Abstract
Histophilus somni, a causative agent of the bovine respiratory disease complex, can also cause a variety of systemic disorders, including bronchopneumonia, myocarditis, pericarditis, arthritis, pleuritis, and infectious thrombotic meningoencephalitis. The purpose of this study was to determine if currently circulating strains differ from those of the 1980s by identifying genomic changes. Single nucleotide polymorphisms (SNPs) and insertion and deletion (INDEL) sites were examined by whole-genome sequencing in 12 samples, 6 old and 6 new. The 31 028 SNP/INDELs recorded were compared against the reference genome sequence of the pathogenic H. somni strain 2336. The distribution of about 75% of these SNPs within a specified gene differed between old and new isolates and did not follow any particular pattern. The other 25% clustered into 2 groups containing the same SNPs in various genes: group I included 5 old isolates and 1 new isolate; group II included 5 new isolates and 1 old isolate. For putative virulence genes there were more SNPs in group I compared with strain 2336, itself an older isolate, than in group II. Although only 25% of all the SNPs formed 2 clusters, the results suggest some genetic difference in various genes between old and new strains.
Résumé
Histophilus somni est l’un des agents majeurs du complexe respiratoire bovin (CRB), qui peut aussi causer diverses pathologies dont de la bronchopneumonie, myocardite, péricardite, arthrite, pleurésie et de la méningo-encéphalite thrombotique. L’objectif général de l’étude était de comparer les souches actuellement en circulation avec les souches isolées dans les années 80. Plus spécifiquement les changements génétiques survenus entre des isolats récents et des isolats collectés il y a une trentaine d’années ont été analysés. Les polymorphismes d’un seul nucléotide (single nucleotide polymorphism, SNP) ont été examinés en utilisant une approche de séquençage global de tout le génome pour 12 échantillons, six anciens et six nouveaux. Un total de 31 028 SNPs a été identifié et une analyse comparative de ces SNPs avec la séquence génomique de référence de la souche pathogène 2336 de H. somni a été effectuée. La distribution génique d’environ 75 % de ces SNPs entre les souches anciennes et récentes est différente et ne suit pas de tendance particulière. Toutefois, 25 % des SNPs se répartissent rapidement en deux groupes distincts. Le groupe I inclut cinq isolats anciens et un récent alors que le groupe II comprend cinq isolats récents et un isolat ancien qui se regroupent ensemble pour de mêmes SNPs dans plusieurs gènes. La présence des SNPs dans des gènes potentiellement liés à la virulence est plus manifeste dans le groupe I, comparé à l’ancien isolat 2336, que dans le groupe II. Bien que seulement 25 % des SNPs totaux se répartissent en deux groupes, les résultats suggèrent des variations génétiques significatives entre souches anciennes et récentes dans les séquences de nombreux gènes.
(Traduit par Docteur François Meurens)
Introduction
Histophilus somni, previously known as Haemophilus somnus, is a commensal opportunistic Gram-negative bacterium that is a causative agent of the bovine respiratory disease (BRD) complex (1,2). Globally the organism is an economically important pathogen, responsible for significant annual losses to the livestock industry, especially through the BRD complex in North America (3). Among cattle H. somni causes a variety of clinical scenarios, including reproductive disorders, septicemia, myocarditis, pericarditis, pleuritis, bronchopneumonia, arthritis, and infectious thrombotic meningoencephalitis (ITME) (4–6). The organism has also been detected in domestic sheep and American bison (7–10). Since its first isolation in 1956, factors that may contribute towards its virulence, such as genes involved in lipooligosaccharide (LOS) biosynthesis, expression of surface immunoglobulin-binding proteins, serum resistance, induction of endothelial cell apoptosis, antigenic phase variation in LOS epitopes, and biofilm formation, have been reported (11–16). Antigenic phase variation in LOS epitopes may arise because of alterations in structure or composition or because of substitution of glycoses such as phosphorylcholine, which contributes to the adhesion of H. somni to respiratory epithelial cells (17). The other most common virulence-associated factors are attachment to host cells, obtaining iron and other nutrients from the host, and interaction with phagocytic cells that results in inhibition of phagocytic cell function (18–23). The rapid advancement in bioinformatics and high-throughput next-generation DNA sequencing has made it possible for fast whole-genome sequencing (24). Recently, sequencing of the complete genome of the pathogenic H. somni strain 2336 (GenBank accession number NC_010519, National Center for Biotechnology Information, Bethesda, Maryland, USA) resulted in the identification of a number of genes presumably involved in virulence, including those coding for adhesins, filamentous hemagglutinins, outer membrane proteins, LOSs, iron transport and utilization proteins, TonB-dependent proteins, and exopolysaccharides (11,25,26).
Vaccination for the prevention of H. somni infection has been practised for decades. Most current products are based on either killed cells or outer membrane proteins that help reduce the risk of ITME and BRD, respectively (27). It is possible that current vaccines protect against only the planktonic form of the bacteria and not when the organisms are in a biofilm (16,28). The formation of biofilms by H. somni in host tissues, particularly during myocarditis, protects the bacteria against host defences as well as antibiotics (28). In addition, the antigenic profile of the bacteria in the host may be different (for example, owing to decoration of some components with sialic acid) from that of the bacteria used to manufacture the vaccines under artificial conditions (such as shaking in a culture flask) (16); limited vaccine efficacy may result from such differences in antigens. Finally, small, noncoding RNAs (sRNAs) found in the virulent strain of H. somni 2336 and not in the avirulent strain 129Pt (GenBank accession number 008309) may contribute to virulence and inefficiency of current vaccines (29).
Almost all of these vaccines use strains that were isolated 10 to 20 y ago. The current study was undertaken to determine H. somni genome changes by studying strains isolated during the 1980s and samples collected in 2012–2013.
Materials and methods
Bacterial isolation
Histophilus somni was isolated from tissue samples obtained from 6 feedlot calves that died during 2012–2013 in Alberta. Amies transport medium with charcoal was used to collect, transport, and maintain the microorganisms. Heart, lung, liver, synovial fluid, and swabs were cultured for H. somni by streaking on trypticase soy agar (TSA) with 5% sheep blood (10,29) and incubation for 24 to 48 h in 5% CO2 at 37°C. Putative H. somni colonies were restreaked on blood agar plates and identified positively from the appearance of colonies, growth characteristics, and pigmentation. A single colony was then used to inoculate brain–heart infusion medium with 0.1% Trisma base and 0.01% thiamine monophosphate (BHITT), which was incubated overnight at 37°C with shaking (10,30). Frozen stocks of the overnight culture were made using 30% heat-inactivated fetal calf serum and 15% glycerol. Six isolates from the 1980s, which had been stored at −80°C, were obtained from lung samples.
