Abstract
Basal cell carcinoma (BCC) is the most common malignancy. Exposure to sunlight is the most important risk factor. Most, if not all, cases of BCC demonstrate overactive Hedgehog signaling. A variety of treatment modalities exist and are selected based on recurrence risk, importance of tissue preservation, patient preference, and extent of disease. The pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management of BCC will be discussed in this review.
Keywords: basal cell carcinoma, BCC, electrodesiccation and curettage, ED&C, surgical excision, Mohs surgery
Basal cell carcinoma (BCC) is the most common cancer. Although it rarely results in death or metastatic disease, BCC can cause significant morbidity due to destructive local spread. The accessibility of skin and the high prevalence of BCC have allowed a thorough characterization of pathogenesis, clinical presentation, and histopathology. Management remains largely surgical in the form of electrodesiccation and curettage, surgical excision, and Mohs surgery. Topical therapies are effective for the treatment of certain low-risk BCCs. Radiation therapy is an option when surgery is contraindicated. Knowledge of the genetic changes underlying BCC has paved the way for the development of targeted therapies for advanced disease.
Pathogenesis
The Sonic Hedgehog Pathway
The nevoid basal cell carcinoma syndrome (NBCCS) is an autosomal dominant disorder that manifests as multiple BCCs, pits of the palms and soles, jaw keratocysts, various other tumors, and developmental abnormalities [1]. The location of the candidate gene was narrowed down to chromosome 9q22.3 [2-4]. Subsequently, loss of heterozygosity in the same region was discovered to be important for the pathogenesis of sporadic BCCs [5]. This pattern was consistent with the gene being a tumor suppressor. Molecular studies revealed the gene to be a human homolog of Drosophila patched [6,7]. Now called PTCH, the gene encodes a receptor for the Sonic hedgehog (SHH) pathway, a pathway important for patterning and growth during vertebrate development (Figure 1) [8]. The SHH ligand binds to the PTCH receptor, inhibiting it and allowing signaling through the pathway. As an inhibitory protein, PTCH allows overactivation of the SHH pathway in the setting of inactivating mutations. Animal studies later showed that mice overexpressing SHH in the context of normal PTCH develop multiple BCCs and features of NBCCS [9,10]. Activating somatic mutations in Smoothened (SMO), a seven-transmembrane protein immediately downstream of PTCH, were found in a selection of sporadic BCCs and transgenic mice overexpressing mutant SMO that developed skin abnormalities similar to BCCs [11]. These findings indicate SMO serves as a proto-oncogene. Overexpression of GLI proteins — transcription factors activated by SMO — in mouse models has been shown to induce BCCs [12-14]. Furthermore, continued SHH signaling has been shown to be required for BCC carcinogenesis because mice engineered to conditionally express GLI-2 show BCC regression when GLI-2 expression is inactivated [14]. All of these studies support the concept that overactivation of SHH signaling is necessary and perhaps sufficient for the development of BCCs.
Figure 1.
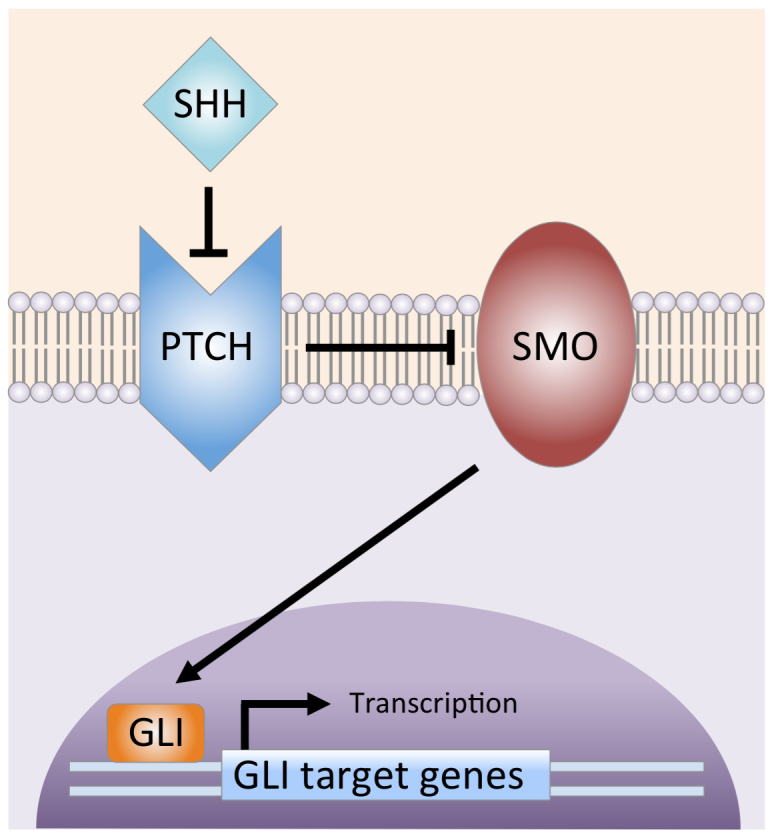
Sonic hedgehog signaling pathway. SHH ligand binds to and inhibits the PTCH transmembrane protein. The inhibition of PTCH relieves suppression of SMO (Smoothened), which then activates the GLI transcription factors. The GLI proteins translocate from the cytoplasm to the nucleus, where they drive gene transcription. (Courtesy of Alexander G. Marzuka, MD)
Other Genetic Changes
The TP53 gene is the most commonly mutated tumor suppressor in cancer. Point mutations in the TP53 gene are the second most common genetic alteration in BCCs, occurring in at least 50 percent of cases [15]. Microdissection of biopsy samples has shown that within a BCC, subclones with second, third, or even more TP53 mutations can be seen arising adjacent to a dominant cell clone [16]. Mutations in the CDKN2A locus and in members of the ras gene family (H-ras, K-ras, and N-ras) also have been identified in a smaller number of sporadic BCCs [15].
Cell of Origin
The identity of the cell of origin for BCC remains controversial because of conflicting evidence. In a mouse model, constitutively active SMO mutant induced formation of BCCs when conditionally expressed in basal keratinocytes of the interfollicular epidermis but not when expressed in hair follicle stem cells [17]. A later study showed induction of uncontrolled SHH signaling in mice by overexpression of GLI-2 transcription factor, resulting in the development of BCC-like tumors whose clinicopathologic type depended on the cellular compartment in the skin where GLI-2 was selectively overexpressed [18]. Superficial BCC-like tumors arose from interfollicular epidermis, whereas nodular BCC-like tumors developed from hair follicle stem cells. A recent study using cell fate tracking showed that X-ray-induced BCCs in PTCH (+/-) mice originate from keratin 15-expressing stem cells of the follicular bulge [19,20]. However, conditional loss of p53 also produced BCCs from basal keratinocytes of the interfollicular epidermis in addition to enhancing development of BCCs from the bulge. These data indicate that depending on the circumstances, BCCs may arise from basal keratinocytes of the interfollicular epidermis or of the hair follicle. Further research is needed to clarify what those circumstances are.
Epidemiology
BCC is the most common malignancy. Cancer registries do not collect data on this skin cancer, so the prevalence and incidence is difficult to estimate. According to the American Cancer Society, more than 2 million people were treated in 2006 for non-melanoma skin cancer (NMSC), mostly BCC [21]. A recent study predicted the total number of NMSCs in those 2 million people during the same time period to be approximately 3.5 million [22]. The lifetime risk for the development of skin cancer is estimated to be 1 in 5 with more than 97 percent being NMSC [23].
A population-based study in Rochester, Minnesota, estimated the age-standardized annual incidence for BCC in both Caucasian men and women to be 146 cases per 100,000 persons [24]. Another population-based study in Kauai, Hawaii, estimated the combined incidence in Caucasian residents to be 422 cases per 100,000 people, the highest documented incidence in the United States at the time of publication in 1993 [25]. Both studies showed rate increased with age and men had a significantly higher incidence of BCC than women. The incidence among Americans younger than 40 appears to be increasing, particularly among women [26].
The risk of developing a subsequent BCC after an initial diagnosis of NMSC is substantial [27-31]. Patients with one index BCC developed one, two, or three new BCCs in 33 percent, 14 percent, and 7 percent of cases, respectively, within 1 year, the time frame in which the highest risk was observed during a 3-year study [27]. Patients with an index squamous cell carcinoma also were at increased risk of developing a BCC (36 percent of cases within 1 year). Other studies have shown the 5-year cumulative risk of a new BCC among patients with at least one previous BCC to be 41 to 45 percent, compared to a risk of only 5 percent in the general Caucasian population [28,29]. A more recent meta-analysis suggested the risk may be even higher. It estimated the 3-year cumulative risk is 44 percent or at least a tenfold increase in incidence compared to the general comparable population [30]. Patients with NMSC also have been shown to be at substantial increased risk for development melanoma with a relative risk of 17 percent compared to the general Caucasian population [32].
UV Exposure as Main Risk Factor for BCC Development
Sun exposure is the most important environmental cause of BCC (Table 1). The risk of BCC appears to depend on the nature of this exposure. A population-based, case-control study conducted in Alberta, Canada, revealed an increased risk with recreational sun exposure in childhood and adolescence, suggesting that these life periods may be critical for establishing adult risk for BCC [33]. This relationship was most pronounced among sun-sensitive subjects with tendency to burn rather than tan. In contrast, mean annual cumulative summer sunlight exposure appeared to have no effect on risk. The study also showed a positive association between BCC and light skin color, severe sunburns and freckling in childhood, and Northern European ethnic origin. Other studies have corroborated that intermittent, intense sun exposure appears to increase risk, whereas cumulative, long-term UV exposure does not [34,35].
