Abstract
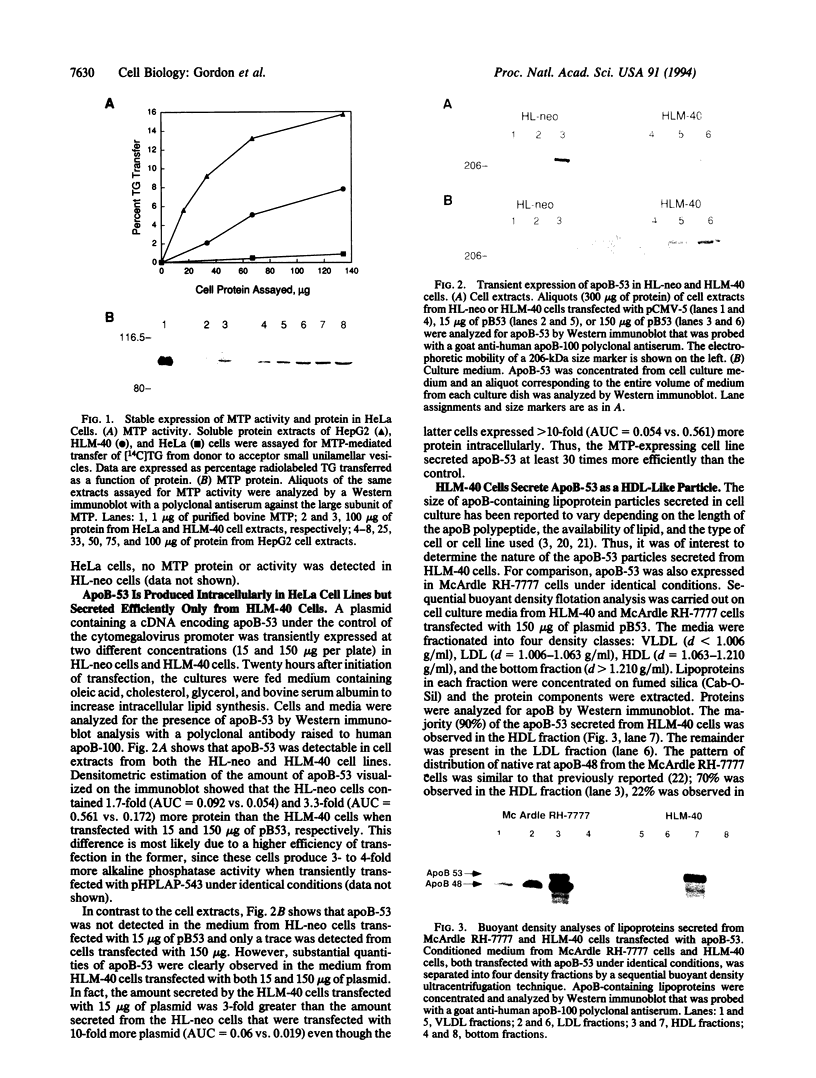
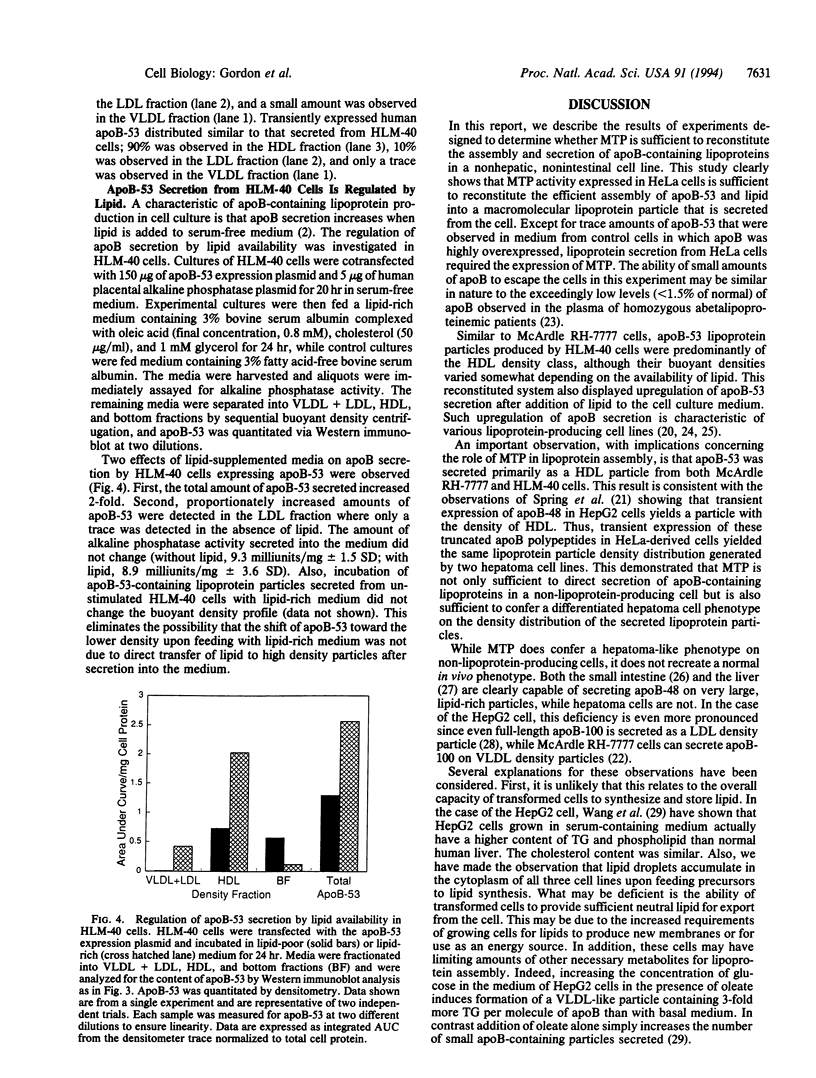
To elucidate the role of the microsomal triglyceride transfer protein (MTP) in lipoprotein assembly, MTP and apolipoprotein B-53 (apoB 53; the N-terminal 53% of apoB) were expressed in HeLa cells. The results showed that apoB-53 could be expressed in HeLa cells with or without expression of MTP. In contrast, efficient secretion of apoB-53 required expression of MTP. Ultracentrifugal density flotation analysis showed that apoB-53 was secreted predominantly as a particle with the density of high density lipoprotein. An essentially identical apoB-53 particle density distribution was obtained after transient expression of apoB-53 in McArdle RH-7777 rat hepatoma cells. The mass of apoB-53 secreted was greater, and the flotation density was lower, from cells fed lipid, suggesting that apoB secretion in HeLa cells was regulated by lipid availability, similar to what has been described for lipoprotein-producing cell lines. These results indicate that MTP is necessary and sufficient to direct the regulated secretion of apoB-53 in HeLa cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander C. A., Hamilton R. L., Havel R. J. Subcellular localization of B apoprotein of plasma lipoproteins in rat liver. J Cell Biol. 1976 May;69(2):241–263. doi: 10.1083/jcb.69.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson S., Davis D. L., Dahlbäck H., Jörnvall H., Russell D. W. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J Biol Chem. 1989 May 15;264(14):8222–8229. [PubMed] [Google Scholar]
- Arbeeny C. M., Meyers D. S., Bergquist K. E., Gregg R. E. Inhibition of fatty acid synthesis decreases very low density lipoprotein secretion in the hamster. J Lipid Res. 1992 Jun;33(6):843–851. [PubMed] [Google Scholar]
- Berger J., Hauber J., Hauber R., Geiger R., Cullen B. R. Secreted placental alkaline phosphatase: a powerful new quantitative indicator of gene expression in eukaryotic cells. Gene. 1988 Jun 15;66(1):1–10. doi: 10.1016/0378-1119(88)90219-3. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986 Apr 4;232(4746):34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- Davis R. A., Prewett A. B., Chan D. C., Thompson J. J., Borchardt R. A., Gallaher W. R. Intrahepatic assembly of very low density lipoproteins: immunologic characterization of apolipoprotein B in lipoproteins and hepatic membrane fractions and its intracellular distribution. J Lipid Res. 1989 Aug;30(8):1185–1196. [PubMed] [Google Scholar]
- Dixon J. L., Ginsberg H. N. Regulation of hepatic secretion of apolipoprotein B-containing lipoproteins: information obtained from cultured liver cells. J Lipid Res. 1993 Feb;34(2):167–179. [PubMed] [Google Scholar]
- Edman J. C., Ellis L., Blacher R. W., Roth R. A., Rutter W. J. Sequence of protein disulphide isomerase and implications of its relationship to thioredoxin. Nature. 1985 Sep 19;317(6034):267–270. doi: 10.1038/317267a0. [DOI] [PubMed] [Google Scholar]
- Huang L. S., Jänne P. A., de Graaf J., Cooper M., Deckelbaum R. J., Kayden H., Breslow J. L., Decklebaum R. J. Exclusion of linkage between the human apolipoprotein B gene and abetalipoproteinemia. Am J Hum Genet. 1990 Jun;46(6):1141–1148. [PMC free article] [PubMed] [Google Scholar]
- Kane J. P. Apolipoprotein B: structural and metabolic heterogeneity. Annu Rev Physiol. 1983;45:637–650. doi: 10.1146/annurev.ph.45.030183.003225. [DOI] [PubMed] [Google Scholar]
- Menzel H. J., Dieplinger H., Lackner C., Hoppichler F., Lloyd J. K., Muller D. R., Labeur C., Talmud P. J., Utermann G. Abetalipoproteinemia with an ApoB-100-lipoprotein(a) glycoprotein complex in plasma. Indication for an assembly defect. J Biol Chem. 1990 Jan 15;265(2):981–986. [PubMed] [Google Scholar]
- Moberly J. B., Cole T. G., Alpers D. H., Schonfeld G. Oleic acid stimulation of apolipoprotein B secretion from HepG2 and Caco-2 cells occurs post-transcriptionally. Biochim Biophys Acta. 1990 Jan 16;1042(1):70–80. doi: 10.1016/0005-2760(90)90058-6. [DOI] [PubMed] [Google Scholar]
- Pullinger C. R., North J. D., Teng B. B., Rifici V. A., Ronhild de Brito A. E., Scott J. The apolipoprotein B gene is constitutively expressed in HepG2 cells: regulation of secretion by oleic acid, albumin, and insulin, and measurement of the mRNA half-life. J Lipid Res. 1989 Jul;30(7):1065–1077. [PubMed] [Google Scholar]
