Abstract
Objective
To evaluate change in sedentary behavior (SB) and physical activity (PA) over three years following bariatric surgery.
Methods
A subset of participants in an observational study (n=473 of 2458; 79% female, median body mass index 45kg/m2) wore an activity monitor pre-surgery and at 1–3 annual post-surgery assessments.
Results
Over the first year, on average, sedentary time decreased from 573 (95%CI 563–582) to 545 (95%CI 534–555) min/d and moderate-to-vigorous intensity PA (MVPA) increased from 77 (95%CI: 71–84) to 106 (95%CI: 98–116) min/wk, or 7 (95%CI: 5–10) to 24 (95%CI: 18–29) min/wk in MVPA bouts ≥10 minutes. There were no changes in these parameters from years 1 to 3 (P for all>.05). The percentage of participants achieving ≥150 min/wk of bout-related MVPA was not different at year 3 [6.5% (95%CI: 3.1–12.7)] vs. pre-surgery [3.4% (95%CI: 1.8–5.0); p=.45]. Most participants followed SB and PA trajectories that paralleled mean change and were consistent with their pre-surgery position in relation to the group.
Conclusions
On average, bariatric surgical patients make small reductions in SB and increases in PA during the first post-surgery year, which are maintained through 3 years. Still, post-surgery PA levels fall short of PA guidelines for general health or weight control.
Keywords: physical activity, sedentary behavior, longitudinal, bariatric surgery, Roux-en-Y gastric bypass
INTRODUCTION
Bariatric surgical procedures usually result in greater weight loss and improvement in related comorbidities compared to lifestyle interventions (1;2) and drug therapy (3). However, weight regain and return of comorbidities are not uncommon (4;5). A sedentary lifestyle with limited participation in moderate-to-vigorous intensity physical activity (MVPA) may play a role (6).
Physical Activity (PA) Guidelines for Americans (7) recommend that adults engage in at least 150 minutes/week of moderate intensity (e.g., brisk walking), or 75 minutes of vigorous intensity aerobic PA, performed in bouts of at least 10 minutes, for health, with additional benefits gained from increasing the frequency, duration and intensity of PA. In particular, greater participation in MVPA is recommended for weight control. The Obesity Society and the 2013 American College of Cardiology/American Heart Association Task Force on Practice Guidelines recommend 200–300 minutes/week to maintain lost weight or minimize weight regain in the long term (8), while the International Association for the Study of Obesity suggests that prevention of weight regain in formerly obese adults requires 60–90 minutes of moderate intensity PA (or lesser amounts of vigorous intensity PA) on most days of the week (9).
Results from studies with objective assessment of PA provide evidence that the vast majority of adults who undergo bariatric surgery have low levels of PA prior to surgery, and contrary to self-report, do not make substantial changes to their PA behavior following surgery (6). In particular, participation in MVPA in bouts of at least 10 minutes remains low (10–15). Recent studies suggest that adults undergoing bariatric surgery also spend more time in sedentary behavior (SB; i.e. waking behaviors performed while sitting or reclining that require very low energy expenditure) (16), compared to normal weight controls or the general population pre- (17) and post-surgery (10;18). This is significant as SB (both the time and the pattern of accumulation) may play an important role in weight status and cardio-metabolic health, independent of participation in MVPA (19).
To date, most studies reporting objective assessment of SB and PA in adults undergoing bariatric surgery have had small sample sizes and been limited in duration, and all have only included one post-surgery assessment, so that the natural progression of PA over time is unknown. Additionally, there has been only one report of objectively-measured pre- to post-surgery change in SB (15). The Longitudinal Assessment of Bariatric Surgery-2 (LABS-2) study, a large multi-center observational cohort study with pre- and annual post-surgery objective assessment of PA (20), provides an unique opportunity to examine changes in PA and SB throughout, and beyond, the active weight loss period that typically extends through the first two years following surgery (4;21). Utilizing data through the third post-surgery year, this investigation examines changes in total ambulatory PA, MVPA and SB, and identifies clusters of individuals following similar trajectories of PA and SB over time.
METHODS
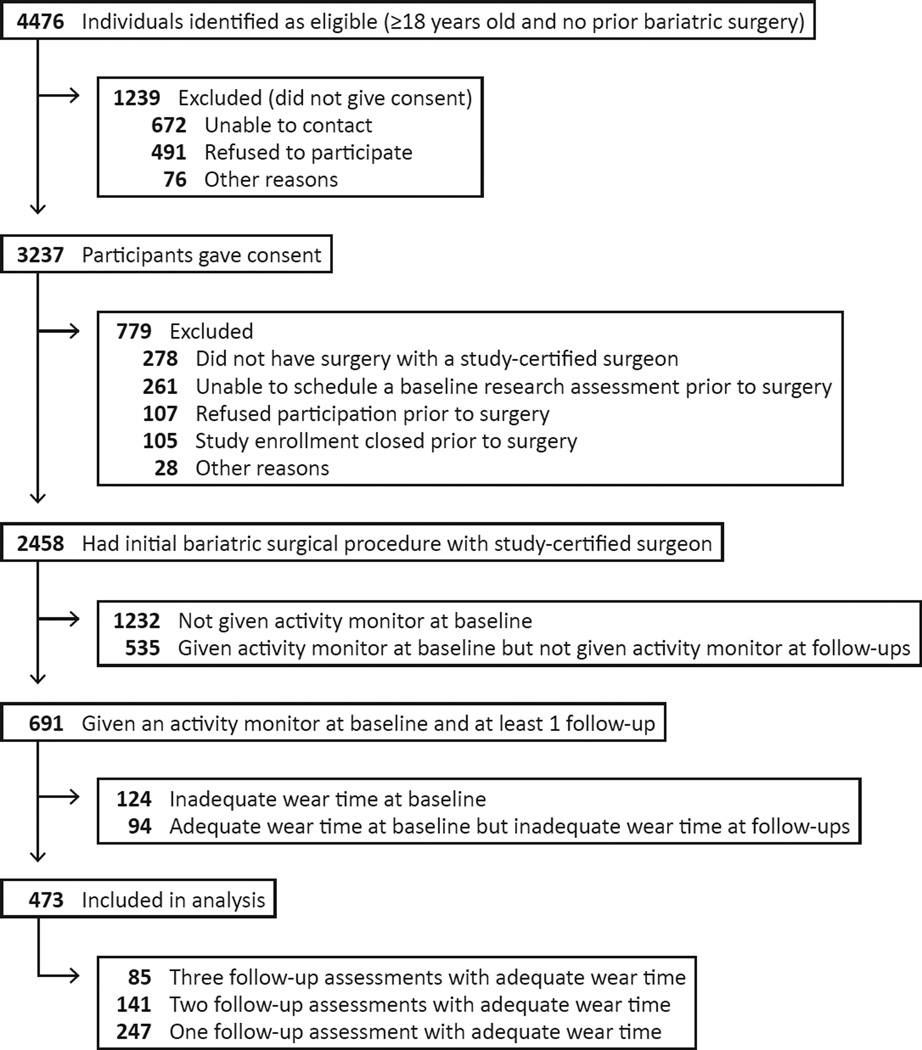
Between February 2006 and February 2009, patients at least 18 years old preparing to undergo their first bariatric surgical procedure from participating surgeons at 10 centers throughout the United States, were recruited to participate in LABS-2 (Figure 1). Characteristics of the overall LABS-2 study sample (n=2458) who underwent a bariatric surgical procedure by April 2009 have been previously reported (20). The institutional review boards at each center approved the protocol and all participants gave written informed consent to participate in the study. The LABS study is registered at ClinicalTrials.gov (NCT00465829).
