Abstract
Objective
Examine the uptake of services and behaviors in the prevention of mother-to-child HIV transmission (PMTCT) cascade in Zimbabwe and determine factors associated with MTCT and maternal antiretroviral therapy (ART) or antiretroviral (ARV) prophylaxis.
Design
Analysis of cross-sectional data from mother-infant pairs.
Methods
We analyzed baseline data collected in 2012 as part of the impact evaluation of Zimbabwe's Accelerated National PMTCT Program. Using multi-stage cluster sampling, eligible mother-infant pairs were randomly sampled from the catchment areas of 157 facilities in five provinces, tested for HIV infection, and interviewed about PMTCT service utilization.
Results
Of 8,800 women, 94% attended ≥1 antenatal care (ANC) visit, 92% knew their HIV serostatus during pregnancy, 77% delivered in a health facility, and 92% attended the 6-8 week postnatal visit. Among 1,075 (12%) HIV-infected women, 59% reported ART/ARV prophylaxis and 63% of their HIV-exposed infants received ARV prophylaxis. Among HIV-exposed infants, maternal receipt of ART/ARV prophylaxis was protective against MTCT (adjusted prevalence ratio (PRa): 0.41, 95% confidence interval (CI): 0.23, 0.74). Factors associated with receipt of maternal ART/ARV prophylaxis included ≥4 ANC visits (PRa: 1.18, 95%CI: 1.01,1.38), institutional delivery (PRa: 1.31, 95%CI: 1.13, 1.52), and disclosure of serostatus (PRa: 1.30, 95%CI: 1.12, 1.49).
Conclusions
These data from women in the community indicate gaps in the PMTCT cascade prior to the accelerated program, which may have been missed by examination of health facility data alone. These gaps were especially noteworthy for services targeted specifically to HIV-infected women and their infants, such as maternal and infant ART/ARV prophylaxis.
Keywords: Prevention of mother-to-child transmission of HIV, vertical HIV transmission, PMTCT cascade, HIV infection, Zimbabwe
Introduction
The number of children born with HIV-infection in 2011 declined 24% since 2009, a decline partly attributable to antiretroviral (ARV) prophylaxis preventing 409,000 children from acquiring HIV infection in low- and middle-income countries during this period.1 However, despite this substantial achievement in the prevention of mother-to-child transmission (PMTCT), there are still significant challenges facing the global effort to achieve UNAIDS' goal of “virtual elimination” of mother-to-child transmission (eMTCT) by 2015.2 To prevent transmission to their infants, HIV-positive pregnant women need to receive a series of services including antenatal care (ANC), HIV testing, disease staging, and antiretroviral therapy (ART) or ARV prophylaxis. In addition, HIV-exposed infants should receive ARV prophylaxis, be tested for HIV infection, be exclusively breastfed for the first 6 months of life, and receive cotrimoxazole prophylaxis to prevent opportunistic infections.3 Although these services have been shown to reduce the risk of MTCT to <5% in breastfeeding populations and <2% in non-breastfeeding populations, in sub-Saharan Africa, 49% of HIV-infected pregnant women are lost between ANC registration and delivery and miss some or all of the essential PMTCT services.3-5
Preventing attrition from each step in the cascade is therefore essential to achieve the World Health Organization's (WHO) goal to provide HIV testing, counseling and ARVs to pregnant women living with HIV to eliminate MTCT.1,2,6 Central to this objective is ensuring that mother-infant pairs receive the full spectrum of PMTCT services by stimulating demand and strengthening linkages between PMTCT, HIV care and treatment services, and maternal and child health services. This focus on a continuous spectrum of care is critical, as the detrimental cumulative impact of attrition at each step in the cascade is significant: in sub-Saharan Africa, only 53–66% percent of pregnant women living with HIV receive ART or ARV prophylaxis, one of the key outcomes of the cascade.1 Increasing retention in the cascade is particularly critical in high-burden countries like Zimbabwe, where 15.9% of pregnant women attending ANC are estimated to be HIV-infected.7
Between 2007 and 2011, Zimbabwe made remarkable progress scaling up PMTCT services, including increasing the percentage of HIV-positive pregnant women attending a health facility who received ARV prophylaxis from 22% to 86%.9 In 2011, the Zimbabwe Ministry of Health and Child Care (MoHCC) began scale up of the Accelerated National PMTCT Program based on the 2010 WHO PMTCT guidelines.3 At that time, Zimbabwe adopted ‘Option A’, which recommended that all eligible (i.e., CD4≤350 or WHO clinical stage 3-4) HIV-positive pregnant women receive lifelong ART for their own health, and that HIV-positive women not eligible for ART and their exposed infants receive ARV prophylaxis in the pre- and perinatal period and during breastfeeding.3,10 To determine the population-level impact of the accelerated program on MTCT and HIV-free child survival, we initiated a concurrent impact evaluation consisting of serial cross-sectional community surveys of mother/caregiver-infant pairs residing in five of Zimbabwe's ten provinces.11 The analyses presented here use data from the baseline survey to describe engagement in PMTCT services before implementation of the 2010 guidelines. Our objectives were to: 1) compare the uptake of services in the maternal and infant PMTCT cascade in 2011-2012 using data from the community-based survey and the National Health Information System; 2) determine factors associated with MTCT and key gaps in the cascade among HIV-infected mothers, and 3) examine patterns of service utilization among HIV-exposed infants.
Methods
Study Population
The baseline survey was conducted between April and September 2012 and targeted mothers and caregivers of infants who were born 9 to 18 months prior in order to capture MTCT occurring during pregnancy, delivery and in the first 9-18 months of breastfeeding. Women were eligible if they were ≥16 years old and biological mothers or caregivers of eligible infants (alive or deceased). For this analysis, we restricted the overall sample of 9,018 mothers and caregivers to 8,662 biological mothers and their eligible infants by excluding 356 (3.9%) caregiver/infant pairs.
