Abstract
Background
Among patients with HIV-infection, changes in the kidney filtration marker cystatin C after initiation of antiretroviral therapy (ART) may be related to changes in body composition or biomarkers of inflammation.
Methods
ACTG A5224s was a substudy of A5202 which randomly assigned ART-naïve HIV-infected subjects to blinded abacavir/lamivudine (ABC/3TC) or tenofovir/emtricitabine (TDF/FTC) with open-label efavirenz (EFV) or atazanavir/ritonavir (ATV/r). This analysis explored changes in cystatin C from 0 to 96 weeks.
Results
Of the 269 subjects, 85% were male and 66% White non-Hispanics; baseline mean CD4 count was 236 cells/mm3 and cystatin C was 0.89 mg/L. Cystatin C decreased significantly within each arm; however, ATV/r attenuated the beneficial effects of ART on cystatin C compared to EFV. Compared to ABC/3TC, TDF/FTC led to a marginally significant attenuation for percent change analyses only. Higher baseline BMI and HIV RNA were associated with larger reductions in cystatin C in multivariable models. At baseline, cystatin C was positively correlated with high sensitivity C-reactive protein (Spearman r=0.25), interleukin-6 (r=0.34), soluble intercellular adhesion molecule (r=0.36), soluble vascular cell adhesion molecule (r=0.54), tumor necrosis factor-α (r=0.57), and soluble TNF-α receptor-I (r=0.70, all p<0.001). Reductions in cystatin C from 0 to 96 weeks correlated with reductions in all inflammatory biomarkers (r=0.39 to 0.58, p<0.001) except for hs-CRP (r=0.01, p=0.89) and IL-6 (r=0.08, p=0.24).
Conclusions
The beneficial effect of ART on cystatin C concentrations is attenuated by boosted ATV when compared to EFV. Reductions in cystatin C after ART are associated with reductions in systemic inflammation.
Keywords: kidney, glomerular filtration rate, cystatin C, antiretroviral therapy, inflammation
Introduction
The cysteine protease inhibitor cystatin C has been extensively studied as a marker of glomerular filtration rate (GFR). In the general population, Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) estimates of glomerular filtration rate based on the combination of creatinine and cystatin C (eGFRcr-cys) are more accurate than estimates based on either marker alone (eGFRcys or eGFRcr)1. These equations account for the effects of age, sex, and race on cystatin C and creatinine levels.
Interestingly, cystatin C and eGFRcys have been more strongly associated with mortality—particularly cardiovascular mortality—when compared to creatinine, eGFRcr, or the combined eGFRcr-cys2–5. The reasons for this potent association are unclear, but may be due to residual non-GFR determinants of creatinine (e.g. diet and muscle mass6) that weaken creatinine’s association with mortality or to non-GFR determinants of cystatin C (e.g. inflammation and obesity7–9) that strengthen cystatin C’s association.
In subjects with chronic HIV infection, plasma cystatin C concentrations are elevated compared to uninfected controls10,11 and related to HIV viremia12, HCV co-infection10, generalized inflammation10,13 and traditional markers of cardiovascular risk10,12. In contrast to the general population, however, cystatin C based estimates of GFR were not more accurate than eGFRcr in studies that have compared the CKD-EPI equations to measured GFR among subjects on antiretroviral therapy (ART)14–16. Yet, cystatin C is still a powerful predictor of cardiovascular events and mortality in multiple HIV-infected cohorts17–19. There is evidence that this may be related to non-GFR determinants such as inflammation16, though it remains controversial20.
We have previously shown that initiation of ART regimens containing ritonavir-boosted atazanavir (ATV/r) or tenofovir disoproxil fumarate/emtricitabine (TDF/FTC) led to sustained declines in eGFRcr over 96 weeks when compared to efavirenz (EFV) or abacavir/lamivudine (ABC/3TC), respectively. Furthermore, there was a significant treatment interaction, such that TDF/FTC use led to significant declines compared to ABC/3TC within the ATV/r arm but not the EFV arm21. Curiously, ATV/r and TDF/FTC were associated with improvements in renal function as estimated by cystatin C equations (eGFRcys). Whether these divergent effects on kidney markers are related to rapid reductions in HIV-viremia and inflammation is unknown.
In this study, we therefore aimed to examine the 96 week effect of ART initiation on plasma cystatin C concentration alone (without transformation to eGFRcys) and to explore whether these changes in cystatin C are related to body composition or biomarkers of inflammation.
Methods
Study Design
A5224s was a metabolic substudy of the AIDS Clinical Trials Group A5202 trial of ART initiation in treatment naïve subjects. Subjects older than 16 years with HIV RNA >1000 copies/ml were randomized to blinded co-formulations of TDF/FTC versus ABC/3TC, along with open-label EFV versus ATV/r. Enrollment exclusion criteria included screening creatinine clearance <60 ml/min (by Cockcroft-Gault), untreated hypogonadism or thyroid disease, Cushing syndrome, diabetes mellitus, and the use of growth hormone, anabolic steroids, or glucocorticoids. The study was approved by all local institutional review boards at participating ACTG sites and written informed consent was obtained from each participant.
Biomarkers
Fasting plasma samples from week 0, 24, and 96 were stored at −80°C without thawing prior to all biomarker measurements. Cystatin C concentration was measured centrally by Quest Diagnostics (Madison, NJ, USA) on a Siemens (Munich, Germany) BNII Nephelometer using the N Latex immunonephelometric assay. High-sensitivity C-reactive protein (hs-CRP), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), soluble TNF-α receptors (sTNF-RI and sTNF-RII), soluble vascular cellular adhesion molecule (sVCAM-1) and soluble intercellular adhesion molecule (sICAM-1) were measured at Johns Hopkins Bayview Advanced Chemistry Laboratory (Baltimore, MD, USA). Hs-CRP was measured using a highly sensitive ELISA (ALPCO Diagnostics; Windham, NH, USA) and other markers were measured using enzyme-immunosorbent assays (R&D Systems; Minneapolis, MN, USA). Intra- and inter-assay coefficients of variation ranged from 1.3—7.6% and 1.83—8.95%, respectively.
Body Composition
Body mass index was calculated from baseline height and from weight obtained at each study visit. Whole body dual-energy X-ray absorptiometry (DEXA) was performed at baseline and the 96 week visit at each local site using a standardized protocol and a Hologic or Lunar scanner. Lean and adipose tissue mass were measured in the anteroposterior view. All scans were read centrally at Tufts University by personnel blinded to subject characteristics.
Statistical Analysis
The current study was a pre-specified secondary analysis to compare changes from baseline to week 96 in cystatin C between pooled, randomized NRTI components (ABC/3TC versus TDF/FTC) and NNRTI/PI components (ATV/r versus EFV). All analyses were initially performed using the intent-to-treat principle based on randomized treatment assignment in which all available data were included and modifications to randomized treatment were ignored; no imputations were made for missing values. Supplemental as-treated analyses were performed in which values were excluded after a change in the randomized NRTI component (when comparing NRTI components) or NNRTI/PI component (when comparing NNRTI/PI components).