Figure 1.
Recruitment and follow-up of participants
As this was an observational study, participants received usual care from their respective surgical center, which may or may not have included advice or counseling related to physical activity. Research assessments were conducted by LABS-trained and certified personnel within 30 days prior to scheduled surgery dates and annually following surgery. This report utilizes follow-up data through the third post-surgery year. To be included in the analysis sample, participants had to have physical activity data at baseline and at one or more follow-up assessments (n=473).
Physical activity (PA)
The StepWatch™ 3 Activity Monitor (OrthoCare Innovations, Washington, D.C.) is a small (75×50×20 mm) lightweight (38g) microprocessor-controlled biaxial activity monitor, worn above the ankle, that combines acceleration, position, and timing information to count strides (i.e. single leg-steps). It is accurate in lean and obese individuals at “slow” (i.e., 1.6 km/h) and “purposeful walking” (i.e., 3.2–5.6 km/h) paces and with a variety of gait styles, with accuracy typically exceeding 98% (22). The monitor was programmed to double count all strides in 1 minute intervals with sensitivity settings appropriate to a participant’s height, gait, and cadence using accompanying software. Participants were asked to wear the monitor for the seven consecutive days following their research assessment. The participant was given the option to remove the monitor during water-based activities (i.e., bathing, swimming) or sleeping. Participants who primarily used a wheel chair, had a walking limitation not directly related to obesity such as multiple sclerosis, or a temporary injury such as a sprained ankle, were excluded from activity monitor assessment.
Data from the manufacturer software were exported to a SAS version 9.3 dataset (SAS Institute Inc, Cary, NC). Sleep and non-wear periods, identified by intervals of at least 120 minutes with no activity (23), were removed. Minutes with a step count greater than 80 were considered MVPA (22). A MVPA bout was defined as 10 or more consecutive MVPA minutes, with allowance for interruptions of no more than 2 minutes below threshold (22;24). Minutes with a step count of 0 were considered sedentary time (22). A bout of SB was defined as a duration of continous sedentary minute(s), with no minimum duration (25). Days with fewer than 10 hours of wear were eliminated. Among those with at least three valid days (26;27), mean steps/d, MVPA min/d, bout-related MVPA min/d, sedentary min/d, sedentary bouts/d, and duration of sedentary bouts (i.e., min/bout) were calculated. To address the prolonged nature of SB, sedentary measures were also calculated based on minimum bout durations of at least 10 minutes and at least 30 minutes (25).
Despite minimum monitor wear time requirements, differences in wear time across time points can have a significant effect on estimated change in SB. Therefore, percentage of wear time that was sedentary (100*sedentary time/wear time) was calculated for each participant at each time point. Additionally, sedentary time and the number of sedentary bouts were standardized to a common wear time across time points as follows: value at “X” assessment (i.e., baseline, 1-, 2-, or 3-year follow-up) times (average wear time across all assessments/wear time at “X” assessment) (28).
For comparison with U.S. PA recommendations, mean daily MVPA and bout-related MVPA were multiplied by 7 for weekly estimates, and the percentage of participants achieving at least 150 minutes/week of bout-related MVPA was determined (7).
Participant characteristics
With bare feet and in light-weight clothing participants’ weight was measured to the nearest pound and height was measured to the nearest inch with a wall-mounted stadiometer. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Weight change was calculated as (follow-up weight minus baseline weight) divided by baseline weight. Age, sex, race, ethnicity, marital status, education, employment status, household income, smoking status, walking aid use, and severe walking limitation (defined as the inability to walk 200 feet (61 M) without assistance) were assessed with study-specific questionnaires (29). Surgeons recorded the surgical procedure at time of surgery. Due to the distribution of procedures (see table 2), procedures other than Roux-en-Y gastric bypass [RYGB] and laparoscopic adjustable gastric band [LAGB] were grouped as “other procedure” for analyses.
Table 2.
Physical activity and sedentary parameters prior to and 1, 2, and 3 years following bariatric surgery (n=473).