Sampling Strategy
Five provinces (Harare, Mashonaland West, Mashonaland Central, Manicaland, and Matabeleland South) were purposefully selected in order to include Zimbabwe's capital, rural communities with high and low HIV prevalence, and both major ethnic groups in Zimbabwe (i.e., Shona, Ndebele). The two-stage sampling strategy has been previous described.11,12 In brief, we randomly selected 157 catchment areas from 699 health facilities offering PMTCT services, proportionate to the number of facilities per district. In each catchment area, all eligible infants were identified and a pre-determined proportion was sampled, depending on the size of the catchment area. Potentially eligible infants and their mothers/caregivers were identified by pooling information from: 1) community health workers, 2) immunization registers from both sampled and nearby health facilities, and 3) peer referral. Together, this approach efficiently identified eligible participants without screening all households and captured mother-infant pairs who did not utilize any health services and those who may have accessed care outside of their area of residence.
Data Collection
Mothers providing written informed consent completed an anonymous interviewer-administered survey which collected information about maternal and household demographics, health services accessed by the mother and her infant during pregnancy and after delivery, and health behaviors germane to MTCT (e.g., breastfeeding, contraception).
Uptake of PMTCT Services: The Maternal and Infant Cascades
The “maternal cascade” included: ANC (any and the WHO-recommended ≥4 visits13), HIV testing during ANC or labor and delivery or prior knowledge of one's HIV-positive status, receipt of HIV test results or prior knowledge of one's HIV-positive status, institutional delivery, postnatal visit attendance (6-8 weeks after birth), current use of a modern contraceptive method,14 and, among HIV-infected mothers, receipt of an CD4+ T-lymphocyte cell count. To determine whether mothers were on ART or whether they or their infant received ARV prophylaxis, a service likely affected by recall and social desirability bias, we asked several questions using various formats and response options, consistent with the principles of good questionnaire design.15 This strategy also included naming specific drugs (i.e., nevirapine), examination of the maternal and infant health cards, when available, and asking some ARV-related questions regardless of serostatus (“were you given a pill to take at the onset of your labor? Why?”). The “infant cascade” among HIV-exposed infants included: ≥1 immunization visit, receipt of cotrimoxazole, exclusive breastfeeding for ≥6 months,16 HIV testing, receipt of results, and treatment (if infected).
Assessment of HIV Status
Living mothers and infants provided blood spot samples for HIV testing, which were air-dried onto filter papers and stored at room temperature until transported biweekly to the laboratory. Maternal samples were tested for HIV-1 antibody using AniLabsytems EIA kit (AniLabsystems Ltd, OyToilette 3, FIN-01720, Vantaa, Finland) with all positive specimens confirmed using Enzygnost Anti-HIV 1/2 Plus ELISA (Dade Behring, Marburg, Germany) and discrepant results resolved by Western Blot. Samples of infants born to HIV-positive mothers or mothers with unavailable samples were tested for HIV with DNA polymerase chain reaction (Roche Amplicor HIV-1 DNA Test, version 1.5). HIV test results were available for 97.8% of women and 97.2% of HIV-exposed infants, and were linked to questionnaire data through a unique code. HIV test results were used for all analyses of maternal and infant HIV serostatus.
Data Analyses
We first performed descriptive analyses, including a comparison of socio-demographic characteristics stratified by maternal HIV status. To construct the maternal and infant PMTCT cascades, we computed the proportion of women or infants completing each step using the total weighted number of women or infants as the denominator. When receipt of the service or engagement in the behavior was unknown or missing, women or infants were classified as not having completed the cascade step; however, no more than 1% of any service or behavior was missing. In addition to the overall cascades, we compared service utilization between groups with an F test adjusted for the study design and alpha=0.05.
We compared the PMTCT cascades generated from the community-based survey to facility-based data collected in 2011 and 2012 by the National Health Information Unit of the MoHCC from all health facilities offering maternal and child health services in Zimbabwe. Each month, health facilities submit summary reports of patient data to the district office, where data are verified and cleaned prior to entry into the District Health Information System and transmission to the national office. We computed the proportion of pregnant women attending health facilities who: received antenatal care (any and ≥4 visits), received HIV testing or had prior knowledge of one's HIV-positive status, received HIV test results or had prior knowledge of one's HIV-positive status, delivered in a health facility, attended the postnatal visit, used a modern method of contraception, and, among HIV-infected mothers, received an CD4 cell count and maternal and infant ARV prophylaxis.
We used the community-based survey data to understand factors associated with MTCT and gaps in the cascade. We first explored factors associated with MTCT among HIV-exposed infants using a Poisson regression model limited to HIV-infected mothers with MTCT as the binary outcome. With cross-sectional data, the exponentiated parameter estimates represent prevalence ratios (PR), a conservative and more interpretable measure of association than odds ratios.17-19 Based on this hypothesis-generating model, we then identified the factor most strongly associated with MTCT and constructed a second model to understand determinants of the use of this service. The fully adjusted models contain all covariates specified a priori for inclusion (see below) and key services or behaviors not hypothesized to lie on the causal pathway. Covariates with variance inflation factors >10 (indicating multicollinearity) were excluded.20 We present PRs and 95% confidence intervals (CI) computed with linearized standard errors to account for the sample design.
Several covariates, which likely preceded pregnancy, were considered for inclusion in models as potential confounders: province, mother's age, religion, being married or having a regular sexual partner, education, and parity. Additionally, we created a household asset index, divided into quartiles, using principal component analysis with a polychoric correlation matrix.21-23 Food security (food secure, food insecure, and food insecure with hunger) was determined with a subset of questions from the Household Food Insecurity Access Scale.24,25 We also included three binary covariates to measure whether the women disclosed her HIV status to others, whether concern that health providers may be unfriendly or hostile is a problem when seeking health care, and whether getting permission to seek healthcare was a problem, a proxy for relationship power – factors previously shown to be associated with PMTCT services in Zimbabwe.26,27 No more than 1% of any covariate was missing.
