Abstract
Although decisions regarding end-of-life care are personal and important, they may be influenced by the ways in which options are presented. To test this hypothesis, we randomly assigned 132 seriously ill patients to complete one of three types of advance directives. Two types had end-of-life care options already checked—a default choice—but one of these favored comfort-oriented care, and the other, life-extending care. The third type was a standard advance directive with no options checked. We found that most patients preferred comfort-oriented care, but the defaults influenced those choices. For example, 77 percent of patients in the comfort-oriented group retained that choice, while 43 percent of those in the life-extending group rejected the default choice and selected comfort-oriented care instead. Among the standard advance directive group, 61 percent of patients selected comfort-oriented care. Our findings suggest that patients may not hold deep-seated preferences regarding end-of-life care. The findings provide motivation for future research examining whether using default options in advance directives may improve important outcomes, including patients’ receipt of wanted and unwanted services, resource use, survival, and quality of life.
Most seriously ill patients value comfort and dignity over life extension,1 but routine care often leads to treatment oriented toward extending life.2,3 Deviating from this life-extending norm requires that someone actively request or suggest doing so.4–6
Specifying one’s goals of care in the living will component of an advance directive provides patients with an opportunity to counter this tendency.7 However, the text and structure of commonly used advance directives carry some of the same implicit biases that tend to favor life extension in the absence of advance directives.2 For example, in the widely used “Five Wishes” document,8 the option “I want to have life support” is listed first in all three clinical scenarios, despite evidence that the ordering of choices influences the choices selected and that the one presented first often dominates.9,10
Federal law encourages people to complete advance directives,11 and their use appears to be increasing.12 Given the importance of the choices embedded in advance directives, it is essential to understand how the structure of advance directives affects patients’ stated preferences. In studies providing hypothetical directives to college students13 and elderly out-patients,14 Laura Kressel and colleagues found that people were significantly more likely to indicate preferences to forgo life-sustaining interventions when completing advance directives in which forgoing these interventions was the default than when they had to actively choose to forgo the interventions.14
These findings using hypothetical scenarios raise the possibility that people might not have well-formulated, strongly held views on what forms of care at the end of life will best promote their values. Indeed, insights from behavioral economics suggest that preferences for end-of-life care are likely to be “constructed” at the moment people are asked to express them, rather than reflective of deeply ingrained preferences, because such choices are made infrequently and often without opportunity for feedback on whether the choices made promoted patients’ interests.2,15 We tested this hypothesis by examining whether default options influence the choices of seriously ill patients in real advance directives, even after patients were alerted to the default option and their responses to it.
Study Data And Methods
DESIGN OVERVIEW, SETTING, AND PARTICIPANTS
We randomly assigned real advance directives, which differed only in their embedded default options, to outpatients who were at least fifty years old, lacked prior advance directives, had incurable diseases of the chest, and were not being considered for lung transplantation (Exhibit 1 and online Appendix Exhibit A).16 Patients were recruited in the thoracic oncology and pulmonary outpatient clinics at the Hospital of the University of Pennsylvania from May 2010 through January 2012. The University of Pennsylvania Institutional Review Board approved this study.
EXHIBIT 1.
Characteristics Of Patients In The Study To Assess The Affect Of Default Options In End-Of-Life Care Planning
| Characteristic | Life-extension default (n =49)
|
Standard advance directive (n=43)
|
Comfort default (n=40)
|
|||
|---|---|---|---|---|---|---|
| Number | Percent | Number | Percent | Number | Percent | |
| Age (mean years) | 64.6 | — | 64.4 | — | 64.8 | — |
|
SEX | ||||||
| Male | 24 | 49.0 | 15 | 34.9 | 17 | 42.5 |
| Female | 25 | 51.0 | 28 | 65.1 | 23 | 57.5 |
|
RACE | ||||||
| Black or African American | 11 | 22.4 | 14 | 32.6 | 10 | 25.0 |
| White or Caucasian | 34 | 69.4 | 29 | 67.4 | 28 | 70.0 |
| Other/unknown | 4 | 8.2 | 0 | 0.0 | 2 | 5.0 |
|
RELIGION | ||||||
| Catholic | 12 | 24.5 | 10 | 23.3 | 12 | 30.0 |
| Protestant | 15 | 30.6 | 13 | 30.2 | 13 | 32.5 |
| Other Christian | 1 | 2.0 | 4 | 9.3 | 2 | 5.0 |
| Jewish | 2 | 4.1 | 3 | 7.0 | 1 | 2.5 |
| Other faiths | 13 | 26.5 | 10 | 23.3 | 6 | 15.0 |
| Unaffiliated | 6 | 12.2 | 3 | 7.0 | 6 | 15.0 |
|
DIAGNOSIS | ||||||
| Non–small cell lung cancer/other thoracic malignancya | 18 | 36.7 | 16.9 | 37.2 | 13 | 32.5 |
| Chronic obstructive pulmonary disease | 14 | 28.6 | 15 | 34.9 | 14 | 35.0 |
| Idiopathic pulmonary fibrosis | 8 | 16.3 | 3 | 7.0 | 5 | 12.5 |
| Other incurable fibrotic lung diseases | 6 | 12.2 | 7 | 16.3 | 5 | 12.5 |
| Otherb | 3 | 6.1 | 2 | 4.7 | 3 | 7.5 |
SOURCE Authors’ analysis.
Other thoracic malignancies include malignant pleural effusion (for example, from breast cancer) and mesothelioma.