Table 1. Risk factors for the development of BCC. Adapted from text.
| Risk factors associated with increased environmental or artificial UV exposure |
| Intermittent, intense sun exposure (especially in childhood and adolescence) |
| Northern European ethnic origin |
| Light skin color |
| Tendency to burn rather than tan |
| Proximity to the equator |
| History of blistering sunburns in childhood |
| Use of tanning beds |
| Other risk factors |
| Exposure to therapeutic ionizing radiation |
| Immunosuppression in organ transplant recipients |
| HIV seropositivity |
| Genetic syndromes: nevoid basal cell carcinoma, xeroderma pigmentosum, Bazex |
There is a geographic variation in incidence of BCC with a positive correlation between rate and proximity to the equator. This observation can be explained by higher UV radiation exposure at lower latitudes, such as Hawaii, compared to higher latitudes, as in the Midwest [24,25].
Although psoralen and ultraviolet A (PUVA) therapy, a highly effective treatment modality for psoriasis, has been associated with a greatly increased risk of squamous cell carcinomas when administered at high doses (more than 350 treatments), the effect on the risk of BCC even at high doses has been reported as modest [36]. The use of tanning beds has been associated with a significantly increased risk of NMSC, particularly with use early in life. A meta-analysis demonstrated the relative risk for the development of BCC after indoor tanning before age 25 years to be 1.40 (95% CI = 1.29-1.52). This translates into a 40 percent increased risk compared to a control population [37]. The risk has been shown to increase in a dose-dependent fashion with years using indoor tanning devices [38]. Photosensitizing medications, such as tetracyclines, thiazide diuretics, NSAIDs, and retinoids, have the ability to produce phototoxic or photoallergic reactions upon UV exposure, increasing the vulnerability of the skin to UV-induced damage. The use of photosensitizing medications, especially antimicrobials, has been associated with an increased risk of BCC, in particular early-onset BCC (OR = 1.5 for age 50 years or younger, 95% CI = 1.1-2.1) [39]. This observation is attributed to the use of tetracyclines for the treatment of acne in adolescence, a time period in which UV exposure has been associated with increased adult risk of BCC [33].
Other Risk Factors
Ionizing radiation in the form of radiotherapy is associated with the development of BCCs. In a study of 1,690 patients previously treated with radiation therapy for reasons other than NMSC, the relative risk of total BCC tumors was 2.3 (95% CI = 1.7-3.1), with marginally higher risk associated with younger age at exposure and time since initial treatment [40]. In particular, radiotherapy for acne, an outdated treatment modality, increased risk of BCC to a greater extent than treatment for other conditions (RR = 3.3; 95% CI = 2.1-5.2).
The consumption of arsenic-contaminated water and arsenic-containing medications has been associated with an increased risk of BCC [41,42]. The most common source of arsenic in diet is seafood [43]. The effect of dietary arsenic intake on risk of BCC is unknown.
Immunosuppression in organ transplant recipients increases the risk of NMSC in proportion to the duration of immunosuppressive therapy [44]. In contrast to the general population, squamous cell carcinoma is the most common skin cancer in transplant recipients, occurring 65 to 250 times as frequently as in the general population. The incidence of BCCs increases tenfold in transplant recipients. Other causes of immune dysfunction have been associated with the development of BCCs. A study showed HIV seropositivity doubled the risk of BCC [45]. There was no significant trend of higher risk with lower recent CD4 counts, indicating a lack of association between BCCs and immunodeficiency in HIV-positive patients.
Certain genetic syndromes are associated with the development of BCCs. The most common is NBCCS, in which patients can develop hundreds of BCCs and a variety of developmental abnormalities.
Prevention
The main strategy to reduce the risk of BCC appears to be protection from UV exposure, especially in childhood and adolescence. Behaviorally, this means avoiding sunburns, tanning beds, and prolonged direct sun exposure between 10 a.m. and 4 p.m., as well as wearing protective clothing, such as wide-brimmed hats and long-sleeved shirts, while outdoors during the day. The benefit of applying sunscreen in the prevention of BCCs is unclear. A mathematical model based on epidemiological data estimated that the regular use of sunscreen with SPF of 15 during the first 18 years of life would reduce the lifetime incidence of BCC by 78 percent [48]. However, a community-based randomized trial involving 1,383 participants in Australia showed subjects who applied SPF 15+ sunscreen daily demonstrated a decreased incidence of squamous cell carcinomas but not BCCs after 4.5 years of follow-up [49]. Similarly, a study in organ transplant patients showed regular use of sunscreen to be effective in the prevention of squamous cell carcinomas and their precursor lesions, actinic keratoses, but only weakly effective on decreasing the risk of BCCs [50]. Although the benefit of regular sunscreen use in adults in the prevention of BCCs appears to be minimal at best, there is a theoretical benefit of sunscreen use in childhood and adolescence, time periods in which adult risk for BCC appears to be established [33]. However, there is a dearth of clinical trials evaluating sunscreen use and subsequent risk of skin cancer in patients younger than 18 years.
Clinical Features
The main clinical subtypes of BCC are nodular, superficial, and morpheaform (Figure 2). Combinations of the latter two types with nodular BCC may occur. Occasionally, variable amounts of melanin may be present within these tumors, which are often referred to as pigmented BCCs. Most cases of BCC occur on the face, consistent with the causative role of UV radiation [51]. The rest of the cases arise on the trunk and extremities and only rarely is BCC encountered on non-hair-bearing sites, such as the genital mucosa.
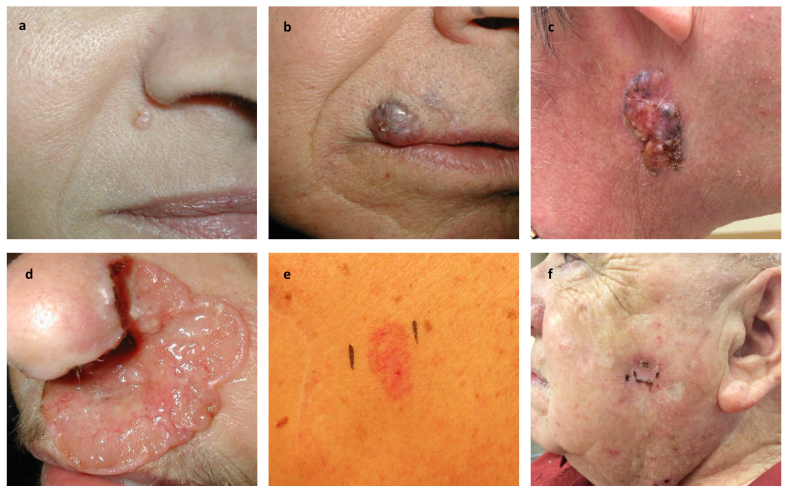
Figure 2.
Clinical variants of basal cell carcinoma. a) Nodular basal cell carcinoma with characteristic pearly surface and telangiectasias located lateral to the right alar crease. b) Pigmented variant located on the skin above the right upper lip and extending past the vermilion border into the lip. c) Large nodular basal cell carcinoma with characteristic telangiectasias and pigmented areas on the right side of the neck. d) Ulcerated aggressive basal cell carcinoma, otherwise known as “rodent ulcer.” This basal cell carcinoma spread locally into the nose, causing extensive destruction of the left nasal ala. e) Superficial basal cell carcinoma appearing as a red patch on the trunk. f) Recurrent basal cell carcinoma at the site of ED&C. (Courtesy of Samuel E. Book, MD)
Nodular Basal Cell Carcinoma
Nodular BCC is the most common clinical subtype, accounting for 50 to 79 percent of all BCCs [51,52]. Lesions consist of papules or nodules with a pearly, shiny quality and small arborizing telangiectasias. The tumor may enlarge and crusting may appear over a central depression. Bleeding with minor trauma is frequent. With time, the lesion may ulcerate (rodent ulcer), but a rolled border usually remains, serving as a clue to the diagnosis. Nodular BCCs predominate on the head, especially the cheeks, nasolabial folds, forehead, and eyelids. In a study, 90 percent of nodular BCCs occurred on the head [51].
The differential diagnosis for a non-ulcerated lesion includes molluscum contagiosum, sebaceous hyperplasia, amelanotic melanoma, intradermal melanocytic nevus, Merkel cell carcinoma, fibrous papule of the nose, trichoepithelioma, and other adnexal neoplasms. Ulcerated lesions may be difficult to distinguish from squamous cell carcinomas and keratoacanthomas.
Superficial Basal Cell Carcinoma
Superficial BCC is the second most common clinical subtype, accounting for up to 15 percent of cases [51,53]. A lesion typically appears as a well-circumscribed, scaly, pink-to-red macule, patch, thin papule, or thin plaque. It may demonstrate crust or a thin rolled border consisting of fine translucent small papules. Areas of spontaneous regression can occur, leaving behind atrophic, hypopigmented areas. Variable amounts of melanin pigment may be present. Superficial BCCs favor the trunk and extremities, in contrast to the other subtypes, which favor the head and neck [46,54]. It tends to occur in younger patients than the other subtypes, with a mean age at diagnosis of 57 years [54].
The differential diagnosis includes inflammatory dermatoses, such as psoriasis and nummular dermatitis, as well as lichenoid keratosis, actinic keratosis, Bowen’s disease (SCC in situ), and early amelanotic melanoma.