Our final objective was to examine the relationship between patterns of service utilization among HIV-exposed infants and MTCT, using the mother's reports of services received. We focused on three services/behaviors that are directly linked to the likelihood of MTCT: maternal ART and ARV prophylaxis, infant ARV prophylaxis, and exclusive breastfeeding. All analyses were conducted with STATA 12 (College Station, Texas) and were weighted to account for the varying sampling fraction by catchment area and 1.1% survey non-response.
Human Subjects Protection
The Medical Research Council of Zimbabwe and the ethical review boards at the University of California, Berkeley and University College London approved this study.
Results
Participant Characteristics
The weighted population included 8,800 mothers of infants who were or would have been between 9 and 18 months of age at the time of the survey (based on 8,662 observations). The average age of women was 26.7 years, 92.7% were married or had a regular sexual partner, 51.1% lived in food insecure households, and they had an average of 2.7 lifetime births (Table 1). Overall, 1,075 women (12.4% of those with test results) were HIV-infected. Among 1,072 HIV-exposed infants with complete testing data, 93 (8.7%) were HIV-infected.
TABLE 1.
Sociodemographic characteristics of study participants, Zimbabwe, 2012. All women were ≥16 years old and biological mothers of infants (alive or deceased) born 9 to 18 months prior to the interview.a
| Characteristic | Total | Maternal HIV statusb | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HIV-infected | HIV-uninfected | |||||
|
|
|
|||||
| N | (%) | N | (%) | N | (%) | |
| Total | 8800 | (100) | 1,075 | (100) | 7,599 | (100) |
| Province | ||||||
| Harare | 1,536 | (17.5) | 167 | (15.6) | 1,319 | (17.4) |
| Manicaland | 3,564 | (40.5) | 373 | (34.7) | 3,157 | (41.5) |
| Mashonaland Central | 1,504 | (17.1) | 158 | (14.7) | 1,325 | (17.4) |
| Mashonaland West | 1,341 | (15.2) | 194 | (18.0) | 1,139 | (15.0) |
| Matabeleland South | 856 | (9.7) | 183 | (17.0) | 659 | (8.7) |
| Age, years (mean, SE) | 26.7 (0.09) | 28.9 (0.23) | 26.4 (0.10) | |||
| Married or has a regular sexual partner | 8,156 | (92.7) | 937 | (87.2) | 7,098 | (93.5) |
| Education, highest completed | ||||||
| No education | 274 | (3.1) | 42 | (3.9) | 230 | (3.0) |
| Primary school (Standard 7) | 2,458 | (27.9) | 341 | (31.7) | 2,096 | (27.6) |
| Some secondary school | 2,469 | (28.1) | 331 | (30.8) | 2,107 | (27.7) |
| “O” Level or higher (Grade 11) | 3,595 | (40.9) | 361 | (33.6) | 3,161 | (41.6) |
| Asset Index (quartile) | ||||||
| 1st (lowest) | 2,464 | (28.0) | 330 | (30.7) | 2,117 | (27.9) |
| 2nd | 1,624 | (18.5) | 166 | (15.4) | 1,438 | (18.9) |
| 3rd | 2,022 | (23.0) | 248 | (23.1) | 1,749 | (23.0) |
| 4th (highest) | 2,685 | (30.5) | 331 | (30.8) | 2,291 | (30.2) |
| Food Security | ||||||
| Food secure | 4,305 | (49.0) | 414 | (38.5) | 3,811 | (50.2) |
| Food insecure | 2,906 | (33.1) | 358 | (33.3) | 2,518 | (33.2) |
| Food insecure with hunger | 1,578 | (18.0) | 304 | (28.3) | 1,261 | (16.6) |
| Lifetime births (mean, SE) | 2.7 (0.04) | 3.1 (0.09) | 2.6 (0.04) | |||
| Infant alive | 8,737 | (99.3) | 1,058 | (98.4) | 7,556 | (99.4) |
| Infant's age or potential age, months (mean, SE)c | 13.7 (0.04) | 13.7 (0.10) | 13.7 (0.04) | |||
SE: Linearized standard error
Weighted counts and proportions presented in the table. Numbers may not sum to column totals due to missing data. Percentages may not add to 100 due to rounding.
Limited to mothers with an HIV test result.
Age of infants who were alive at the time of the survey as well as the age deceased infants would have been at the time of the survey.
PMTCT Service Utilization and Engagement in Prevention Behaviors
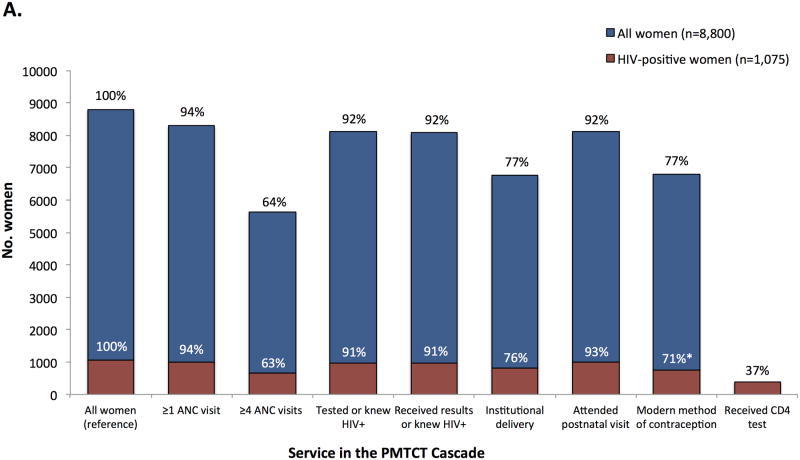
Although 8,287 (94%) of all women attended ≥1 ANC visit during pregnancy, only 64% attended ≥4 ANC visits (Figure 1, Panel A). Ninety-two percent of women reported HIV testing during pregnancy and either received their results or had prior knowledge of their HIV-positive serostatus, 6,753 (77%) women delivered their infants in a health facility, and 8,113 (92%) attended the 6-8 week postnatal visit. The uptake of services or behaviors intended for all pregnant or postpartum women did not differ significantly between women who were HIV-infected and women who were HIV-negative or of unknown status, with the exception of postpartum use of modern contraception (71% among HIV-infected women vs. 78% among HIV-uninfected women, p<0.01).