Polymerase chain reaction (PCR) of 16S ribosomal RNA (rRNA)
With a previously described procedure (31), PCR was also used to identify H. somni. The 16S rRNA sequence of H. somni was generated with the use of forward primer HS-453F (5′-GAAGGCGATTAGTTTAAGAG-3′) and reverse primer HS-860R (5′-TTCGGGCACCAAGTRTTCA-3′) (31). Samples were processed in a PTC-100 Thermal Cycler (Bio-Rad Laboratories, Hercules, California, USA) with 35 cycles of the following steps: initial denaturation at 94°C for 5 min, denaturation at 94°C for 1 min, annealing at 55°C for 1 min, and extension at 72°C for 1.5 min. The samples were then subjected to electrophoresis in a 1% agarose gel. A DNA fragment of 400 base pairs (bp) was detected in all the samples.
Genomic DNA isolation and whole-genome sequencing
The frozen culture stocks were plated on TSA with 5% sheep blood and incubated for 48 h at 37°C in a 5% CO2 atmosphere (10,29,30). Bacterial growth was scraped and transferred to BHITT and grown overnight on a shaker at 37°C. Genomic DNA was extracted from the cell pellet with a genomic-tip (Qiagen Canada, Toronto, Ontario) as described by the manufacturer. Genome sequencing of the 12 isolates was completed by means of the MiSeq desktop sequencer (Illumina, San Diego, California, USA) with paired-end 150-bp read type at Cofactor Genomics, St. Louis, Missouri, USA. Single nucleotide polymorphisms (SNPs) and insertion and deletion (INDEL) sites were constructed by Cofactor Genomics with reference to the genome sequence of the pathogenic H. somni strain 2336. Comparative SNP/INDEL analysis was conducted by aligning the reads generated from each genome sample and the reference strain. A SNP was generated with at least 4 reads covering the site and 2 reads covering the non-reference base. Of all the reads covering the SNP site, at least 10% supported the nonreference allele. A threshold of 8 times or more was used to produce a SNP value. The percentage SNP call against the reference strain for each sample with at least 8 times coverage was then calculated (http://cofactorgenomics.com). The SNPs/INDEL value ranged from 0.00 to 1.00.
Statistical analysis
The data were analyzed with a t-test for 2 independent means and a 2-tailed hypothesis. The level of significance was set at P < 0.05.
Results
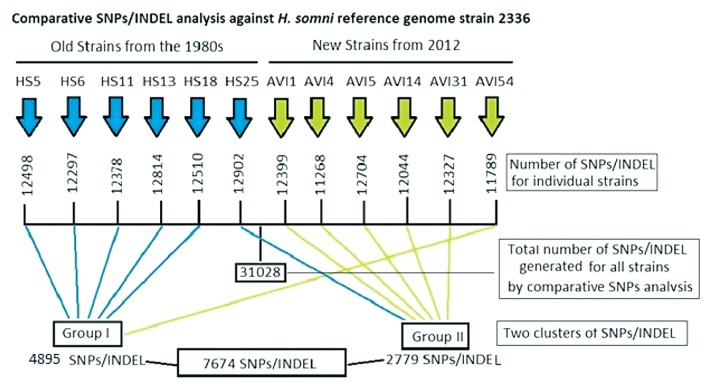
Comparative SNP analysis generated 31 028 SNPs or INDEL sites having a SNP score ranging from 0.00 to 1.00 that resulted from at least 8 times coverage for the 12 genome samples (including intergenic regions) with reference to the genome sequence of pathogenic H. somni strain 2336. The SNP/INDELs of each genome were randomly combined in clusters to detect if the percentage SNP call against the reference genome sequence clustered into 2 groups that contained the greatest number of old or new isolates with the highest total number of SNP/INDELs within a group. From the 31 028 SNP/INDELs with a score of 0.00 to 1.00, 2 groups were formed, the highest total number of SNPs corresponding to the greatest number of old or new isolates that clustered together being 7674 (25%) (Figure 1). Group I consisted of 5 old isolates and 1 new isolate with 4895 SNP/INDELs (16%) for the same genes and same positions; the remaining isolates scored a value of zero. In the same manner group II contained 5 new isolates and 1 old isolate with 2779 SNP/INDELs (9%) for the same genes. Comparing the 2 groups, the old isolates contained more SNPs than the new isolates. However, if recent isolate AVI14 was removed, the total number of SNPs for group II increased from 2779 (9%) to 4504 (15%). Again, the emphasis was to cluster all isolates rather than to look at any individual isolate.
Figure 1.
Overview of the number of single nucleotide polymorphisms (SNPs) and insertion–deletion (INDEL) sites with a score of 0.00 to 1.00 in the genome of 6 isolates of Histophilus somni from the 1980s (“Old”) and 6 from 2012–2013 (“New”) generated by comparative analysis with reference to the genome of pathogenic H. somni strain 2336 (GenBank sequence accession number NC_010519, National Center for Biotechnology Information, Bethesda, Maryland, USA).