Morpheaform Basal Cell Carcinoma (Sclerosing, Infiltrating)
Morpheaform BCC accounts for a low proportion of cases, estimated at 5 to 10 percent [51,55]. It is called morpheaform or sclerosing due its clinical resemblance to an indurated plaque of morphea, or localized scleroderma. Lesions present as pink-to-ivory-white, shiny, smooth, scar-like, indurated plaques or depressions with ill-defined borders. Frequently, there is associated atrophy. Telangiectasias, erosions, or small crusts may sometimes develop. Lesions are notorious for their subtlety. Also known as infiltrating BCC, morpheaform BCC is usually more aggressive than nodular and superficial BCC as it tends to exhibit subclinical spread with the potential for extensive local destruction.
Morpheaform BCC may be confused with scar, morphea (localized scleroderma), dermatofibrosarcoma protuberans, Merkel cell carcinoma, amelanotic melanoma, microcystic adnexal carcinoma, and other adnexal neoplasms.
Diagnosis
After visually inspecting a lesion in search of characteristic features of BCC, clinicians can rely on dermatoscopy to further refine the differential diagnosis. Dermatoscopy, or the use of a magnifying device that emits polarized light to examine the skin, can detect reliable and robust diagnostic parameters, including arborizing telangiectasias, leaflike areas, foci of microulceration, multiple blue/gray globules, and large blue/gray ovoid nests [56,57]. Pigmented BCCs can be a diagnostic challenge because they can demonstrate dermatoscopic features suggestive of melanocytic lesions, such as multiple brown-to-black dots/globules, blue/white veil-like structures, and nonarborizing vessels [56].
A biopsy is the only way to definitively diagnose BCC and decide on appropriate treatment. No suspected lesion should be treated without histopathologic confirmation. Various biopsy techniques may be used, including excisional, incisional, shave, and punch biopsies. Punch and shave biopsies have been shown to have similar diagnostic accuracy [58,59]. Even so, in most patients, the preferred biopsy method is a shave biopsy that incorporates the full depth of the lesion [52]. In patients with obvious BCC and for whom cosmetic outcome is not a priority, an excisional biopsy involving removal of the whole lesion can be done for histologic diagnosis and definitive therapy. Punch biopsy, despite some advantages, has limitations. The diagnostic accuracy of punch biopsy, defined as the proportion of histologic diagnoses made by punch biopsy that are subsequently confirmed after surgical excision of primary BCCs, has been reported in various studies to be 61 to 82 percent [58-61]. In the case of aggressive-growth subtypes of BCC (defined as single or mixed histology tumors containing morpheaform, infiltrative, micronodular, or basosquamous components), the accuracy of punch biopsy was 62 to 72 percent in two of these studies [60,61]. This means that 28 to 38 percent of aggressive BCCs were misdiagnosed by punch biopsy as indolent-growth BCCs. Although aggressive-growth subtypes correspond to a smaller proportion of all primary BCCs compared to indolent-growth subtypes, the missed diagnoses of aggressive-growth subtypes corresponded to 8 to 15 percent of all cases evaluated. One of the main determinants for choice of treatment of BCC is the aggressiveness of the tumor, meaning that 1 in 7 to 1 in 14 patients will be undertreated because of a missed histologic diagnosis of aggressive BCC.
Histopathology
BCCs also can be classified into two broad categories on the basis of histopathologic features: indolent-growth and aggressive-growth subtypes [62-64]. Indolent-growth subtypes include nodular and superficial, corresponding to the clinical nodular and superficial subtypes, respectively, previously described (Figure 3). Aggressive-growth subtypes, which have a higher recurrence rate and tend to cause extensive local destruction, include morpheaform, infiltrative, micronodular, and basosquamous (Figure 4). Combinations of these histopathologic patterns may be found in a single specimen, which is then referred to as a mixed histology tumor. Mixed histology tumors account for approximately 40 percent of primary BCCs [62].
Figure 3.
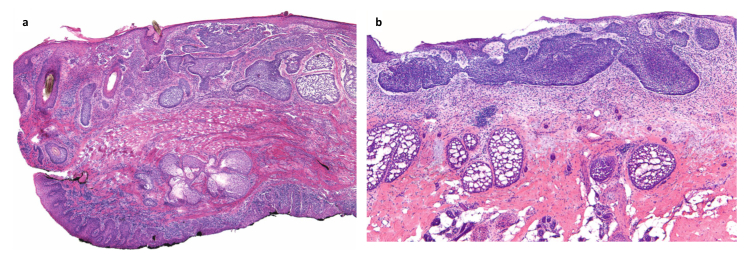
Indolent-growth histologic subtypes [64]. a) Nodular basal cell carcinoma: Medium-power view of a section of eyelid showing both the cutaneous surface at the top and the conjunctiva in the lower inked portion. Nodular basal cell carcinoma is present throughout the dermis. Note the large, nodular aggregates of dark-blue-staining basaloid keratinocytes showing peripheral palisading and clefting. b) Superficial basal cell carcinoma: Medium-power view of superficial basal cell carcinoma, showing basaloid aggregates emanating from the epidermis and growing along an axis parallel to the epidermis. Peripheral palisading and clefting is present.
Figure 4.
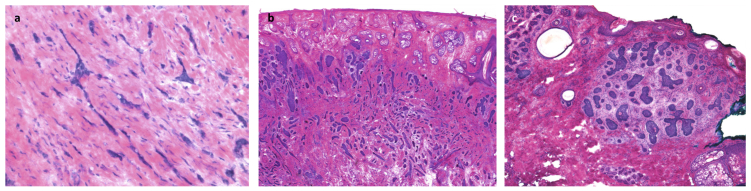
Aggressive-growth histologic subtypes [64]. a) Morpheaform basal cell carcinoma: High-power view of small irregular (sharply angulated) tongues of basaloid, neoplastic cells, ranging from one to four cells thick, embedded within a heavily collagenized stroma. b) Infiltrative basal cell carcinoma: Low-power view of basaloid aggregates of various sizes and shapes, many with angulated appearance, that decrease in size from superficial to the deep portion of the neoplasm. c) Micronodular basal cell carcinoma: small, nodular, irregular aggregates of basaloid neoplastic cells surrounded by cellular myxoid stroma. Focal clefting is appreciated between the neoplastic aggregates and the stroma.
All subtypes of BCC share in common the presence of aggregations of basaloid keratinocytes that are surrounded by stromal tissue and typically demonstrate a connection to the epidermis. Basaloid cells resemble the basal keratinocytes of normal epidermis and are characterized by intensely basophilic (blue-staining), large, relatively uniform nuclei, and scant cytoplasm. In many cases, artifactual retraction of the stroma around tumor islands creates microscopically visible clefts. Tumor aggregates may demonstrate peripheral palisading of nuclei. Apoptotic cells are common.
Nodular Basal Cell Carcinoma
This form of BCC is characterized by large nests of basaloid cells in the papillary or reticular dermis accompanied by peritumoral retraction from the stroma and peripheral palisading. Ulceration may be present. Cystic spaces may develop within larger tumor islands secondary to necrosis. If mucin pools in these central spaces, the tumor may be referred to as nodulocystic.
Superficial Basal Cell Carcinoma
In superficial BCC, basaloid cells proliferate along an axis parallel to the epidermal surface and no more deeply than the papillary dermis. Slit-like retraction of palisaded basal cells from the subjacent stroma may be observed. These tumors may be described as multifocal if multiple discrete foci of proliferation are present. These foci, however, appear to connect in a net-like pattern, such that most superficial BCCs are not truly multifocal.
Morpheaform Basal Cell Carcinoma
The typical architectural growth pattern of morpheaform BCC is strands of basaloid cells one to five cells thick extending between dense collagen bundles. The tumor is poorly demarcated and may show widespread invasion of the reticular dermis and even penetration into the subcutaneous fat. Retraction artifact is uncommon and peripheral palisading is absent.
Infiltrative Basal Cell Carcinoma
Like morpheaform BCC, infiltrative BCC demonstrates heavy stromal fibrosis with dense collagen bundles, grows in a poorly circumscribed fashion, and may show invasion of the subcutis. However, tumor cells form large nodules with irregular contours in addition to strands and cords.
Micronodular Basal Cell Carcinoma
Like nodular BCC, micronodular BCC shows round or oval tumor nests. These nests, however, are smaller and widely dispersed, extending deeper into the dermis, and, in some cases, even penetrating the subcutis. There is associated stromal proliferation.
Basosquamous Carcinoma
Also known as metatypical BCC, basosquamous carcinoma shows infiltrating jagged cords of tumor cells, some with an abortive peripheral palisade and clear-cut basaloid morphology, as well as areas with intercellular bridge formation and cytoplasmic keratinization.
Management
The goal of treatment of BCC is to completely remove the tumor and maximally preserve function and cosmesis at the site of treatment. Choice of treatment depends in large part on the risk of lesion recurrence, which in turn depends on the presence or absence of aggressive clinical and histopathologic features (Table 2) [65]. Another important consideration for treatment choice is location of the lesion, as preservation of function and cosmesis is paramount in certain locations, such as the face. Accurate assessment of a lesion’s risk for recurrence and selection of appropriate management is important to avoid overtreatment of low-risk lesions and under-treatment of high-risk lesions. Patient preference after a discussion of the risks and benefits of various modalities is also an important consideration in treatment selection.