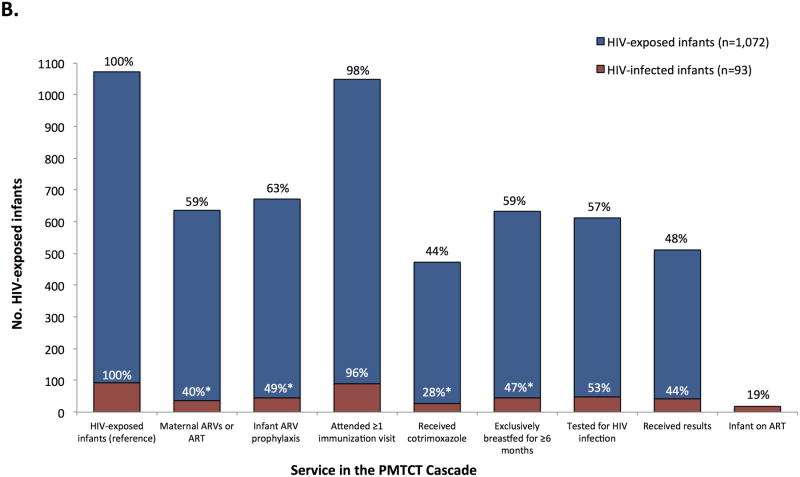
FIGURE 1.
Uptake of health services and engagement in health behaviors recommended for pregnant and postpartum women (Panel A) and HIV-exposed infants (Panel B) in the PMTCT cascade, stratified by HIV serostatus at the time of the survey, Zimbabwe, 2012. Percentages shown on the chart are the proportion of the weighted population (N=8,800) or the weighted subset of HIV-infected mothers (N=1,075, Panel A) or HIV-exposed infants (N=1,072, Panel B) who reported receiving the service or engaging in the behavior (the percentages are not conditional on the prior service or behavior). Asterisks (*) indicate p<0.05 for the design-based F test of HIV-infected versus HIV-uninfected/unknown status mothers (Panel A) or HIV-infected versus HIV-exposed and uninfected infants (Panel B).
Among HIV-infected women, 59% reported receiving ART or ARV prophylaxis and 37% reported receiving a CD4 test. Among their 1,072 HIV-exposed infants (Figure 1, Panel B), 671 (63%) received ARV prophylaxis, 473 (44%) received cotrimoxazole, 633 (59%) were exclusively breastfed for ≥6 months, and 611 (57%) were tested for HIV infection. Among HIV-infected infants, 18 (19%) were on ART at the time of the survey. There were some differences between HIV-infected infants and their mothers compared to HIV-exposed but uninfected infants: mothers of HIV-infected infants were less likely to report maternal ART or ARV prophylaxis (40% vs. 61%, p<0.01), infant ARV prophylaxis (49% vs. 64%, p=0.02), that their infant received cotrimoxazole (28% vs. 46%, p<0.01), and exclusive breastfeeding for ≥6 months (47% vs. 60%, p=0.03).
Compared to the community-based survey, national data from all maternal and child health facilities indicated a similar proportion of pregnant women who knew or learned their HIV serostatus (94% in 2012 vs. 92% in the survey) and who delivered in a health facility (74% in 2012 vs. 77% in the survey, Table 2). However, the proportion of pregnant women receiving antenatal care, CD4 counts (if HIV-infected), ART or ARV prophylaxis, and modern contraception were substantially higher when estimated from the facility-based data compared to the community survey. Likewise, infant ARV prophylaxis was higher in the facility-based data compared to the community survey (87% in 2012 vs. 63% in the survey).
TABLE 2.
Uptake of health services recommended for PMTCT by women attending maternal and child health facilities in 2011 and 2012, Zimbabwe. Data were collected by the National Health Information Unit of the Ministry of Health and Child Care.a
| Service | 2011 | 2012 | ||
|---|---|---|---|---|
|
|
|
|||
| N | % | N | % | |
| Estimated pregnancies | 412,120 | 100% | 420,364 | 100% |
| ANC booking or ≥1 ANC visit | 403,938 | 98% | 414,859 | 99% |
| ≥4 ANC visits | --b | --b | 337,552 | 80% |
| Tested or known HIV-infected | 406,815 | 99% | 416,273 | 99% |
| Received results or known HIV-infected | 341,725 | 83% | 395,081 | 94% |
| HIV-infected | 42,082c | 10% | 60,105 | 14% |
| Institutional delivery | 320,939 | 78% | 309,744 | 74% |
| Attended postnatal visit | 212,242 | 52% | --b | --b |
| Modern method of contraception | 350,617 | 85% | --b | --b |
| Received CD4 test, if HIV-infected | 23,567 | 56% | 28,729 | 48% |
| Received ARV prophylaxis/therapy | 39,091 | 93% | 55,758 | 93% |
| Infant ARV prophylaxis | 38,748 | 92% | 52,163 | 87% |
PMTCT: prevention of mother-to-child transmission; ANC: antenatal care; NHIS: National Health Information System; ARV: antiretroviral
The denominator for each indicator was the estimated number of pregnant women attending maternal and child health facilities in 2011 or 2012.
Data missing in NHIS during this period
In 2011, the number of HIV-infected pregnant women excluded women who were known to be living with HIV infection.
