Abstract
Indirect enzyme-linked immunosorbent assays (ELISAs) are frequently run as endpoint ELISAs (e-ELISAs). However, kinetic ELISAs (k-ELISAs) have certain advantages over e-ELISAs. The objective of this study was to understand the relationship between e-ELISA and k-ELISA results. Specifically, to determine whether it was possible to run both k-ELISA and e-ELISA on the same plate and establish an appropriate time interval for k-ELISA measurements. A normalization method for k-ELISA slopes (slope ratio) is proposed. Using an indirect e-ELISA test measuring antibodies against Ostertagia ostertagi in milk from dairy cattle, we found that running a k-ELISA had no effect on optical density ratio results of an e-ELISA on the same plate, and that agreement was very strong at 10, 15, and 28 min, allowing for a reduction in the total processing time for ELISA tests.
Résumé
Les épreuves immunoenzymatiques indirectes (ELISA) sont fréquemment effectuées comme ELISA avec un point limite (e-ELISA). Toutefois, une épreuve ELISA cinétique (k-ELISA) a certains avantages par rapport à une e-ELISA. L’objectif de la présente étude était de comprendre la relation entre les résultats d’une e-ELISA et ceux d’une k-ELISA. Spécifiquement, déterminer s’il est possible de réaliser simultanément sur la même plaque une épreuve k-ELISA et une épreuve e-ELISA et d’établir un intervalle de temps approprié pour les mesures de k-ELISA. Une normalisation de la méthode pour les pentes des k-ELISA (rapport de pente) est proposée. En utilisant une épreuve e-ELISA indirecte pour mesurer les anticorps dirigés contre Ostertagia ostertagi dans le lait de bovins laitiers, nous avons trouvé que d’effectuer une k-ELISA n’avait aucun effet sur les résultats des ratios de densité optique d’une e-ELISA effectuée sur la même plaque, et que l’accord était très élevé à 10, 15, et 28 min, permettant ainsi une réduction du temps de traitement total pour les épreuves ELISA.
(Traduit par Docteur Serge Messier)
Introduction
Indirect enzyme-linked immunosorbent assays (ELISAs) are frequently run as endpoint ELISAs (e-ELISAs) (1–3). Antibodies against Ostertagia ostertagi in milk from dairy cattle are measured and a high result from the e-ELISA (Svanovir; Svanova Veterinary Diagnostics, Uppsala, Sweden) indicates a high level of intestinal parasitism. This has been used to predict milk production losses due to an undetermined level of parasitism at the individual cow or herd level (4–6).
An e-ELISA allows the substrate to react and change color for a set amount of time before a stop solution is added arresting the chromatogenic reaction. The plate is read by a spectrophotometer within a few minutes of adding the stop solution to determine the optical density (OD) of the samples.
An e-ELISA has some disadvantages. Firstly, the addition of a stop solution does not necessarily arrest color change since the chemical reaction can continue without functional enzymes (7). Secondly, the relationship between endpoint color intensity and antibody concentration need not be linear, especially at high and low antibody concentrations (8,9). Lastly, the chemical reaction is only approximately linear with the enzymatic concentration in the well during a brief period at the initial phase of the reaction and provided there is an abundant amount of substrate (10). Therefore, an e-ELISA is incapable of distinguishing between a moderate and large increase in antibody concentration when it lies in the upper regions of the linear scale of the OD, unless the sample undergoes predetermined dilutions.
An e-ELISA can become a k-ELISA if the OD is recorded at regular short intervals (e.g., 45 s) starting as soon as the chromatogenic reaction begins. A k-ELISA does not require a stop solution, thus eliminating the problem of continued color change. The measurements from a k-ELISA are taken in real-time, allowing the necessary information to be gathered much sooner than an e-ELISA. Theoretically, k-ELISA results can quantify the initial approximate linear enzymatic reaction and thus be a truly quantitative test (10).
A normalization method to reduce variation between plates is often used for e-ELISA (including Svanovir®), with results being reported as optical density ratios (ODR) (11–13). The ODR is calculated as follows:
The OD measured at predetermined intervals in k-ELISA can be similarly normalized. However, k-ELISA results are usually presented as slopes, not as OD. We propose a similar normalization equation to the slope, resulting in a slope ratio (SR):
The advantages of the k-ELISA led us to investigate whether e-ELISA can be run as a k-ELISA. The objectives of this study were to: i) determine whether it is possible to run both a k-ELISA and an e-ELISA on the same plate without interfering with e-ELISA results; ii) establish an appropriate specific time interval and duration for the k-ELISA measurements; and iii) understand the relationship between the e-ELISA and the k-ELISA results.
