase inhibitors, anti-interleukin-5 and anti-CD52 antibodies.12,13 The use of alemtuzumab, an anti-CD52 antibody approved as therapy for chronic lymphocytic leukemia, in CEL-NOS and I-HES is justified by the biologic evidence of CD52 expression on eosinophils.14,15 Its efficacy in refractory HES, at the dosage of 30 mg weekly, was reported for the first time in 2004.16 A second case report described a patient with HES relapsing after allogeneic BMT, who achieved complete remission with alemtuzumab and was on a maintenance regimen of 30 mg every 3 weeks.17 In 2009, we published an observational study of 11 patients (9 with I-HES and 2 with CEL-NOS) treated with alemtuzumab as salvage therapy.18 Ten patients achieved a complete hematologic response (CHR), with resolution of bone marrow eosinophilia in 4 of 7 cases (but no cytogenetic response in CEL-NOS cases). Clinical symptoms disappeared in 9 of 10 patients who achieved CHR. Observed adverse effects were infusion related (fever, shortness of breath), lymphopenia, 2 cytomegalovirus (CMV) reactivations, 1 Epstein-Barr virus positive orbital diffuse large B-cell lymphoma, and 1 periorbital cellulitis. Seven of 10 patients relapsed (5 while off therapy and 2 while on therapy), and most obtained a second remission after alemtuzumab rechallenge. These data have highlighted a possible role for alemtuzumab as salvage therapy in patients with relapsed-refractory I-HES and CEL-NOS, but data about its efficacy and safety in a long-term setting and the role of maintenance therapy are still lacking. Here we provide an update on long-term outcome of 9 previously reported and 3 new patients treated with alemtuzumab at our institution.
Methods and Patients
This is a retrospective analysis, based on the institutional review board approved retrospective chart review protocol, of the efficacy and safety of alemtuzumab therapy in 12 patients with either I-HES or CEL-NOS treated at MD Anderson until 2012 (Tables 1 and 2). A diagnosis of I-HES and CEL-NOS was established according to the World Health Organization criteria.3 Cytogenetic analysis and molecular screening for FIPL1-PDGFRα, and T-cell receptor rearrangements were performed before treatment. Informed consent for therapy was obtained from all patients before alemtuzumab (Genzyme Corporation, Sanofi Company, Paris, France) administration. During and for approximately 2 months after treatment with alemtuzumab, all the patients received prophylaxis with both trimethoprim/sulfamethoxazole 800/160 mg orally twice daily 3 times a week and valacyclovir 500 mg orally daily. CMV antigenemia was assessed at baseline and approximately every 3 months both during treatment and for 2 months after alemtuzumab discontinuation. CHR was defined as normalization of the absolute eosinophil count. Partial hematologic remission (PHR) was defined as a reduction in peripheral blood eosinophilia by at least 50% from baseline. CHR and PHR were assessed during and after 1 cycle (4 weeks) of therapy. Any alemtuzumab therapy provided after 1 cycle (4 weeks) was considered as maintenance therapy. Time to progression (TTP) was defined as the time from CHR achievement until the absolute eosinophil count increase over the normal range and was calculated by using Kaplan-Meier estimates with SPSS version 19 (SPSS Inc, Chicago, IL). Three patients had CEL-NOS (Tables 1 and 2) due to abnormal karyotype: del (12) (q24.1q24.3), t(5;6) (q22; q21), and +8, respectively. Previous therapies included corticosteroids (in 10 patients), HU (6), imatinib (8), nilotinib (1), dasatinib (3), interferon alfa (2), methotrexate (1), cladribine plus cytarabine (1), and splenectomy (1). The main patient disease-related symptoms before starting therapy were skin rash (7), constitutional symptoms (2), and gastrointestinal tract symptoms (2).
Table 1.
Patient Baseline Characteristics
| Median (range), Number (%) | |
|---|---|
| Age, y | 51 (26–81) |
| Sex, No. | |
| Men | 8 (67%) |
| Women | 4 (33%) |
| Previous Treatments, No. | 2 (1–4) |
| Absolute Eosinophil Count, K/uL | 5.8 (1–40) |
| Eosinophil Count (%) | 24 (7–74) |
| White Blood Cell Count, K/uL | 18 (6.9–113) |
| Hemoglobin, g/dL | 12 (8.8–15.9) |
| Abnormal Karyotype, No. | 3 (25%) |
Table 2.