The unadjusted effects of the NRTI component and the NNRTI/PI component on changes were evaluated separately using two-sample t-tests. Linear regression was used to test for pre-specified interactions between NRTI and NNRTI/PI components and to evaluate potential interactions between treatment components and baseline characteristics. Correlations of cystatin C with biomarkers of inflammation and endothelial activation were evaluated using Spearman’s rank correlation test. P-values below 0.05 (<0.10 for assessing interactions) were considered statistically significant. Analyses that explored associations with baseline factors used linear regression. Univariate associations with a p-value <0.20 were included in a multivariable model which utilized backwards selection and only factors with a p-value <0.05 were retained; NRTI component and NNRTI/PI component were retained regardless of p-value. Analyses were performed using SAS, version 9.2 (SAS Institute).
Results
Overall, 269 eligible subjects were randomized to one of the four regimens in A5224s. These subjects were used for all baseline analyses. Their characteristics are displayed in Table 1 and were balanced by study group assignment. Mean (standard deviation, SD) age was 38 (10) years; 85% were male and 47% were White non-Hispanics. Mean (SD) CD4+ T-cell count was 236 (165) cells/mm3.
Table 1.
Baseline characteristics of study subjects
| EFV + TDF/FTC (N=69) |
EFV + ABC/3TC (N=70) |
ATV/r + TDF/FTC (N=65) |
ATV/r + ABC/3TC (N=65) |
Total (N=269) |
||
|---|---|---|---|---|---|---|
| Age, years | 39 (10) | 39 (10) | 38 (10) | 37 (10) | 38 (10) | |
| Sex | ||||||
| Male | 58 (84) | 56 (80) | 56 (86) | 59 (91) | 229 (85) | |
| Female | 11 (16) | 14 (20) | 9 (14) | 6 (9) | 40 (15) | |
| Race/Ethnicity | ||||||
| White Non-Hispanic | 37 (54) | 34 (49) | 26 (40) | 29 (45) | 126 (47) | |
| Black Non-Hispanic | 22 (32) | 20 (29) | 21 (32) | 27 (42) | 90 (33) | |
| Hispanic | 8 (12) | 14 (20) | 14 (22) | 8 (12) | 44 (16) | |
| Other | 2 (2) | 2 (2) | 4 (6) | 1 (1) | 9 (4) | |
| Baseline HIV RNA, log10 copies/mL | 4.6 (0.7) | 4.6 (0.6) | 4.6 (0.7) | 4.7 (0.7) | 4.6 (0.7) | |
| Baseline CD4 count | 248 (160) | 231 (167) | 226 (142) | 238 (189) | 236 (165) | |
| HBV surface antigen positive | 5 (7) | 3 (4) | 0 (0) | 1 (2) | 9 (3) | |
| HCV antibody positive | 5 (7) | 8 (11) | 3 (5) | 7 (11) | 23 (9) | |
| Baseline BMI, kg/m2 | 25 (4.0) | 26 (4.6) | 26 (5.4) | 26 (4.5) | 26 (4.7) | |
| Baseline total lean body mass, kg | 54 (9.8) | 53 (9.1) | 56 (9.9) | 56 (8.1) | 55 (9.3) | |
| Baseline trunk fat, kg | 9.5 (5.8) | 10.1 (5.5) | 10.8 (7.1) | 10.0 (5.8) | 10.1 (6.1) | |
| Baseline limb fat, kg | 7.7 (3.9) | 8.8 (5.5) | 8.8 (5.5) | 8.1 (5.0) | 8.3 (5.0) | |
| Baseline eGFR†, ml/min/1.73m2 | ||||||
| eGFRcr | 107 (15) | 106 (19) | 106 (21) | 109 (17) | 107 (18) | |
| eGFRcr-cys | 105 (16) | 100 (17) | 103 (19) | 105 (17) | 103 (17) | |
| eGFRcys | 102 (19) | 95 (20) | 100 (21) | 101 (21) | 99 (20) | |
| Urine dipstick proteinuria | 16 (24) | 22 (31) | 20 (33) | 17 (27) | 75 (29) | |
Data presented as mean (standard deviation) for continuous variables or number (%) for categorical variables
eGFR calculated using Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations1.
EFV, efavirenz; TDF, tenofovir; FTC, emtricitabine; ABC, abacavir; 3TC, lamivudine; ATV/r, ritonovir-boosted atazanavir; HBV, hepatitis B virus; HCV, hepatitis C virus; GRF, glomerular filtration rate; BMI, body mass index
Two hundred and ten subjects (78%) had cystatin C concentrations measured at both 0 and 96 weeks. Reasons for early discontinuation have been described previously22. These 210 subjects who were used for longitudinal analyses were more commonly of white non-Hispanic race (50% vs. 34%, p=0.022), less commonly infected with hepatitis C (6% vs. 17%, p=0.025), and had higher baseline CD4+ T-cell count (mean 247 vs. 199 cells/mm3, p=0.049) compared to the 59 subjects who did not have week 0 and 96 cystatin C measurements. By week 96, 184 (88%) of the 210 subjects had achieved viral suppression (HIV RNA <50 copies/ml) with no differences in the probability of virologic suppression between the NRTI components or between the NNRTI/PI components.
ART treatment effects on plasma cystatin C
Baseline mean (SD) plasma cystatin C concentration was 0.89 (0.17) mg/L. Concentrations declined rapidly with ART initiation and were sustained through week 96 (Figure 1). Overall, mean 0 to 96 week absolute and percentage decreases were 0.12 (0.14) mg/dL and 12.1 (14.4) %, respectively. There were no statistically significant interactions between the NRTI and NNRTI/PI components (p>0.4 for both absolute and percentage change). Compared to EFV, ATV/r assignment led to a significant attenuation of the beneficial effects of ART on cystatin C. Compared to ABC/3TC, TDF/FTC assignment led to a marginally significant attenuation for percent change analyses only. The intention-to-treat analyses are displayed in Figure 1. As treated analysis results were similar (data not shown).
Figure 1. Mean (95% CI) change in plasma cystatin C concentration from 0–96 weeks by treatment group [(A) percentage change from baseline and (B) absolute cystatin C concentrations].