Factors Associated with MTCT and Gaps in the Cascade
In multivariable analyses, among HIV-exposed infants, maternal receipt of ART or ARV prophylaxis was strongly associated with MTCT (PRa: 0.41, 95% CI: 0.23, 0.74, Table 3), although there was no association between MTCT and infant ARV prophylaxis (PRa: 1.22, 95% CI: 0.67, 2.21) or exclusive breastfeeding for ≥6 months (PRa: 0.68, 95% CI: 0.42, 1.09). Factors positively associated with receipt of maternal ART or ARV prophylaxis, the outcome of interest for the second model, included ≥4 ANC visits (PRa: 1.18, 95% CI: 1.01,1.38, Table 3), institutional delivery (PRa: 1.31, 95% CI: 1.13, 1.52), and disclosure of serostatus (PRa: 1.30, 95% CI: 1.12, 1.49). Getting permission for health care and concern over unfriendly or hostile providers were not associated with maternal ART or ARV prophylaxis.
TABLE 3.
The association between PMTCT-related services and behaviors and 1) mother-to-child transmission among HIV-exposed infants, and 2) use of ART or ARV prophylaxis for PMTCT among HIV-infected pregnant women.a,b
| Service or Behavior | Frequency | (1) Infant HIV-infected at 9-18 months of age (MTCT) | (2) Maternal ART or ARV prophylaxis | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| N | % | PRa | 95% CI | PRa | 95% CI | |
| Disclosed HIV test result to someone | 768 | (72.2) | 1.22 | (0.76, 1.97) | 1.30 | (1.12, 1.49)** |
| Permission for healthcare is a problem | 53 | (5.0) | 0.87 | (0.37, 2.01) | 0.83 | (0.59, 1.16) |
| Concern about unfriendly or hostile healthcare providers | 191 | (17.7) | 1.37 | (0.72, 2.62) | 1.00 | (0.87, 1.15) |
| ≥4 Antenatal care visits | 676 | (63.4) | 0.97 | (0.64, 1.46) | 1.18 | (1.01, 1.38)* |
| Institutional Delivery | 812 | (75.5) | 0.68 | (0.42, 1.11) | 1.31 | (1.13, 1.52)** |
| Maternal ART or ARV prophylaxis | 639 | (59.5) | 0.41 | (0.23, 0.74)** | --- | |
| Infant ARV prophylaxis | 673 | (62.9) | 1.22 | (0.67, 2.21) | ---c | |
| ≥6 months exclusive breastfeeding | 634 | (59.2) | 0.68 | (0.42, 1.09) | --- c | |
PRa: adjusted prevalence ratio; CI: confidence interval; MTCT: mother-to-child transmission
Regression models of 1,052 (Model 1) and 1,063 (Model 2) HIV-infected women, adjusted for the clustered study design. In addition to the PMTCT-related services and behaviors listed in the table, the models include province, maternal age, religion, whether the woman has a husband or regular partner, education, lifetime births, an asset index created using a principal component analysis with a polychoric correlation matrix, and food security status. The binary indicator of ANC and being tested for HIV infection were excluded from regression models, services which were utilized by more than 90% of women and were strongly correlated.
+p<0.10, *p<0.05, **p<0.01
Excluded from the model as this service/behavior temporally succeeded the outcome (maternal ART or prophylactic ARVs).
PMTCT Service Utilization Among HIV-Infected Pregnant Women and their Infants and MTCT
Eighteen percent of HIV-exposed infants received neither maternal nor infant ARV prophylaxis and were not exclusively breastfed for ≥6 months (Pattern 1, Table 4). In contrast, 38% of HIV-exposed infants received all three services (Pattern 2). Overall, 33% of infants received neither maternal nor infant ARV prophylaxis, irrespective of breastfeeding status (Patterns 1 and 4). The MTCT rate was 4.5% among infants receiving all three services compared to 12.8% among infants receiving neither maternal nor infant ARV prophylaxis and who were not exclusively breastfed for ≥6 months (p<0.01).
TABLE 4.
Patterns of service utilization, prevention behavior, and MTCT, Zimbabwe, 2012. Black boxes indicate that the service was utilized or the behavior occurred; white boxes indicate that the service was not received or the behavior did not occur. For each service utilization pattern, the number and proportion of HIV-exposed infants who were HIV-infected at 9-18 months (MTCT) is shown.
| Service Utilization Pattern | Service or behavior targeted to HIV-infected women and their infants | Population | |||
|---|---|---|---|---|---|
| Maternal ART or ARV prophylaxis | Infant ARV prophylaxis | ≥6 months exclusive breastfeeding | All HIV-exposed infants (N=1,063) N (%) | HIV-infected infants at 9-18 months of age (MTCT rate)a N (%) | |
| 1 | 188 (18%) | 24 (12.8%) | |||
| 2 | 403 (38%) | 18 (4.5%) | |||
| 3 | 191 (18%) | 14 (7.3%) | |||
| 4 | 167 (16%) | 19 (11.4%) | |||
| 5 | 40 (4%) | 7 (17.5%) | |||
| 6 | 37 (4%) | 7 (18.9%) | |||
| 7 | 21 (2%) | 0 (0%) | |||
| 8 | 17 (2%) | 5 (29.4%) | |||
MTCT: mother-to-child HIV transmission
Row percentages shown for each service utilization pattern.
Discussion
Our community-level data indicate that Zimbabwe has made significant progress towards elimination of MTCT,9,28 however, in 2011-2012, prior to implementation of the Accelerated National PMTCT Program, gaps remained in the uptake of maternal and infant PMTCT services. We estimate that 36% percent of women did not attend at least 4 ANC visits, 23% did not deliver in a health facility, 41% of HIV-infected women reported neither ART nor ARV prophylaxis, and more than one-third of HIV-exposed infants did not receive ARV prophylaxis. Predictably, many of our estimates of service uptake were lower than national estimates from facility-based programmatic data, which is limited to women who access services. Thus, although Zimbabwe's MTCT rate in this study was 8.7%, considerably lower than the 17-30% estimated by previous modeling studies,29-31 there is still scope to increase engagement and retention in all steps in the PMTCT cascade. This is especially true for maternal receipt of ART or ARV prophylaxis, which was the strongest predictor of MTCT in our analysis and is also important for maternal health.