Individual Clinical Characteristics and Response to Alemtuzumab in 12 Patients With I-HES and CEL-NOS
| First Inductiona | Response/Time to Response (weeks) | BM while in CHR | First Maintenance | Response Duration/Time Off Therapy Before Relapse (weeks) | Second Induction | Response/Time to Response (weeks) | Second Maintenance | Response Duration/Time Off Therapy Before Relapse (weeks) | Current Status |
|---|---|---|---|---|---|---|---|---|---|
| 30 mg I.V. t.i.w. × 12 doses | CHR/3.5 | CR | 6/2 | 30 mg I.V. QW × 4 doses | PHR/3 | 5/2 | Given nilotinib; died of PD | ||
| 30 mg s.c. t.i.w. × 12 doses | CHR/0.5 | ND | 6/2 | Died of PD | |||||
| 30 mg I.V. t.i.w. × 12 doses | CHR/0.5 | ND | 16/12 | 30 mg s.c. t.i.w. × 12 doses | CHR/2 | 30 mg s.c. QW × 2 doses | 41/35 (ongoing CHR) | Died while in CHR | |
| 30 mg s.c. t.i.w. × 12 doses | CHR/8 | PR | 30 mg s.c. Q2W × 133 doses | 292/44 (ongoing CHR) | Alive on no therapy | ||||
| 10 mg I.V. t.i.w. × 12 doses | CHR/0.5 | CR | 10 mg I.V. QW × 1 dose | 11/6 | Died of TRM after BMT | ||||
| 10 mg I.V. t.i.w. × 12 doses | CHR/5 | PR | 10 mg s.c. t.i.w. × 30 doses | 39/29 | 10 mg s.c. QW × 4 doses | CHR/3 | 10 mg I.V. b.i.w. × 14 doses | 240/229 (ongoing CHR) | Alive on no therapy |
| 30 mg I.V. t.i.w. × 12 doses | PHR/2 | NR | 5/1 | Alive after HU and imatinib | |||||
| 30 mg I.V. t.i.w. × 12 doses | CHR/1 | ND | 10 mg I.V. Qmonth × 15 doses | 66/0 | 30 mg I.V. t.i.w. × 12 doses | CHR/5 | 30 mg I.V. QW × 1 dose | 7/0 | Alive on therapy for DLBCL |
| 30 mg s.c. t.i.w. × 24 doses | PHR/10.5 | PR | 15/7 | Died of PD | |||||
| 30 mg I.V. t.i.w. × 12 doses | CHR/1 | CR | 19/15 | 30 mg I.V. b.i.w. × 8 doses | CHR/3 | 14/10 | Alive on maintenance p.r.n. | ||
| 30 mg I.V. t.i.w. × 12 doses | CHR/2 | NR | 30 mg s.c. b.i.w. × 40 doses | 68/4 | 30 mg s.c. b.i.w. × 4 doses | CHR/2 | 17/15 | Alive on maintenance p.r.n. | |
| 30 mg s.c. t.i.w. × 12 doses | CHR/1 | ND | 5/1 | Alive after BMT |
Abbreviations: b.i.w. = twice a week; BM = bone marrow; BMT = bone marrow transplantation; CEL-NOS = chronic eosinophilic leukemia–not otherwise specified; CHR = complete hematologic remission; CR = complete remission; DLBCL = diffuse large B-cell lymphoma; HES = hypereosinophilic syndrome; HU = hydroxyurea; I.V. = intravenous; ND = not done; NR = no response; p.r.n. = as needed; PD = progressive disease; PHR = partial hematologic remission; PR = partial remission; Q2W = every 2 weeks; Qmonth = every month; QW = every week; s.c. = subcutaneous; t.i.w. = 3 times a week; TRM = transplantation-related mortality.
The patients were given alemtuzumab initially at the dose of 5 mg I.V. on day 1, then 10 mg I.V. on day 2 and 30 mg I.V. on day 3 if tolerated.