Chronic obstructive asthma, bronchiectasis, cystic fibrosis, chronic pulmonary heart disease, other pulmonary insufficiency not elsewhere classified, other respiratory abnormalities, radiation pneumonitis, beryllium disease, lung involvement in systemic sclerosis, SLE (systemic lupus erythematosis), RA (rheumatoid arthritis).
Each week a research nurse screened electronic health records to identify eligible patients and obtained permission from these patients’ physicians to recruit them. The research nurse then met with potentially eligible patients in person, described the study, reviewed the potential benefits of completing advance directives, answered all questions, and provided patients with a written informed-consent document. The consent form and nurse’s spoken guidance informed patients that different types of advance directives would be assigned by chance, that patients in all groups could select or decline the same interventions and treatment goals, and that patients could change their choices at any time.
INTERVENTIONS
Consenting patients were randomly assigned to complete one of three advance directives. All three were modified slightly from the professionally endorsed directive published by the Allegheny County Medical Society.17 Each was deemed consistent with Pennsylvania law by the University of Pennsylvania Office of the General Counsel. Each included an identical section for the designation of a durable power of attorney for health care and a living will section that was altered among the three versions, as described below. Facsimiles of all of the advance directive forms used in this study can be found in the online Appendix.16
In all versions, patients were shown the exact same options. The versions differed in whether or not they contained a default—that is, whether a particular option was already marked with an X. When such a preselected default was used, that choice was placed first of the three options. Patients first were asked to choose an overall plan of care that prioritized extending life or one that prioritized minimization of pain and suffering.
The precise language used was adapted from that used by William Knaus in the Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments (SUPPORT), a landmark clinical trial.18 Patients could expand upon or clarify their choices by writing in the additional space provided.
Patients also were asked to choose whether or not they wished to receive five potentially life-sustaining interventions, such as feeding tube insertion, if they became unable to make decisions themselves. Again, patients could opt not to make these choices, and they could expand upon their choices in the additional space provided.
Allocation of individual patients was determined by electronic number generation, with assignment probabilities of 33.3 percent to each group. The research nurse recruiting patients used a numbered packet containing the assigned advance directive for each sequentially randomized patient.
One-third of patients were assigned to receive a “comfort default advance directive” that defaulted to the goal of relief of pain and suffering and nonreceipt of life-sustaining interventions. Patients were instructed to make other choices if they preferred by crossing out the default options and initialing lines next to their selections.
Another one-third of patients, in the “life-extension default advance directive” group, received a directive that defaulted to the goal of life extension and receipt of potentially life-sustaining interventions. Again, patients were shown how to make alternative selections.
Patients in a third group were assigned to receive a “standard advance directive” that required patients to actively choose their goals of care or preferences for specific interventions. As in usual practice, if patients did not make active choices, surrogates and clinicians would make decisions if patients lost capacity.
All patients were encouraged to involve their family members and physicians in completing their advance directives and to return them. If completed directives were not returned within ten days, the nurse telephoned patients up to three times to remind them and, if desired, to schedule a clinic visit specifically for help in completing the advance directive. For an advance directive to be considered complete, the signatures of two witnesses or a notary were required, as per Pennsylvania law.
DEBRIEFING
After patients returned completed advance directives, one investigator called patients to debrief19 them about the precise differences between their assigned advance directive and the advance directives that other study participants received. The investigator used an Institutional Review Board–approved debriefing script.16 After explaining the goals of the study, including the concept of default options, the investigator read patients’ choices back to them and asked if they wished to make any changes.
OUTCOMES
The primary outcome was the proportion of patients across the three intervention groups who selected a comfort-oriented goal of care. Given the propensity of the health care system to try to extend life in the absence of a directive otherwise,3,4,7 patients who selected a life-extending goal of care and those who did not select an overall goal of care were jointly considered to have not selected a comfort-oriented goal.
We also assessed patients’ satisfaction with their advance care planning two months after the debriefing. One of two authors blinded to patients’ group assignments contacted patients by phone and administered the Canadian Healthcare Evaluation Project (CANHELP) questionnaire. This thirteen-item questionnaire has been validated for assessing satisfaction with end-of-life care planning.20,21
STATISTICAL ANALYSIS
Our protocol specified a primary analysis in which we included only patients who returned completed advance directives. This analysis assessed the efficacy of default options in advance directives among patients who completed them.
Because such analyses are susceptible to selection effects, intention-to-treat analyses were also conducted in which all patients who were randomly assigned an advance directive were included in the analyses. These intention-to-treat analyses were considered secondary because they are heavily biased toward the null. Specifically, they make the implausible assumption that all patients who did not complete advanced directives chose not to receive comfort-oriented plans of care and chose not to forgo any potentially life-sustaining interventions.
Chi-square tests, Cochran-Armitage tests of trend, and t tests were used as appropriate for two-group binary, three-group binary, and continuous outcomes data, respectively. In secondary analyses, logistic regression models were created to adjust for chance imbalance across arms in patient-level variables.
The clinic where patients were recruited for the study was modeled as a random effect to adjust for potentially correlated outcomes within clinics and to prevent confounding by clinic.22 Analyses were performed using the software Stata, version 11.0, except for the Cochrane-Armitage tests, which were performed using SAS, version 9.3.