Table 2. Risk factors for recurrence of basal cell carcinoma from the National Comprehensive Cancer Network (NCCN) [65].
| H&P | Low-Risk | High-Risk |
| Location/size | Area L < 20 mm | Area L ≥ 20 mm |
| Area M < 10 mm | Area M ≥ 10 mm | |
| Area H < 6 mma | Area H ≥ 6 mma | |
| Borders | Well defined | Poorly defined |
| Primary vs. recurrent | Primary | Recurrent |
| Immunosuppression | (-) | (+) |
| Site of prior RT | (-) | (+) |
| Pathology | ||
| Subtype | Nodular, superficial | Aggressive-growth pattern |
| Perineural involvement | (-) | (+) |
Area L = trunk and extremities. Area M = cheeks, forehead, scalp, and neck. Area H = “mask areas” of face (central face, eyelids, eyebrows, periorbital, nose, lips [cutaneous and vermilion], chin, mandible, preauricular and postauricular skin/sulci, temple, ear), genitalia, hands, and feet. aLocation independent of size may constitute high risk in certain clinical settings.
A variety of surgical and medical therapies are available for the treatment of BCC [66]. BCCs at low risk for recurrence are most commonly managed with electrodesiccation and curettage (ED&C) or surgical excision. Other less frequently used treatments include topical 5-fluorouracil (5-FU) or imiquimod, cryosurgery, intralesional injection, and photodynamic therapy for low-risk lesions. Mohs surgery, a specialized method of surgical removal, provides the highest cure rate. It is indicated for lesions at increased risk of recurrence and where functional and anatomic relations need to be preserved. Excision, without the benefit of the Mohs microscopically controlled method, and radiotherapy may be appropriate alternatives in the right clinical setting. ED&C is not indicated for the management of high-risk BCCs because of the high probability of recurrence.
Electrodesiccation and Curettage
ED&C is a technique that consists of superficial ablation combined with surgical scraping of the affected skin with a loop (curette). While the traditional approach to ED&C is to perform three rounds of curettage and electrodesiccation, the authors prefer to electrofulgurate the lesion (i.e., the cautery tip does not come into contact with the tissue) and then curette the devitalized epidermis [66]. This may be repeated once. If the tumor requires more curettage, it should be excised. A side effect of ED&C is the development of a hypopigmented scar at the site of treatment.
One of the largest efficacy studies of ED&C involved the review of 2,314 primary BCCs treated with this technique between 1955 and 1982. Subgroup analysis of 521 primary BCCs treated between 1973 and 1982 revealed that location and size were important determinants of 5-year recurrence rates. Lesions in the neck, trunk, and extremities had a recurrence rate of 3 percent regardless of diameter. BCCs in the scalp, forehead, preauricular and postauricular areas, or the cheeks had a recurrence rate of 5 percent for lesions less than 10 mm in diameter and 23 percent for lesions greater than 10 mm. Tumors in the nose, paranasal area, nasolabial fold, ear, chin, mandibular area, perioral region, or periocular sites recurred in 5 percent of cases if the lesion was less than 6 mm or in 18 percent of instances if the lesion was greater than 6 mm. BCCs with low recurrence rates (≤ 5 percent) largely meet current NCCN criteria for low-risk BCC based on anatomic location and tumor size, while those with unacceptably high recurrence rates correspond to high-risk BCC. The effect of histologic subtype on risk of recurrence was not evaluated. In the same study, recurrent BCCs, an NCCN criterion for high-risk tumors, had a recurrence rate of 18 percent. A recurrence rate at 5 years of 40 percent has been reported for recurrent BCCs treated with ED&C [67].
Other studies have confirmed the efficacy of ED&C. A large prospective cohort study involving 361 BCCs and squamous cell carcinomas showed that in appropriately selected patients ED&C has a cure rate of 95.1 percent (95% CI = 92.6-97.7) with a median follow-up of 7 years [68]. Although treatment of primary low-risk BCCs with ED&C is effective and achieves low recurrence rates, lesions with aggressive features on histology should not be treated with ED&C because of significantly higher recurrence rates compared to Mohs micrographically controlled surgery and, when conservation of tissue is not a priority, surgical excision. A population-based retrospective case study reviewed 37 primary infiltrative, morpheaform, or micronodular BCCs that were treated with ED&C alone. The reported cure rate was 73 percent with median follow-up of 6.5 years [69].
There is evidence that curettage alone is as effective as ED&C for the treatment of nonaggressive BCCs [70]. Data is limited, however, and further studies are needed before this technique can safely become part of standard clinical practice.
In summary, ED&C is most appropriate for the treatment of low-risk superficial and nodular primary BCCs on the extremities and trunk. The preferred technique is one cycle of electrofulguration followed by curettage. Many dermatologists do not favor the use of ED&C for the treatment of low-risk primary BCC on the face for a variety of reasons. First, it may result in an unattractive, hypopigmented scar. Second, recurrent BCCs initially treated with ED&C grow within scar tissue and may have a more aggressive biological behavior, potentially causing significant destruction.
Surgical Excision
Surgical excision is a routine office-based procedure performed under local anesthesia. It involves elliptical surgical removal of the BCC. The surgical defect is typically immediately repaired by side-side closure. Healing by second intention is appropriate under certain circumstances. Variable amounts of normal tissue must be sacrificed to achieve acceptable cure rates. The excised specimen is fixed in formalin and sent to the pathology laboratory, where it is embedded in paraffin, processed, and stained for evaluation of tissue margins. Less than 1 percent of the tissue margin is analyzed in this manner. The most commonly used excisional margin is 4 mm because, in cases where tissue conservation is not a priority, this width totally eradicates the tumor in more than 95 percent of cases for tumors with a diameter of less than 2 cm [71]. A recent systematic analysis revealed that a margin of 3 mm may be equally effective [72].
The 5-year cure rate for primary BCC treated with surgical excision has been reported at 95.2 percent in one study [73]. Tumor location on the head was an independent risk factor for recurrence. BCCs on the head smaller than 6 mm had a low recurrence rate of 3.2 percent. Larger lesions had higher recurrence rates with tumors 6 to 9 mm in diameter recurring in 8 percent of cases and tumors of at least 10 mm reappearing in 9 percent of instances. Excision of facial BCCs with standard 4-mm margins is often not feasible because of cosmetic and functional concerns. A study found that facial, nodular BCCs excised with 1 to 3 mm margins were associated with positive margins on histology in 20 percent of cases, requiring additional excision [74]. Because of this, nodular BCCs on the face should be excised with the standard 4-mm margins or with tissue-sparing Mohs micrographically controlled surgery, depending on the specific location and tumor size. Locations associated with the highest recurrence rates after conventional excision are the nose, periocular and paranasal regions, and scalp [75,76]. Recurrent BCCs treated with surgical excision have 5-year recurrence rates of 11 to 17 percent and should therefore not be treated with this technique [63,73,77].
Aggressive-growth histologic patterns are associated with poorer margin clearance than indolent-growth tumors after surgical excision. A study of 1,039 BCCs correlated the various histologic patterns with adequacy of margins of surgical excision. Nodular and superficial BCCs were completely removed by conventional surgical excision in a high percentage of cases (94 percent and 96 percent, respectively). In contrast, micronodular, infiltrative, and morpheaform BCCs had a high incidence of positive tumor margins (19 percent, 26 percent, and 33 percent, respectively).
In summary, surgical excision is effective for most primary BCCs on the trunk or extremities where conservation of tissue is not a priority.
Mohs Micrographically Controlled Surgery
Mohs micrographically controlled surgery (MMCS) is a specialized office-based surgical procedure that combines staged resection under local anesthesia with frozen section evaluation of the complete epidermal and deep surgical margins (Figure 5). This technique achieves the lowest recurrence rates of all treatment modalities and maximally preserves tissue. For primary BCC, the 5-year recurrence rate has been reported to be 1.4 percent, while for recurrent tumors it has been estimated at 4 percent [78]. The most common site of recurrence was the nose. Most of the tumors in this study were located on the head and neck. There is compelling data supporting MMCS as the treatment of choice for recurrent BCC [67,77]. At 5.6 percent, MMCS results in the lowest 5-year recurrence rate out of all treatment modalities [67]. In contrast, surgical excision, ED&C, and radiation therapy show recurrence rates of 17.4 percent, 40 percent, and 9.8 percent, respectively. BCCs with aggressive-growth histologic subtype have been shown to exhibit extensive subclinical spread and are therefore particularly appropriate for treatment with MMCS [79]. Margin control with MMCS also has been shown to be of benefit in the setting of BCCs demonstrating perineural involvement, a histologic marker associated with aggressive-growth histologic subtypes [80]. Perineural involvement is more commonly associated with squamous cell carcinoma than BCC, but altogether this is an infrequent feature of NMSC. Another advantage of MMCS is its tissue-sparing capability. A study showed that the median area of the surgical defects produced during MMCS for the treatment of nodular BCCs, most of them on the head and neck, was significantly smaller than that of conventional surgical excision (116.6 mm2 versus 187.7 mm2, p < 0.001) [81]. The cost of MMCS is comparable to the cost of office-based conventional surgical excision with permanent section margin control and lower than the cost of excisions performed in surgical centers or hospital operating rooms [82].
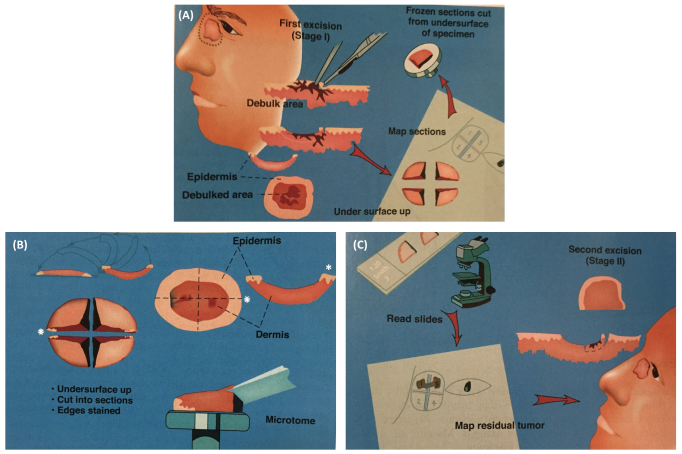
Figure 5.