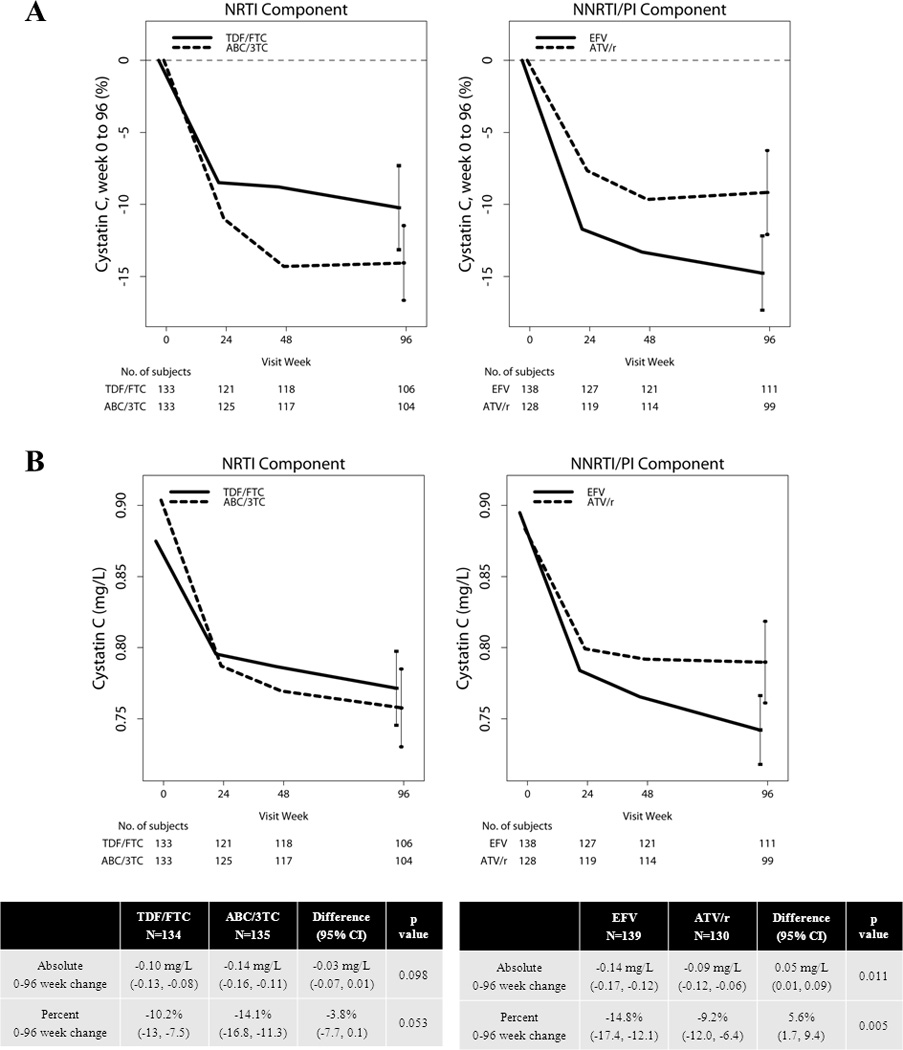
The intention-to-treat analysis is displayed below the graphs (p value for between-group difference).
Association of HIV viremia and body composition with cystatin C
In a model to assess the independent associations of baseline factors with cystatin C changes (Table 2), higher baseline HIV RNA was independently associated with a larger percent decrease in cystatin C. In general, higher baseline BMI was associated with larger cystatin C declines in the adjusted model; however, there was a significant ABC/3TC * BMI interaction, such that higher BMI was associated with smaller declines in cystatin C among ABC/3TC subjects. There is evidence that the NRTI effect differs by BMI, with larger differences between the arms in the lower BMI (<25 kg/m2) group (Table 3). Additional interactions assessed included ABC/3TC * baseline weight and ABC/3TC * screening HIV RNA, but these were not statistically significant. A model of absolute changes in cystatin C yielded similar results (data not shown).
Table 2.
Associations of percent change in cystatin C concentration from 0 to 96 weeks
| Univariate Analyses | Multivariable Analysis | |||||
|---|---|---|---|---|---|---|
| Covariate/Level* | Reference | Estimated mean difference (95% CI) |
p- value |
Estimated mean difference (95% CI) |
p- value |
|
| TDF/FTC | ABC/3TC | 3.8 (0.1, 7.7) | 0.053 | 4.1 (0.4, 7.8) | 0.028 | |
| ATV/r | EFV | 5.6 (1.7, 9.4) | 0.005 | 6.4 (2.7, 10.1) | 0.001 | |
| Age (years) | Continuous (per 1 year higher) | −1.4 (−3.5, 0.6) | 0.16 | |||
| Male | Female | 1.6 (−3.7, 6.9) | 0.56 | |||
| Race/Ethnicity | 0.65 | |||||
| Black Non-Hispanic | White Non-Hispanic | 1.1 (−3.3, 5.5) | ||||
| Hispanic | White Non-Hispanic | 2.6 (−3.0, 8.2) | ||||
| HIV RNA screening ≥100,000 (copies/mL) | <100,000 (copies/mL) | −3.4 (−7.3, 0.6) | 0.098 | |||
| Baseline HIV RNA (log10 copies/mL) | Continuous (per 1 log10 copies/mL higher) | −4.7 (−7.6, −1.8) | 0.001 | −5.2 (−8.0, −2.4) | <0.001 | |
| Baseline CD4 count | Continuous (per 50 cll/mm3 higher) | 0.7 (0.1, 1.3) | 0.017 | |||
| Baseline HCV or HBV co-infection | Negative | −5.0 (−11.6, 1.6) | 0.14 | |||
| Baseline BMI (kg/m2) | Continuous (per 1kg/m2 higher) | −0.0 (−0.5, 0.4) | 0.86 | −0.6 (−1.2, −0.0) | 0.041 | |
| Baseline Total Lean Body Mass (kg) | Continuous (per 1kg higher) | 0.1 (−0.1, 0.3) | 0.41 | |||
| Baseline Total Limb Fat (kg) | Continuous (per 1kg higher) | −0.0 (−0.4, 0.4) | 0.90 | |||
| Baseline Total Trunk Fat (kg) | Continuous (per 1kg higher) | 0.1 (−0.3, 0.4) | 0.74 | |||
| ABC/3TC * baseline weight (kg) | Continuous (per 1kg higher) | 2.4 (−0.2, 5.0) | 0.071 | |||
| ABC/3TC * baseline BMI (kg/m2) | Continuous (per 1kg/m2 higher) | 0.9 (0.1, 1.7) | 0.037 | 0.9 (0.1, 1.7) | 0.022 | |
| ABC/3TC * HIV RNA screening ≥100,000 (copies/mL) | Not ABC/3TC & screening HIV RNA <100,000 (copies/mL) | 5.8 (−2.1, 13.7) | 0.15 | |||
| ATV/r * HIV RNA screening ≥100,000 (copies/mL) | Not ATV/r & Screening HIV RNA <100,000 (copies/mL) | −5.8 (−13.6, 2.0) | 0.14 | |||
Interactions between NRTI and NNRTI/PI components were tested along with interactions between treatment components and the following baseline characteristics: age, sex, race/ethnicity, HIV RNA, HIV RNA strata (<100,000 vs. ≥100,000 copies/mL), CD4+ T-cell count, weight, BMI, lean body mass, and HCV/HBV co-infection. Interactions with p >0.2 are not shown.
EFV, efavirenz; TDF, tenofovir; FTC, emtricitabine; ABC, abacavir; 3TC, lamivudine; ATV/r, ritonavir-boosted atazanavir; HBV, hepatitis B virus; HCV, hepatitis C virus; GFR, glomerular filtration rate; BMI, body mass index; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor
Table 3.