Materials and methods
The milk samples were acquired from herds participating in the Canadian Bovine Mastitis Research Network (14). Details on the animals used were previously published by Reyher et al (14). Samples were run according to the manufacturer’s specifications (Svanova Veterinary Diagnostics, Uppsala, Sweden). These milk samples were part of a larger study investigating the effects of intestinal parasites on productivity of dairy cattle. Positive and negative controls, included in the ELISA kits (Svanovir; Svanova Veterinary Diagnostics), were run in triplicates. For k-ELISA procedures, the initial steps in the instructions were performed as recommended by the manufacturers (Svanova Veterinary Diagnostics), after the addition of the substrate the plate was then placed directly in the spectrophotometer (405 nm and 492 nm wavelengths, SpectraMax; MDS Incorporated, Sunnyvale, California, USA). The spectrophotometer and software (SoftMax, Release Pro 5; MDS Incorporated) were programmed to shake to homogenize the color within each well for 3 s before every reading and read the plate every 45 s until the end of the program. The software automatically calculated slope values for each well at the end of the k-ELISA program; however, slopes were also derived separately from regression coefficients between the OD and time for each well.
Concordance correlation coefficients (CCC) were calculated using computer software (Stata Statistical Software, Release 10.1; Stata Corporation LP, College Station, Texas, USA) and used to evaluate the agreement between the e-ELISA ODR and k-ELISA SR. Values close to 1 indicated very good agreement while values approaching zero reflected very poor agreement (15). Within the CCC analysis, a Bradley-Blackwood F-test compares the mean and variance between the 2 series (16), where P < 0.05 indicates that either the mean or the variance (or both) are unequal between the series.
To investigate the effect that a k-ELISA might have on e-ELISA results on the same plate, a total of 6 plates (3 pairs) with 96 wells were used (n = 276 wells). Each pair of plates was identical, having the same controls and milk samples repeated in their respective wells (i.e., well 1 in the first plate had the same milk sample as well 1 in the second plate). Within each pair of plates, one plate underwent a kinetic process for 15 min and after an additional 15 min the stop solution was added and the endpoint ODR (eODR) recorded (30 min after the reaction started). The corresponding plate in the pair underwent a standard e-ELISA, giving a total of 261 paired sample observations. One well was contaminated; therefore, 260 pairs of wells were included in the CCC analysis.
In order to confirm that 15 min of k-ELISA readings were sufficient for accuracy, a separate single plate with samples was allowed to undergo the k-ELISA procedure for 28 min, allowing 2 min to remove the plate from the spectrophotometer before the stop solution was added (at 30 min) to complete the e-ELISA.
To assess the relationship between the e-ELISA and the k-ELISA (Svanovir; Svanova), a total of 27 plates underwent both methods of testing. Each plate, loaded with diagnostic milk samples, started with the k-ELISA procedure for 15 min and ended with the recommended e-ELISA procedure at 30 min, giving results for both real-time k-ELISA and e-ELISA for the same wells. To evaluate whether results of a k-ELISA and an e-ELISA were comparable, the CCC between the normalized SR of the k-ELISA and the normalized ODR of the e-ELISA (eODR) was calculated. Additionally, to explore other time periods for the k-ELISA, a CCC analysis between the eODR and SR at each 45 s time interval was done in these 27 plates.
Results
The CCC between eODR from kinetic and endpoint series was very high (0.953) with no significant difference of mean or variance between the 2 series (P = 0.195, Bradley-Blackwood F-test). Therefore, it is reasonable to assume that the k-ELISA methods have no effect on the endpoint ODR, so that k-ELISA and e-ELISA can be safely done on the same plate with minimal influence on e-ELISA results. Laboratories that want to run a real-time k-ELISA and an e-ELISA on the same samples are able to do so without affecting the final e-ELISA results.