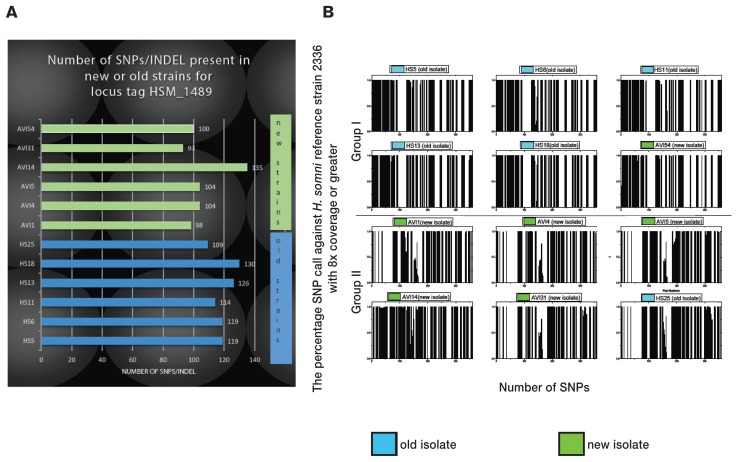
Comparing SNP/INDELs of H. somni strains isolated during the 1980s with those isolated in 2012–2013 might identify genomic changes more relevant to virulence. The average bacterial gene size is approximately 1 kb (32). In contrast, in H. somni, for example, locus tag HSM_1489 (GenBank accession number CP000947) contains a 12.3-kb immunoglobulin-binding protein A gene (ibpA) with a total of 360 SNP/INDELs for all 12 isolates. However, when each isolate was considered individually, the average number of SNP/INDELs was 113. The numbers of SNP/INDELs for the ibpA gene for each old or new strain are shown in Figure 2A. The highest and lowest numbers as a group were in new isolates AVI14 (135) and AVI31 (93). The 6 old isolates did not contain a significantly higher number of SNP/INDELs than the 6 new isolates. With reference to strain 2336, for locus tag HSM_1489 the distribution and location of all SNP/INDELs for 5 old strains (HS5, HS6, HS11, HS13, and HS18) and 1 new strain (AVI54) were similar; these isolates formed group I (Figure 2B). In the same manner, 5 new strains (AVI1, AVI4, AVI5, AVI14, and AVI31) and 1 old strain (HS25) clustered to form group II on the basis of the distribution of all SNP/INDELs within locus tag HSM_1489 (Figure 2B). For locus tag HSM_1489, only 43 SNP/INDELs scored higher than the required threshold and were present in all the isolates of group I, the remaining isolates scoring zero (Table I). In the same manner, only 21 SNP/INDELs scored higher than the required threshold and were present in all the isolates of group II (Table II). These 2 groups had the highest number of SNP/INDELs clustering the maximum number of old or new isolates compared with other combinations for locus tag HSM_1489. Group I, which contained most of the old strains (5 old strains plus 1 new strain), had a significantly higher number of SNP/INDELs (P < 0.05) than group II, which contained most of the new strains (5 new strains plus 1 old strain).
Figure 2.
A — Numbers of SNP/INDELs in the immunoglobulin-binding protein A gene (ibpA) of the new (green) and old (blue) isolates. B — Distribution of SNP/INDELs in the 2 groups of isolates formed on the basis of percentage of SNP calls against the reference genome with 8 times coverage or greater. The reference strain was H. somni strain 2336, the locus tag containing ibpA being represented by HSM_1489 (GenBank accession number CP000947) annotated in the reference strain.
Table I.
Distribution of single nucleotide polymorphisms (SNPs) and insertion–deletion (INDEL) sites in the immunoglobulin-binding protein A gene (ibpA) of 5 Histophilus somni isolates from the 1980s and 1 isolate from 2012–2013 scoring higher than the threshold with reference to pathogenic H. somni strain 2336 and locus tag HSM_1489 and clustering to form group I (highlighted in pink)
| SNP/INDEL number | Positiona | Reference baseb | SNP/INDEL base | HS5 | HS6 | HS11 | HS13 | HS18 | HS25 | AVI1 | AVI4 | AVI5 | AVI14 | AVI31 | AVI54 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1701111 | C | T | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 2 | 1701144 | A | G | 1 | 1 | 0.96 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0.94 |
| 3 | 1701258 | A | T | 1 | 0.99 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 4 | 1701259 | G | T | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 5 | 1701399 | A | G | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 6 | 1701405 | C | T | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 7 | 1701894 | T | C | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 8 | 1702260 | T | C | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 9 | 1702440 | C | T | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 10 | 1702591 | A | G | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 11 | 1702599 | G | A | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 12 | 1702602 | G | A | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 13 | 1702956 | C | T | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 14 | 1703031 | C | T | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0.89 |
| 15 | 1703033 | A | G | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0.89 |
| 16 | 1703036 | G | A | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0.9 |
| 17 | 1703185 | G | A | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 18 | 1703197 | C | T | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 19 | 1703201 | T | C | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 20 | 1705756 | G | T | 1 | 1 | 1 | 1 | 0.99 | 0 | 0 | 0 | 0 | 0 | 0 | 0.99 |
| 21 | 1705827 | T | C | 0.96 | 0.88 | 0.97 | 1 | 0.9 | 0 | 0 | 0 | 0 | 0 | 0 | 0.93 |
| 22 | 1705857 | A | G | 0.86 | 0.68 | 0.91 | 1 | 0.83 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 23 | 1706622 | A | G | 0.92 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 24 | 1707471 | C | T | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 25 | 1707579 | A | G | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 26 | 1707849 | A | T | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 27 | 1707915 | C | T | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 28 | 1708038 | T | C | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 29 | 1708224 | C | T | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 30 | 1708227 | G | A | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 31 | 1708287 | C | T | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 32 | 1708371 | G | A | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 33 | 1710171 | A | G | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 34 | 1710207 | T | C | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 35 | 1710222 | A | G | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 36 | 1710225 | T | C | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 37 | 1710864 | C | T | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 38 | 1711853 | C | T | 1 | 1 | 0.98 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 39 | 1711864 | T | C | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 40 | 1711872 | C | G | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 41 | 1712736 | T | C | 1 | 1 | 1 | 0.91 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 42 | 1713162 | G | C | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 43 | 1713164 | A | C | 1 | 0.99 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Position of SNP/INDEL is from the reference sequence of H. somni 2336 (GenBank accession number NC_010519, National Center for Biotechnology Information, Bethesda, Maryland, USA), used for the comparative SNP/INDEL analysis.
Reference base is from the reference sequence of H. somni 2336 (GenBank accession number NC_010519, National Center for Biotechnology Information, Bethesda, Maryland, USA), used for the comparative SNP/INDEL analysis.
Table II.