Percent change in cystatin C from 0 to 96 weeks by treatment group and stratified by baseline weight, body-mass index, and lean body mass subgroups.
| NRTI Component | NNRTI/PI Component | ||||||
|---|---|---|---|---|---|---|---|
| ABC/3TC | TDF/FTC | p-value for interaction* |
ATV/r | EFV | p-value for interaction* |
||
| Weight tertile | |||||||
| <69.5 kg | −17 (−22, −12) | −8.9 (−14, −4.1) | 0.071 | −10 (−15, −5) | −15 (−20, −11) | 0.81 | |
| 69.5 – 83.2 kg | −16 (−20, −11) | −9.5 (−14, −4.6) | −8.0 (−13, −3.3) | −18 (−22, −13) | |||
| > 83.2 kg | −9.5 (−14, −4.9) | −12 (−17, −7.6) | −9.6 (−14, −4.8) | −12 (−16, −7.6) | |||
| BMI category† | |||||||
| < 25kg/m2 | −16 (−20, −12) | −9.3 (−13, −5.5) | 0.037 | −8.4 (−12, −4.5) | −17 (−20, −13) | 0.90 | |
| 25 – 30kg/m2 | −12 (−17, −7.3) | −12 (−16, −7) | −12 (−16, −6.7) | −12 (−17, −7.9) | |||
| > 30kg/m2 | −12 (−19, −5.4) | −9.5 (−17, −1.7) | −6.6 (−14, 0.6) | −16 (−23, −8.3) | |||
| Lean body mass tertile | |||||||
| < 50.74 kg | −16 (−21, −11) | −13 (−18, −8.1) | 0.51 | −11 (−16, −5.6) | −16 (−21, −12) | 0.75 | |
| 50.74 – 58.69 kg | −15 (−19, −10) | −7.9 (−13, −2.7) | −7.8 (−12, −3.3) | −17 (−22, −12) | |||
| > 58.69 kg | −12 (−17, −6.8) | −9.4 (−14, −4.9) | −9.0 (−14, −4.1) | −12 (−16, −7.2) | |||
Data expressed as mean (95% confidence interval)
Baseline BMI categories were defined according to United States Centers for Disease Control recommendations (n=106, 74, and 30 for <25, 25–30, and >30kg/m2, respectively).
p-value for interaction is based on the continuous covariate
Abbreviations as in Table 2
Baseline HIV RNA was positively correlated with baseline cystatin C (r=0.30, p <0.001); however, differences in cystatin C changes between those who achieved week 96 viral suppression (HIV RNA <50 copies/ml; n=184) and those who did not (n=26) were not statistically different (p=0.19 for absolute change and p=0.17 for percent change). Baseline and changes in BMI and DEXA-derived measures of body composition (limb fat, trunk fat, and lean body mass) did not correlate with baseline or changes in cystatin C, respectively (all p >0.3).
Cystatin C and Inflammation
At baseline, cystatin C concentrations consistently showed a positive relationship with biomarkers of inflammation and endothelial activation (Figure 2, all p <0.001), with hs-CRP having the weakest correlation (Spearman’s r=0.25) and sTNF-RI the strongest (r=0.70). Additionally, changes in cystatin C from 0 to 96 weeks positively correlated with changes in inflammatory biomarkers, except for hs-CRP and IL-6. Correlation plots for three biomarkers representing different pathways of inflammation and endothelial activation (IL-6, sTNF-RI, and sVCAM-1) are displayed in Figure 2 along with a table of baseline, week 96, and change correlations with all biomarkers.
Figure 2. Correlations of baseline, week 96, and 0–96 week changes in cystatin C with baseline, week 96, and 0–96 week changes in biomarkers of inflammation.
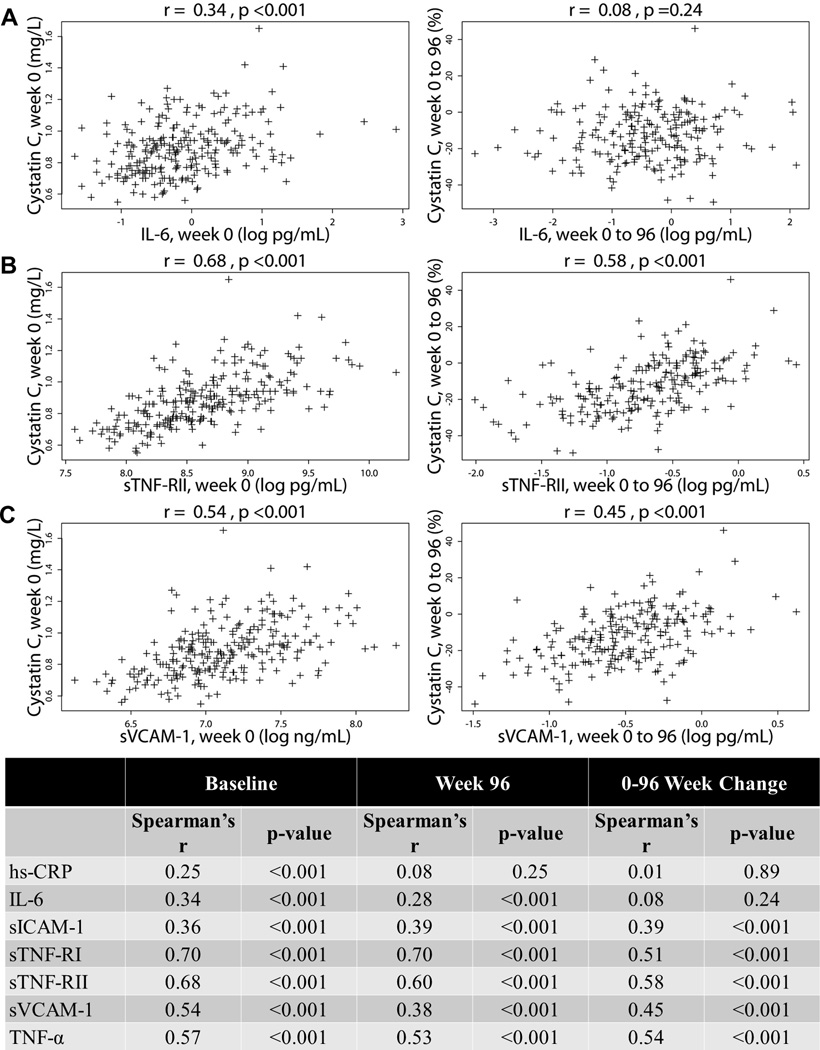
Three representative scatter plots of inflammatory cytokines [(A) interleukin-6 and (B) soluble TNF-α receptor II] and a biomarker of endothelial activation [(C) souble vascular cellular adhesion molecule] are shown with a table of correlation coefficients. IL-6, interleukin-6; hs-CRP, high sensitivity C-reactive protein; TNF-α, tumor necrosis factor-α; sTNF-RI and sTNF-RII, soluble TNF-α receptors I & II; sVCAM-1, souble vascular cellular adhesion molecule; sICAM-1, soluble intercellular adhesion molecule.
Discussion
In this study of HIV-infected subjects naïve to ART, initiation of therapy was associated with rapid and sustained declines in plasma cystatin C levels over 96 weeks with an estimated mean decline of 12%. Importantly, plasma cystatin C even declined with the use of drugs with known renal toxicities (ATV/r and TDF/FTC), though the effect was attenuated by ATV/r compared to EFV-containing regimens. We have also shown, for the first time in a randomized trial of ART initiation, that reductions in cystatin C correlate with reductions in multiple biomarkers of inflammation and endothelial activation, suggesting that inflammation may be an important non-GFR determinant of cystatin C levels in this population.
Our study was prompted by a prior analysis of A5224s, in which eGFRcr declined in all 4 treatment arms over 96 weeks despite improvements in eGFRcys. These divergent findings were most prominent in the ATV/r +TDF/FTC arm [mean change in eGFRcr vs. eGFRcys, −1.1 vs. +12.7 mL/min/1.732 for EFV + TDF/FTC, −0.2 vs. +18.0 for EFV + ABC/3TC, −12.8 vs. +8.9 for ATV/r + TDF/FTC, and −4.3 vs. +10.7 for ATV/r + ABC/3TC]21. These findings were surprising in light of multiple studies that have previously described the modest renal risks of ATV/r and TDF/FTC, especially when used in combination23–26. The mechanism of the ATV/r + TDF/FTC interaction has been attributed to increased tenofovir plasma concentrations with boosted and unboosted protease inhibitors27, as eGFRcr decline is associated with higher trough concentrations of TDF28. Early changes in eGFRcr may be attributed to TDF inhibition of tubular creatinine excretion29, but long-term declines and risk of chronic kidney disease (CKD)30 may be due to true toxicity.