| Time point | Adjusted P Valuea | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline (BL) | 1 Year (Y) | 2 Years | 3 Years | BL vs 1 Y | BL vs 2 Y | BL vs 3 Y | 1 Y vs 3 Y | |
| Modeled means and 95% confidence intervals | ||||||||
| Steps/day | 7687.5 (7400.7–7974.2) | 8958.8 (8623.4–9294.2) | 9214.3 (8847.6–9581.0) | 8935.4 (8565.1–9305.7) | <.0001 | <.0001 | <.0001 | 0.99 |
| MVPA min/wk | 77.3 (70.9–84.2) | 106.0 (97.8–116.4) | 112 .5 (99.8–123.0) | 98.5 (88.0–110.0) | <.0001 | <.0001 | <.0 001 | 0.65 |
| in ≥10 min bouts | 7.2 (5.2–9.6) | 24.3 (18.4–29.3) | 24.9 (19.1–32.7) | 17.1 (12.8–23.6) | <.0001 | <.0001 | <. 0001 | 0.11 |
| ≥150 min/wk in ≥10 min bouts (%) | 3.4 (1.8–5.0) | 10.6 (7.2–14.0) | 13.6 (9.5–17.8) | 6.5 (3.1–12.7) | <.0001 | <.0001 | 0.45 | 0.64 |
| % sedentary time | 64.6 (63.7–65.5) | 61.4 (60.3–62.4) | 61.7 (60.7–62.8) | 61.5 (60.3–62.7) | <.0001 | <.0001 | <. 0001 | 0.99 |
| Sedentary min/db | 572.9 (563.8–582.0) | 544.5 (534.4–554.6) | 548.4 (538 .2–558.6) | 547.3 (535.5–559.1) | <.0001 | <.0001 | <.0001 | 0.96 |
| in ≥10 min bouts | 407.3 (396.4–418.3) | 380.5 (369.3–391.8) | 388.2 (376.2–400.3) | 386.2 (372.7–399.7) | <.00 01 | <.01 | <.01 | 0.82 |
| in ≥30 min bouts | 217.5 (207.8–227.2) | 192.9 (183.5–202.3) | 200.8 (190.5–211.1) | 198.8 (187.3–210.4) | <.00 01 | 0.01 | <.01 | 0.43 |
| Sedentary bouts/db | 73.1 (71.5–74.7) | 72.4 (70.8–74.1) | 72.8 (70.8–74.7) | 73.3 (71.5–75.2) | 0.78 overall | |||
| ≥10 min bouts | 15.8 (15.5–16.1) | 15.3 (14.9–15.7) | 15.5 (15.1–15.9) | 15.2 (14.8–15.6) | 0.02 | 0.46 | 0.02 | 0.97 |
| ≥30 min bouts | 4.2 (44.4) | 3.8 (3.6–4.0) | 3.9 (3.7–4.1) | 3.8 (3.6–4.0) | <.0001 | 0.03 | <.01 | 0.98 |
| Sedentary min/boutb | 8.5 (8.2–8.8) | 7.9 (7.7–8.2) | 8.0 (7.7–8.3) | 8.0 (7.7–8.3) | <.0001 | 0.06 | 0.03 | 0.99 |
| ≥10 min bouts | 25.5 (25.1–26.0) | 24.6 (24.2–25.0) | 25.1 (24.6–25.6) | 25.1 (24.6–25.6) | <.0001 | 0.39 | 0.41 | 0.28 |
| ≥30 min bouts | 50.8 (50.2–51.4) | 50.3 (49.5–51.1) | 50.1 (49.3–50.9) | 51.1 (50.3–52.0) | 0.67 | 0.41 | 0.89 | 0.43 |
MVPA, moderate-to-vigorous intensity physical activity.
Linear mixed models (for mean steps/d, MVPA min/wk, % sedentary time, sedentary min/d, sedentary bouts/d, duration/sedentary bout), a random effects two-part model (for MVPA min/wk in ≥10 min bouts), and a Poisson mixed model with robust error variance (for % ≥150 MVPA min/wk in ≥10 min bouts) were adjusted for multiple comparisons using simulation.
Adjusted for differences in wear time across assessments as follows: value at “X” assessment (i.e., baseline, 1-, 2-, or 3-year follow-up) × (average wear time across all assessments /wear time at “X” assessment).
Analysis
Analyses were conducted using SAS version 9.3 (SAS Institute Inc, Cary, NC). All reported P values are two-sided and P values less than 0.05 were considered to be statistically significant. Descriptive statistics summarize baseline characteristics. Frequencies and percentages are reported for categorical data. Medians, 25th and 75th percentiles are reported for continuous data.
Data were assumed to be missing at random. Generalized linear mixed models were used to identify baseline characteristics related to missing follow-up assessments. Age and site were identified (data not shown). Thus longitudinal analyses controlled for these factors. Change in PA parameters over time was assessed with linear mixed models and Poisson mixed model with robust error variance using all available data. Given the high prevalence of participants with no bout-related MVPA (63.4% at baseline, 41.3–49.4% in years 1–3), a random effects two-part model was fit, which considered both the probability of zero and distribution of values greater than zero (30). Pairwise comparisons were made between baseline and each follow-up assessment and between the first and last follow-up assessments. Modeled means and 95% confidence intervals (CI), and p values adjusted for multiple comparisons using simulation are reported.
Group-based trajectory models were used to describe the trajectories of three PA parameters (steps, MVPA, and % sedentary time) over the course of three years. MVPA, rather than bout-related MVPA, was selected because many participants did not accumulate any bout-related MVPA. Group-based trajectory models are based on change in PA as a function of time with growth or decline in PA represented by latent factors capturing the intercept (i.e. estimated baseline score), slope (i.e. estimated change over time), and a parameter representing a latent (unobserved) categorical variable that identifies homogenous classes varying in level or rate of change in PA. Several factors were used to determine the optimal number of trajectory groups: the acceptable minimum number of participants in a group, entropy values and average probabilities of the most likely trajectory group as indicators of classification, and Bayesian Information Criteria (BIC) values for evaluation of model fit. Fit of linear and nonlinear models were compared. The modeled trajectories of each class are plotted along with bars indicating the interquartile range (IQR) of the observed data for the individuals within a class.
To determine whether it was appropriate to report results for all surgical procedures combined, a sensitivity analysis was performed to determine whether surgical procedure (entered as RYGB, LAGB or “other procedure”) predicted change in PA parameters or trajectory class membership.
RESULTS
The analysis sample includes 473 (19.2%) of 2458 LABS-2 participants (Figure 1). Of the 1892 potential PA assessments among the analysis sample (i.e., 473 LABS-2 participants times four assessments), valid PA data was available for 1257 (66%) assessments. Reasons for missing PA data include: monitor not available (51%), refusal (17%), missed research assessment (16%), failure to return monitor with ≥3 valid days (7%), ineligible (6%), monitor data could not be read or was lost (2%)). Of those who were given a monitor, 76–81% met the wear time requirement of at least 3 days with at least 10 hours of wear at each assessment.