Distribution of SNP/INDELs in ibpA of 1 H. somni isolate from the 1980s and 5 isolates from 2012–2013 scoring higher than the threshold with reference to H. somni 2336 and locus tag HSM_1489 and clustering to form group II (highlighted in yellow)
| SNP/INDEL number | Positiona | Reference baseb | SNP/INDEL base | HS5 | HS6 | HS11 | HS13 | HS18 | HS25 | AVI1 | AVI4 | AVI5 | AVI14 | AVI31 | AVI54 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1704765 | G | A | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| 2 | 1704785 | C | T | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| 3 | 1704813 | T | C | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| 4 | 1704842 | C | A | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| 5 | 1705255 | T | C | 0 | 0 | 0 | 0 | 0 | 1 | 0.89 | 1 | 1 | 1 | 1 | 0 |
| 6 | 1705264 | G | C | 0 | 0 | 0 | 0 | 0 | 1 | 0.88 | 1 | 1 | 1 | 1 | 0 |
| 7 | 1705267 | T | C | 0 | 0 | 0 | 0 | 0 | 1 | 0.86 | 1 | 1 | 1 | 1 | 0 |
| 8 | 1705836 | T | G | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0.37 | 1 | 0 |
| 9 | 1705846 | C | T | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0.55 | 1 | 0 |
| 10 | 1708200 | G | T | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| 11 | 1708203 | T | C | 0 | 0 | 0 | 0 | 0 | 1 | 0.99 | 1 | 0.99 | 1 | 0.99 | 0 |
| 12 | 1708407 | T | G | 0 | 0 | 0 | 0 | 0 | 0.99 | 1 | 1 | 1 | 1 | 1 | 0 |
| 13 | 1708410 | A | G | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| 14 | 1708902 | A | C | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| 15 | 1708917 | G | A | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| 16 | 1709642 | T | C | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| 17 | 1709672 | G | C | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| 18 | 1711453 | C | T | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0.95 | 0 |
| 19 | 1711731 | G | A | 0 | 0 | 0 | 0 | 0 | 0.99 | 1 | 1 | 1 | 0.75 | 1 | 0 |
| 20 | 1711786 | T | G | 0 | 0 | 0 | 0 | 0 | 0.98 | 1 | 1 | 1 | 1 | 0.98 | 0 |
| 21 | 1711790 | T | G | 0 | 0 | 0 | 0 | 0 | 0.98 | 1 | 1 | 1 | 1 | 0.94 | 0 |
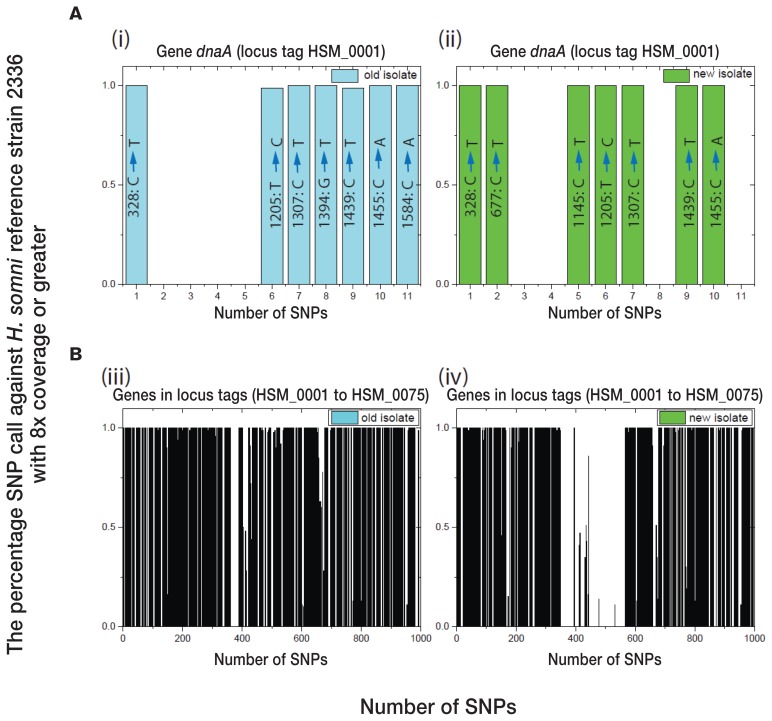
Information regarding the distribution pattern of SNPs within a gene is important for genotype characteristics (33). To analyze how the SNPs were distributed within a single isolate, and to compare H. somni isolated during the 1980s with the strains recently isolated, we randomly selected 2 samples, 1 old strain from group I and 1 new strain from group II, and plotted the number of SNPs and the percentage SNP call against H. somni reference strain 2336 for each sample having 8 times coverage or greater. For example, the chromosomal-initiation protein gene dnaA (locus tag HSM_0001) had 11 SNPs, but only 7 were distributed in the 2 samples selected (Figure 3A). Among these 7 SNPs, 5 (positions 328[C→T], 1205[T→C], 1307[C→T], 1439[C→T], and 1455[C→A]) were present in both samples. For the old isolate the other 2 SNPs were at positions 1394(G→T) and 1584(C→A), whereas for the new isolate they were at positions 677(C→T) and 1145(C→T). Locus tags ranging from HSM_0001 to HSM_0075 were plotted in the same manner and found to include approximately 1000 SNPs (Figure 3B). Finally, all 31 028 SNP/INDELs were studied for their distribution within genes of these 2 isolates. The results (data not shown) suggested that the distribution within a specified gene differs between old and new isolates and does not follow any particular pattern.
Figure 3.
Distribution of SNPs in an old isolate (left panels) and a new isolate (right panels) with reference to H. somni 2336, the numbers of SNPs being plotted against the percentage of SNP calls against the reference genome with 8 times coverage or greater. A — Distribution for the chromosomal-initiation protein gene dnaA (locus tag HSM_0001). B — Distribution for the first 1000 SNPs representing locus tags HSM_0001 to HSM_0075.
The 16S rRNA gene sequence is important for phylogenetic studies, especially within the Pasteurellaceae family (34). In reference strain H. somni 2336 the 16S rRNA genes are contained in locus tags HSM_R0014, HSM_R0019, HSM_R0027, HSM_R0038, and HSM_R0060. These genes are commonly used for molecular bacterial diagnostic assays. In our study the 16S rRNA gene contained SNP/INDELs for both groups I and II, as shown in Table III. For locus tag HSM_R0060, a SNP of A→G at position 2118616 was recorded for group I and not for group II. For locus tag HSM_R0019, in group I SNPs T→G and T→A were present at positions 629791 and 629818, respectively, but in group II SNPs T→C and T→ + GA were detected at positions 629802 and 629818, respectively. Overall, for SNP/INDEL values that ranged from 0.5 to 1.00 for the 16S rRNA genes, the old isolates contained a significantly higher number of SNPs (P < 0.05) than the new isolates.
Table III.