If cystatin C concentrations are influenced by inflammation, then differential effects of ART regimens on cystatin C levels may also be influenced by differential effects on inflammation and immune activation. Indeed, data have emerged to suggest that there may be some differential effect of ART on inflammation and immune activation. For example,we recently found that initiation of ART with the intergarse inhibitor elvitegravir led to greater decreases in markers of immune activation and inflammation when compared to initiation of EFV31. In ACTG 5260s, treatment with ATV/r led to greater declines in hsCRP and proinflammatory monocytes when compared to treatment with darunavir/ritonavir32. Importantly, in our prior study of A5224s, ATV/r was associated with greater reductions in inflammation markers compared to EFV33. In that study, endothelial activation and TNF-α receptors decreased uniformly across all groups, but TDF/FTC and ATV/r were associated with greater reductions in hs-CRP and IL-6 compared to ABC/3TC and EFV, respectively. Thus, the discordant effects of ART on inflammation versus GFR may explain why observed differences between ART regimens were more pronounced for creatinine than for cystatin C21. Because immune activation markers such as soluble CD163 or sCD14 were not measured in A5224s, we cannot exclude that our findings may be partially explained by differential effects on immune activation.
In the context of these prior studies, a reasonable explanation of our findings is that reductions in plasma cystatin C after ART result from both reductions in generalized inflammation and from early improvements in GFR related to the effect of HIV viral suppression on glomerular function34, but that antiretroviral drugs with renal toxicity or adverse effects on inflammation markers may attenuate this benefit.
In the general population, CKD is characterized by elevated systemic inflammation35 which is associated with higher risk of cardiovascular and all-cause mortality36. To what degree inflammation is a cause or consequence of kidney disease is, however, unclear. The fact that certain biomarkers of inflammation (e.g. sTNF-α and its soluble receptors) are primarily cleared by the kidney37 while others (e.g. CRP) are cleared by the liver38 is evidence that it may partly be a consequence of impaired filtration and explains the stronger correlations of cystatin C and TNF-α receptors in our study and others. In the Multi-Ethnic Study of Atherosclerosis (MESA), for example, the correlation between cystatin C and sTNF-RI was stronger in subjects with CKD versus those without CKD35. Additionally, correlations of inflammatory markers were consistently stronger with cystatin C than with creatinine in both patient subgroups. In MESA, IL-6 was independently predictive of rapid GFR decline (by both cystatin C and creatinine-based measures) and incident eGFR <60 mL/min/1.73m239. In a study of subjects with Type I diabetes, sTNF-α receptors predicted GFR loss40. These findings suggest that inflammation may indeed cause renal decline.
Given the plausible bidirectional links between inflammation and glomerular function, the question of whether cystatin C is related to inflammation independent of GFR is understandably complex and controversial. In a pooled cross-sectional analysis of subjects with CKD, cystatin C was associated with hs-CRP independent of measured GFR8. This was confirmed in a recent study that also included subjects without CKD9. Since chronic HIV-infection is characterized by high levels of systemic inflammation, it is not surprising then that cystatin C levels would be elevated compared to control subjects10 or that eGFRcys would not be more accurate than eGFRcr compared to measured GFR14,16. Inker et al.14 found that eGFRcys was more biased and less accurate than eGFRcr when measured GFR was <60 ml/min/1.73m2, suggesting that non-GFR determinants of cystatin C are more important in this subgroup. On the other hand, findings were similar when divided into subgroups of CRP < or ≥ 4.8mg/dL. Bhasin et al.16 did not find any differences by measured GFR strata (< or ≥ 90 ml/min/1.73m2), but did note that eGFRcys was significantly less accurate in HIV-infected subjects with > median CD4+ and CD8+ T-cell activation. Unfortunately, these studies8,9,14,16 did not measure inflammatory markers that tend to be more strongly correlated with cystatin C in cross-sectional studies, such as TNF-α receptors like in our study and in MESA35.
Ours is the second study to provide longitudinal evidence that changes in inflammation are correlated with changes in cystatin C among HIV-infected participants. In the Strategies for Management of Antiretroviral Therapy (SMART) trial13, short-term changes in hs-CRP and IL-6 were modestly correlated with changes in cystatin C in the overall study cohort; however, there are important differences between A5224s and SMART. Since SMART was a trial of treatment interruption versus treatment continuation and since most subjects (~75%) were on ART at baseline, changes in viremia and inflammation were quantitatively less than in A5224s, where HIV RNA was not suppressed in the untreated subjects at baseline and declined rapidly after ART initiation. Secondly, ART regimens were randomized and limited to only four in A5224s, allowing more controlled analysis of the effect of specific drugs on renal function and inflammation.
We also explored the relationship between body composition and cystatin C in this study. As reported previously, both limb fat and visceral abdominal fat increased in all arms of A5224s over 96 weeks, without statistically significant differences between groups22. These increases in adipose tissue were accompanied by adverse effects on glucose metabolism41. In this current study, higher baseline BMI—but not lean mass, trunk fat, or peripheral fat—was independently associated with reductions in cystatin C, but a significant interaction suggested the opposite relationship for subjects taking ABC/3TC. Fat mass has been proposed as another non-GFR determinant of cystatin C8,9. Others have shown that increased cathepsin s (a cysteine protease) activity during preadipocyte differentiation is accompanied by an increased release of its endogenous inhibitor—cystatin C42. Obesity is a growing problem in subjects with HIV infection, particularly among racial and ethnic minorities43; however, the relationship between BMI and adiposity is confounded by lipodystrophy44 and adipose tissue dysfunction45. These relationships of body composition and cystatin C changes should be explored in future studies of physical activity and weight reduction in this population.
Cystatin C based estimates of GFR are strong predictors of cardiovascular events and mortality among subjects with HIV infection17–19. In light of these studies, our findings support the net benefit of antiretroviral therapy to reduce cardiovascular mortality in this population. In the Study of Fat Redistribution and Metabolic Change in HIV Infection (FRAM)17, eGFRcys <60 ml/min/1.73m2 was associated with over 2-fold higher mortality after adjustment for demographics, traditional CVD risk factors, CD4+ count, and PI exposure. Further adjustment for CRP and fibrinogen attenuated the association, though it remained significant; whereas eGFRcr was associated with mortality in unadjusted and demographic adjusted models only. In the Women’s Interagency HIV Study (WIHS)19, eGFRcys 60–90 and <60 ml/min/1.73m2 were associated with adjusted mortality risk in a graded fashion, whereas eGFRcr was associated with mortality only at <60 ml/min/1.73m2. In adjusted models that explored factors associated with the magnitude of difference between eGFRcr and eGFRcys, current smoking, HDL, waist circumference, HIV RNA, HCV infection, and current CD4 <200 cells/µL were all significantly associated. The authors point out that these are all risk factors for kidney disease; however, it is important to note that most are also important risk factors for cardiovascular disease and mortality independent of kidney function. Causes of death were not known for either the FRAM or WIHS cohorts. Cardiovascular events, however, were adjudicated in SMART, and were associated with eGFRcys (but not eGFRcr or eGFRcr-cys) even after adjustment for inflammation and coagulation markers (hs-CRP, IL-6, and D-dimer)18.