We targeted a sample size of ninety-three patients. If evenly distributed across the three ordered groups, this sample would yield 81 percent power to declare significance at p = 0.05 for a difference in the proportion of patients selecting comfort-oriented goals of care of 35 percent. This calculation assumed that the proportion in the standard advance directive group (the middle group) would be roughly equidistant between the proportions in the life-extension and comfort default groups.
LIMITATIONS
This study was designed to enroll a relatively small number of patients from a single health system. Although it was a randomized trial, the sample size does not allow us to rule out the possibility that results were confounded by unmeasured variables, such as how well patients understood their illnesses or how often they spoke with their physicians about prognosis.
Second, we did not randomly assign the ordering of options within the standard advance directive but instead listed the comfort-oriented goal of care and the options to forgo specific interventions first for all patients in that arm. Because the first-listed option tends to be more commonly selected,9,10 the observed differences in selections between the comfort-default advance directive and standard advance directive may actually underestimate the magnitude of the default effect.
Third, we enrolled only patients with serious thoracic diseases—primarily lung cancer and obstructive and restrictive lung diseases. The findings from this group of patients might not be generalizable to all patients or to patients with other specified health conditions.
Study Results
One thousand and sevety-nine patients were screened and determined to be eligible for this study based upon reviews of their electronic health records. Of these, physicians requested that 43 not be contacted for study enrollment at the time of their visit, and 332 missed or rescheduled their visit or were not approached by the research nurse because of scheduling conflicts. Of the remaining 704 patients, 391 (55.5 percent) were deemed ineligible when in-person questioning revealed that they had existing advance directives.16 Thus, there were 313 fully eligible patients, 132 (42.2 percent) of whom consented to participate.
One patient was excluded because he completely rewrote the assigned advance directive, making choices that were not classifiable using our coding scheme. The other 131 consented patients were included in intention-to-treat analyses.
Completed advance directives were returned by ninety-five patients (72.0 percent), only two of whom (2.1 percent) elected to reconsider their choices during the debriefing. One of these patients returned a new advance directive in which the only change was his selection of a new durable power of attorney; the other patient did not return a completed advance directive by the end of the study. Thus, ninety-four patients were included in per protocol analyses. Principal diagnoses and demographic characteristics among the 132 patients who consented to participate are shown in Exhibit 1.
GOALS OF CARE
The specific goals selected by patients in each group are shown in online Appendix Exhibit F.16 This exhibit shows that fifty-four (57.4 percent) of the ninety-four patients who returned a completed advance directive made a choice regarding their overall goals that differed from the default option.
Nonetheless, in per protocol analyses, advance directive default options significantly influenced the proportions of patients who chose comfort-oriented goals of care. Among the comfort default group, 77 percent retained comfort as their overall goal of care; 61 percent in the standard advance directive group chose comfort as their goal; and 43 percent of those in the life-extension default group rejected the default choice and indicated comfort as their primary goal (p < 0.01 for test of trend; Exhibit 2).
EXHIBIT 2.
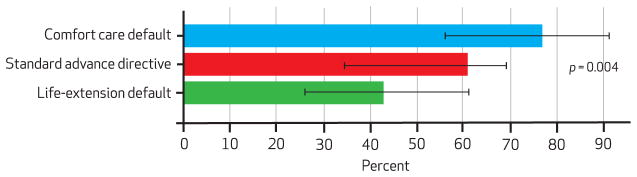
Percentage Of Patients Choosing A Comfort-Oriented Goal Of Care (Per Protocol Population)
SOURCE Authors’ analysis. NOTES Standard error bars denote 95% confidence intervals..
Intention-to-treat analyses produced a similar trend in proportions: 50 percent in the comfort default group, 47 percent in the standard advance directive group, and 31 percent in the life-extension default group (p = 0.04).16
In secondary analyses adjusting for race, sex, age, marital status, and the recruiting research nurse, group assignment remained significantly associated with selections of comfort-oriented goals of care in per-protocol analyses (odds ratio: 2.12; 95% confidence interval: 1.21–3.72; p < 0.01). Intention-to-treat analyses yielded similar but nonsignificant results (odds ratio: 1.51; 95% confidence interval: 0.96–2.37; p = 0.07). These results were robust to different modeling strategies.16
Among the thirty-six patients in whom education level was measured, patients who had never attended college (10/17, 59 percent) were as likely as patients who had attended college (10/19, 53 percent) to make selections other than the default option (odds ratio: 1.33; 95% confidence interval: 0.26–6.68).
CHOICES TO RECEIVE POTENTIALLY LIFE-SUSTAINING INTERVENTIONS
Patients completing different advance directive versions also had different probabilities of choosing to forgo potentially life-sustaining interventions. For example, the proportions of patients choosing to forgo feeding-tube insertion were 54 percent in the comfort-default group, 45 percent in the standard advance directive group, and 26 percent in the life-extension default group (p = 0.01 for test of trend; Exhibit 3). For cardiopulmonary resuscitation, corresponding proportions were 42 percent, 32 percent, and 20 percent (p = 0.03).
EXHIBIT 3.