Mohs micrographically controlled surgery (MCCS). A) After gentle curettage for debulking of the tumor, the lesion is excised with a minimal margin of clinically normal-appearing tissue and the specimen is precisely mapped and processed immediately by frozen section for microscopic examination. B) The whole specimen is transected, inverted, and inked. The white asterisks indicate the 3 o’clock position of the lateral margin. Each segment of the whole specimen is processed by frozen section with the use of a microtome, which cuts from the undersurface of the segments. C) The deep and lateral margins of each segment are viewed with a microscope in search for the presence of tumor cells (positive margin). Precise mapping allows for directed extirpation of any remaining tumor. (Courtesy of David J. Leffell, MD, Manual of Skin Surgery, Second Edition, PMPH; 2011.)
In summary, MMCS is the preferred treatment for BCCs at high risk for recurrence [65,83,84]. BBCs at high risk for recurrences include those in high-risk sites of the face (also known as the H-zone, which includes central face, eyelids, eyebrows, periorbital area, nose, lips, chin, mandible, preauricular and postauricular skin, temple, and ears), tumors with clinically poorly defined borders or with aggressive-growth histologic patterns (morpheaform, infiltrative, micronodular, basosquamous), and recurrent lesions. MMCS is also the preferred treatment for sites in which tissue conservation is crucial and there is a need for reliable clear margins.
Radiotherapy
Radiation therapy has been reported to result in low recurrence rates for both primary BCC (7.4 percent) and recurrent BCC (9.5 percent) [85]. It is the primary option for the treatment of BCCs if surgery is contraindicated. Because of potential long-term complications such as chronic radiation dermatitis, alopecia, and even radiation-induced cutaneous malignancies, patients over the age of 60 are preferred candidates. Poor cosmetic results in the form of permanent areas of hypopigmentation and hyperpigmentation, dryness, epidermal atrophy, telangiectasias, and dermal fibrosis (chronic radiation dermatitis) are common, occurring in 37 percent of patients treated with radiation therapy in one study [85].
Other Treatment Modalities
Topical imiquimod 5% cream is approved by the FDA for the treatment of superficial BCCs in low-risk sites. Imiquimod is a TLR-7 (toll-like receptor 7) agonist that induces interferon and other cytokines and is thought to promote T-cell-mediated apoptosis of tumor cells by circumventing survival mechanisms [86]. Pooled results of two identical multicenter randomized phase III trials proved the efficacy of imiquimod for the treatment of superficial BCC. Treatment with topical imiquimod 5% cream once daily, five times per week for 6 weeks resulted in a histologic clearance rate of 82 percent 12 weeks after treatment [87]. The vast majority of the lesions were located on the trunk or extremities. Adverse side effect included localized erythema, erosion, and scabbing or crusting. Topical imiquimod appears to be less effective for the treatment of nodular BCCs. A randomized phase III trial showed that thrice-weekly topical imiquimod for either 8 or 12 weeks for the treatment of low-risk nodular BCC resulted in complete histopathological clearance upon subsequent surgical excision of the lesion and margin evaluation in only 64 percent of tumors [88]. In summary, topical imiquimod 5% cream is best used for the treatment of primary superficial BCCs in low-risk sites where recurrence is unlikely to result in significant morbidity. Patients with multiple BCCs secondary to an underlying genetic syndrome, such as NBCCS or xeroderma pigmentosum, have been shown in case reports to also benefit from topical imiquimod treatment, obviating the need for multiple, disfiguring surgeries [89-92]. Topical 5-fluorouracil (5-FU, 5% formulation) is an FDA-approved alternative to topical imiquimod for the treatment of superficial BCCs in low-risk sites. It is rarely used, however, as imiquimod has largely replaced it for this indication.
Photodynamic therapy (PDT) consists of using light and porphyrins to induce tumor destruction. The FDA has not approved this modality for the treatment of BCC. Most studies have come from Europe because PDT is approved for the treatment of BCC in many countries in that continent. The lesions most responsive to therapy appear to be superficial BCCs with cure rates ranging from 72 to 100 percent [93-96].
Cryosurgery, or the use of liquid nitrogen to freeze the tumor, is a rarely used therapy for BCC. Treatment can result in a hypopigmented scar and a high recurrence rate.
Intralesional injection of pro-inflammatory agents is a practically obsolete method formerly used to treat certain BCCs. Intralesional injection of interferon-α-2b has been shown to be effective for the treatment of superficial and nodular BCCs but involved frequent painful injections over 3 weeks. Topical imiquimod is much simpler to use than intralesional agents and achieves similar results.
Management of Locally Advanced and Metastatic Basal Cell Carcinoma
If left untreated, BCC can become locally destructive to the point where surgical resection is not feasible because of the large size of the tumor or the proximity to vital or functionally important structures. Also, radiation therapy may be ineffective. Until recently, there were limited options for the treatment of locally advanced BCC. Metastasis of BCC is extremely rare, occurring in approximately 0.003 percent to 0.1 percent of cases [97]. On January 30, 2012, the FDA approved vismodegib, a small molecule inhibitor of the Smoothened receptor in the hedgehog pathway, for the treatment of metastatic and locally advanced BCC. The approval was based on the results of a phase II clinical trial (ERIVANCE) which showed objective responses in 30 percent of patients with metastatic BCC and in 43 percent of patients with locally advanced BCC with a median duration of response of 7.6 months in both cohorts [98]. Common adverse events included muscle spasms, alopecia, taste disturbance, weight loss, and fatigue. The effectiveness of vismodegib in the treatment of advanced BCC has been corroborated in an expanded access study, in which 46.4 percent of patients with locally advanced BCC and 30.8 percent of patients with metastatic BCC showed objective responses [99]. Patients with NBCCS have also been shown to benefit from treatment with vismodegib. A randomized, double-blind, placebo-controlled trial in patients with NBCCS showed vismodegib reduces BCC tumor burden and blocks growth of new BCCs in patients with this genetic syndrome [100]. Given the preclinical and clinical success of vismodegib and similar small molecules, topical formulations of hedgehog pathway inhibitors are under investigation for the treatment of BCC [101,102]. Although vismodegib is the first effective medical treatment for advanced BCC, several challenges remain, including understanding the genetic underpinnings of clinical response and developing strategies to circumvent acquired resistance.
Acknowledgments
Dr. David J. Leffell for his guidance in the revision of this manuscript.