Results
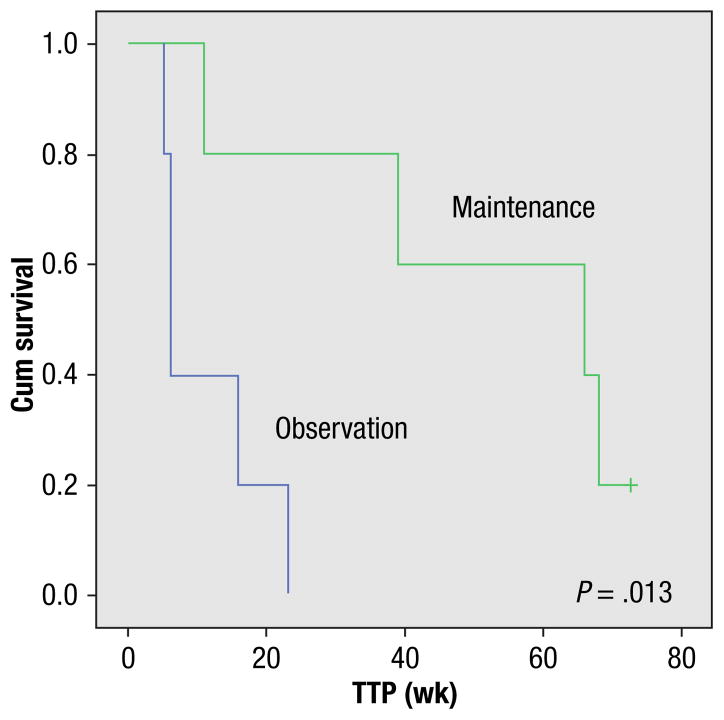
Ten patients achieved CHR, with the elimination of disease-related symptoms, and 2 achieved PHR (with persistence of symptoms). The median time to CHR was 1 week (range, 0.5–3.5 weeks), and the median duration of CHR was 66 weeks (range, 11–73 weeks). Five patients were given maintenance with alemtuzumab for a median duration of 20 weeks (range, 1–266 weeks). Patients with CHR who received maintenance (n = 5) had a significantly longer TTP than the patients who were simply observed (n = 5) (16 vs. 2 weeks; P =.01) (Figure 1). Bone marrow was repeated in 8 patients: 3 normalized eosinophil percentage (complete remission), and 3 had more than 50% reduction in eosinophil percentage (partial remission). No cytogenetic response was observed in patients with CEL-NOS. Eleven (92%) patients relapsed; only one was on maintenance therapy at the time of relapse. One patient who has not relapsed yet (in CHR now for close to 300 weeks) has CEL-NOS with 12q-abnormality. Signs and symptoms at relapse were skin rash (4), fatigue (1), gastrointestinal tract symptoms (2), and renal failure due to eosinophilic infiltrate (1). Two (18%) patients died after relapse without further therapy. Three were given different therapies: BMT, cladribrine plus cytarabine followed by HU and BMT, HU followed by imatinib, respectively (1 died in complete remission of transplantation-related complications, and the other 2 are still alive).
Figure 1.
Time to Progression in Patients Provided Maintenance Alemtuzumab vs. Observation Only
Six were rechallenged with alemtuzumab, and 5 (83%) achieved a second CHR after a median of 3.5 weeks (range, 1.9–5 weeks); 3 of the 5 patients were given maintenance therapy with alemtuzumab for a median of 2 weeks (range, 1–7 weeks). The median duration of the second CHR was 123 weeks (range, 5–240 weeks): those given maintenance (n = 3), although very short, again had longer TTP than the ones who were simply observed (n = 2; P = .04). Of the 5 responders, 1 died while in CHR and one is in ongoing CHR for more than 240 weeks. The remaining 3 relapsed (only 1 patient while still on therapy), 2 patients were rechallenged with alemtuzumab and are still alive and in CHR.
Therapy-related adverse effects were seen in 10 (83%) patients: CMV reactivation (2), zoster reactivation (1), pneumonia (3), rash (1), Epstein-Barr virus–related diffuse large B cell lymphoma (1), fever (4), conjunctivitis (1), and mild renal failure with creatinine 1.7 mg/dL (1). Among hematologic toxicities, lymphopenia was the only significant toxicity, seen in 11 of 12 patients. The intravenous route of administration was used in 8 patients, and the subcutaneous route was used in 4; no difference in treatment efficacy or observed toxicity was seen.