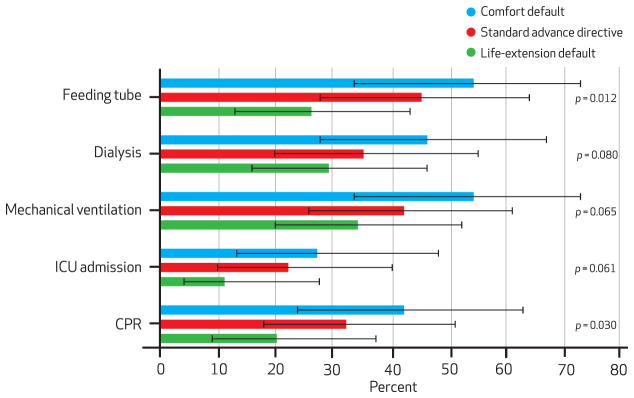
Percentage Of Patients Choosing To Forgo Each Intervention, By Type Of Advance Directive (Per Protocol Population)
SOURCE Authors’ analysis. NOTES Standard error bars denote 95% confidence intervals. CPR is cardiopulmonary resuscitation.
Similar but nonsignificant trends were noted for intensive care unit admission (p = 0.06), mechanical ventilation (p = 0.06), and hemodialysis (p = 0.08). Similar trends were also noted in intention-to-treat analyses, although only the test for feeding-tube insertion was statistically significant.16
SATISFACTION WITH END-OF-LIFE CARE PLANNING
Assessments of patients’ satisfaction with end-of-life care planning were completed for seventy-eight of the ninety-four patients who completed advance directives (83.0 percent). Of the remaining sixteen patients, at least five died within two months; the remainder were lost to follow-up, and it was not known with certainty that they were still living. Global and average satisfaction scores were high across the three intervention groups (greater than 4.5 out of a possible 5) and no significant between- or among-group differences were identified.16
Discussion
Default options, or the events or conditions that will be set into place if no alternative is actively chosen,23,24 have been shown to influence decisions in domains as diverse as drivers’ insurance,25 retirement savings,26,27 influenza vaccination,28 and organ donation.29,30 A hallmark of defaults is that they lead gently, without restricting any options.
Thus, when people have strong preferences, such as for low-deductible health care insurance, they commonly make choices that counter the default. This is precisely what happened, for example, with the roll-out of Medicare Part D drug coverage, when despite the default annual deductible of $250, most Americans chose plans with no deductible at all.24
The study on which we report here shows that default options have large influences on seriously ill patients’ actual choices for health care interventions at the end of life. Overall, most patients with terminal illnesses stated preferences for comfort-oriented care when offered the opportunity to state these preferences in real advance directives, but the proportions of patients choosing this option differed markedly as a function of how the default was set.
Importantly, these effects manifested even after patients were made aware of the defaults and shown how they had responded to them, and after it was made easy to choose counter to the default, which many patients did, particularly in the life-extension default and standard advance directive groups.16 Only 2.1 percent of patients in this study elected to reconsider their selections after being alerted to the manipulation of the default option, but ultimately these patients did not change their original selections. Furthermore, intentionally setting defaults was not associated with any changes in patients’ satisfaction with their choices, which suggests that patients were content to be guided in such decisions.
Although one might expect patients to hold strong prior preferences about end-of-life treatments, the findings that people were heavily swayed by defaults, and content to be swayed, suggest that many seriously ill patients lack deep-seated preferences about their end-of-life care. Despite the importance of end-of-life health care decisions, it should come as no surprise that many patients lack well-established preferences in this domain. People commonly lack prior preferences for decisions that are made infrequently and provide few opportunities for learning after the fact whether the choices made did or did not promote their goals.15 These are precisely the characteristics of end-of-life care choices.
The power of defaults in determining stated end-of-life care preferences underlines the importance of selecting defaults carefully without limiting patients’ options.24,31 At the same time, clinicians should recognize that it is often difficult to avoid defaults, and they should therefore consider carefully the predictable consequences of which defaults are used or allowed to remain.2,24,32
Indeed, there is a default option embedded in the standard approach to advance care planning: If patients do not actively choose specific goals in advance directives, clinicians and surrogates must make decisions for patients who lose capacity. Because many patients do not complete advance directives or merely designate a durable power of attorney, this “default to surrogate decision making” not only is prevalent, but also carries important bereavement consequences for family members.33–35
Given that most patients place a high priority on not burdening their loved ones35–39 and that most patients in our study selected comfort-oriented goals even in the standard advance directive group, there is reason to believe that the current systemic default of life extension might not optimally promote patients’ wishes and values.
These results can also be interpreted as evidence that advance directive forms, absent a well-structured conversation among patients, family members, and providers, will not meaningfully promote patients’ values.40 A preferable approach to advance care planning may be one that relies not on forms but on carefully structured conversations that explore patients’ values in the presence of their potential surrogate decision makers.41
For particular patients and families cared for by particular clinicians—for example, patients with good access to physicians well trained in end-of-life communication and with family members experienced in advance care planning—such coordinated communication may indeed prove optimal. But it is uncertain whether this approach could be implemented across diverse populations with differential access to skilled clinicians and experienced family members.
By contrast, designing an advance directive that would help the majority of patients make decisions that promote their goals could provide a way to improve end-of-life care more broadly, for all Americans. Recent evidence provides substantial motivation to try, as observational studies in the United States show that patients who complete advance directives less commonly die in a hospital,12,42–44 more often receive care consistent with their preferences,12 have surrogates who are less likely to report concerns with communication near the end of life,43 and, in certain regions, receive less costly care.44
This study shows that using default options in advance directives strongly influences the end-of-life care choices that people make without affecting their satisfaction with their advance care planning. Furthermore, because the effects of defaults were identical even after patients were directly told about the default, this study suggests that for many patients, “preferences” for end-of-life care are not deeply held.