Our study has several limitations. Most importantly, we did not directly measure GFR and cannot, therefore, determine if the associations of cystatin C changes with changes in inflammation are related to true changes in GFR. Our study may have been underpowered to detect a small but clinically significant NRTI effect (TDF/FTC versus ABC/3TC). These findings may not extend to groups who would have been excluded from trial participation, such as those with creatinine clearance <60 ml/min, diabetes, etc. Finally, only 78% of subjects in our study had measures of cystatin C at both 0 to 96 weeks. Comparing these subjects to those without week 0 and 96 data, there were differences of race, hepatitis C status, and CD4+ T-cell count which may have affected the results.
In conclusion, initiation of ART is associated with rapid and sustained declines in plasma cystatin C levels over 96 weeks in HIV-infected subjects naïve to treatment, though this beneficial effect on cystatin C is attenuated by use of ATV/r compared to EFV-containing regimens. Reductions in cystatin C after ART may partially result from improved glomerular filtration rate, but may also be due to decreased systemic inflammation. Future studies should examine whether adjunctive treatment with anti-inflammatory agents such as statins or low-dose methotrexate can further reduce cystatin C concentration, a surrogate marker of cardiovascular events and mortality in this population.
Acknowledgements
The authors would like to thank the study subjects and each of the AIDS Clinical Trials Group sites that participated in A5224s:
Sadia Shaik, MD and Ruben Lopez, MD – Harbor-UCLA Medical Center (Site 603) CTU Grant #: AI0694241; CTSI Grant #: UL1TR000124.
Susan L. Koletar, MD and Diane Gochnour, RN – The Ohio State University Medical Center (Site 2301) CTU Grant #: AI069474.
Geyoul Kim, RN and Mark Rodriguez, RN –Washington University (Site 2101) CTU Grant #: U01AI069495; GCRC Grant: UL1 RR024992.
Elizabeth Lindsey, RN and Tamara James, BS – Alabama Therapeutics CRS (Site 5801) CTU Grant #: U01AI069452.
Ann C. Collier, MD and Jeffrey Schouten, MD, JD – University of Washington (Site 1401) CTU Grant #: AI069434; UL1 RR025014.
Jorge L. Santana Bagur, MD and Santiago Marrero, MD– Puerto Rico-AIDS Clinical Trials Unit (Site 5401) CTU Grant #: 5 U0I AI069415-03.
Jenifer Baer, RN, BSN and Carl Fichtenbaum, MD – University of Cincinnati (Site 2401) CTU Grant #: AI069513.
Patricia Walton, BSN, RN and Barbara Philpotts, BSN, RN – Case Western Reserve (Site 2501) CTU Grant#: AI69501.
Princy Kumar, MD and Joseph Timpone, MD – Georgetown University (Site 1008) CTU Grant#: ACTG grant #: 5U01AI069494.
Donna Pittard, RN BSN and David Currin, RN – University of North Carolina (Site 3201) CTU Grant #: 5-U01 AI069423-03; UNC CFAR #: P30 AI050410(-11); UNC CTRC #: UL 1RR 025747.
Julie Hoffman, RN and Edward Seefried, RN – San Diego Medical Center UC (Site 701) CTU Grant #: AI69432.
Susan Swindells, MBBS and Frances Van Meter, APRN – University of Nebraska (Site 1505) CTU Grant #: AI 27661.
Deborah McMahon, MD and Barbara Rutecki, MSN, MPH, CRNP – University of Pittsburgh (Site 1001) CTU Grant #: 1 U01 AI069494-01.
Michael P. Dube, MD and Martha Greenwald, RN, MSN – Indiana University (Site 2601) CTU Grant #: 5U01AI025859; GCRC #: M01 RR00750.
Ilene Wiggins, RN, and Eric Zimmerman, RN – Johns Hopkins University (Site 201) CTU Grant #: AI27668; CTSA Grant #: UL1 RR025005.
Judith Aberg, MD and Margarita Vasquez, RN – New York University/NYCHHCat Bellevue Hospital Center (Site 401) CTU Grant #: AI27665, New grant number: AI069532.
Martin McCarter and M. Graham Ray, RN, MSN – Colorado AIDS Clinical Trials Unit, (Site 6101) CTU Grant #: AI69450; RR025780.
Mamta Jain, MD – PI and Tianna Petersen, MS – University of Texas Southwestern Medical Center (Site 3751) CTU Grant #: 3U01AI046376-05S4.
Emily Stumm, BS and Pablo Tebas, MD – University of Pennsylvania, Philadelphia (Site 6201) CTU Grant #: P30-AI0450008-11; CFAR Grant #: UO1-AI069467-04.
Mary Albrecht, MD and Neah Kim, NP – Beth Israel Deaconess (Partners/Harvard) CRS (Site 103) CTU Grant #: U01 AI069472-04.
Paul Edward Sax, MD and Joanne Delaney, RN – Brigham and Women’s Hospital (Site 107) CTU Grant #: UOI AI 069472.
Christine Hurley, RN and Roberto Corales, DO – AIDS Care (Site 1108) CTU Grant #: U01AI069511-02 (as of 2/12/08); GCRC: UL1 RR 024160.
Keith Henry, MD and Bette Bordenave, RN – Hennepin County Medical Center (Site 1502) CTU Grant #: N01 AI72626.
Wendy Armstrong, MD and Ericka R. Patrick, RN, MSN, CCRC – Emory University HIV/AIDS Clinical Trails Unit (Site 5802) CTU Grant #: UO1Al69418-01/CFAR Grant Number: P30Al050409.
Jane Reid RNC MS and Mary Adams RN MPh – University of Rochester (Site 1101) CTU Grant #: U01AI069511-02 (as of 2/12/08); GCRC: UL1 RR 024160.
Gene D. Morse, PharmD, FCCP, BCPS, SUNY – Buffalo, Erie County Medical Ctr. (Site 1102) CTU Grant #: AI27658.
Michael P. Dube, MD and Martha Greenwald, RN, MSN – Wishard Memorial Hospital Indiana University (Site 2603) CTU Grant #: 5U01AI025859; GCRC #: M01 RR00750.
Kimberly Y. Smith, MD, MPH and Joan A. Swiatek, APN – Rush University Medical Center (Site 2702) CTU Grant #: U01 AI069471.
Nancy Hanks, RN, and Debra Ogata-Arakaki, RN – University of Hawaii at Manoa, Leahi Hospital (Site 5201) CTU Grant #: AI34853.
Ardis Moe, MD and Maria Palmer, PA-C – UCLA Medical Center (Site 601) CTU Grant #: 1U01AI069424-01.
Jeffery Meier, MD and Jack T. Stapleton, MD – University of Iowa Hospitals and Clinics (Site 1504) CTU Grant #: UL1RR024979.
Gary Matthew Cox, MD and Martha Silberman, RN – Duke University Medical Center Adult CRS (Site 1601) CTU Grant #: 5U01 AI069 484-02.
2705 – Cook County Hospital
Gerianne Casey, RN and William O’Brien MD – University of Texas, Galveston (Site 6301) CTU Grant #: AI32782.