Sample characteristics
Baseline characteristics of the analysis sample are shown in Table 1. The median (IQR) number of days that participants wore the monitor was 6 (5–7) days across assessments; 90% of participants wore the monitor on at least 1 weekend day. Average daily wear time was significantly higher at 2 years vs. baseline (902 min/day (95%CI: 890–913) vs. 881 min/day (95%CI: 873–890), respectively), but did not differ between other time points (p for all>.05).
Table 1.
Baseline characteristics of the analysis sample (n=473a)
| Characteristics | |
|---|---|
| Female, n (%) | 372 (78.6) |
| Age, years, median (25th, 75th percentile) | 47 (37,55) |
| Race, n (%) | |
| White | 420 (89.2) |
| Black | 38 (8.1) |
| Other | 13 (2.8) |
| Hispanic/Latino ethnicity, n (%) | 31 (6.6) |
| Education, n (%) | |
| High School or less | 97 (21.0) |
| Some college | 170 (36.7) |
| College degree | 196 (42.3) |
| Employed fulltime, n (%) | 346 (74.7) |
| Household income, n (%) | |
| < $25,000 | 59 (13.0) |
| $25,000–$49,000 | 118 (26.0) |
| $50,000–$74,999 | 112 (24.7) |
| $75,000–$99,999 | 77 (17.0) |
| ≥$100,000 | 88 (19.4) |
| Married/living as married, n (%) | 302 (65.5) |
| Current/recent smoker, n (%) | 53 (11.3) |
| Body mass index, kg/m2, median (25th, 75th percentile) | 45.4 (41.9,51.2) |
| Walking aid use, n (%) | 57 (11.3) |
| Severe walking limitation, n (%) | 13 (3.2) |
| Surgical procedure, n (%) | |
| Roux-en-Y Gastric Bypass | 329 (69.6) |
| Laparoscopic Adjustable Gastric Band | 108 (22.8) |
| Biliopancreatic Diversion with Duodenal Switch | 7 (1.5) |
| Sleeve Gastrectomy | 26 (5.5) |
| Banded Gastric Bypass | 3 (0.6) |
Missing: race (n=1), education (n=10), employment (n=10), household income (n=19), marital status (n=12), smoking status (n=2), walking aid use (n=12), severe walking limitation (n=69).
Weight loss
Mean weight loss following bariatric surgery was 29% (95%CI: 29–30) at 1 year, 30% (95%CI: 29–31) at 2 years, and 29% (95%CI: 28–30) at 3 years.
PA and SB by time point
Sensitivity analysis revealed that surgical procedure was not a significant predictor of change in PA or SB parameters or trajectory membership (data not shown) so results are presented for the total sample. PA and SB parameters are presented by time point in Table 2. On average, participants were more active at all post-surgery assessments compared to baseline (p for all<.0001), with one exception; after an initial increase in the percentage of participants achieving at least 150 minutes/week of bout-related MVPA (i.e., baseline vs. year 1 and baseline vs. year 2; p for all<.0001), the percentage of participants meeting this threshold at year 3 did not differ from baseline (p=.45). There were no statistically significant changes in PA parameters from 1 to 3 years.
On average, sedentary time decreased by 28 min/day (95%CI: 18–39) from pre-surgery to 1 year (p<.0001). While the number of sedentary bouts, on average, did not change from pre- to post-surgery (p=.78), the mean duration of sedentary bouts was shorter at year 1 and 3 vs. baseline (p for both<.05). In particular, time spent in prolonged sedentary bouts of at least 10 minutes and of at least 30 minutes was shorter at all post-surgery assessments vs. baseline (p≤.01).
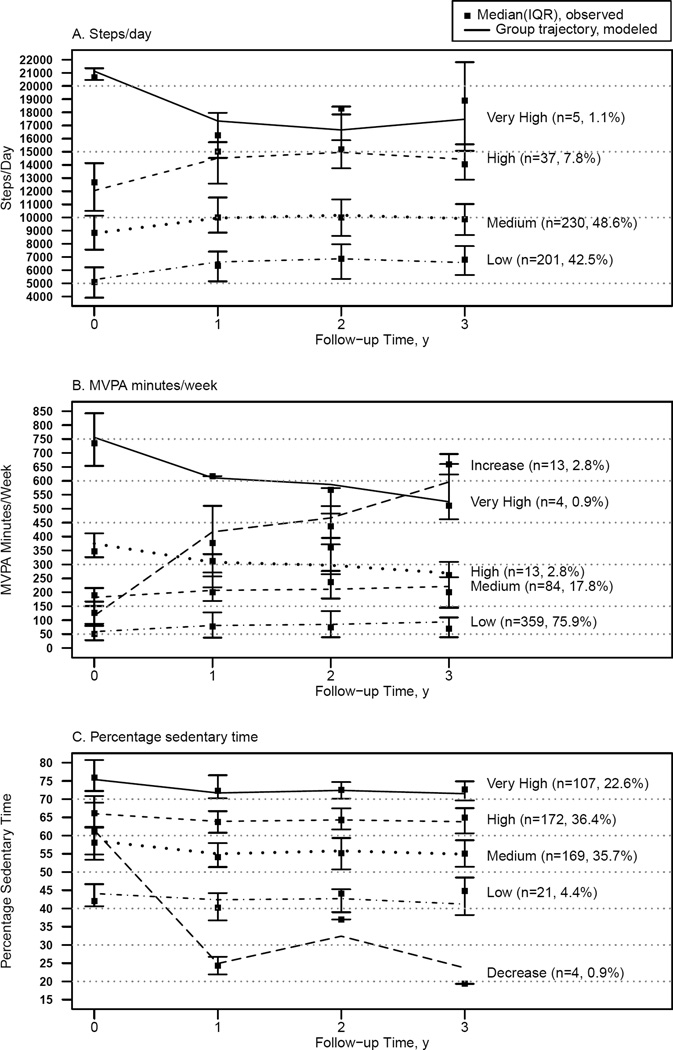
Longitudinal PA and SB Trajectories
To evaluate common patterns of change in PA behavior from baseline to 3 years, steps, MVPA and % sedentary time trajectory groups were identified (Figure 2A–C). The slopes in the nonlinear group-based trajectory models differed from zero (p<.0001) for all three PA parameters, thus, nonlinear models were selected. All three final models had entropy values of 0.72 –0.93, indicating medium-high classification (31), and average probabilities of the most likely trajectory group were greater than 0.9, indicating good classification (32).