Contrary to some previous reports, these results suggest that most pregnant and postpartum women are not completely lost from the cascade.4 We found that >90% of women attended ≥1 ANC visit, the postnatal visit, and an immunization visit, with no significant differences by maternal serostatus (similar to previous reports from Zimbabwe32). There is much lower uptake, however, of services and behaviors targeted specifically to HIV-infected mothers and their HIV-exposed infants. For example, although 92% of HIV-infected women reported knowing their HIV status by delivery, only 59% reported receiving ART or ARV prophylaxis themselves, only 63% of their HIV-exposed infants received ARV prophylaxis, and among those infants who were infected, only 19% were reported to be on treatment. Thus, although HIV-infected women stay connected to the healthcare system after delivery, they appear to miss key services that prevent and treat HIV infection. These gaps are consistent with data from UNAIDS, which reported that between 50-74% of HIV-infected pregnant women in Zimbabwe received combination ARV regimens in 2011,1 but these data are much lower than the estimate from the MoHCC PMTCT 2011 Annual Report, which reported that 86% of HIV-infected women and 85% of HIV-exposed infants, respectively, received ARVs.9,33 This discrepancy may reflect the fact that the MoHCC report was restricted to women who had already engaged in care, as was the case with the facility-based estimates of service uptake in this study, which were higher than those in the community. Nevertheless, multiple estimates from different sources and data collection methodologies are valuable for monitoring progress towards eMTCT.
One explanation for sub-optimal uptake of HIV-specific services found in the community-based survey could be lack of knowledge about the benefits of ART or ARV prophylaxis. However, the 2010-2011 DHS found that 79% of women in Zimbabwe knew that HIV can be transmitted by breastfeeding and that the risk of MTCT can be reduced by taking special drugs during pregnancy.8 Similar findings were revealed in the Zimbabwe National Behavior Change Programme survey in 2011-12.34 Another explanation is that supply-side factors, like poor provider knowledge or attitudes, lack of trained providers, user fees,35,36 or stock-outs33 restricted women and their infants' receipt of ART and ARV prophylaxis. For example, although 94% of facilities in our in survey were offering PMTCT services, high levels of staff absenteeism may mean that a trained heath care worker is not always present.37
An alternative explanation for this pattern of service utilization could be related to women's concerns about inadvertent disclosure of their HIV-positive status and stigma.38 In our study, HIV-infected women who had disclosed their serostatus were 30% more likely to report receipt of ART or ARV prophylaxis, even after adjusting for ANC and institutional delivery, the other significant predictors of maternal ART or ARV prophylaxis in our study and in others.39 When asked to whom they disclosed, 92% of women reported disclosing to their husband or partner (data not shown), underscoring the critical role of male support for eMTCT.40,41 In addition to stigma and fear of disclosure, a recent qualitative study in Zimbabwe highlighted the important role of mistrust and fear of healthcare staff in preventing engagement with health services. For example, HIV-positive women who registered late for ANC were perceived as “bad clients” who might be treated poorly at delivery – perpetuating a dangerous cycle in which women who delayed receiving care continue to avoid care.26,27 Although our measure of concern over unfriendly or hostile clinic staff was not predictive of MTCT or maternal ARVs, it was asked in general terms and not specifically about ANC. The midline impact evaluation survey (in progress) will include more precise questions about the perception or experience of poor treatment by healthcare staff.
Our study analyzed cross-sectional data from a PMTCT impact evaluation that was not specifically designed to examine the utilization of PMTCT services; hence, we did not verify self-reported receipt of services against medical records. However, self-reports of engagement with PMTCT services were inversely associated with MTCT, suggesting the validity of the self-reported data. For example, HIV-exposed infants who did not receive maternal or infant ARV prophylaxis and who were not exclusively breastfed for at least 6 months were more than twice as likely to be HIV-infected than HIV-exposed infants who received all three services. In addition, although our data are representative of the communities from which the sample was selected, they are not representative of all regions in Zimbabwe.
Another notable limitation is that women's and infant's HIV status was measured 9-18 months postpartum. Thus, although we have assumed that women who were HIV-infected at the time of the survey were also HIV-infected during their pregnancy, it is possible that a small proportion were infected during pregnancy or postpartum. Assuming that HIV incidence during pregnancy and postpartum is 4.8 per 100 woman-years,42 approximately 83 of the 1075 HIV-infected women could have acquired their infection after they became pregnant and may have (appropriately) not received HIV-specific services like ARV prophylaxis. Likewise, a small proportion of infants may have been HIV-infected before interventions such as ARV prophylaxis or exclusive breastfeeding were recommended, and infants who were still breastfeeding at the time of the survey remained at risk of MTCT, so we may not have captured all possible infant infections. Furthermore, we were unable to measure whether women and their infants were adherent to the ARV/ART regimens they received.
Zimbabwe's remarkable progress in preventing pediatric HIV infections implies that it may be on track to achieve elimination of MTCT if the gaps in the cascade we have described are closed. The transition to Option B+ is changing the service delivery paradigm as ART initiation services are expanded to all 1,560 health facilities offering PMTCT services. Alongside the tremendous opportunity this offers for eMTCT and maternal and infant health more broadly, our analysis supports previous mathematical models29 which demonstrate that efforts to engage and retain HIV-infected pregnant and postpartum women in HIV-specific services are essential to achieve eMTCT. This may include special attention to strategies to reduce stigma, protect patient confidentiality, and enhance women's ability to safely disclose their HIV status to their partners. In addition, our analysis highlights the value of community-based data: examination of program data, which only includes women who access services, may have missed areas where intensified efforts to optimize uptake is crucial in order to eliminate mother-to-child HIV transmission.