- Sharp D., Blinderman L., Combs K. A., Kienzle B., Ricci B., Wager-Smith K., Gil C. M., Turck C. W., Bouma M. E., Rader D. J. Cloning and gene defects in microsomal triglyceride transfer protein associated with abetalipoproteinaemia. Nature. 1993 Sep 2;365(6441):65–69. doi: 10.1038/365065a0. [DOI] [PubMed] [Google Scholar]
- Spring D. J., Chen-Liu L. W., Chatterton J. E., Elovson J., Schumaker V. N. Lipoprotein assembly. Apolipoprotein B size determines lipoprotein core circumference. J Biol Chem. 1992 Jul 25;267(21):14839–14845. [PubMed] [Google Scholar]
- Talmud P. J., Lloyd J. K., Muller D. P., Collins D. R., Scott J., Humphries S. Genetic evidence from two families that the apolipoprotein B gene is not involved in abetalipoproteinemia. J Clin Invest. 1988 Nov;82(5):1803–1806. doi: 10.1172/JCI113795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe S., Sherman H., Smith L., Yang L. A., Fleming R., Hay R. Biogenesis of plasma lipoproteins in rat hepatoma McA-RH7777: importance of diffusion-mediated events during cell growth. In Vitro Cell Dev Biol. 1989 Dec;25(12):1129–1140. doi: 10.1007/BF02621264. [DOI] [PubMed] [Google Scholar]
- Thrift R. N., Drisko J., Dueland S., Trawick J. D., Davis R. A. Translocation of apolipoprotein B across the endoplasmic reticulum is blocked in a nonhepatic cell line. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9161–9165. doi: 10.1073/pnas.89.19.9161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrift R. N., Forte T. M., Cahoon B. E., Shore V. G. Characterization of lipoproteins produced by the human liver cell line, Hep G2, under defined conditions. J Lipid Res. 1986 Mar;27(3):236–250. [PubMed] [Google Scholar]
- Vance D. E., Weinstein D. B., Steinberg D. Isolation and analysis of lipoproteins secreted by rat liver hepatocytes. Biochim Biophys Acta. 1984 Jan 17;792(1):39–47. doi: 10.1016/0005-2760(84)90280-7. [DOI] [PubMed] [Google Scholar]
- Wang S. R., Pessah M., Infante J., Catala D., Salvat C., Infante R. Lipid and lipoprotein metabolism in Hep G2 cells. Biochim Biophys Acta. 1988 Aug 12;961(3):351–363. doi: 10.1016/0005-2760(88)90082-3. [DOI] [PubMed] [Google Scholar]