This study also provides motivation for future research examining whether using default options in advance directives may improve important outcomes, including patients’ receipt of wanted and unwanted services, survival, quality of life, resource use, and family members’ bereavement. If such research shows that setting defaults in advance directives improves such outcomes while adhering to ethical standards for default setting (including assurances that patients are aware of the decisions to be made and that countering the default can be done easily),3,24,45,46 then the clinical use of default options in advance directives may provide a novel way to improve end-of-life care for large populations of seriously ill patients.
Acknowledgments
This work was supported by research grants from the Greenwall Foundation, Kornfeld Foundation, Leonard Davis Institute of Health Economics at the University of Pennsylvania, and Center for Excellence in Cancer Communication Research of the Universities of Michigan and Pennsylvania. The authors thank Susan Metzger for her assistance with patient recruitment.
Biographies

Scott D. Halpern is the director of the Fostering Improvement in End-of-Life Decision Science Program, University of Pennsylvania.
In this month’s Health Affairs, Scott Halpern and coauthors report on their study testing whether end-of-life care decisions are influenced by the ways in which options are presented. They randomly assigned 132 seriously ill patients to complete one of three types of advance directives that had either different “default” choices already selected for comfort-oriented care or life-extending care, or no options checked at all. The results indicated that the defaults clearly influenced patients’ choices and that patients might not hold deep-seated preferences regarding end-of-life care, the authors write. The authors recommend further research to determine whether use of advance directives with these default options leads to outcomes important to patients, families, and society.
Halpern is the director of the Fostering Improvement in End-of-Life Decision Science Program and deputy director of the Center for Health Incentives and Behavioral Economics at the Leonard Davis Institute of Health Economics, Wharton School, University of Pennsylvania. He is also an assistant professor of medicine, specializing in pulmonary and critical care, epidemiology, and medical ethics and health policy at the university.
Halpern is a member of the Secretary of Health and Human Services Advisory Committee on Blood and Tissue Safety and Availability and of the Technical Advisory Committee to the Scientific Registry of Transplant Recipients. He received a Robert Wood Johnson Foundation Young Leader Award in 2012. Halpern earned master’s degrees in bioethics and clinical epidemiology, a doctorate in epidemiology, and a medical degree from the University of Pennsylvania.

George Loewenstein is the Herbert A. Simon Professor of Economics and Psychology, Carnegie Mellon University.
George Loewenstein is the Herbert A. Simon Professor of Economics and Psychology at Carnegie Mellon University and one of the founders of the fields of behavioral economics and neuroeconomics. He is the former president of the Society for Judgment and Decision Making and a member of the Russell Sage Foundation Behavioral Economics Roundtable, and he served on the board of the Society for Neuroeconomics. Loewenstein earned a doctorate in economics from Yale University.

Kevin G. Volpp is the director of the Center for Health Incentives and Behavioral Economics.
Kevin Volpp is the director of the Center for Health Incentives and Behavioral Economics and of the National Institute on Aging–funded Penn–Carnegie Mellon University Roybal P30 Center on Behavioral Economics and Health at the University of Pennsylvania. He is also a tenured professor of medicine in the Perelman School of Medicine and of the Wharton School’s Health Care Management Department, both at Penn, as well as a staff physician at the Philadelphia Veterans Affairs (VA) Medical Center and core faculty member of its Center for Health Equity Research and Promotion. Volpp earned a doctorate in health economics, public policy, and management and a medical degree from the University of Pennsylvania.

Elizabeth Cooney is the assistant director of the Fostering Improvement in End-of-Life Decision Science Program.
Elizabeth Cooney is the assistant director of the Fostering Improvement in End-of-Life Decision Science Program. She has managed several clinical trials addressing public health issues, including end-of-life care and decision making, substance abuse, and college student health. Cooney earned a master’s degree in public health from Boston University.

Kelly Vranas is a hospitalist in the medical intensive care unit at the Philadelphia VA Medical Center.
Kelly Vranas is a hospitalist in the medical intensive care unit at the Philadelphia VA Medical Center. She completed her internship and residency in internal medicine at the Hospital of the University of Pennsylvania and will start her fellowship in pulmonary and critical care medicine at Stanford University in June 2013. She earned a medical degree from Cornell University.

Caroline M. Quill is a fellow at the Hospital of the University of Pennsylvania.
Caroline Quill is a fellow in the Division of Pulmonary, Allergy, and Critical Care at the Hospital of the University of Pennsylvania, where she also completed her internship and residency in internal medicine. Her research focuses on sources of variation in end-of-life care, particularly among intensive care units and providers. She earned a medical degree from the University of Rochester and is in the process of completing a master’s degree in health policy research at the University of Pennsylvania.

Mary S. McKenzie is a fellow at the Hospital of the University of Pennsylvania.
Mary McKenzie is also a fellow in the Division of Pulmonary, Allergy, and Critical Care at the Hospital of the University of Pennsylvania. She completed her internship and residency in internal medicine at the Beth Israel–Deaconess Medical Center. McKenzie holds a master’s degree in death and society from the University of Reading, England, and a medical degree from Oregon Health and Science University. She is in the process of earning a master’s degree in health policy research at the University of Pennsylvania.

Michael O. Harhay is a doctoral student in epidemiology, University of Pennsylvania.
Michael Harhay is a doctoral student in epidemiology at the University of Pennsylvania. His research focuses on methodological strategies to improve the design and analysis of clinical trials and observational studies in critical care medicine. He holds master’s degrees in demography, health policy, and bioethics, all from the University of Pennsylvania.