Acknowledgments
We are grateful to all of the women and children who participated in the study. We are also indebted to Dr. Maya Petersen and Dr. Mi-Suk Kang Dufour for their valuable contributions to the impact evaluation. SM, RB, NP, TM, and FC collaboratively designed the impact evaluation. SM and RB conducted the data analysis, which was iteratively refined after discussion with all authors. SM drafted the initial manuscript and all authors participated in reviewing the draft for intellectual content and assisting with revisions. All authors approved the final version of the manuscript.
The evaluation of Zimbabwe's Accelerated National PMTCT Program was supported by the Children's Investment Fund Foundation (CIFF). Dr. McCoy is supported by Award Number K01MH094246 from the National Institute of Mental Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
Sources of Support: The evaluation of Zimbabwe's Accelerated National PMTCT Program was supported by the Children's Investment Fund Foundation (CIFF). Dr. McCoy is supported by Award Number K01MH094246 from the National Institute of Mental Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
Footnotes
Previous Presentations: The preliminary results from this study were presented in 2013 by Dr. McCoy at the 7th IAS Conference on HIV Pathogenesis, Treatment and Prevention in Kuala Lumpur, Malaysia.
References
- 1.UNAIDS. Report on the Global AIDS Epidemic. Geneva: 2012. [Google Scholar]
- 2.Joint United Nations Programme on HIV/AIDS (UNAIDS) Global Plan Towards the Elimination of New HIV Infections Among Children By 2015 and Keeping their Mothers Alive, 2011-2015. Geneva: 2011. [Google Scholar]
- 3.World Health Organization. Antiretroviral Drugs for Treating Pregnant Women and Preventing HIV Infection in Infants: Recommendations for a public health approach. Geneva: 2010. [PubMed] [Google Scholar]
- 4.Sibanda EL, Weller IV, Hakim JG, Cowan FM. The magnitude of loss to follow-up of HIV-exposed infants along the prevention of mother-to-child HIV transmission continuum of care: a systematic review and meta-analysis. Aids. 2013 Nov 13;27(17):2787–2797. doi: 10.1097/QAD.0000000000000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paintsil E, Andiman WA. Update on successes and challenges regarding mother-to-child transmission of HIV. Curr Opin Pediatr. 2009;21:94–101. doi: 10.1097/MOP.0b013e32831ec353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization, UNICEF, Interagency Task Team of Prevention of HIV Infection in Pregnant Women M, and their Children. Guidance on Global Scale-Up of the Prevention of Mother to Child Transmission of HIV. Geneva: 2007. [Google Scholar]
- 7.Zimbabwe Ministry of Health and Child Care. National Survey of HIV and Syphilis Prevalence Among Pregnant women Attending Antenatal Clinics, 2012. Harare: 2013. [Google Scholar]
- 8.Zimbabwe National Statistics Agency (ZIMSTAT) and ICF International. Zimbabwe Demographic and Health Survey 2010-2011. Calverton, Maryland: ZIMSTAT and ICF International, Inc.; 2012. [Google Scholar]
- 9.Zimbabwe Ministry of Health and Child Welfare. [Accessed December 10, 2014];Global AIDS Response Country Progress Report: Zimbabwe 2014. 2014 http://www.unaids.org/sites/default/files/country/documents/ZWE_narrative_report_2014.pdf.
- 10.World Health Organization. Rapid Advice: Use of Antiretroviral Drugs for Treating Pregnant Women and Preventing HIV Infection in Infants. Geneva: 2009. [PubMed] [Google Scholar]
- 11.Buzdugan R, McCoy SI, Petersen M, et al. Feasibility of population-based cross-sectional surveys for estimating vertical HIV transmission: data from Zimbabwe; Paper presented at: 7th IAS Conference on HIV Pathogenesis, Treatment and Prevention; 2013; Kuala Lumpur. [Google Scholar]
- 12.McCoy SI, Buzdugan R, Ralph LJ, et al. Unmet Need for Family Planning, Contraceptive Failure, and Unintended Pregnancy among HIV-Infected and HIV-Uninfected Women in Zimbabwe. PLoS One. 2014;9(8):e105320. doi: 10.1371/journal.pone.0105320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Antenatal Care Randomized Trial: Manual for the Implementation of the New Model. Geneva: 2002. [Google Scholar]
- 14.United Nations Department of Economic and Social Affairs. [Accessed January 24, 2012];World Contraceptive Use 2011. 2011 http://www.un.org/esa/population/publications/contraceptive2011/wallchart_front.pdf.