Figure 2.
Physical activity trajectories of adults undergoing bariatric surgery
Steps over time was best described with four trajectory groups (Figure 2A). The vast majority of participants (91%) were in the two lowest steps groups which had modest increases (improvements) in the first year and then fairly stable levels through year 3. The very high steps trajectory group (1% of participants) experienced a decrease in steps following surgery. However, this group remained the most active through year 3.
MVPA over time was best described with five trajectory groups (Figure 2B). Most participants (94%) were in the two least active MVPA trajectory groups, which followed similar patterns to the least active steps groups. The most common trajectory (76%) remained well below the threshold of 150 minutes/week, even though total MVPA (i.e., not just bout-related MVPA) was considered. A small fraction of participants (3%) had a dramatic increase in MVPA, and an even smaller fraction (1%), which started with very high MVPA participation, had a decrease over three years, but remained very active.
Percent sedentary time over time was best described with five trajectory groups (Figure 2C). Four of the groups accounting for 99% of participants showed a similar pattern, with a modest decrease (improvement) in % sedentary time in the first year, followed by fairly stable levels through year 3. A very small minority (1%) of participants had a large decrease in % sedentary time in the first year.
DISCUSSION
The current study is the first to objectively evaluate longer-term changes in PA and SB following bariatric surgery. Our findings suggest that, on average, this cohort of adults made modest favorable changes in total ambulatory PA, MVPA, bout-related MVPA and SB from pre- to 1-year post-surgery, and maintained these small changes through the third year. Changes in PA and SB mirror post-surgery weight loss in that the vast majority of weight change following bariatric surgery occurs in the first year, with relatively little change in the second and third post-surgery years (21). Another similarity is that despite improvements, just as most patients remain overweight or obese following bariatric surgery, these data suggest that most post-surgery patients continue to have low levels of PA.
While post-surgery PA and SB improved compared to pre-surgery levels, the majority of participants made insufficient changes in activity to meet current MVPA recommendations. By year one only 10.6% of participants met the PA recommendation (7) of at least 150 minutes/week of bout-related MVPA for general health benefits; only 6.5% by year 3. Thus, even fewer achieved the higher PA levels recommended for weight loss maintenance (i.e., 200–300 (8) to 300–450 (9) min/week). At present, there are not specific recommendations regarding SB.
Recent laboratory-based studies show that interrupting prolonged bouts of SB with brief PA breaks can have acute beneficial effects on glucose and lipid metabolism, blood pressure and energy expenditure (33–35). Additionally, several population-based studies have found associations between higher cardiometabolic risk factors and fewer transitions from sedentary to active minutes (described as breaks in SB, but perhaps more representative of sedentary bouts (25)) independent of sedentary time (36–39). Thus, the finding that participants, on average, decreased their sedentary time following surgery by reducing the amount of time they spent in prolonged bouts of SB is encouraging. In particular, by year 1 there was an average decrease of almost 30 min/day in sedentary time accumulated in bouts of at least 10 minutes or at least 30 minutes in duration. However, the durability of this effect is uncertain, as the average decreases from baseline were only 16–21 min/day in years 2 and 3 (although still significant). The only other study to objectively assess SB pre- and post-surgery reported no change in sedentary time (−9.5 ± 129.4 min/day; p=.58) in a sample of 56 women who wore an Actigraph GT3X+ accelerometer 3 months pre- and 9 months post-RYGB (15).
A consistent finding across parameters of PA behavior was that the trajectories representing the vast majority of participants (>90%) followed similar patterns to the mean change over time, consistent with their pre-surgery position in relation to the group as a whole. This finding is consistent with our previous work, which found that the best predictor of 1 year PA level is pre-surgery PA level (11), and provides further support for incorporating strategies within clinical care to increase PA and reduce SB in the pre-surgery period. While few studies, to date, have tested interventions to increase PA and reduce SB among adults undergoing bariatric surgery in the pre- or post-surgery period, a recent randomized clinical trial among surgical candidates had encouraging results; a 6-week behavioral intervention with individualized instruction in strategies to increase walking exercise was effective at producing marked increases in objectively-measured bout-related MVPA (40) prior to surgery.
Major strengths of this study are that data were collected on a large sample using standardized definitions and procedures by trained evaluators in a multicenter, geographically diverse cohort, with objective assessment of PA at four time points over three years. While only a subset of LABS-2 participants was included in the analysis sample, a previous comparison of LABS-2 participants with and without PA data indicated that the analysis sample is generally representative of the larger cohort (11). In addition, the vast majority of reasons for missing follow-up data among the analysis sample did not appear to be related to PA participation, and compliance was high among those given an activity monitor; four in five participants met the minimum monitor wear time requirement, with the majority far exceeding it (i.e., the majority wore the monitor for 6–7 days; average wear time was almost 15 hours per day). Together these findings minimize the concern of selection bias.
Several limitations should be considered when interpreting results. Primarily, this study relied on indirect measures of MVPA and SB derived from measuring ambulatory activity (i.e. steps) recorded in 1 minute intervals. MVPA performed at an ambulation ≤80 steps/minute (including non-ambulatory activity) would be misclassified as non-MVPA and vice versa. Likewise, SB during which steps were erroneously registered (e.g., from heel tapping) would be misclassified as non-SB. Although it should be noted that the Stepwatch has sensitivity settings to distinguish fidgeting from actual steps (23). Furthermore, because the Stepwatch does not directly measure posture it is likely that some minutes spent standing with zero steps were misclassified as SB. It is also possible that sedentary bouts greater than 120 minutes were misclassified as non-wear periods (23). Finally, it is possible that monitors were removed for water-based activities. However, our previous investigation revealed that swimming was uncommon among study participants (3% at baseline and 2% at year 1) (11). While these limitations do not preclude comparing MVPA and SB over time, the precision of estimates of MVPA and SB are unknown. Thus, comparisons with similar PA parameters derived from other monitors should be made with caution. It should also be noted that the majority of participants underwent RYGB. Although surgical procedure was not an independent predictor of change in PA or SB parameters or step, MVPA or % sedentary trajectory class membership, it is unclear whether results are generalizable to all bariatric surgical procedures.