Nicole B. Gabler is a biostatistician in the Center for Clinical Epidemiology and Biostatistics.
Nicole Gabler is a biostatistician in the Center for Clinical Epidemiology and Biostatistics at the University of Pennsylvania. Her research interests include critical care health services research, treatment-effect heterogeneity, pulmonary arterial hypertension, and end-of-life decision making. She earned master’s degrees in public health and health administration from Tulane University and a doctorate in epidemiology from the University of California, Davis.

Tatiana Silva is a doctoral student in economics, University of Mannheim.
Tatiana Silva is a doctoral student in economics at the University of Mannheim. She previously worked as a research coordinator at the Perelman School of Medicine and the Wharton School, both at Penn. She has a master’s degree in public administration from the University of Pennsylvania.

Robert Arnold is the director of the Institute for Doctor-Patient Communication, University of Pittsburgh.
Robert Arnold is the director of the Institute for Doctor-Patient Communication and codirector of the Institute to Enhance Palliative Care, both at the University of Pittsburgh. He is also a professor in the Division of General Internal Medicine, Department of Medicine, University of Pittsburgh, and in the university’s Center for Bioethics and Health Law. Arnold earned a medical degree from the University of Missouri–Kansas City.

Derek C. Angus is the director of the Clinical Research, Investigation, and Systems Modeling of Acute Illnesses Center, University of Pittsburgh.
Derek Angus is a professor in and chair of the Department of Critical Care Medicine and director of the Clinical Research, Investigation, and Systems Modeling of Acute Illnesses Center at the University of Pittsburgh. He holds secondary appointments in medicine, health policy and management, and clinical and translational science at the university. He is editor of the Journal of the American Medical Association’s Caring for the Critically Ill section. Angus earned a master’s degree in public health from the University of Pittsburgh and a medical degree from the University of Glasgow, Scotland.

Cindy Bryce is an associate professor of health policy and management and of medicine, University of Pittsburgh.
Cindy Bryce is an associate professor of health policy and management and of medicine at the University of Pittsburgh. She is also a core member of the university’s Center for Research on Health Care and of the Clinical Research, Investigation, and Systems Modeling of Acute Illnesses Center. She holds both a master’s degree and a doctorate in policy analysis from Carnegie Mellon University.
Footnotes
Some portions of this article were previously presented at the 33rd Annual Meeting of the Society for Medical Decision-Making, Chicago, IL, October 22–24, 2011, and at the AcademyHealth Annual Research Meeting, Orlando, FL, June 24–26, 2012.
Contributor Information
Scott D. Halpern, Email: shalpern@exchange.upenn.edu, Director of the Fostering Improvement in End-of-Life Decision Science Program, University of Pennsylvania, in Philadelphia
George Loewenstein, Herbert A. Simon Professor of Economics and Psychology, Carnegie Mellon University, in Pittsburgh, Pennsylvania.
Kevin G. Volpp, Director of the Center for Health Incentives and Behavioral Economics, Leonard Davis Institute of Health Economics, Wharton School, at the University of Pennsylvania
Elizabeth Cooney, Assistant director of the Fostering Improvement in End-of-Life Decision Science Program.
Kelly Vranas, Hospitalist in the medical intensive care unit at the Philadelphia Veterans Affairs Medical Center, in Pennsylvania.
Caroline M. Quill, Fellow in the Division of Pulmonary, Allergy, and Critical Care at the Hospital of the University of Pennsylvania, in Philadelphia
Mary S. Mckenzie, Fellow in the Division of Pulmonary, Allergy, and Critical Care at the Hospital of the University of Pennsylvania
Michael O. Harhay, Doctoral student in epidemiology, University of Pennsylvania
Nicole B. Gabler, Biostatistician in the Center for Clinical Epidemiology and Biostatistics, University of Pennsylvania
Tatiana Silva, Doctoral student in economics, University of Mannheim, in Germany.
Robert Arnold, Director of the Institute for Doctor-Patient Communication, University of Pittsburgh, in Pennsylvania.
Derek C. Angus, Director of the Clinical Research, Investigation, and Systems Modeling of Acute Illnesses Center, University of Pittsburgh
Cindy Bryce, Associate professor of health policy and management and of medicine, University of Pittsburgh.