Distribution of SNP/INDELs in the 16S ribosomal RNA gene of H. somni isolated during the 1980s (highlighted in blue) or in 2012–2013 (highlighted in green) with reference to H. somni 2336 and locus tag HSM_1489
| Gene (and locus tag) | SNP/INDEL position | Reference base | SNP/INDEL base | HS5 | HS6 | HS11 | HS13 | HS18 | HS25 | AVI1 | AVI4 | AVI5 | AVI14 | AVI31 | AVI54 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cyaY (HSM_R0060) | 2118616 | A | T | 0.5 | 0.36 | 0 | 0.58 | 0.29 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| cyaY (HSM_R0060) | 2118616 | A | G | 0.5 | 0.64 | 1 | 0.42 | 0.71 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| glyQ (HSM_R0038) | 985721 | A | T | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| hemG (HSM_R0014) | 513361 | A | G | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| rpoZ (HSM_R0019) | 629791 | T | G | 0.5 | 0.91 | 0.46 | 1 | 0.75 | 0 | 0 | 0 | 0 | 0 | 0 | 0.78 |
| rpoZ (HSM_R0019) | 629818 | T | A | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| rpoZ (HSM_R0019) | 629802 | T | C | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| rpoZ (HSM_R0019) | 629818 | T | +GA | 0 | 0 | 0 | 0 | 0 | 0.95 | 0.86 | 1 | 0.87 | 0.8 | 0.83 | 0 |
Putative virulent gene products and factors that contribute to the virulence of H. somni have been clearly defined (11–25). The results of our comparative SNP/INDEL analysis of some of these genes in groups I and II are shown in Table IV. The immunoglobulin-binding proteins A and B (locus tags HSM_1489 and HSM_1490) show homology with other virulence proteins found in Pasteurella multocida, Haemophilus ducreyi, and Bordetella pertussis (11,25). Vaccination with 3 recombinant subunit proteins from IbpA was successfully used to protect mice against experimental infection with H. somni (22). In our study, groups I and II had 43 (12%) and 21 (6%), respectively, of the 360 SNP/INDELs identified in the ibpA gene (Table IV). Changes in strains circulating in the field between the 1980s and 2012–2013 may have emerged because of different virulence factors. One of the new isolates (AVI14) in group II had a SNP distribution pattern that did not match that of the other isolates in this group (Figure 2B). If this recent isolate was removed from group II, the resulting total number of SNPs increased from 21 to 81 for locus tag HSM_1489.
Table IV.
Number of SNP/INDELs in groups I and II for gene products that may be involved in the virulence of H. somni with reference to H. somni 2336
| Proteina | Locus tag and reference sequence numbera | Homologs | Number of SNP/INDELs | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Total | In group I | In group II | In group II after removal of isolate AVI14 | |||
| Cysteine protease | HSM_1489 (YP_001784809.1) | Immunoglobulin-binding protein A | 360 | 43 | 21 | 81 |
| SmpA/OmlA domain-containing protein | HSM_0095 (YP_001783450.1) | OmlA | 6 | 0 | 0 | 0 |
| HSM_1317 (YP_001784639.1) | 16 | 4 | 5 | 1 | ||
| TonB-dependent lactoferrin and transferrin receptor | HSM_0750 (YP_001784088.1) | TbpA | 73 | 20 | 5 | 15 |
| HSM_1988 (YP_001785302.1) | 37 | 0 | 0 | 0 | ||
| Tbp | HSM_0749 (YP_001784087.1) | TbpB | 24 | 7 | 1 | 4 |
| TonB-dependent receptor plug | HSM_0932 (YP_001784264.1) | TbpA | 14 | 3 | 0 | 0 |
| TonB-dependent receptor | HSM_0047 (YP_001783402.1)b | TonB-dependent heme/hemoglobin receptor family protein | 65 | 21 | 1 | 3 |
| HSM_0931 (YP_001784263.1) | 36 | 1 | 0 | 9 | ||
| HSM_1372 (YP_001784692.1) | 70 | 7 | 6 | 14 | ||
| HSM_1962 (YP_1707182576.1) | 33 | 8 | 6 | 0 | ||
| TonB family protein | HSM_1100 (YP_001784430.1) | TonB protein | 15 | 6 | 0 | 3 |
| TonB-dependent hemoglobin/transferrin/lactoferrin family receptor | HSM_1168 (YP_001784498.1) | 62 | 9 | 16 | 0 | |
| TonB system energizer | HSM_1102 (YP_001784432.1) | Outer membrane transport energization protein ExbB | 9 | 2 | 0 | 1 |
| ExbB type 2 and TonB system transporter ExbD type 2 | HSM_1101 (YP_001784431.1) | 4 | 0 | 0 | 2 | |
| Lipoprotein A | HSM_0191 (YP_001783542.1) | Lipoprotein LppA | 10 | 3 | 3 | 1 |
| Rare lipoprotein B | HSM_0468 (YP_001783814.1) | 9 | 1 | 6 | 0 | |
| Oml LolB | HSM_1476 (YP_001784796.1) | 11 | 1 | 1 | 1 | |
| LppC family lipoprotein | HSM_1207 (YP_001784531.1) | 13 | 0 | 0 | 3 | |
| Filamentous hemagglutinin Omp | HSM_0268 (YP_001783619.1) | 108 | 26 | 0 | 19 | |
| Hemagglutinin/hemolysin-like protein | HSM_0270 (YP_001783621.1) | 26 | 3 | 0 | 18 | |
| Adhesin | HSM_0274 (YP_001783625.1) | 9 | 2 | 0 | 0 | |
| Yersinia adhesin A domain-containing protein | HSM_0077 (YP_001783432.1) | Hemagglutinin domain protein or Hag repeat-containing protein | 171 | 40 | 10 | 29 |
| HSM_0338 (YP_001783687.1) | 139 | 32 | 17 | 11 | ||
| HSM_0346 (YP_001783695.1) | 85 | 10 | 5 | 8 | ||
| HSM_0377 (YP_001783726.1) | 123 | 0 | 6 | 1 | ||
| HSM_0394 (YP_001783743.1) | 70 | 0 | 0 | 5 | ||
| HSM_0708 (YP_001784046.1) | 163 | 16 | 3 | 21 | ||
| HSM_0844 (YP_001784176.1) | 158 | 16 | 5 | 21 | ||
| HSM_1022 (YP_001784352.1) | 248 | 38 | 18 | 43 | ||
| HSM_1257 (YP_001784581.1) | 245 | 37 | 6 | 45 | ||
| HSM_1484 (YP_001784804.1) | 72 | 32 | 0 | 2 | ||
| HSM_1542 (YP_001784862.1) | 116 | 6 | 20 | 21 | ||
| HSM_1571 (YP_001784891.1) | 214 | 57 | 2 | 46 | ||
| HSM_1793 (YP_001785113.1) | 100 | 17 | 2 | 5 | ||
| Lipooligosaccharide sialyltransferase | HSM_1476 (YP_001784746.1) | Sialyltransferase | 14 | 1 | 1 | 1 |
| Glycosyltransferase family protein | HSM_0978 (YP_001784308.1)c | Lipooligosaccharide galactosyltransferase II | 23 | 1 | 0 | 0 |
| HSM_0977 (YP_001784307.1)d | 28 | 0 | 0 | 1 | ||
| Cell wall biogenesis glycosyltransferase-like protein | HSM_0976 (YP_001784306.1)e | N-acetylglucosamine glycosyltransferase | 5 | 0 | 0 | 0 |
Annotation is from the reference sequence of H. somni 2336 (GenBank accession number NC_010519, National Center for Biotechnology Information, Bethesda, Maryland, USA), used for the comparative SNP/INDEL analysis.