Abbreviations
- BCC
basal cell carcinoma
- NBCCS
nevoid basal cell carcinoma syndrome
- NMSC
nonmelanoma skin cancer
- ED&C
electrodesiccation and curettage
- SHH
Sonic hedgehog
- SMO
Smoothened
- PUVA
psoralen and ultraviolet A
- MMCS
Mohs micrographically controlled surgery
- PDT
photodynamic therapy
References
- Gorlin RJ, Goltz RW. Multiple nevoid basal-cell epithelioma, jaw cysts and bifid rib. A syndrome. N Engl J Med. 1960;262:908–912. doi: 10.1056/NEJM196005052621803. [DOI] [PubMed] [Google Scholar]
- Gailani MR, Bale SJ, Leffell DJ, DiGiovanna JJ, Peck GL, Poliak S. et al. Developmental defects in Gorlin syndrome related to a putative tumor suppressor gene on chromosome 9. Cell. 1992;69(1):111–117. doi: 10.1016/0092-8674(92)90122-s. [DOI] [PubMed] [Google Scholar]
- Farndon PA, Del Mastro RG, Evans DG, Kilpatrick MW. Location of gene for Gorlin syndrome. Lancet. 1992;339(8793):581–582. doi: 10.1016/0140-6736(92)90868-4. [DOI] [PubMed] [Google Scholar]
- Reis A, Kuster W, Linss G, Gebel E, Hamm H, Fuhrmann W. et al. Localisation of gene for the naevoid basal-cell carcinoma syndrome. Lancet. 1992;339(8793):617. doi: 10.1016/0140-6736(92)90903-g. [DOI] [PubMed] [Google Scholar]
- Gailani MR, Leffell DJ, Ziegler A, Gross EG, Brash DE, Bale AE. Relationship between sunlight exposure and a key genetic alteration in basal cell carcinoma. J Natl Cancer Inst. 1996;88(6):349–354. doi: 10.1093/jnci/88.6.349. [DOI] [PubMed] [Google Scholar]
- Hahn H, Wicking C, Zaphiropoulous PG, Gailani MR, Shanley S, Chidambaram A. et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85(6):841–851. doi: 10.1016/s0092-8674(00)81268-4. [DOI] [PubMed] [Google Scholar]
- Johnson RL, Rothman AL, Xie J, Goodrich LV, Bare JW, Bonifas JM. et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272(5268):1668–1671. doi: 10.1126/science.272.5268.1668. [DOI] [PubMed] [Google Scholar]
- Stone DM, Hynes M, Armanini M, Swanson TA, Gu Q, Johnson RL. et al. The tumour-suppressor gene patched encodes a candidate receptor for Sonic hedgehog. Nature. 1996;384(6605):129–134. doi: 10.1038/384129a0. [DOI] [PubMed] [Google Scholar]
- Fan H, Oro AE, Scott MP, Khavari PA. Induction of basal cell carcinoma features in transgenic human skin expressing Sonic Hedgehog. Nat Med. 1997;3(7):788–792. doi: 10.1038/nm0797-788. [DOI] [PubMed] [Google Scholar]
- Oro AE, Higgins KM, Hu Z, Bonifas JM, Epstein EH Jr., Scott MP. Basal cell carcinomas in mice overexpressing sonic hedgehog. Science. 1997;276(5313):817–821. doi: 10.1126/science.276.5313.817. [DOI] [PubMed] [Google Scholar]
- Xie J, Murone M, Luoh SM, Ryan A, Gu Q, Zhang C. et al. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391(6662):90–92. doi: 10.1038/34201. [DOI] [PubMed] [Google Scholar]
- Grachtchouk M, Mo R, Yu S, Zhang X, Sasaki H, Hui CC. et al. Basal cell carcinomas in mice overexpressing Gli2 in skin. Nat Genet. 2000;24(3):216–217. doi: 10.1038/73417. [DOI] [PubMed] [Google Scholar]
- Nilsson M, Unden AB, Krause D, Malmqwist U, Raza K, Zaphiropoulos PG. et al. Induction of basal cell carcinomas and trichoepitheliomas in mice overexpressing GLI-1. Proc Natl Acad Sci USA. 2000;97(7):3438–3443. doi: 10.1073/pnas.050467397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchin ME, Kariapper MS, Grachtchouk M, Wang A, Wei L, Cummings D. et al. Sustained Hedgehog signaling is required for basal cell carcinoma proliferation and survival: conditional skin tumorigenesis recapitulates the hair growth cycle. Genes Dev. 2005;19(2):214–223. doi: 10.1101/gad.1258705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole EA, Ponten F, Lundeberg J, Asplund A. In: Dermatology. 3rd edition. Bolognia JL, Jorizzo JL, Schaffer JV, editors. Saunders; 2012. Principles of Tumor Biology and Pathogenesis of BCCs and SCCs; pp. 1759–1772. [Google Scholar]
- Ponten F, Berg C, Ahmadian A, Ren ZP, Nister M, Lundeberg J. et al. Molecular pathology in basal cell cancer with p53 as a genetic marker. Oncogene. 1997;15(9):1059–1067. doi: 10.1038/sj.onc.1201435. [DOI] [PubMed] [Google Scholar]
- Youssef KK, Van Keymeulen A, Lapouge G, Beck B, Michaux C, Achouri Y. et al. Identification of the cell lineage at the origin of basal cell carcinoma. Nat Cell Biol. 2010;12(3):299–305. doi: 10.1038/ncb2031. [DOI] [PubMed] [Google Scholar]
- Grachtchouk M, Pero J, Yang SH, Ermilov AN, Michael LE, Wang A. et al. Basal cell carcinomas in mice arise from hair follicle stem cells and multiple epithelial progenitor populations. J Clin Invest. 2011;121(5):1768–1781. doi: 10.1172/JCI46307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GY, Wang J, Mancianti ML, Epstein EH Jr.. Basal cell carcinomas arise from hair follicle stem cells in Ptch1(+/-) mice. Cancer Cell. 2011;19(1):114–124. doi: 10.1016/j.ccr.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seykora JT, Cotsarelis G. Keratin 15-positive stem cells give rise to basal cell carcinomas in irradiated Ptch1(+/-) mice. Cancer Cell. 2011;19(1):5–6. doi: 10.1016/j.ccr.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Facts & Figures 2010. American Cancer Society [Internet] Available from: http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2010/index .
- Rogers HW, Weinstock MA, Harris AR, Hinckley MR, Feldman SR, Fleischer AB. et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146(3):283–287. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
- Rigel DS, Friedman RJ, Kopf AW. Lifetime risk for development of skin cancer in the U.S. population: current estimate is now 1 in 5. J Am Acad Dermatol. 1996;35(6):1012–1013. doi: 10.1016/s0190-9622(96)90139-5. [DOI] [PubMed] [Google Scholar]
- Chuang TY, Popescu A, Su WP, Chute CG. Basal cell carcinoma. A population-based incidence study in Rochester, Minnesota. J Am Acad Dermatol. 1990;22(3):413–417. doi: 10.1016/0190-9622(90)70056-n. [DOI] [PubMed] [Google Scholar]
- Reizner GT, Chuang TY, Elpern DJ, Stone JL, Farmer ER. Basal cell carcinoma in Kauai, Hawaii: the highest documented incidence in the United States. J Am Acad Dermatol. 1993;29(2 Pt 1):184–189. doi: 10.1016/0190-9622(93)70165-p. [DOI] [PubMed] [Google Scholar]
- Christenson LJ, Borrowman TA, Vachon CM, Tollefson MM, Otley CC, Weaver AL. et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294(6):681–690. doi: 10.1001/jama.294.6.681. [DOI] [PubMed] [Google Scholar]
- Schreiber MM, Moon TE, Fox SH, Davidson J. The risk of developing subsequent nonmelanoma skin cancers. J Am Acad Dermatol. 1990;23(6 Pt 1):1114–1118. doi: 10.1016/0190-9622(90)70343-g. [DOI] [PubMed] [Google Scholar]
- Karagas MR, Stukel TA, Greenberg ER, Baron JA, Mott LA, Stern RS. Risk of subsequent basal cell carcinoma and squamous cell carcinoma of the skin among patients with prior skin cancer. Skin Cancer Prevention Study Group. JAMA. 1992;267(24):3305–3310. [PubMed] [Google Scholar]
- Marghoob A, Kopf AW, Bart RS, Sanfilippo L, Silverman MK, Lee P. et al. Risk of another basal cell carcinoma developing after treatment of a basal cell carcinoma. J Am Acad Dermatol. 1993;28(1):22–28. doi: 10.1016/0190-9622(93)70003-c. [DOI] [PubMed] [Google Scholar]
- Marcil I, Stern RS. Risk of developing a subsequent nonmelanoma skin cancer in patients with a history of nonmelanoma skin cancer: a critical review of the literature and meta-analysisa. Arch Dermatol. 2000;136(12):1524–1530. doi: 10.1001/archderm.136.12.1524. [DOI] [PubMed] [Google Scholar]
- Schinstine M, Goldman GD. Risk of synchronous and metachronous second nonmelanoma skin cancer when referred for Mohs micrographic surgery. J Am Acad Dermatol. 2001;44(3):497–499. doi: 10.1067/mjd.2001.110646. [DOI] [PubMed] [Google Scholar]
- Marghoob AA, Slade J, Salopek TJ, Kopf AW, Bart RS, Rigel DS. Basal cell and squamous cell carcinomas are important risk factors for cutaneous malignant melanoma. Screening implications. Cancer. 1995;75(2 Suppl):707–714. doi: 10.1002/1097-0142(19950115)75:2+<707::aid-cncr2820751415>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Gallagher RP, Hill GB, Bajdik CD, Fincham S, Coldman AJ, McLean DI. et al. Sunlight exposure, pigmentary factors, and risk of nonmelanocytic skin cancer. I. Basal cell carcinoma. Arch Dermatol. 1995;131(2):157–163. [PubMed] [Google Scholar]
- Zanetti R, Rosso S, Martinez C, Nieto A, Miranda A, Mercier M. et al. Comparison of risk patterns in carcinoma and melanoma of the skin in men: a multi-centre case-case-control study. Br J Cancer. 2006;94(5):743–751. doi: 10.1038/sj.bjc.6602982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kricker A, Armstrong BK, English DR, Heenan PJ. Does intermittent sun exposure cause basal cell carcinoma? a case-control study in Western Australia. Int J Cancer. 1995;60(4):489–494. doi: 10.1002/ijc.2910600411. [DOI] [PubMed] [Google Scholar]