Discussion
In our series of patient with I-HES and CEL-NOS, alemtuzumab proved to be very effective in eliminating blood eosinophils and controlling disease-related signs and symptoms. The response is brisk (usually within a week) and, with maintenance therapy, can be durable. Bone marrow clearance of eosinophils, however, has not been achieved as often; however, it did not correspond to a clinical response (blood count, signs, and symptoms), and, therefore, its value is questionable. In everyday practice, it may not be necessary to perform a bone marrow biopsy after alemtuzumab therapy for the purpose of a response assessment. Alemtuzumab again was shown to be very effective upon patient rechallenge, which resulted in almost all patients again achieving CHR (with a slightly longer median time to response, of 3.5 weeks). In a few patients, we used alemtuzumab for a third time, again with an excellent response. This is important, given the difficulty of reinducing a complete remission (hematologic and clinical) in patients with relapsing refractory HES with traditional therapies.11
The use of a maintenance treatment after the initial 4 weeks of therapy provided a significant advantage in terms of TTP, both after the first and the second trial of alemtuzumab (Figure 1). The decision to provide a given patient maintenance therapy or not was in the hands of the treating physician, based on his or her evaluation of the patient’s current status, ability to provide continuous therapy, and other factors. Although alemtuzumab apparently can provide significant benefit to a patient several times (rechallenges are effective), use of alemtuzumab at a lower dose and in a less-aggressive schedule as maintenance may provide optimal benefit of long-term good control of disease signs and symptoms. Our data certainly encourage the use of alemtuzumab in a maintenance setting.
Most of the reported alemtuzumab-related adverse effects were either transfusion related or related to infectious problems, and were seen early in the therapy course. Despite appropriate prophylaxis, cases of CMV and zoster were seen, related to lymphopenia and elimination of T lymphocytes. Lymphopenia was the only hematologic toxicity, notwithstanding the recent discovery of CD52 expression also on neutrophils.15 Interestingly, it subsided almost completely during the maintenance phase and, concordantly, adverse effects were less frequent. One case of Epstein-Barr virus–related diffuse large B-cell lymphoma was recorded, which highlights the risk of long-term adverse effects, likely related to long-lasting immunodeficiency. Both in terms of efficacy and toxicity, no difference was reported for the 2 different routes of administration (intravenous vs. subcutaneous), which allowed both to be used according to the preferential clinical setting.
Conclusion
Alemtuzumab is an effective treatment for I-HES and CEL-NOS in terms of both CHR achievement (even after repeated rechallenges) and duration (particularly if provided as a maintenance therapy). It is reserved as therapy for patients with relapsed refractory disease to standard therapies because it carries a possibility of significant early adverse effects, lymphopenia related, such as viral infections (which requires patients to take prophylactic antibiotics), and late complications associated with prolonged immunosuppression, such as secondary lymphoma. Our data may provide the impetus for the performance of larger more comprehensive studies of alemtuzumab in the salvage setting. The maker of alemtuzumab has recently withdrawn alemtuzumab from the United States and European markets to prepare for the drug’s relaunch in a lower-dose form as a therapy for patients with relapsed or refractory multiple sclerosis. Therefore, future treatment of patients with HES/CEL with alemtuzumab will be possible through a compassionate-use program.
Clinical Practice Points.
Patients who have relapsed, refractory I-HES and CEL-NOS do not have many effective durable therapeutic options.
Alemtuzumab, an anti-CD52 monoclonal antibody, can provide CHR and elimination of disease-related signs and symptoms in almost all patients in this setting.
Resistance to alemtuzumab appears not to develop as rechallenge with alemtuzumab in relapsing patient provides again excellent result.
Introduction of the maintenance alemtuzumab therapy may significantly contribute to the durability of the response, as TTP is significantly prolonged.
Alemtuzumab is an effective treatment for I-HES and CEL-NOS, in terms of both CHR achievement (even after repeated rechallenges) and duration (particularly if provided as a maintenance therapy). This treatment is reserved to relapsing and refractory patients, because of potential side effects, particularly lymphopenia-related viral infections (patients are required to take prophylactic antibiotics), and long-term risk of lymphoma.
Footnotes
Disclosure
The authors have stated that they have no conflicts of interest.