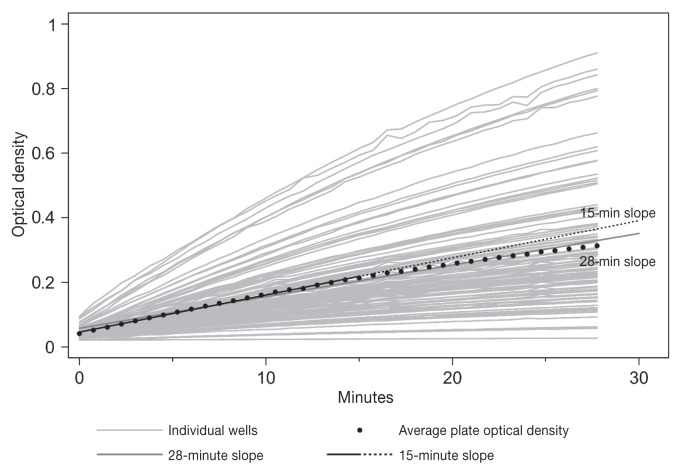
Figure 1 shows the OD in real time over 28 min for each individual well and for the average of the plate. After 28 min, the wells had not reached their maximum OD and would have continued to rise, if given the chance. Based on a linear regression model predicting OD readings and accounting for the quadratic effect of time, the estimated time to reach a plateau would have been approximately 50 min. The overall slope decreased with time, for instance, the slopes at 15 and 28 min were 1.15 × 10−2 and 0.98 × 10−2 OD units/min, respectively. This time-dependency in the slopes makes it necessary to normalize the slopes before evaluating the CCC between the values at any time interval and the eODR (30 min).
Figure 1.
Optical densities from kinetic-ELISA procedures for all wells, from one plate, that were followed for 28 min (light grey lines). The 15-minute slope (solid/dashed black line) was extended to 30 min for comparison with the 28-minute slope (dark grey).
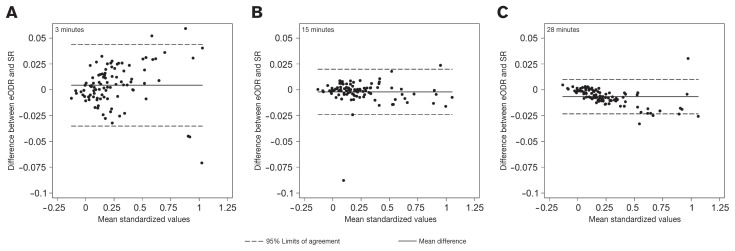
Bland and Altman (17) plots (Figure 2) were created to compare eODR with SR at 15 min, the time point presumed for the k-ELISA, and 2 other extreme time points (3 and 28 min). The CCC between eODR and SR at 3, 15, and 28 min were 0.997, 0.999, and 0.999, respectively. The SR from the k-ELISA at 15 min were nearly identical to the eODR values (CCC = 0.999) for this plate, thus a k-ELISA program set for 15 min should yield satisfactory results. The variation in the difference between eODR and SR decreased with time, as seen with smaller 95% limits of agreement intervals. The 95% limits of agreement interval at 15 min was empirically better than at 3 min, but only marginally lower at 28 min, suggesting that a 15-min k-ELISA would be adequate.
Figure 2.
Bland-Altman plots of the limits of agreement between slope ratios (SR) and the endpoint optical density ratio (eODR) for (A) 3, (B) 15, and (C) 28 min.
While running the 28-min plate, it was noticed that the OD recorded at 30 min, after the stop solution was added, were lower than OD at 28 min. Given that OD should have continued rising until 50 min had elapsed (based on the regression model mentioned above), the 30-minute OD should be higher than the 28-minute OD. However, the decrease in OD at 30 min was likely artificial due to the addition of 50 μL of transparent stop solution. Fortunately, the normalization equation adjusts for this dilution effect because it incorporates the similarly affected controls from the same plate.