Five old strains in group I had a deletion of TATGATAGGA at position 39651.
INDELs of (CT)n were at position 1116054 for many of the old and new isolates.
INDELs were present in the gene.
All 5 genetic changes were insertions of (C)n.
Smp — small outer-membrane lipoprotein; Oml — outer-membrane lipoprotein; Tbp — transferrin-binding protein.
Further analysis of putative virulence genes showed that the transferring-binding protein genes tbpA (locus tag HSM_0750) and tbpB (locus tag HSM_0749) had 73 and 24 SNPs, respectively; 20 and 7 SNPs belonged to group I, and 5 and 1 SNPs to group II, respectively. The gene encoding the small outer-membrane lipoprotein A/outer-membrane lipoprotein A domain-containing protein (locus tags HSM_0095 and HSM_1317) had 6 and 16 SNPs, respectively, 4 for group I and 5 for group II. The gene for the TonB-dependent receptor plug (locus tag HSM_0932) had 14 SNPs, with only 3 in group I. The gene encoding the TonB-dependent receptor (locus tags HSM_0047, HSM_0931, HSM_1372, and HSM_1962) had more SNPs in group I than in group II; for example, HSM_0047 had 65 SNPs, of which 21 belonged to group I, whereas only a single SNP belonged to group II. For most of the other genes represented in Table IV there were also more SNPs in group I than in group II. Although the emphasis was to cluster all isolates rather than look at the individual isolate, removing the recent isolate AVI14 from group II resulted in an increase in SNPs in group II to a number greater than that in group I for some locus tags. Finally, comparison of the number of SNPs in known virulence genes and the housekeeping gene that encodes uridine diphosphate-glucose 4-epimerase (Table V) showed that for the locus tags of the TonB family protein, fimbrial subunit, and TonB-dependent lactoferrin and transferrin receptor the old isolates contained significantly more SNPs (P < 0.05) than the new isolates.
Table V.
Number of SNP/INDELs in the housekeeping gene that encodes uridine diphosphate (UDP)-glucose 4-epimerase in comparison with known virulence genes of H. somni strains isolated during the 1980s (highlighted in blue) or in 2012–2013 (highlighted in green) with reference to H. somni 2336
| Gene product | Locus tag | Number of base pairs | P-value | Number of SNP/INDELs | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| HS5 | HS6 | HS11 | HS13 | HS18 | HS25 | AVI1 | AVI4 | AVI5 | AVI14 | AVI31 | AVI54 | ||||
| UDP-glucose 4-epimerase | HSM_1256 | 1017 | 0.051281 | 13 | 18 | 14 | 18 | 16 | 26 | 21 | 27 | 28 | 21 | 18 | 18 |
| Tbp | HSM_0749 | 1989 | 0.081602 | 9 | 8 | 10 | 8 | 8 | 4 | 6 | 5 | 6 | 1 | 6 | 10 |
| Lipooligosaccharide sialyltransferase | HSM_1426 | 903 | 0.354694 | 5 | 5 | 6 | 5 | 5 | 6 | 6 | 6 | 6 | 9 | 3 | 4 |
| Glycosyltransferase | HSM_0148 | 783 | 0.055693 | 2 | 4 | 4 | 4 | 2 | 3 | 4 | 3 | 4 | 5 | 4 | 4 |
| TonB family protein | HSM_1100 | 753 | 0.009998a | 11 | 11 | 11 | 11 | 11 | 3 | 3 | 3 | 3 | 4 | 3 | 11 |
| SmpA/OmlA domain-containing protein | HSM_0095 | 414 | 0.170447 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 0 |
| Fimbrial subunit | HSM_0123 | 459 | 0.019260a | 8 | 7 | 7 | 6 | 8 | 6 | 6 | 4 | 5 | 2 | 4 | 8 |
| Lipoprotein A | HSM_0191 | 414 | 0.188687 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 6 | 5 | 5 |
| TonB-dependent lactoferrin and transferrin receptor | HSM_0750 | 2916 | 0.004261a | 37 | 41 | 42 | 43 | 44 | 32 | 32 | 31 | 32 | 18 | 31 | 35 |
Significantly higher number of SNPs (P < 0.05) in the old isolates than in the new.
Discussion
Current H. somni vaccines that contain bacteria-free suspensions of outer membrane proteins have been shown to help reduce the risk of respiratory disease but may not protect cattle against other disease manifestations, such as myocarditis and arthritis. This may be due to the formation of biofilms during myocarditis, differences in the antigens present on bacteria in the host as compared with the vaccines, and sRNAs found in virulent strains (16,28,29). Research into the development of new vaccines with IbpA and outer membrane lipoproteins has provided an insight into potentially protective antigens (22,35). The current study was based on the possibility that comparative SNP analysis might shed light on changes in genomic characteristics between isolates from the 1980s and those from 2012–2013. The reference genome strain, H. somni 2336, contains 2065 genes that produce 1980 proteins containing locus tags HSM_0001 to HSM_2021 (NC_010519) (11,25). With reference to this genome, our comparative SNP analysis generated 31 028 SNP/INDELs with a SNP score of 0.00 to 1.00. Each genome isolated from the 1980s contributed approximately 40% to 42% SNP call against the H. somni reference strain with 8 times coverage or greater, whereas the more recent isolates had 36% to 41% SNP call. The distribution of these SNPs within a gene is different for each genome isolate, which in turn may cause variations in gene products associated with the changes in sequence. The distribution of about 75% of these SNPs within a specified gene differed between the old and new isolates and did not follow any particular pattern. The remaining 25% of SNPs (7674) clustered into 2 groups that contained the highest number of old or new strains. Group I (16%) included 5 old isolates and 1 new isolate, whereas group II (9%) contained 5 new isolates and 1 old isolate, which suggested some genetic variation between old and new isolates. However, if 1 new isolate (AVI14) was removed from group II, the total number of SNPs for this group increased to 4504 (15%), indicating a 6% increase in genetic variation between old and new isolates. Even though the SNPs may not be present in the clustered group, they may be present in individual isolates, old and/or new. In addition, as shown in Table IV, there were more SNPs among putative virulence genes in group I compared with pathogenic H. somni strain 2336, itself an older isolate, than in group II, which could be due to a number of factors, ranging from geographic origin of the isolate to bacterial biotype (36). Again, when strain AVI14 was removed from group II, the number of SNPs increased in some putative virulent genes, such as those encoding IbpA, TonB-dependent receptor, TonB system transporter, hemagglutinin/hemolysin-like protein, and Yersinia adhesion A domain-containing protein (locus tags HSM_1489, HSM_1372, HSM_1101, HSM_0270, HSM_0708, HSM_0844, HSM_1022, HSM_1257, and HSM_1542) in group II compared with group I, which suggested the accumulation of more SNPs in new strains than in old strains.