- 15.Fowler F. Improving Survey Questions: Design and Evaluation. Vol. 38. Thousand Oaks, California: Sage Publications, Inc.; 1995. [Google Scholar]
- 16.World Health Organization. Guidelines on HIV and infant feeding: Principles and recommendations for infant feeding in the context of HIV and a summary of evidence. 2010 [PubMed] [Google Scholar]
- 17.Zocchetti C, Consonni D, Bertazzi PA. Relationship between prevalence rate ratios and odds ratios in cross-sectional studies. Int J Epidemiol. 1997 Feb;26(1):220–223. doi: 10.1093/ije/26.1.220. [DOI] [PubMed] [Google Scholar]
- 18.Greenland S. Interpretation and choice of effect measures in epidemiologic analyses. Am J Epidemiol. 1987 May;125(5):761–768. doi: 10.1093/oxfordjournals.aje.a114593. [DOI] [PubMed] [Google Scholar]
- 19.Barros AJ, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC medical research methodology. 2003 Oct 20;3:21. doi: 10.1186/1471-2288-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kutner MH, Nachtsheim CJ, Neter J, Li W. Applied Linear Statistical Models. Fifth. New York, New York: McGraw-Hill/Irwin; 2005. [Google Scholar]
- 21.Kolenikov S, Angeles G. CPC/MEASURE Working paper. Chapel Hill, North Carolina: MEASURE Evaluation; 2004. The Use of Discrete Data in Principal Component Analysis With Applications to Socio-Economic Indices. [Google Scholar]
- 22.Vyas S, Kumaranayake L. Constructing socio-economic status indices: how to use principal components analysis. Health Policy Plan. 2006 Nov;21(6):459–468. doi: 10.1093/heapol/czl029. [DOI] [PubMed] [Google Scholar]
- 23.Filmer D, Pritchett L. Estimating Wealth Effect Without Expenditure Data—Or Tears: An Application to Educational Enrollments in States of India. Demography. 2001;38:115–132. doi: 10.1353/dem.2001.0003. [DOI] [PubMed] [Google Scholar]
- 24.Coates J, Swindale A, Bilinsky P. Household Food Insecurity Access Scale (HFIAS) for Measurement of Food Access: Indicator Guide. Washington D.C.: United States Agency for International Development; 2007. [Google Scholar]
- 25.Coates J. Experience and Expression of Food Insecurity Across Cultures: Practical Implications for Valid Measurement. Washington D.C.: Food and Nutrition Technical Assistance Project, Academy for Educational Development; 2004. [Google Scholar]
- 26.Sibanda EL, Weller I, Bernays S, Hakim J, Cowan F. Facilitators and barriers to cotrimoxazole and nevirapine prophylaxis among HIV exposed babies: a qualitative study from Harare, Zimbabwe; Paper presented at: International Congress on Drug Therapy in HIV Infection; 2012; Glasgow, Scotland. [Google Scholar]
- 27.Sibanda EL, Weller IVD, Hakim JG, Cowan FM. An investigation of barriers to seeking antenatal care among women in Harare, Zimbabwe: a qualitative study; Paper presented at: International AIDS Society; 2013; Kuala Lumpur. [Google Scholar]
- 28.UNAIDS. A progress report on the global plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive. Geneva: 2012. [Google Scholar]
- 29.Ciaranello AL, Perez F, Keatinge J, et al. What will it take to eliminate pediatric HIV? Reaching WHO target rates of mother-to-child HIV transmission in Zimbabwe: a model-based analysis. PLoS Med. 2012 Jan;9(1):e1001156. doi: 10.1371/journal.pmed.1001156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ciaranello AL, Perez F, Maruva M, et al. WHO 2010 guidelines for prevention of mother-to-child HIV transmission in Zimbabwe: modeling clinical outcomes in infants and mothers. PLoS One. 2011;6(6):e20224. doi: 10.1371/journal.pone.0020224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Republic of Zimbabwe. Zimbabwe National HIV and AIDS Strategic Plan, 2011-2015. Harare: 2011. [Google Scholar]
- 32.Maruva M, Gwavuya A, Marume M, Musarandega R, Madzingira N. Knowledge of HIV Status at ANC and Utilization of Maternal Health Services in the 2010-11 Zimbabwe Demographic and Health Survey; Zimbabwe Working Papers; Accessed December 16, 2014.2014. http://dhsprogram.com/pubs/pdf/WP107/WP107.pdf.
- 33.Zimbabwe Ministry of Health and Child Welfare. PMTCT 2011 Annual Report. Harare: 2011. [Google Scholar]
- 34.Buzdugan R, Watadzaushe C, Dirawo J, et al. Positive attitudes to pediatric HIV testing: findings from a nationally representative survey from Zimbabwe. PLoS One. 2012;7(12):e53213. doi: 10.1371/journal.pone.0053213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simkhada B, Teijlingen ER, Porter M, Simkhada P. Factors affecting the utilization of antenatal care in developing countries: systematic review of the literature. J Adv Nurs. 2008 Feb;61(3):244–260. doi: 10.1111/j.1365-2648.2007.04532.x. [DOI] [PubMed] [Google Scholar]
- 36.Duff P, Kipp W, Wild TC, Rubaale T, Okech-Ojony J. Barriers to accessing highly active antiretroviral therapy by HIV-positive women attending an antenatal clinic in a regional hospital in western Uganda. Journal of the International AIDS Society. 2010;13:37. doi: 10.1186/1758-2652-13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldstein M, Zivin JG, Habyarimana J, Pop-Eleches C, Thirumurthy H. The Effect of Absenteeism and Clinic Protocol on Health Outcomes: The Case of Mother-to-Child Transmission of HIV in Kenya. American economic journal Applied economics. 2013;5(2):58–85. doi: 10.1257/app.5.2.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turan JM, Nyblade L. HIV-related stigma as a barrier to achievement of global PMTCT and maternal health goals: a review of the evidence. AIDS Behav. 2013 Sep;17(7):2528–2539. doi: 10.1007/s10461-013-0446-8. [DOI] [PubMed] [Google Scholar]
- 39.Lerebo W, Callens S, Jackson D, Zarowsky C, Temmerman M. Identifying factors associated with the uptake of prevention of mother to child HIV transmission programme in Tigray region, Ethiopia: a multilevel modeling approach. BMC Health Serv Res. 2014;14:181. doi: 10.1186/1472-6963-14-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aluisio A, Richardson BA, Bosire R, John-Stewart G, Mbori-Ngacha D, Farquhar C. Male antenatal attendance and HIV testing are associated with decreased infant HIV infection and increased HIV-free survival. J Acquir Immune Defic Syndr. 2011 Jan 1;56(1):76–82. doi: 10.1097/QAI.0b013e3181fdb4c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kalembo FW, Zgambo M, Mulaga AN, Yukai D, Ahmed NI. Association between male partner involvement and the uptake of prevention of mother-to-child transmission of HIV (PMTCT) interventions in Mwanza district, Malawi: a retrospective cohort study. PLoS One. 2013;8(6):e66517. doi: 10.1371/journal.pone.0066517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drake AL, Wagner A, Richardson B, John-Stewart G. Incident HIV during Pregnancy and Postpartum and Risk of Mother-to-Child HIV Transmission: A Systematic Review and Meta-Analysis. PLoS Med. 2014 Feb;11(2):e1001608. doi: 10.1371/journal.pmed.1001608. [DOI] [PMC free article] [PubMed] [Google Scholar]