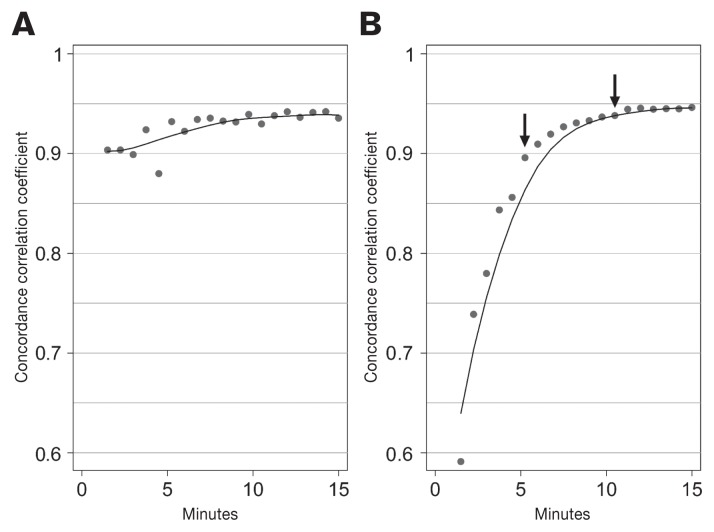
The CCC between the SR of the k-ELISA at 15 min and the eODR of the e-ELISA for the 27 plates was calculated (0.946) and indicated excellent agreement between the 2 measurements. The CCC of the SR versus eODR were low at the beginning of the reaction, however, increased with time (Figure 3B). Specifically, the CCC values were ≤ 0.856 before 5.25 min and ≥ 0.944 after 10.5 min (Figure 3B). Figure 3B also shows the SR following a more predictable curve compared to the ODR for each time point. The pattern may be due to the additive properties of a slope, summarizing more information for the calculated slope as time progresses. This is different from the ODR, where the estimate was derived from a specified time point without using previous information. It is, therefore, recommended that SR rather than ODR be used with k-ELISA, provided SR are measured for at least 10 min. The data also suggest that k-ELISA readings could be reduced to as little as 10 min without losing information; therefore, switching from an e-ELISA to a k-ELISA could reduce the total time by as much as 20 min without requiring a stop solution.
Figure 3.
A — Concordance correlation coefficients (CCC) between optical density ratios (ODR), calculated at readings from 1.5 to 15 min, and the endpoint-ODRs (eODR), calculated at 28 min for a total of 27 plates. B — Similarly, CCC were calculated between slope ratios (SR) for readings from 1.5 to 15 min, and eODR at 28 min, for a total of 27 plates. Arrows are placed at 5.25 and 10.5 min. The CCC show the level of agreement between the various reduced processing times, calculated as either ODR or SR, and the conventional protocol for eODR measured at 28 min. Locally weighted scatterplot smoothing curves (black lines) show the trending patterns for the CCC over time.
Discussion
Enzyme concentrations are only linear with respect to their reactions during the initial phase of reaction and when the proportion of substrate concentration greatly surpasses that of the enzymes (18). As such, Tsang et al (10) theorized that k-ELISA could quantify the initial approximate linear enzymatic reaction, provided there is sufficient substrate concentration. Consequently, a k-ELISA may be able to better discriminate between high ODR (i.e., differentiate between those samples that have very high concentrations of antibodies with those that have high levels, but have similarly elevated OD on the linear scale) by using SR from the early phase of the reaction. If this is true, there would potentially be no need to dilute samples with high antibody levels if they could be quantified by their initial slopes. It is well-known that serum samples have higher concentrations of antibodies than milk, and may therefore benefit from k-ELISA readings, although the ELISA (Svanova) we used was strictly designed to the test milk samples. Our average eODR for the 27 plates was too low (0.272) to investigate the potential ability for k-ELISA to better discriminate between high OD, though increased variance was observed in the lowest ODR percentiles. It is likely that samples with higher antibody concentrations and shorter initial k-ELISA reading intervals (e.g., 15 or 30 s) will be able to answer this question.
The CCC between ODR for each time interval and the eODR did improve as time increased (Figure 3A); however, the CCC did not stabilize nearly as much as between SR and the eODR (Figure 3B). These results suggest that the e-ELISA could be stopped prematurely, after 7 min instead of the recommended 30 min, and yield similar results to the 30 min interval, but with more variability than using results from a k-ELISA that was prematurely stopped.
Overall, our study found that running a k-ELISA had no effect on ODR from an e-ELISA on the same plate. The strong agreement of k-ELISA results (SR) at various time points with e-ELISA ODR demonstrates the validity of using k-ELISA and thus reduces the total processing time. Although we found that e-ELISA processing time can also be reduced, k-ELISA offers better agreement with less variability in its results.
Acknowledgments
The authors thank Natasha Robinson and Judy Sheppard for their technical assistance, and Dr. María Forzán for her critical review of the manuscript. Svanovir ELISA kits were kindly supplied at no charge by Svanova.