CONCLUSION
During the first post-surgery year, on average, bariatric surgery patients make small but significant increases in ambulatory PA and MVPA. Similarly they make small reductions in time spent sedentary, largely through accumulating less time in prolonged sedentary bouts of at least 30 minutes in duration. For the most part these changes are maintained through 3 years, but the percentage of participants meeting the guideline of at least 150 minutes/week of bout-related MVPA is not different from baseline levels by year 3. Despite changes, most patients continue to have low levels of activity and be highly sedentary through the first 3 years following surgery, suggesting there is a need to incorporate effective pre- and post-surgery PA counseling into clinical care. Guidance on PA counseling strategies specific to this population is available (6). However, more studies are needed to elucidate the specific barriers and facilitators of changing PA and SB in surgical patients, as well as the most time and cost-effective mechanisms for increasing PA and reducing SB in surgical patients.
What is already known about this subject?
Participation in habitual moderate-to-vigorous intensity physical activity is a key contributor to behaviorally-induced weight loss maintenance, and is hypothesized to contribute to long-term weight loss maintenance following bariatric surgery.
The vast majority of adults who undergo bariatric surgery are highly sedentary and have low participation in moderate-to-vigorous intensity physical activity as compared to physical activity guidelines for health and weight control.
No study has objectively assessed sedentary behavior and physical activity pre-surgery and at multiple post-surgery time points to describe the natural progression of these behaviors over time.
What does your study add?
This study is the first to show that on average, bariatric surgical patients make small reductions in sedentary behavior and increases in physical activity (moderate-to-vigorous intensity physical activity and total ambulation) during the first year following surgery, which are maintained through 3 years (i.e., these behaviors are stable from 1–3 years post-surgery).
Most patients follow sedentary behavior and physical activity group-based trajectories that parallel mean change and are consistent with their pre-surgery position in relation to the group, indicating pre-surgery sedentary behavior and physical activity highly influence these behaviors post-surgery.
Participation in moderate-to-vigorous intensity physical activity during the first, second and third post-surgery year falls short of guidelines for general health benefits and weight control.
ACKNOWLEDGEMENTS
LABS personnel contributing to the study include: Columbia University Medical Center, New York, NY: Paul D. Berk, MD, Marc Bessler, MD, Amna Daud, Harrison Lobdell IV, Jemela Mwelu, Beth Schrope, MD, PhD, Akuezunkpa Ude, MD Cornell University Medical Center, New York, NY: Michelle Capasso, BA, Ricardo Costa, BS, Greg Dakin, MD, Faith Ebel RD, MPH, Michel Gagner, MD, Jane Hsieh BS, Alfons Pomp, MD, Gladys Strain, PhD Mt. Sinai Medical Center, New York, NY: W. Barry Inabnet, MD East Carolina Medical Center, Greenville, NC: Rita Bowden, RN, William Chapman, MD, FACS, Lynis Dohm, PhD, John Pender MD, Walter Pories, MD, FACS Neuropsychiatric Research Institute, Fargo, ND: Jennifer Barker, MBA, Luis Garcia, MD, FACS, MBA, Kathy Lancaster, BA, Erika Lovaas, BS, James E. Mitchell, MD, Oregon Health & Science University: Chelsea Cassady, BS, Clifford Deveney, MD, Katherine Elder, PhD, Andrew Fredette, BA, Stefanie Greene, Jonathan Purnell, MD, Robert O’Rourke, MD, Lynette Rogers, Chad Sorenson, Bruce M. Wolfe, MD, Legacy Good Samaritan Hospital, Portland, OR: Emma Patterson, MD, Mark Smith, MD, William Raum, MD, Lisa VanDerWerff, PAC, Jason Kwiatkowski, PAC, Jamie Laut, MEd Sacramento Bariatric Medical Associates, Sacramento, CA: Iselin Austrheim-Smith, CCRP, Laura Machado, MD University of Pittsburgh Medical Center, Pittsburgh, PA: Chris Costa, BA Anita P. Courcoulas, MD, MPH, FACS, Jessie Eagleton, BS, George Eid, MD, William Gourash, MSN, CRNP, Lewis H. Kuller, MD, DrPH, Carol A. McCloskey, MD, Ramesh Ramanathan, MD, Rebecca Search, MPH, Eleanor Shirley, MA University of Washington, Seattle, WA: David E. Cummings, MD, E. Patchen Dellinger, MD, Hallie Ericson, BA, David R. Flum, MD, MPH, Katrina Golub, MPH, CCRC, Brant Oelschlager, MD, Skye Steptoe, MS, CCRC, Tomio Tran, Andrew Wright, MD Virginia Mason Medical Center, Seattle, WA: Lily Chang, MD, Stephen Geary, RN, Jeffrey Hunter, MD, Anne MacDougall, BA Ravi Moonka, MD, Olivia A. Seibenick, CCRC, Richard Thirlby, MD Data Coordinating Center, Graduate School of Public Health at the University of Pittsburgh, Pittsburgh, PA: Abi Adenijii, PhD, Steven H. Belle, PhD, MScHyg, Lily (Jia-Yuh) Chen, MS, Nicholas Christian, PhD, Michelle Fouse, BS, Jesse Hsu, PhD, Wendy C. King, PhD, Kevin Kip, PhD, Kira Leishear, PhD, Laurie Iacono, MFA, Debbie Martin, BA, Rocco Mercurio, MBA, Faith Selzer, PhD, Abdus Wahed, PhD National Institute of Diabetes and Digestive and Kidney Diseases: Mary Evans, Ph.D, Mary Horlick, MD, Carolyn W. Miles, PhD, Myrlene A. Staten, MD, Susan Z. Yanovski, MD National Cancer Institute: David E. Kleiner, MD, PhD
Funding: This clinical study was a cooperative agreement funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Grant numbers: DCC -U01 DK066557; Columbia - U01-DK66667 (in collaboration with Cornell University Medical Center CTRC, Grant UL1-RR024996); University of Washington - U01-DK66568 (in collaboration with CTRC, Grant M01RR-00037); Neuropsychiatric Research Institute - U01-DK66471; East Carolina University – U01-DK66526; University of Pittsburgh Medical Center – U01-DK66585 (in collaboration with CTRC, Grant UL1-RR024153); Oregon Health & Science University – U01-DK66555.