Valery Hughes, FNP and Todd Stroberg, RN – Cornell CRS (Site 7803, 7804) – CTU Grant#: U01 AI069419; CTSC #: UL1 RR024996.
Nyef El-Daher, MD – McCree McCuller Wellness Center at the Connection (Site 1107) CTU Grant #: U01AI069511-02 (as of 2/12/08); GCRC: UL1 RR 024160.
Rebecca J. Basham, BS and Husamettin Erdem, MD – Vanderbilt Therapeutics CRS (Site 3652) CTU Grant #: AI46339-01; MO1 RR 00095.
Funding: This work was supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (award numbers U01 AI068636, U01 AI68634, AI065348, AI38855, AI69501, AI069472, AI0450008, and AI069424). GlaxoSmithKline and Gilead funded the DEXA scans. Study medications were provided by Abbott Pharmaceuticals, Bristol-Myers Squibb, Gilead Sciences, and GlaxoSmithKline. The study is registered with clinicaltrials.gov, number NCT00118898.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
CTL has received grants from Bristol-Myers Squibb and the Medtronic Foundation. PES has received research grants from Bristol-Myers Squibb, Gilead Sciences, and GlaxoSmithKline and is a consultant or scientific advisory board member for Abbott, Bristol-Myers Squibb, Gilead Sciences, Merck, and Janssen Pharmaceutics. ESD has received research grants from Bristol-Myers Squibb, Merck, and ViiV Healthcare and has received consultancy/advisory fees from Bristol-Myers Squibb, Gilead Sciences, Janssen Pharmaceuticals, Merck, Abbvie, and ViiV Healthcare. SKG has received research grants from Merck & Co., Inc., Janssen Pharmaceutics, Inc., and Gilead Sciences, Inc., travel support to present data at the 2011 IAS Rome Conference, and has received advisory/consultancy/lecture fees from Bristol-Myers Squibb and Merck & Co., Inc. GAM has served as a scientific advisor or speaker for Bristol-Myers Squibb, GlaxoSmithKline, Tibotec, and Gilead Sciences, has received research grants from Bristol-Myers Squibb, GlaxoSmithKline, and Gilead Sciences, and is currently serving as the DSMB Chair for a Pfizer-sponsored study. CT currently serves on the DSMB of a Tibotec/Janssen-sponsored study.
Footnotes
Conflicts of Interest:
DK has no disclosures.
These data were presented at the 21st Conference on Retroviruses and Opportunistic Infection (Boston, MA, USA; March 2013).
References
- 1.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. The New England journal of medicine. 2012 Jul 5;367(1):20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. The New England journal of medicine. 2005 May 19;352(20):2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 3.Shlipak MG, Wassel Fyr CL, Chertow GM, et al. Cystatin C and mortality risk in the elderly: the health, aging, and body composition study. Journal of the American Society of Nephrology : JASN. 2006 Jan;17(1):254–261. doi: 10.1681/ASN.2005050545. [DOI] [PubMed] [Google Scholar]
- 4.Peralta CA, Shlipak MG, Judd S, et al. Detection of chronic kidney disease with creatinine, cystatin C, urine albumin-to-creatinine ratio and association with progression to end-stage renal disease and mortality. JAMA : the journal of the American Medical Association. 2011 Apr 20;305(15):1545–1552. doi: 10.1001/jama.2011.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shlipak MG, Matsushita K, Arnlov J, et al. Cystatin C versus creatinine in determining risk based on kidney function. The New England journal of medicine. 2013 Sep 5;369(10):932–943. doi: 10.1056/NEJMoa1214234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. The New England journal of medicine. 2006 Jun 8;354(23):2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 7.Knight EL, Verhave JC, Spiegelman D, et al. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney international. 2004 Apr;65(4):1416–1421. doi: 10.1111/j.1523-1755.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 8.Stevens LA, Schmid CH, Greene T, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney international. 2009 Mar;75(6):652–660. doi: 10.1038/ki.2008.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rule AD, Bailey KR, Lieske JC, Peyser PA, Turner ST. Estimating the glomerular filtration rate from serum creatinine is better than from cystatin C for evaluating risk factors associated with chronic kidney disease. Kidney international. 2013 Jun;83(6):1169–1176. doi: 10.1038/ki.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Odden MC, Scherzer R, Bacchetti P, et al. Cystatin C level as a marker of kidney function in human immunodeficiency virus infection: the FRAM study. Archives of internal medicine. 2007 Nov 12;167(20):2213–2219. doi: 10.1001/archinte.167.20.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neuhaus J, Jacobs DR, Jr, Baker JV, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. The Journal of infectious diseases. 2010 Jun 15;201(12):1788–1795. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Longenecker CT, Scherzer R, Bacchetti P, Lewis CE, Grunfeld C, Shlipak MG. HIV viremia and changes in kidney function. Aids. 2009 Jun 1;23(9):1089–1096. doi: 10.1097/QAD.0b013e32832a3f24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mocroft A, Wyatt C, Szczech L, et al. Interruption of antiretroviral therapy is associated with increased plasma cystatin C. Aids. 2009 Jan 2;23(1):71–82. doi: 10.1097/QAD.0b013e32831cc129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inker LA, Wyatt C, Creamer R, et al. Performance of creatinine and cystatin C GFR estimating equations in an HIV-positive population on antiretrovirals. Journal of acquired immune deficiency syndromes. 2012 Nov 1;61(3):302–309. doi: 10.1097/QAI.0b013e31826a6c4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gagneux-Brunon A, Delanaye P, Maillard N, et al. Performance of creatinine and cystatin C-based glomerular filtration rate estimating equations in a European HIV-positive cohort. Aids. 2013 Jun 19;27(10):1573–1581. doi: 10.1097/QAD.0b013e32835fac30. [DOI] [PubMed] [Google Scholar]
- 16.Bhasin B, Lau B, Atta MG, et al. HIV Viremia and T-Cell Activation Differentially Affect the Performance of Glomerular Filtration Rate Equations Based on Creatinine and Cystatin C. PloS one. 2013;8(12):e82028. doi: 10.1371/journal.pone.0082028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi A, Scherzer R, Bacchetti P, et al. Cystatin C, albuminuria, and 5-year all-cause mortality in HIV-infected persons. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2010 Nov;56(5):872–882. doi: 10.1053/j.ajkd.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lucas G, Cozzi-Lepri A, Wyatt C, et al. Glomerular filtration rate estimated using creatinine, cystatin C or both markers and the risk of clinical events in HIV-infected individuals. HIV medicine. 2013 Sep 11; doi: 10.1111/hiv.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Driver TH, Scherzer R, Peralta CA, et al. Comparisons of creatinine and cystatin C for detection of kidney disease and prediction of all-cause mortality in HIV-infected women. Aids. 2013 Sep 10;27(14):2291–2299. doi: 10.1097/QAD.0b013e328362e874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gagneux-Brunon A, Mariat C, Delanaye P. Cystatin C in HIV-infected patients: promising but not yet ready for prime time. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2012 Apr;27(4):1305–1313. doi: 10.1093/ndt/gfs001. [DOI] [PubMed] [Google Scholar]