References
- 1.Klion AD, Bochner BS, Gleich GJ, et al. Approaches to the treatment of hypereosinophilic syndromes: a workshop summary report. J Allergy Clin Immunol. 2006;117:1292–302. doi: 10.1016/j.jaci.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 2.Simon HU, Rothenberg ME, Bochner BS, et al. Refining the definition of hypereosinophilic syndrome. J Allergy Clin Immunol. 2010;126:45–9. doi: 10.1016/j.jaci.2010.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gotlib J. World Health Organization-defined eosinophilic disorders: 2011 update on diagnosis, risk stratification, and management. Am J Hematol. 2011;86:677–88. doi: 10.1002/ajh.22062. [DOI] [PubMed] [Google Scholar]
- 4.Noel P. Eosinophilic myeloid disorders. Semin Hematol. 2012;49:120–7. doi: 10.1053/j.seminhematol.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Baccarani M, Cilloni D, Rondoni M, et al. The efficacy of imatinib mesylate in patients with FIP1L1-PDGFRalpha-positive hypereosinophilic syndrome. Results of a multicenter prospective study. Haematologica. 2007;92:1173–9. doi: 10.3324/haematol.11420. [DOI] [PubMed] [Google Scholar]
- 6.Apperley JF, Gardembas M, Melo JV, et al. Response to imatinib mesylate in patients with chronic myeloproliferative diseases with rearrangements of the platelet-derived growth factor receptor beta. N Engl J Med. 2002;347:481–7. doi: 10.1056/NEJMoa020150. [DOI] [PubMed] [Google Scholar]
- 7.Roufosse F, Cogan E, Goldman M. Lymphocytic variant hypereosinophilic syndromes. Immunol Allergy Clin North Am. 2007;27:389–413. doi: 10.1016/j.iac.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Helbig G, Soja A, Bartkowska-Chrobok A, et al. Chronic eosinophilic leukemia-not otherwise specified has a poor prognosis with unresponsiveness to conventional treatment and high risk of acute transformation. Am J Hematol. 2012;87:643–5. doi: 10.1002/ajh.23193. [DOI] [PubMed] [Google Scholar]
- 9.Ogbogu PU, Bochner BS, Butterfield JH, et al. Hypereosinophilic syndrome: a multicenter, retrospective analysis of clinical characteristics and response to therapy. J Allergy Clin Immunol. 2009;124:1319–25. doi: 10.1016/j.jaci.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tefferi A, Patnaik MM, Pardanani A. Eosinophilia: secondary, clonal and idiopathic. Br J Haematol. 2006;133:468–92. doi: 10.1111/j.1365-2141.2006.06038.x. [DOI] [PubMed] [Google Scholar]
- 11.Kalac M, Quintás-Cardama A, Vrhovac R, et al. A critical appraisal of conventional and investigational drug therapy in patients with hypereosinophilic syndrome and clonal eosinophilia. Cancer. 2007;110:955–64. doi: 10.1002/cncr.22920. [DOI] [PubMed] [Google Scholar]
- 12.Butterfield JH, Weiler CR. Treatment of hypereosinophilic syndromes: the first 100 years. Semin Hematol. 2012;49:182–91. doi: 10.1053/j.seminhematol.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Antoniu SA. Novel therapies for hypereosinophilic syndromes. Neth J Med. 2010;68:304–10. [PubMed] [Google Scholar]
- 14.Elsner J, Höchstetter R, Spiekermann K, Kapp A. Surface and mRNA expression of the CD52 antigen by human eosinophils but not by neutrophils. Blood. 1996;88:4684–93. [PubMed] [Google Scholar]
- 15.Ambrose LR, Morel AS, Warrens AN. Neutrophils express CD52 and exhibit complement-mediated lysis in the presence of alemtuzumab. Blood. 2009;114:3052–5. doi: 10.1182/blood-2009-02-203075. [DOI] [PubMed] [Google Scholar]
- 16.Pitini V, Teti D, Arrigo C, et al. Alemtuzumab therapy for refractory idiopathic hypereosinophilic syndrome with abnormal T cells: a case report. Br J Haematol. 2004;127:477. doi: 10.1111/j.1365-2141.2004.05206.x. [DOI] [PubMed] [Google Scholar]
- 17.Sefcick A, Sowter D, DasGupta E, et al. Alemtuzumab therapy for refractory idiopathic hypereosinophilic syndrome. Br J Haematol. 2004;124:558–9. doi: 10.1046/j.1365-2141.2003.04801.x. [DOI] [PubMed] [Google Scholar]
- 18.Verstovsek S, Tefferi A, Kantarjian H, et al. Alemtuzumab therapy for hypereosinophilic syndrome and chronic eosinophilic leukemia. Clin Cancer Res. 2009;15:368–73. doi: 10.1158/1078-0432.CCR-08-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]