Dr. Courcoulas has received research grants from Allergan Pfizer, Covidien, EndoGastric Solutions, Nutrisystem and is on the Scientific Advisory Board of Ethicon J & J Healthcare System. Dr. Flum has received research grants from Covidien and Sanofi-Aventis and has an advisor role with Pacira Pharmaceuticals. Dr. Patterson is a consultant for Apollo and Ethicon. Dr. Wolfe is a Consultant and Advisor for Covidien, Ethicon, Crospon, Viudico, Medtronics and has received a research grant from Enteromedics.
Footnotes
Disclosures: Drs. Belle, Bond, Cook, Dakin, Inabnet, King, Mitchell, and Ms. Chen have no disclosures to report.
Contributor Information
Wendy C King, University of Pittsburgh, Graduate School of Public Health, Pittsburgh, PA, USA.
Jia-Yuh Chen, University of Pittsburgh, Graduate School of Public Health, Pittsburgh, PA, USA.
Dale S Bond, Warren Alpert Medical School of Brown University, Providence, RI, USA.
Steven H Belle, University of Pittsburgh, Graduate School of Public Health, Pittsburgh, PA, USA.
Anita P Courcoulas, University of Pittsburgh Medical Center, Pittsburgh, PA, USA.
Emma J Patterson, Legacy Good Samaritan Weight Management Institute, Portland, OR, USA.
James E Mitchell, Neuropsychiatric Research Institute, Fargo, ND, USA.
William B Inabnet, Mount Sinai Hospital, New York, NY, USA.
George F Dakin, Weill Cornell Medical Center, New York, NY, USA.
David R Flum, University of Washington, Seattle, WA, USA.
Brian Cook, Neuropsychiatric Institute, Fargo, ND, USA.
Bruce M Wolfe, Oregon Health and Science University, Portland, OR, USA.
References
- 1.Unick JL, Beavers D, Jakicic JM, Kitabchi AE, Knowler WC, Wadden TA, et al. Effectiveness of lifestyle interventions for individuals with severe obesity and type 2 diabetes: results from the Look AHEAD trial. Diabetes Care. 2011;34(10):2152–2157. doi: 10.2337/dc11-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danielsen KK, Svendsen M, Maehlum S, Sundgot-Borgen J. Changes in body composition, cardiovascular disease risk factors, and eating behavior after an intensive lifestyle intervention with high volume of physical activity in severely obese subjects: a prospective clinical controlled trial. J Obes. 2013;2013:325–464. doi: 10.1155/2013/325464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ling H, Lenz TL, Burns TL, Hilleman DE. Reducing the risk of obesity: defining the role of weight loss drugs. Pharmacotherapy. 2013;33(12):1308–1321. doi: 10.1002/phar.1277. [DOI] [PubMed] [Google Scholar]
- 4.Higa K, Ho T, Tercero F, Yunus T, Boone KB. Laparoscopic Roux-en-Y gastric bypass: 10-year follow-up. Surg Obes Relat Dis. 2011;7(4):516–525. doi: 10.1016/j.soard.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 5.Adams TD, Davidson LE, Litwin SE, Kolotkin RL, LaMonte MJ, Pendleton RC, et al. Health benefits of gastric bypass surgery after 6 years. JAMA. 2012;308(11):1122–1131. doi: 10.1001/2012.jama.11164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King WC, Bond DS. The importance of pre and postoperative physical activity counseling in bariatric surgery. Exerc Sport Sci Rev. 2012 doi: 10.1097/JES.0b013e31826444e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.U.S. Department of Health and Human Services. 2008 Physical Activity Guidelines for Americans. Washington, DC: U.S. Department of Health and Human Services; 2008. Physical activity has many health benefits; pp. 7–14. [Google Scholar]
- 8.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63(25 Pt B):2985–3023. doi: 10.1016/j.jacc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Saris WH, Blair SN, van Baak MA, Eaton SB, Davies PS, DiPietro L, et al. How much physical activity is enough to prevent unhealthy weight gain? Outcome of the IASO 1st Stock Conference and consensus statement. Obes Rev. 2003;4(2):101–114. doi: 10.1046/j.1467-789x.2003.00101.x. [DOI] [PubMed] [Google Scholar]
- 10.Chapman N, Hill K, Taylor S, Hassanali M, Straker L, Hamdorf J. Patterns of physical activity and sedentary behavior after bariatric surgery: an observational study. Surg Obes Relat Dis. 2014;10(3):524–530. doi: 10.1016/j.soard.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 11.King WC, Hsu JY, Belle SH, Courcoulas AP, Eid GM, Flum DR, et al. Pre- to Post-operative Changes in Physical Activity: Report from the Longitudinal Assessment of Bariatric Surgery-2. Surg Obes Relat Dis. 2012;8(5):522–532. doi: 10.1016/j.soard.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bond DS, Jakicic JM, Unick JL, Vithiananthan S, Pohl D, Roye GD, et al. Pre- to postoperative physical activity changes in bariatric surgery patients: self report vs. objective measures. Obesity (Silver Spring) 2010;18(12):2395–2397. doi: 10.1038/oby.2010.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramirez-Marrero FA, Miles J, Joyner MJ, Curry TB. Self-reported and objective physical activity in postgastric bypass surgery, obese and lean adults: association with body composition and cardiorespiratory fitness. J Phys Act Health. 2014;11(1):145–151. doi: 10.1123/jpah.2012-0048. [DOI] [PubMed] [Google Scholar]
- 14.Josbeno DA, Jakicic JM, Hergenroeder A, Eid GM. Physical activity and physical function changes in obese individuals after gastric bypass surgery. Surg Obes Relat Dis. 2010;6(4):361–366. doi: 10.1016/j.soard.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Berglind D, Willmer M, Eriksson U, Thorell A, Sundbom M, Udden J, et al. Longitudinal assessment of physical activity in women undergoing Roux-en-Y gastric bypass. Obes Surg. 2015;25(1):119–125. doi: 10.1007/s11695-014-1331-x. [DOI] [PubMed] [Google Scholar]
- 16.Sedentary Behaviour Research Network. Letter to the editor: standardized use of the terms "sedentary" and "sedentary behaviours". Appl Physiol Nutr Metab. 2012;37(3):540–542. doi: 10.1139/h2012-024. [DOI] [PubMed] [Google Scholar]