Focal myocarditis related to H. somni has been observed in fall-placed cattle in western provinces and states of North America (37). Clinical evaluations and results from postmortem inspections showed that 42% of deaths in 2007 were due to myocardial disease in the US state of Wyoming (38). An increase in rates of illness and death due to myocarditis has been reported in fall-placed calves in western Canada since the 1980s (38–41) and at present is the most common fatal form of the disease. However, current vaccines may protect against only the planktonic form of the bacteria and have limited efficacy against the myocardial form of the disease complex, suggesting a need for improved vaccines (16,28,29,42). At present, whole-genome sequencing of bacterial strains is an important tool to identify regulatory genes and, more importantly, genes involved in virulence. In particular, the identification of unique gene products could be used to formulate new vaccines against H. somni infection, resulting in protection against new strains circulating in the field.
Acknowledgments
This work was supported by the Alberta Livestock and Meat Agency and the Alberta Cattle Feeders Association.
Footnotes
Disclosure of potential conflicts of interest
No potential conflict of interest was disclosed.
The authors declare that there are no conflicts of interest or financial conflicts of interest.
References
- 1.Stephens LR, Little PB, Wilkie BN, Barnum DA. Infectious thromboembolic meningoencephalitis in cattle: A review. J Am Vet Med Assoc. 1981;178:378–384. [PubMed] [Google Scholar]
- 2.Corbeil LB, Widders PR, Gogolewski R, Arthur J, Inzana TJ, Ward AC. Haemophilus somnus: Bovine reproductive and respiratory disease. Can Vet J. 1986;27:90–93. [PMC free article] [PubMed] [Google Scholar]
- 3.Gagea MI, Bateman KG, van Dreumel T, et al. Diseases and pathogens associated with mortality in Ontario beef feedlots. J Vet Diagn Invest. 2006;18:18–28. doi: 10.1177/104063870601800104. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy PC, Biberstein EL, Howarth JA, Frazier LM, Dungworth DL. Infectious meningo-encephalitis in cattle, caused by a Haemophilus-like organism. Am J Vet Res. 1960;21:403–409. [PubMed] [Google Scholar]
- 5.Humphrey JD, Stephens LR. ‘Haemophilus somnus:’ A review. Vet Bull. 1983;53:987–1004. [Google Scholar]
- 6.Guichon PT, Pritchard J, Jim GK. Haemophilus somnus myocarditis in a feedlot steer. Can Vet J. 1988;29:1012–1013. [PMC free article] [PubMed] [Google Scholar]
- 7.Philbey AW, Glastonbury JRW, Rothwell JT, Links IJ, Searson JE. Meningoencephalitis and other conditions associated with Histophilus ovis infection in sheep. Aust Vet J. 1991;68:387–390. doi: 10.1111/j.1751-0813.1991.tb03104.x. [DOI] [PubMed] [Google Scholar]
- 8.Ward ACS, Dyer NW, Corbeil LB. Characterization of putative Haemophilus somnus from tonsils of American bison (Bison bison) Can J Vet Res. 1999;63:166–169. [PMC free article] [PubMed] [Google Scholar]
- 9.Kennedy PC, Frazier LM, Theilen GN, Biberstein EL. A septicemic disease of lambs caused by Haemophilus agni (new species) Am J Vet Res. 1958;19:645–654. [PubMed] [Google Scholar]
- 10.Ward ACS, Weiser GC, Anderson BC, Cummings PJ, Arnold KF, Corbeil LB. Haemophilus somnus (Histophilus somni) in bighorn sheep. Can J Vet Res. 2006;70:34–42. [PMC free article] [PubMed] [Google Scholar]
- 11.Siddaramappa S, Challacombe JF, Duncan AJ, et al. Horizontal gene transfer in Histophilus somni and its role in the evolution of pathogenic strain 2336, as determined by comparative genomic analyses. BMC Genomics. 2011;12:570–590. doi: 10.1186/1471-2164-12-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Y, McQuiston JH, Cox A, Pack TD, Inzana TJ. Molecular cloning and mutagenesis of a DNA locus involved in lipooligosaccharide biosynthesis in Haemophilus somnus. Infect Immun. 2000;68:310–319. doi: 10.1128/iai.68.1.310-319.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cole SP, Guiney DG, Corbeil LB. Molecular analysis of a gene encoding a serum-resistance-associated 76 kDa surface antigen of Haemophilus somnus. J Gen Microbiol. 1993;139:2135–2143. doi: 10.1099/00221287-139-9-2135. [DOI] [PubMed] [Google Scholar]
- 14.McQuiston JH, McQuiston JR, Cox AD, Wu Y, Boyle SM, Inzana TJ. Characterization of a DNA region containing 5′-(CAAT)(n)-3′ DNA sequences involved in lipooligosaccharide biosynthesis in Haemophilus somnus. Microb Pathog. 2000;28:301–312. doi: 10.1006/mpat.1999.0351. [DOI] [PubMed] [Google Scholar]
- 15.Inzana TJ, Gogolewski RP, Corbeil LB. Phenotypic phase variation in Haemophilus somnus lipooligosaccharide during bovine pneumonia and after in vitro passage. Infect Immun. 1992;60:2943–2951. doi: 10.1128/iai.60.7.2943-2951.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sandal I, Inzana TJ, Molinaro A, et al. Identification, structure, and characterization of an exopolysaccharide produced by Histophilus somni during biofilm formation. BMC Microbiology. 2011;11:186–201. doi: 10.1186/1471-2180-11-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elswaifi SF, Scarratt WK, Inzana TJ. The role of lipooligosaccharide phosphorylcholine in colonization and pathogenesis of Histophilus somni in cattle. Vet Res. 2012;43:49–62. doi: 10.1186/1297-9716-43-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corbeil LB, Gogolewski RP, Stephens LR, Inzana TJ. Haemophilus somnus: Antigen analysis and immune responses. In: Donachie W, editor. Haemophilus, Actinobacillus, and Pasteurella. New York, New York: Plenum Press; 1995. pp. 63–73. [Google Scholar]