- Stern RS. PUVA Follow-Up Study. The risk of squamous cell and basal cell cancer associated with psoralen and ultraviolet A therapy: a 30-year prospective study. J Am Acad Dermatol. 2012;66(4):553–562. doi: 10.1016/j.jaad.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Wehner MR, Shive ML, Chren MM, Han J, Qureshi AA, Linos E. Indoor tanning and non-melanoma skin cancer: systematic review and meta-analysis. BMJ. 2012;345:e5909. doi: 10.1136/bmj.e5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci LM, Cartmel B, Molinaro AM, Leffell DJ, Bale AE, Mayne ST. Indoor tanning and risk of early-onset basal cell carcinoma. J Am Acad Dermatol. 2012;67(4):552–562. doi: 10.1016/j.jaad.2011.11.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SN, Zens MS, Perry AE, Spencer SK, Duell EJ, Karagas MR. Photosensitizing agents and the risk of non-melanoma skin cancer: a population-based case-control study. J Invest Dermatol. 2013;133(8):1950–1955. doi: 10.1038/jid.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagas MR, McDonald JA, Greenberg ER, Stukel TA, Weiss JE, Baron JA. et al. Risk of basal cell and squamous cell skin cancers after ionizing radiation therapy. For The Skin Cancer Prevention Study Group. J Natl Cancer Inst. 1996;88(24):1848–1853. doi: 10.1093/jnci/88.24.1848. [DOI] [PubMed] [Google Scholar]
- Karagas MR, Tosteson TD, Blum J, Morris JS, Baron JA, Klaue B. Design of an epidemiologic study of drinking water arsenic exposure and skin and bladder cancer risk in a U.S. population. Environ Health Perspect. 1998;106 Suppl 4:1047–1050. doi: 10.1289/ehp.98106s41047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonchai W, Green A, Ng J, Dicker A, Chenevix-Trench G. Basal cell carcinoma in chronic arsenicism occurring in Queensland, Australia, after ingestion of an asthma medication. J Am Acad Dermatol. 2000;43(4):664–669. doi: 10.1067/mjd.2000.107939. [DOI] [PubMed] [Google Scholar]
- Tao SS, Bolger PM. Dietary arsenic intakes in the United States: FDA Total Diet Study, September 1991-December 1996. Food Addit Contam. 1999;16(11):465–472. doi: 10.1080/026520399283759. [DOI] [PubMed] [Google Scholar]
- Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Engl J Med. 2003;348(17):1681–1691. doi: 10.1056/NEJMra022137. [DOI] [PubMed] [Google Scholar]
- Silverberg MJ, Leyden W, Warton EM, Quesenberry CP Jr., Engels EA, Asgari MM. HIV infection status, immunodeficiency, and the incidence of non-melanoma skin cancer. J Natl Cancer Inst. 2013;105(5):350–360. doi: 10.1093/jnci/djs529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: A study of 596 cases. J Am Acad Dermatol. 2000;42(2 Pt 1):263–268. doi: 10.1016/S0190-9622(00)90136-1. [DOI] [PubMed] [Google Scholar]
- Rosen H, Schmidt B, Lam HP, Meara JG, Labow BI. Management of nevus sebaceous and the risk of Basal cell carcinoma: an 18-year review. Pediatr Dermatol. 2009;26(6):676–681. doi: 10.1111/j.1525-1470.2009.00939.x. [DOI] [PubMed] [Google Scholar]
- Stern RS, Weinstein MC, Baker SG. Risk reduction for nonmelanoma skin cancer with childhood sunscreen use. Arch Dermatol. 1986;122(5):537–545. [PubMed] [Google Scholar]
- Green A, Williams G, Neale R, Hart V, Leslie D, Parsons P. et al. Daily sunscreen application and betacarotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: a randomised controlled trial. Lancet. 1999;354(9180):723–729. doi: 10.1016/S0140-6736(98)12168-2. [DOI] [PubMed] [Google Scholar]
- Ulrich C, Jurgensen JS, Degen A, Hackethal M, Ulrich M, Patel MJ. et al. Prevention of non-melanoma skin cancer in organ transplant patients by regular use of a sunscreen: a 24 months, prospective, case-control study. Br J Dermatol. 2009;161 Suppl 3:78–84. doi: 10.1111/j.1365-2133.2009.09453.x. [DOI] [PubMed] [Google Scholar]
- Scrivener Y, Grosshans E, Cribier B. Variations of basal cell carcinomas according to gender, age, location and histopathological subtype. Br J Dermatol. 2002;147(1):41–47. doi: 10.1046/j.1365-2133.2002.04804.x. [DOI] [PubMed] [Google Scholar]
- Soyer HP, Rigel DS, Wurm EM. In: Dermatology. 3rd edition. Bolognia JL, Jorizzo JL, Schaffer JV, editors. Saunders; 2012. Basal Cell Carcinoma and Squamous Cell Carcinoma; pp. 1773–1793. [Google Scholar]
- Christensen SR, Leffell DJ. In: DeVita, Hellman, and Rosenberg’s Cancer: Principles & Practice of Oncology. 10th edition. DeVita VT, Lawrence TS, Rosenberg SA, editors. LWW; 2014. Cancer of the Skin; pp. 1314–1323. [Google Scholar]
- McCormack CJ, Kelly JW, Dorevitch AP. Differences in age and body site distribution of the histological subtypes of basal cell carcinoma. A possible indicator of differing causes. Arch Dermatol. 1997;133(5):593–596. [PubMed] [Google Scholar]
- Wu PA. Epidemiology and clinical features of basal cell carcinoma 2014. UpToDate [Internet] Available from: http://www.uptodate.com/contents/epidemiology-and-clinical-features-of-basal-cell-carcinoma .
- Altamura D, Menzies SW, Argenziano G, Zalaudek I, Soyer HP, Sera F. et al. Dermatoscopy of basal cell carcinoma: morphologic variability of global and local features and accuracy of diagnosis. J Am Acad Dermatol. 2010;62(1):67–75. doi: 10.1016/j.jaad.2009.05.035. [DOI] [PubMed] [Google Scholar]
- Zalaudek I, Kreusch J, Giacomel J, Ferrara G, Catricala C, Argenziano G. How to diagnose nonpigmented skin tumors: a review of vascular structures seen with dermoscopy: part II. Nonmelanocytic skin tumors. J Am Acad Dermatol. 2010;63(3):377-86; quiz 87-8. doi: 10.1016/j.jaad.2009.11.697. [DOI] [PubMed] [Google Scholar]
- Russell EB, Carrington PR, Smoller BR. Basal cell carcinoma: a comparison of shave biopsy versus punch biopsy techniques in subtype diagnosis. J Am Acad Dermatol. 1999;41(1):69–71. doi: 10.1016/s0190-9622(99)70409-3. [DOI] [PubMed] [Google Scholar]
- Haws AL, Rojano R, Tahan SR, Phung TL. Accuracy of biopsy sampling for subtyping basal cell carcinoma. J Am Acad Dermatol. 2012;66(1):106–111. doi: 10.1016/j.jaad.2011.02.042. [DOI] [PubMed] [Google Scholar]
- Roozeboom MH, Mosterd K, Winnepenninckx VJ, Nelemans PJ, Kelleners-Smeets NW. Agreement between histological subtype on punch biopsy and surgical excision in primary basal cell carcinoma. J Eur Acad Dermatol Venereol. 2013;27(7):894–898. doi: 10.1111/j.1468-3083.2012.04608.x. [DOI] [PubMed] [Google Scholar]
- Wolberink EA, Pasch MC, Zeiler M, van Erp PE, Gerritsen MJ. High discordance between punch biopsy and excision in establishing basal cell carcinoma subtype: analysis of 500 cases. J Eur Acad Dermatol Venereol. 2013;27(8):985–989. doi: 10.1111/j.1468-3083.2012.04628.x. [DOI] [PubMed] [Google Scholar]
- Sexton M, Jones DB, Maloney ME. Histologic pattern analysis of basal cell carcinoma. Study of a series of 1039 consecutive neoplasms. J Am Acad Dermatol. 1990;23(6 Pt 1):1118–1126. doi: 10.1016/0190-9622(90)70344-h. [DOI] [PubMed] [Google Scholar]
- Crowson AN. Basal cell carcinoma: biology, morphology and clinical implications. Mod Pathol. 2006;19 Suppl 2:S127–S147. doi: 10.1038/modpathol.3800512. [DOI] [PubMed] [Google Scholar]
- Aasi SZ, Leffell DJ, Lazova RZ. Atlas of Practical Mohs Histopathology. Springer; 2013. 320 p [Google Scholar]
- Miller SJ, Alam M, Andersen J, Berg D, Bichakjian CK, Bowen G. et al. Basal cell and squamous cell skin cancers. J Natl Compr Canc Netw. 2010;8(8):836–864. doi: 10.6004/jnccn.2010.0062. [DOI] [PubMed] [Google Scholar]
- Neville JA, Welch E, Leffell DJ. Management of nonmelanoma skin cancer in 2007. Nat Clin Pract Oncol. 2007;4(8):462–469. doi: 10.1038/ncponc0883. [DOI] [PubMed] [Google Scholar]
- Rowe DE, Carroll RJ, Day CL Jr.. Mohs surgery is the treatment of choice for recurrent (previously treated) basal cell carcinoma. J Dermatol Surg Oncol. 1989;15(4):424–431. doi: 10.1111/j.1524-4725.1989.tb03249.x. [DOI] [PubMed] [Google Scholar]
- Chren MM, Linos E, Torres JS, Stuart SE, Parvataneni R, Boscardin WJ. Tumor recurrence 5 years after treatment of cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2013;133(5):1188–1196. doi: 10.1038/jid.2012.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blixt E, Nelsen D, Stratman E. Recurrence rates of aggressive histologic types of basal cell carcinoma after treatment with electrodesiccation and curettage alone. Dermatol Surg. 2013;39(5):719–725. doi: 10.1111/dsu.12122. [DOI] [PubMed] [Google Scholar]