- 17.Bond DS, Jakicic JM, Vithiananthan S, Thomas JG, Leahey TM, Sax HC, et al. Objective quantification of physical activity in bariatric surgery candidates and normal-weight controls. Surg Obes Relat Dis. 2010;6(1):72–78. doi: 10.1016/j.soard.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gradaschi R, Camerini G, Carlini F, Sukkar S, Sopinaro N, Adami GF. Physical activity after surgically obtained weight loss: study with a SenseWear armband in subjects undergoing biliopancreatic diversion. Obes Surg. 2014;24(2):260–265. doi: 10.1007/s11695-013-1078-9. [DOI] [PubMed] [Google Scholar]
- 19.Ford ES, Caspersen CJ. Sedentary behaviour and cardiovascular disease: a review of prospective studies. Int J Epidemiol. 2012;41(5):1338–1353. doi: 10.1093/ije/dys078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belle SH, Berk PD, Chapman WH, Christian NJ, Courcoulas AP, Dakin GF, et al. Baseline characteristics of participants in the Longitudinal Assessment of Bariatric Surgery-2 (LABS-2) study. Surg Obes Relat Dis. 2013;9(6):926–935. doi: 10.1016/j.soard.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Courcoulas AP, Christian NJ, Belle SH, Berk PD, Flum DR, Garcia L, et al. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA. 2013;310(22):2416–2425. doi: 10.1001/jama.2013.280928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boone DA, Coleman KL. Use of a step activity monitor in determining outcomes. J Prosthet Orthot. 2006;18(1S):86–92. [Google Scholar]
- 23.King WC, Li J, Leishear K, Mitchell JE, Belle SH. Determining activity monitor wear time: an influential decision rule. J Phys Act Health. 2011;8(4):566–580. doi: 10.1123/jpah.8.4.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell I. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 25.Kim Y, Welk GJ, Braun SI, Kang M. Extracting objective estimates of sedentary behavior from accelerometer data: measurement considerations for surveillance and research applications. PLoS One. 2015;10(2):e0118078. doi: 10.1371/journal.pone.0118078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trost SG, McIver KL, Pate RR. Conducting accelerometer-based activity measurements in field-based research. Med Sci Sports Exerc. 2005;37(11 Suppl):S531–S543. doi: 10.1249/01.mss.0000185657.86065.98. [DOI] [PubMed] [Google Scholar]
- 27.Mudge S, Taylor D, Chang O, Wong R. Test-retest reliability of the StepWatch Activity Monitor outputs in healthy adults. J Phys Act Health. 2010;7(5):671–676. doi: 10.1123/jpah.7.5.671. [DOI] [PubMed] [Google Scholar]
- 28.Bond DS, Thomas JG, Raynor HA, Moon J, Sieling J, Trautvetter J, et al. B-MOBILE--a smartphone-based intervention to reduce sedentary time in overweight/obese individuals: a within-subjects experimental trial. PLoS One. 2014;9(6):e100821. doi: 10.1371/journal.pone.0100821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belle SH, Berk PD, Courcoulas AP, Flum DR, Miles CW, Mitchell JE, et al. Safety and efficacy of bariatric surgery: Longitudinal Assessment of Bariatric Surgery. Surg Obes Relat Dis. 2007;3(2):116–126. doi: 10.1016/j.soard.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu L, Strawderman RL, Cowen ME, Shih YC. A flexible two-part random effects model for correlated medical costs. J Health Econ. 2010;29(1):110–123. doi: 10.1016/j.jhealeco.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clark S, Muthen B. Relating latent class analysis results to variables not included in the analysis. 2009 http://www.statmodel.com/download/relatinglca.pdf. Ref Type: Electronic Citation. [Google Scholar]
- 32.Nagin D. Group-Based Modeling of Development. Cambridge, MA: Harvard University Press; 2005. [Google Scholar]
- 33.Dunstan DW, Kingwell BA, Larsen R, Healy GN, Cerin E, Hamilton MT, et al. Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care. 2012;35(5):976–983. doi: 10.2337/dc11-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larsen RN, Kingwell BA, Sethi P, Cerin E, Owen N, Dunstan DW. Breaking up prolonged sitting reduces resting blood pressure in overweight/obese adults. Nutr Metab Cardiovasc Dis. 2014;24(9):976–982. doi: 10.1016/j.numecd.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 35.Swartz AM, Squires L, Strath SJ. Energy expenditure of interruptions to sedentary behavior. Int J Behav Nutr Phys Act. 2011;8:69. doi: 10.1186/1479-5868-8-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Healy GN, Dunstan DW, Salmon J, Cerin E, Shaw JE, Zimmet PZ, et al. Breaks in sedentary time: beneficial associations with metabolic risk. Diabetes Care. 2008;31(4):661–666. doi: 10.2337/dc07-2046. [DOI] [PubMed] [Google Scholar]
- 37.Bankoski A, Harris TB, McClain JJ, Brychta RJ, Caserotti P, Chen KY, et al. Sedentary activity associated with metabolic syndrome independent of physical activity. Diabetes Care. 2011;34(2):497–503. doi: 10.2337/dc10-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Healy GN, Matthews CE, Dunstan DW, Winkler EA, Owen N. Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003–06. Eur Heart J. 2011;32(5):590–597. doi: 10.1093/eurheartj/ehq451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henson J, Yates T, Biddle SJ, Edwardson CL, Khunti K, Wilmot EG, et al. Associations of objectively measured sedentary behaviour and physical activity with markers of cardiometabolic health. Diabetologia. 2013;56(5):1012–1020. doi: 10.1007/s00125-013-2845-9. [DOI] [PubMed] [Google Scholar]
- 40.Bond DS, Vithiananthan S, Thomas JG, Trautvetter J, Unick JL, Jakicic JM, et al. Bari-Active: A randomized controlled trial of a preoperative intervention to increase physical activity in bariatric surgery patients. Surg Obes Relat Dis. 2014 doi: 10.1016/j.soard.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]