NOTES
- 1.Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. N Engl J Med. 2002;346(14):1061–6. doi: 10.1056/NEJMsa012528. [DOI] [PubMed] [Google Scholar]
- 2.Halpern SD. Shaping end-of-life care: behavioral economics and advance directives. Semin in Respir Crit Care Med. 2012;33:393–400. doi: 10.1055/s-0032-1322403. [DOI] [PubMed] [Google Scholar]
- 3.Blinderman CD, Krakauer EL, Solomon MZ. Time to revise the approach to determining cardiopulmonary resuscitation status. JAMA. 2012;307(9):917–8. doi: 10.1001/jama.2012.236. [DOI] [PubMed] [Google Scholar]
- 4.White DB, Arnold RM. The evolution of advance directives. JAMA. 2011;306(13):1485–6. doi: 10.1001/jama.2011.1430. [DOI] [PubMed] [Google Scholar]
- 5.Schroeder SA. Personal reflections on the high cost of American medical care. Arch Intern Med. 2011;171:722–7. doi: 10.1001/archinternmed.2011.149. [DOI] [PubMed] [Google Scholar]
- 6.Neuman MD. Surgeons’ decisions and the financial and human costs of medical care. N Engl J Med. 2010;363(25):2382–3. doi: 10.1056/NEJMp1009621. [DOI] [PubMed] [Google Scholar]
- 7.Halpern SD, Emanuel EJ. Advance directives and costs of care: greater clarity and perpetual confusion. Arch Intern Med. 2012;172:266–8. doi: 10.1001/archinternmed.2011.1399. [DOI] [PubMed] [Google Scholar]
- 8.Aging with Dignity. Five wishes [Internet] Tallahassee (FL): Aging with Dignity; [cited 2012 Mar 13]. Available from: http://www.agingwithdignity.org/forms/5wishes.pdf. [Google Scholar]
- 9.Krosnick JA, Alwin DF. An evaluation of a cognitive theory of response-order effects in survey measurement. Public Opinion Quarterly. 1987;51(2):201–19. [Google Scholar]
- 10.Bishop GF, Smith AE. Response-order effects in public opinion surveys: the plausibility of rival hypotheses [Internet]. Papers presented at: 52nd Annual Conference of the American Association for Public Opinion Research, Survey Research Methods Section; Norfolk, Virginia. 1997 May 15–18; [cited 2013 Jan 22]. Available from: http://www.amstat.org/sections/srms/Proceedings/papers/1997_179.pdf. [Google Scholar]
- 11.Wolf SM, Boyle P, Callahan D, Fins JJ, Jennings B, Nelson JL, et al. Sources of concern about the Patient Self-Determination Act. N Engl J Med. 1991;325(23):1666–71. doi: 10.1056/nejm199112053252334. [DOI] [PubMed] [Google Scholar]
- 12.Silveira MJ, Kim SYH, Langa KM. Advance directives and outcomes of surrogate decision making before death. N Engl J Med. 2010;362(13):1211–8. doi: 10.1056/NEJMsa0907901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kressel LM, Chapman GB. The default effect in end-of-life medical treatment preferences. Med Decis Making. 2007;27(3):299–310. doi: 10.1177/0272989X07300608. [DOI] [PubMed] [Google Scholar]
- 14.Kressel LM, Chapman GB, Leventhal E. The influence of default options on the expression of end-of-life treatment preferences in advance directives. J Gen Intern Med. 2007;22:1007–10. doi: 10.1007/s11606-007-0204-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slovic P. The construction of preference. Am Psychol. 1995;50:364–71. [Google Scholar]
- 16.To access the Appendix, click on the Appendix link in the box to the right of the article online.
- 17.Allegheny County Medical Society. Advance health care directive: health care power of attorney and living will [Internet] Pittsburgh (PA): The Society; 2009. [cited 2013 Jan 9]. Available from: http://www.acba.org/Public/Legal-information/LivingWillPoweroAttyform.pdf. [Google Scholar]
- 18.Knaus WA, Connors AF, Dawson NV, Desbiens NA, Fulkerson WJ, Goldman L, et al. A controlled trial to improve care for seriously ill hospitalized-patients—the Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments (SUPPORT) JAMA. 1995;274(20):1591–8. [PubMed] [Google Scholar]
- 19.Miller FG, Gluck JP, Wendler D. Debriefing and accountability in deceptive research. Kennedy Inst Ethics J. 2008;18(3):235–51. doi: 10.1353/ken.0.0196. [DOI] [PubMed] [Google Scholar]
- 20.Heyland DK, Cook DJ, Rocker GM, Dodek PM, Kutsogiannis DJ, Skrobik Y, et al. The development and validation of a novel questionnaire to measure patient and family satisfaction with end of life care: the Canadian Health care Evaluation Project (CANHELP) Questionnaire. Palliat Med. 2010;24(7):682–95. doi: 10.1177/0269216310373168. [DOI] [PubMed] [Google Scholar]
- 21.Heyland DK, Frank C, Tranmer J, Paul N, Pichora D, Jiang XR, et al. Satisfaction with end-of-life care: a longitudinal study of patients and their family caregivers in the last months of life. J Palliat Care. 2009;25(4):245–56. [PubMed] [Google Scholar]
- 22.Localio AR, Berlin J, Ten Have TR, Kimmel SE. Adjustments for center in multicenter studies: an overview. Ann Intern Med. 2001;135(2):112–23. doi: 10.7326/0003-4819-135-2-200107170-00012. [DOI] [PubMed] [Google Scholar]
- 23.Carroll GD, Choi JJ, Laibson D, Madrian BC, Metrick A. Optimal defaults and active decisions. Q J Econ. 2009;124(4):1639–74. doi: 10.1162/qjec.2009.124.4.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halpern SD, Ubel PA, Asch DA. Harnessing the power of default options to improve health care. N Engl J Med. 2007;357(13):1340–4. doi: 10.1056/NEJMsb071595. [DOI] [PubMed] [Google Scholar]