References
- 1.Charlier J, Forbes A, Van Gucht S, Duchateau L, Goddeeris BM, Vercruysse J. Serological evidence of Ostertagia ostertagi infection in dairy cows does not impact the efficacy of rabies vaccination during the housing period. Res Vet Sci. 2013;95:1055–1058. doi: 10.1016/j.rvsc.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Pablos-Tanarro A, Pérez-Cabal MÁ, Ortega-Mora LM, Ferre I. Presence of Ostertagia ostertagi antibodies in bulk tank milk from cattle herds in northern Spain. Vet Parasitol. 2013;197:388–392. doi: 10.1016/j.vetpar.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 3.Vanderstichel R, Dohoo I, Sanchez J, Conboy G. Effects of farm management practices and environmental factors on bulk tank milk antibodies against gastrointestinal nematodes in dairy farms across Canada. Prev Vet Med. 2012;104:53–64. doi: 10.1016/j.prevetmed.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 4.Charlier J, Claerebout E, Duchateau L, Vercruysse J. A survey to determine relationships between bulk tank milk antibodies against Ostertagia ostertagi and milk production parameters. Vet Parasitol. 2005;129:67–75. doi: 10.1016/j.vetpar.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 5.Sanchez J, Dohoo I, Nødtvedt A, et al. A longitudinal study of gastrointestinal parasites in Canadian dairy farms. The value of an indirect Ostertagia ostertagi ELISA as a monitoring tool. Vet Parasitol. 2002;107:209–226. doi: 10.1016/s0304-4017(02)00158-9. [DOI] [PubMed] [Google Scholar]
- 6.Vanderstichel R, Dohoo I, Sanchez J, Sithole F, Keefe G, Stryhn H. Predicting the effect of anthelmintic treatment on milk production of dairy cattle in Canada using an Ostertagia ostertagi ELISA from individual milk samples. Prev Vet Med. 2013;111:63–75. doi: 10.1016/j.prevetmed.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Bullock SL, Walls KW. Evaluation of some of the parameters of the enzyme-linked immunospecific assay. J Infect Dis. 1977;136(Suppl):S279–285. doi: 10.1093/infdis/136.supplement_2.s279. [DOI] [PubMed] [Google Scholar]
- 8.Engvall E, Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972;109:129–135. [PubMed] [Google Scholar]
- 9.Pesce AJ, Mendoza N, Boreisha I, Gaizutis MA, Pollak VE. Use of enzyme-linked antibodies to measure serum anti-DNA antibody in systemic lupus erythematosus. Clin Chem. 1974;20:353–359. [PubMed] [Google Scholar]
- 10.Tsang VC, Wilson BC, Maddison SE. Kinetic studies of a quantitative single-tube enzyme-linked immunosorbent assay. Clin Chem. 1980;26:1255–1260. [PubMed] [Google Scholar]
- 11.Charlier J, Duchateau L, Claerebout E, Vercruysse J. Assessment of the repeatability of a milk Ostertagia ostertagi ELISA and effects of sample preparation. Prev Vet Med. 2005;68:277–288. doi: 10.1016/j.prevetmed.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez J, Dohoo I, Markham F, Leslie K, Conboy G. Evaluation of the repeatability of a crude adult indirect Ostertagia ostertagi ELISA and methods of expressing test results. Vet Parasitol. 2002;109:75–90. doi: 10.1016/s0304-4017(02)00194-2. [DOI] [PubMed] [Google Scholar]
- 13.Vanderstichel R, Dohoo I, Stryhn H. The impact of milk handling procedures on Ostertagia ostertagi antibody ELISA test results. Vet Parasitol. 2010;169:204–208. doi: 10.1016/j.vetpar.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Reyher K, Dufour S, Barkema H, et al. The National Cohort of Dairy Farms — A data collection platform for mastitis research in Canada. J Dairy Sci. 2011;94:1616–1626. doi: 10.3168/jds.2010-3180. [DOI] [PubMed] [Google Scholar]
- 15.Dohoo I, Martin W, Stryhn H. Veterinary Epidemiologic Research. 2nd ed. Charlottetown, Prince Edward Island: AVC Incorporated; 2009. [Google Scholar]
- 16.Bradley E, Blackwood L. Comparing paired data: A simultaneous test for means and variances. Am Stat. 1989;43:234–235. [Google Scholar]
- 17.Bland J, Altman D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 18.Dixon M, Webb E. Enzymes. 2nd ed. New York, New York: Academic Press; 1964. pp. 54–56. [Google Scholar]