- Barlow JO, Zalla MJ, Kyle A, DiCaudo DJ, Lim KK, Yiannias JA. Treatment of basal cell carcinoma with curettage alone. J Am Acad Dermatol. 2006;54(6):1039–1045. doi: 10.1016/j.jaad.2006.01.041. [DOI] [PubMed] [Google Scholar]
- Wolf DJ, Zitelli JA. Surgical margins for basal cell carcinoma. Arch Dermatol. 1987;123(3):340–344. [PubMed] [Google Scholar]
- Gulleth Y, Goldberg N, Silverman RP, Gastman BR. What is the best surgical margin for a Basal cell carcinoma: a meta-analysis of the literature. Plast Reconstr Surg. 2010;126(4):1222–1231. doi: 10.1097/PRS.0b013e3181ea450d. [DOI] [PubMed] [Google Scholar]
- Silverman MK, Kopf AW, Bart RS, Grin CM, Levenstein MS. Recurrence rates of treated basal cell carcinomas. Part 3: Surgical excision. J Dermatol Surg Oncol. 1992;18(6):471–476. doi: 10.1111/j.1524-4725.1992.tb03307.x. [DOI] [PubMed] [Google Scholar]
- Kimyai-Asadi A, Alam M, Goldberg LH, Peterson SR, Silapunt S, Jih MH. Efficacy of narrow-margin excision of well-demarcated primary facial basal cell carcinomas. J Am Acad Dermatol. 2005;53(3):464–468. doi: 10.1016/j.jaad.2005.03.038. [DOI] [PubMed] [Google Scholar]
- Bart RS, Schrager D, Kopf AW, Bromberg J, Dubin N. Scalpel excision of basal cell carcinomas. Arch Dermatol. 1978;114(5):739–742. [PubMed] [Google Scholar]
- Dubin N, Kopf AW. Multivariate risk score for recurrence of cutaneous basal cell carcinomas. Arch Dermatol. 1983;119(5):373–377. [PubMed] [Google Scholar]
- Mosterd K, Krekels GA, Nieman FH, Ostertag JU, Essers BA, Dirksen CD. et al. Mohs’ micrographic surgery for primary and recurrent basal-cell carcinoma of the face: a prospective randomised controlled trial with 5-years’ follow-up. Lancet Oncol. 2008;9(12):1149–1156. doi: 10.1016/S1470-2045(08)70260-2. [DOI] [PubMed] [Google Scholar]
- Leibovitch I, Huilgol SC, Selva D, Richards S, Paver R. Basal cell carcinoma treated with Mohs surgery in Australia II. Outcome at 5-year follow-up. J Am Acad Dermatol. 2005;53(3):452–457. doi: 10.1016/j.jaad.2005.04.087. [DOI] [PubMed] [Google Scholar]
- Batra RS, Kelley LC. Predictors of extensive subclinical spread in nonmelanoma skin cancer treated with Mohs micrographic surgery. Arch Dermatol. 2002;138(8):1043–1051. doi: 10.1001/archderm.138.8.1043. [DOI] [PubMed] [Google Scholar]
- Leibovitch I, Huilgol SC, Selva D, Richards S, Paver R. Basal cell carcinoma treated with Mohs surgery in Australia III. Perineural invasion. J Am Acad Dermatol. 2005;53(3):458–463. doi: 10.1016/j.jaad.2005.04.089. [DOI] [PubMed] [Google Scholar]
- Muller FM, Dawe RS, Moseley H, Fleming CJ. Randomized comparison of Mohs micrographic surgery and surgical excision for small nodular basal cell carcinoma: tissue-sparing outcome. Dermatol Surg. 2009;35(9):1349–1354. doi: 10.1111/j.1524-4725.2009.01240.x. [DOI] [PubMed] [Google Scholar]
- Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39(5 Pt 1):698–703. doi: 10.1016/s0190-9622(98)70041-6. [DOI] [PubMed] [Google Scholar]
- Drake LA, Dinehart SM, Goltz RW, Graham GF, Hordinsky MK, Lewis CW. et al. Guidelines of care for Mohs micrographic surgery. American Academy of Dermatology. J Am Acad Dermatol. 1995;33(2 Pt 1):271–278. doi: 10.1016/0190-9622(95)90261-9. [DOI] [PubMed] [Google Scholar]
- Ad Hoc Task Force. Connolly SM, Baker DR, Coldiron BM, Fazio MJ, Storrs PA. et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67(4):531–550. doi: 10.1016/j.jaad.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Silverman MK, Kopf AW, Gladstein AH, Bart RS, Grin CM, Levenstein MJ. Recurrence rates of treated basal cell carcinomas. Part 4: X-ray therapy. J Dermatol Surg Oncol. 1992;18(7):549–554. doi: 10.1111/j.1524-4725.1992.tb03508.x. [DOI] [PubMed] [Google Scholar]
- Schon M, Bong AB, Drewniok C, Herz J, Geilen CC, Reifenberger J. et al. Tumor-selective induction of apoptosis and the small-molecule immune response modifier imiquimod. J Natl Cancer Inst. 2003;95(15):1138–1149. doi: 10.1093/jnci/djg016. [DOI] [PubMed] [Google Scholar]
- Geisse J, Caro I, Lindholm J, Golitz L, Stampone P, Owens M. Imiquimod 5% cream for the treatment of superficial basal cell carcinoma: results from two phase III, randomized, vehicle-controlled studies. J Am Acad Dermatol. 2004;50(5):722–733. doi: 10.1016/j.jaad.2003.11.066. [DOI] [PubMed] [Google Scholar]
- Eigentler TK, Kamin A, Weide BM, Breuninger H, Caroli UM, Mohrle M. et al. A phase III, randomized, open label study to evaluate the safety and efficacy of imiquimod 5% cream applied thrice weekly for 8 and 12 weeks in the treatment of low-risk nodular basal cell carcinoma. J Am Acad Dermatol. 2007;57(4):616–621. doi: 10.1016/j.jaad.2007.05.022. [DOI] [PubMed] [Google Scholar]
- Kagy MK, Amonette R. The use of imiquimod 5% cream for the treatment of superficial basal cell carcinomas in a basal cell nevus syndrome patient. Dermatol Surg. 2000;26(6):577-8; discussion 8-9. doi: 10.1046/j.1524-4725.2000.00003.x. [DOI] [PubMed] [Google Scholar]
- Stockfleth E, Ulrich C, Hauschild A, Lischner S, Meyer T, Christophers E. Successful treatment of basal cell carcinomas in a nevoid basal cell carcinoma syndrome with topical 5% imiquimod. Eur J Dermatol. 2002;12(6):569–572. [PubMed] [Google Scholar]
- Weisberg NK, Varghese M. herapeutic response of a brother and sister with xeroderma pigmentosum to imiquimod 5% cream. Dermatol Surg. 2002;28(6):518–523. doi: 10.1046/j.1524-4725.2002.01196.x. [DOI] [PubMed] [Google Scholar]
- Ferreres JR, Macaya A, Jucgla A, Muniesa C, Prats C, Peyri J. Hundreds of basal cell carcinomas in a Gorlin-Goltz syndrome patient cured with imiquimod 5% cream. J Eur Acad Dermatol Venereol. 2006;20(7):877–878. doi: 10.1111/j.1468-3083.2006.01552.x. [DOI] [PubMed] [Google Scholar]
- Fantini F, Greco A, Del Giovane C, Cesinaro AM, Venturini M, Zane C. et al. Photodynamic therapy for basal cell carcinoma: clinical and pathological determinants of response. J Eur Acad Dermatol Venereol. 2011;25(8):896–901. doi: 10.1111/j.1468-3083.2010.03877.x. [DOI] [PubMed] [Google Scholar]
- Cosgarea R, Susan M, Crisan M, Senila S. Photodynamic therapy using topical 5-aminolaevulinic acid vs. surgery for basal cell carcinoma. J Eur Acad Dermatol Venereol. 2013;27(8):980–984. doi: 10.1111/j.1468-3083.2012.04619.x. [DOI] [PubMed] [Google Scholar]
- Roozeboom MH, Arits AH, Nelemans PJ, Kelleners-Smeets NW. Overall treatment success after treatment of primary superficial basal cell carcinoma: a systematic review and meta-analysis of randomized and nonrandomized trials. Br J Dermatol. 2012;167(4):733–756. doi: 10.1111/j.1365-2133.2012.11061.x. [DOI] [PubMed] [Google Scholar]
- Arits AH, Mosterd K, Essers BA, Spoorenberg E, Sommer A, De Rooij MJ. et al. Photodynamic therapy versus topical imiquimod versus topical fluorouracil for treatment of superficial basal-cell carcinoma: a single blind, non-inferiority, randomised controlled trial. Lancet Oncol. 2013;14(7):647–654. doi: 10.1016/S1470-2045(13)70143-8. [DOI] [PubMed] [Google Scholar]
- von Domarus H, Stevens PJ. Metastatic basal cell carcinoma. Report of five cases and review of 170 cases in the literature. J Am Acad Dermatol. 1984;10(6):1043–1060. doi: 10.1016/s0190-9622(84)80334-5. [DOI] [PubMed] [Google Scholar]
- Sekulic A, Migden MR, Oro AE, Dirix L, Lewis KD, Hainsworth JD. et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366(23):2171–2179. doi: 10.1056/NEJMoa1113713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AL, Solomon JA, Hainsworth JD, Goldberg L, McKenna E, Day BM. et al. Expanded access study of patients with advanced basal cell carcinoma treated with the Hedgehog pathway inhibitor, vismodegib. J Am Acad Dermatol. 2014;70(1):60–69. doi: 10.1016/j.jaad.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Tang JY, Mackay-Wiggan JM, Aszterbaum M, Yauch RL, Lindgren J, Chang K. et al. Inhibiting the hedgehog pathway in patients with the basal-cell nevus syndrome. N Engl J Med. 2012;366(23):2180–2188. doi: 10.1056/NEJMoa1113538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skvara H, Kalthoff F, Meingassner JG, Wolff-Winiski B, Aschauer H, Kelleher JF. et al. Topical treatment of Basal cell carcinomas in nevoid Basal cell carcinoma syndrome with a smoothened inhibitor. J Invest Dermatol. 2011;131(8):1735–1744. doi: 10.1038/jid.2011.48. [DOI] [PubMed] [Google Scholar]
- Tang T, Tang JY, Li D, Reich M, Callahan CA, Fu L. et al. Targeting superficial or nodular Basal cell carcinoma with topically formulated small molecule inhibitor of smoothened. Clin Cancer Res. 2011;17(10):3378–3387. doi: 10.1158/1078-0432.CCR-10-3370. [DOI] [PMC free article] [PubMed] [Google Scholar]