- 25.Johnson EJ, Hershey JC, Meszaros J, Kunreuther H. Framing, probability distortions, and insurance decisions. J Risk Uncertain. 1993;7:35–51. [Google Scholar]
- 26.Choi JJ, Laibson D, Madrian BC, Metrick A. Defined contribution pensions: plan rules, participant decisions, and the path of least resistance. In: Poterba JM, editor. Tax policy and the economy. Vol. 16. Cambridge (MA): MIT Press; 2002. pp. 67–113. [Google Scholar]
- 27.Madrian BC, Shea DF. The power of suggestion: inertia in 401(k) participation and savings behavior. Q J Econ. 2001;116(4):1149–87. [Google Scholar]
- 28.Chapman GB, Li M, Colby H, Yoon H. Opting in vs. opting out of influenza vaccination. JAMA. 2010;304(1):43–4. doi: 10.1001/jama.2010.892. [DOI] [PubMed] [Google Scholar]
- 29.Horvat LD, Cuerden MS, Kim SJ, Koval JJ, Young A, Garg AX. Informing the debate: rates of kidney transplantation in nations with presumed consent. Ann Intern Med. 2010;153(10):641–68. doi: 10.7326/0003-4819-153-10-201011160-00006. [DOI] [PubMed] [Google Scholar]
- 30.Johnson EJ, Goldstein D. Do defaults save lives? Science. 2003;302(5649):1338–9. doi: 10.1126/science.1091721. [DOI] [PubMed] [Google Scholar]
- 31.Loewenstein G, Brennan T, Volpp KG. Asymmetric paternalism to improve health behaviors. JAMA. 2007;298(20):2415–7. doi: 10.1001/jama.298.20.2415. [DOI] [PubMed] [Google Scholar]
- 32.Camerer C, Issacharoff S, Loewenstein G, O’Donoghue T, Rabin M. Regulation for conservatives: behavioral economics and the case for asymmetric paternalism. Univ PA Law Rev. 2003;151(3):1211–54. [Google Scholar]
- 33.Azoulay E, Pochard F, Kentish-Barnes N, Chevret S, Aboab J, Adrie C, et al. Risk of post-traumatic stress symptoms in family members of intensive care unit patients. Am J Respir Crit Care Med. 2005;171(9):987–94. doi: 10.1164/rccm.200409-1295OC. [DOI] [PubMed] [Google Scholar]
- 34.Wendler D, Rid A. Systematic review: the effect on surrogates of making treatment decisions for others. Ann Intern Med. 2011;154(5):336–46. doi: 10.7326/0003-4819-154-5-201103010-00008. [DOI] [PubMed] [Google Scholar]
- 35.Wright AA, Zhang BH, Ray A, Mack JW, Trice E, Balboni T, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and care-giver bereavement adjustment. JAMA. 2008;300(14):1665–73. doi: 10.1001/jama.300.14.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Terry PB, Vettese M, Song J, Forman J, Haller KB, Miller DJ, et al. End-of-life decision making: when patients and surrogates disagree. J Clin Ethics. 1999;10(4):286–93. [PubMed] [Google Scholar]
- 37.Singer PA, Martin DK, Lavery JV, Thiel EC, Kelner M, Mendelssohn DC. Reconceptualizing advance care planning from the patient’s perspective. Arch Intern Med. 1998;158(8):879–84. doi: 10.1001/archinte.158.8.879. [DOI] [PubMed] [Google Scholar]
- 38.Mead GE, O’Keeffe ST, Jack CI, Maèstri-Banks AM, Playfer JR, Lye M. What factors influence patient preferences regarding cardiopulmonary resuscitation? J R Coll Physicians Lond. 1995;29(4):295–8. [PMC free article] [PubMed] [Google Scholar]
- 39.Steinhauser AE, Christakis NA, Clipp EC, McNeilly M, McIntyre L, Tulsky JA. Factors considered important at the end of life by patients, family, physicians, and other care providers. JAMA. 2000;284(19):2476–82. doi: 10.1001/jama.284.19.2476. [DOI] [PubMed] [Google Scholar]
- 40.Sudore RL, Fried TR. Redefining the “planning” in advance care planning: preparing for end-of-life decision making. Ann Intern Med. 2010;153(4):256–61. doi: 10.1059/0003-4819-153-4-201008170-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kirchhoff KT, Hammes BJ, Kehl KA, Briggs LA, Brown RL. Effect of a disease-specific planning intervention on surrogate understanding of patient goals for future medical treatment. J Am Geriatr Soc. 2010;58(7):1233–40. doi: 10.1111/j.1532-5415.2010.02760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Degenholtz HB, Rhee Y, Arnold RM. Brief communication: the relationship between having a living will and dying in place. Ann Intern Med. 2004 Jul;141(2):113–7. doi: 10.7326/0003-4819-141-2-200407200-00009. [DOI] [PubMed] [Google Scholar]
- 43.Teno JM, Gruneir A, Schwartz Z, Nanda A, Wetle T. Association between advance directives and quality of end-of-life care: a national study. J Am Geriatr Soc. 2007;55(2):189–94. doi: 10.1111/j.1532-5415.2007.01045.x. [DOI] [PubMed] [Google Scholar]
- 44.Nicholas LH, Langa KM, Iwashyna TJ, Weir DR. Regional variation in the association between advance directives and end-of-life medicare expenditures. JAMA. 2011;306(13):1447–53. doi: 10.1001/jama.2011.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blumenthal-Barby JS, Burroughs H. Seeking better health care outcomes: the ethics of using the “nudge”. Am J Bioeth. 2012;12(2):1–10. doi: 10.1080/15265161.2011.634481. [DOI] [PubMed] [Google Scholar]
- 46.Quill CM, Halpern SD. Deciphering the appropriateness of defaults: the need for domain-specific evidence. J Med Ethics. 2012;38(12):721–2. doi: 10.1136/medethics-2012-100724. [DOI] [PubMed] [Google Scholar]