- 21.Gupta SK, Kitch D, Tierney C, et al. Cystatin C-Based Renal Function Changes After Antiretroviral Initiation: A Substudy of a Randomized Trial. Open Forum Infectious Diseases. 2014 Mar 1;1(1) doi: 10.1093/ofid/ofu003. 2014; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McComsey GA, Kitch D, Sax PE, et al. Peripheral and central fat changes in subjects randomized to abacavir-lamivudine or tenofovir-emtricitabine with atazanavir-ritonavir or efavirenz: ACTG Study A5224s. Clin Infect Dis. 2011 Jul 15;53(2):185–196. doi: 10.1093/cid/cir324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young J, Schafer J, Fux CA, et al. Renal function in patients with HIV starting therapy with tenofovir and either efavirenz, lopinavir or atazanavir. Aids. 2012 Mar 13;26(5):567–575. doi: 10.1097/QAD.0b013e32834f337c. [DOI] [PubMed] [Google Scholar]
- 24.Gupta S, Kitch D, Tierney C, et al. Cystatin C-Based Renal Function Changes After Antiretroviral Initiation: A Substudy of a Randomized Trial. Open Forum Infectious Diseases. 2013 doi: 10.1093/ofid/ofu003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper RD, Wiebe N, Smith N, Keiser P, Naicker S, Tonelli M. Systematic review and meta-analysis: renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010 Sep 1;51(5):496–505. doi: 10.1086/655681. [DOI] [PubMed] [Google Scholar]
- 26.Albini L, Cesana BM, Motta D, et al. A randomized, pilot trial to evaluate glomerular filtration rate by creatinine or cystatin C in naive HIV-infected patients after tenofovir/emtricitabine in combination with atazanavir/ritonavir or efavirenz. Journal of acquired immune deficiency syndromes. 2012 Jan 1;59(1):18–30. doi: 10.1097/QAI.0b013e31823a6124. [DOI] [PubMed] [Google Scholar]
- 27.Calcagno A, Gonzalez de Requena D, Simiele M, et al. Tenofovir plasma concentrations according to companion drugs: a cross-sectional study of HIV-positive patients with normal renal function. Antimicrobial agents and chemotherapy. 2013 Apr;57(4):1840–1843. doi: 10.1128/AAC.02434-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poizot-Martin I, Solas C, Allemand J, et al. Renal impairment in patients receiving a tenofovir-cART regimen: impact of tenofovir trough concentration. Journal of acquired immune deficiency syndromes. 2013 Apr 1;62(4):375–380. doi: 10.1097/QAI.0b013e31827ce4ee. [DOI] [PubMed] [Google Scholar]
- 29.Vrouenraets SM, Fux CA, Wit FW, et al. Persistent decline in estimated but not measured glomerular filtration rate on tenofovir may reflect tubular rather than glomerular toxicity. Aids. 2011 Nov 13;25(17):2149–2155. doi: 10.1097/QAD.0b013e32834bba87. [DOI] [PubMed] [Google Scholar]
- 30.Ryom L, Mocroft A, Kirk O, et al. Association between antiretroviral exposure and renal impairment among HIV-positive persons with normal baseline renal function: the D:A:D study. The Journal of infectious diseases. 2013 May 1;207(9):1359–1369. doi: 10.1093/infdis/jit043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hileman CO, Kinley B, Scharen-Guivel V, et al. Elvitegravir Reduces Monocyte Activation and Vascular Inflammation More than Efavirenz in HIV-Infected Adults Starting Antiretrovirals. Journal of Infectious Diseases. 2014 doi: 10.1093/infdis/jiv004. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelesidis T, Trann T, McComsey GA, et al. Comparison of effects of atazanavir, raltegravir, or darunavir with FTC/tenofovir on biomarkers of systemic inflammation, macrophage and T cell activation: ACTG A5260s. 10th International AIDS Conference; Melbourne, Australia. 2014. [Google Scholar]
- 33.McComsey GA, Kitch D, Daar ES, et al. Inflammation markers after randomization to abacavir/lamivudine or tenofovir/emtricitabine with efavirenz or atazanavir/ritonavir. Aids. 2012 Jul 17;26(11):1371–1385. doi: 10.1097/QAD.0b013e328354f4fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalayjian RC, Franceschini N, Gupta SK, et al. Suppression of HIV-1 replication by antiretroviral therapy improves renal function in persons with low CD4 cell counts and chronic kidney disease. Aids. 2008 Feb 19;22(4):481–487. doi: 10.1097/QAD.0b013e3282f4706d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keller C, Katz R, Cushman M, Fried LF, Shlipak M. Association of kidney function with inflammatory and procoagulant markers in a diverse cohort: a cross-sectional analysis from the Multi-Ethnic Study of Atherosclerosis (MESA) BMC nephrology. 2008;9:9. doi: 10.1186/1471-2369-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shlipak MG, Fried LF, Cushman M, et al. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. JAMA : the journal of the American Medical Association. 2005 Apr 13;293(14):1737–1745. doi: 10.1001/jama.293.14.1737. [DOI] [PubMed] [Google Scholar]
- 37.Bemelmans MH, Gouma DJ, Buurman WA. Tissue distribution and clearance of soluble murine TNF receptors in mice. Cytokine. 1994 Nov;6(6):608–615. doi: 10.1016/1043-4666(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 38.Hutchinson WL, Noble GE, Hawkins PN, Pepys MB. The pentraxins, C-reactive protein and serum amyloid P component, are cleared and catabolized by hepatocytes in vivo. The Journal of clinical investigation. 1994 Oct;94(4):1390–1396. doi: 10.1172/JCI117474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hiramoto JS, Katz R, Peralta CA, et al. Inflammation and coagulation markers and kidney function decline: the Multi-Ethnic Study of Atherosclerosis (MESA) American journal of kidney diseases : the official journal of the National Kidney Foundation. 2012 Aug;60(2):225–232. doi: 10.1053/j.ajkd.2012.02.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gohda T, Niewczas MA, Ficociello LH, et al. Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. Journal of the American Society of Nephrology : JASN. 2012 Mar;23(3):516–524. doi: 10.1681/ASN.2011060628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Erlandson KM, Kitch D, Tierney C, et al. Impact of randomized antiretroviral therapy initiation on glucose metabolism. Aids. 2014 Jun 19;28(10):1451–1461. doi: 10.1097/QAD.0000000000000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taleb S, Cancello R, Clement K, Lacasa D. Cathepsin s promotes human preadipocyte differentiation: possible involvement of fibronectin degradation. Endocrinology. 2006 Oct;147(10):4950–4959. doi: 10.1210/en.2006-0386. [DOI] [PubMed] [Google Scholar]
- 43.Taylor BS, Liang Y, Garduno LS, et al. High Risk of Obesity and Weight Gain for HIV-Infected Uninsured Minorities. Journal of acquired immune deficiency syndromes. 2013 Oct 10; doi: 10.1097/QAI.0000000000000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bacchetti P, Gripshover B, Grunfeld C, et al. Fat distribution in men with HIV infection. Journal of acquired immune deficiency syndromes. 2005 Oct 1;40(2):121–131. doi: 10.1097/01.qai.0000182230.47819.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kosmiski LA, Bacchetti P, Kotler DP, et al. Relationship of fat distribution with adipokines in human immunodeficiency virus infection. The Journal of clinical endocrinology and metabolism. 2008 Jan;93(1):216–224. doi: 10.1210/jc.2007-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]