- Wetterau J. R., Aggerbeck L. P., Bouma M. E., Eisenberg C., Munck A., Hermier M., Schmitz J., Gay G., Rader D. J., Gregg R. E. Absence of microsomal triglyceride transfer protein in individuals with abetalipoproteinemia. Science. 1992 Nov 6;258(5084):999–1001. doi: 10.1126/science.1439810. [DOI] [PubMed] [Google Scholar]
- Wetterau J. R., Aggerbeck L. P., Laplaud P. M., McLean L. R. Structural properties of the microsomal triglyceride-transfer protein complex. Biochemistry. 1991 May 7;30(18):4406–4412. doi: 10.1021/bi00232a006. [DOI] [PubMed] [Google Scholar]
- Wetterau J. R., Combs K. A., McLean L. R., Spinner S. N., Aggerbeck L. P. Protein disulfide isomerase appears necessary to maintain the catalytically active structure of the microsomal triglyceride transfer protein. Biochemistry. 1991 Oct 8;30(40):9728–9735. doi: 10.1021/bi00104a023. [DOI] [PubMed] [Google Scholar]
- Wetterau J. R., Combs K. A., Spinner S. N., Joiner B. J. Protein disulfide isomerase is a component of the microsomal triglyceride transfer protein complex. J Biol Chem. 1990 Jun 15;265(17):9800–9807. [PubMed] [Google Scholar]
- Wetterau J. R., Zilversmit D. B. Purification and characterization of microsomal triglyceride and cholesteryl ester transfer protein from bovine liver microsomes. Chem Phys Lipids. 1985 Aug 30;38(1-2):205–222. doi: 10.1016/0009-3084(85)90068-4. [DOI] [PubMed] [Google Scholar]
- White A. L., Graham D. L., LeGros J., Pease R. J., Scott J. Oleate-mediated stimulation of apolipoprotein B secretion from rat hepatoma cells. A function of the ability of apolipoprotein B to direct lipoprotein assembly and escape presecretory degradation. J Biol Chem. 1992 Aug 5;267(22):15657–15664. [PubMed] [Google Scholar]
- Yao Z. M., Blackhart B. D., Linton M. F., Taylor S. M., Young S. G., McCarthy B. J. Expression of carboxyl-terminally truncated forms of human apolipoprotein B in rat hepatoma cells. Evidence that the length of apolipoprotein B has a major effect on the buoyant density of the secreted lipoproteins. J Biol Chem. 1991 Feb 15;266(5):3300–3308. [PubMed] [Google Scholar]