- 19.Sanders JD, Bastida-Corcuera FD, Arnold KF, Wunderlich AC, Corbeil LB. Genetic manipulation of immunoglobulin binding proteins of Haemophilus somnus. Microb Pathog. 2003;34:131–139. doi: 10.1016/s0882-4010(02)00188-2. [DOI] [PubMed] [Google Scholar]
- 20.Gomis SM, Godson DL, Beskorwayne T, Wobeser GA, Potter AA. Modulation of phagocytic function of bovine mononuclear phagocytes by Haemophilus somnus. Microb Pathog. 1997;22:13–21. doi: 10.1006/mpat.1996.0086. [DOI] [PubMed] [Google Scholar]
- 21.Gray-Owen SD, Schryvers AB. Characterization of transferrin binding proteins 1 and 2 in invasive type b and nontypeable strains of Haemophilus influenzae. Infect Immun. 1995;63:3809–3815. doi: 10.1128/iai.63.10.3809-3815.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geertsema RS, Worby C, Kruger RP, et al. Protection of mice against H. somni septicemia by vaccination with recombinant immunoglobulin binding protein subunits. Vaccine. 2008;26:4506–4512. doi: 10.1016/j.vaccine.2008.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoshinoo K, Sasaki K, Tanaka A, Corbeil LB, Tagawa Y. Virulence attributes of Histophilus somni with a deletion mutation in the ibpA gene. Microb Pathog. 2009;46:273–282. doi: 10.1016/j.micpath.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Pareek CS, Smoczynski R, Tretyn A. Sequencing technologies and genome sequencing. J Appl Genet. 2011;52:413–435. doi: 10.1007/s13353-011-0057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandal I, Inzana TJ. A genomic window into the virulence of Histophilus somni: A review. Trends Microbiol. 2010;18:90–99. doi: 10.1016/j.tim.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Challacombe JF, Duncan AJ, Brettin TS, et al. Complete genome sequence of Haemophilus somnus (Histophilus somni) strain 129Pt and comparison to Haemophilus ducreyi 35000HP and Haemophilus influenzae Rd. J Bacteriol. 2007;189:1890–1898. doi: 10.1128/JB.01422-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Novartis Animal Health Trial Report. Basel, Switzerland: Novartis International; 2007. SOMNU-STAR Ph newborn calf vaccination protocols. [Google Scholar]
- 28.Sandal I, Shao JQ, Annadata S, et al. Histophilus somni biofilm formation in cardiopulmonary tissue of the bovine host following respiratory challenge. Microbes Infect. 2009;11:254–263. doi: 10.1016/j.micinf.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 29.Kumar R, Lawrence MK, Watt J, Cooksey AM, Burgess SC, Nanduri B. RNA-seq based transcriptional map of bovine respiratory disease pathogen “Histophilus somni 2336”. PLoS One. 2012;7:e29435. doi: 10.1371/journal.pone.0029435. Epub 2012 Jan 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hellenbrand KM, Forsythe KM, Rivera-Rivas JJ, Czuprynski CJ, Aulik NA. Histophilus somni causes extracellular trap formation by bovine neutrophils and macrophages. Microb Pathog. 2013;54:67–75. doi: 10.1016/j.micpath.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Angen Ø, Ahrens P, Tegtmeier C. Development of a PCR test for identification of Haemophilus somnus in pure and mixed cultures. Vet Microbiol. 1998;63:39–48. doi: 10.1016/s0378-1135(98)00222-3. [DOI] [PubMed] [Google Scholar]
- 32.Casjens S. The diverse and dynamic structures of bacterial genomes. Annu Rev Genet. 1998;32:339–377. doi: 10.1146/annurev.genet.32.1.339. [DOI] [PubMed] [Google Scholar]
- 33.Su YC, Resman F, Hörhold F, Riesbeck K. Comparative genomic analysis reveals distinct genotypic features of the emerging pathogen Haemophilus influenzae type f. BMC Genomics. 2014;15:38–61. doi: 10.1186/1471-2164-15-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanaka A, Hoshinoo K, Hoshino T, Tagawa Y. Differentiation between bovine and ovine strains of Histophilus somni based on the sequences of 16S rDNA and rpoB gene. J Vet Med Sci. 2005;67:255–262. doi: 10.1292/jvms.67.255. [DOI] [PubMed] [Google Scholar]
- 35.Guzmán-Brambila C, Rojas-Mayorquín AE, Flores-Samaniego B, Ortuño-Sahagún D. Two outer membrane lipoproteins from Histophilus somni are immunogenic in rabbits and sheep and induce protection against bacterial challenge in mice. Clin Vaccine Immunol. 2012;19:1826–1832. doi: 10.1128/CVI.00451-12. Epub 2012 Sep 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ward ACS, Jaworski MD, Eddow JM, Corbeil LB. A comparative study of bovine and ovine Haemophilus somnus isolates. Can J Vet Res. 1995;59:173–178. [PMC free article] [PubMed] [Google Scholar]
- 37.O’Toole D, Allen T, Hunter R, Corbeil LB. Diagnostic exercise: Myocarditis due to Histophilus somni in feedlot and backgrounded cattle. Vet Pathol. 2009;46:1015–1017. doi: 10.1354/vp.08-VP-0332-O-DEX. [DOI] [PubMed] [Google Scholar]
- 38.Haines DM, Moline KM, Sargent RA, Campbell JR, Myers DJ, Doig PA. Immunohistochemical study of Hemophilus somnus, Mycoplasma bovis, Mannheimia hemolytica, and bovine viral diarrhea virus in death losses due to myocarditis in feedlot cattle. Can Vet J. 2004;45:231–234. [PMC free article] [PubMed] [Google Scholar]
- 39.Harris FW, Janzen ED. The Haemophilus somnus disease complex (hemophilosis): A review. Can Vet J. 1989;30:816–822. [PMC free article] [PubMed] [Google Scholar]
- 40.Maxie MG, Robinson WF. Cardiovascular system. In: Maxie MG, editor. Pathology of Domestic Animals. 5th ed. Philadelphia, Pennsylvania: Elsevier Saunders; 2007. p. 32. [Google Scholar]
- 41.Orr JP. Haemophilus somnus infection: A retrospective analysis of cattle necropsied at the Western College of Veterinary Medicine from 1970 to 1990. Can Vet J. 1992;33:719–722. [PMC free article] [PubMed] [Google Scholar]
- 42.Van Donkersgoed J, Janzen ED, Harland RJ. Epidemiological features of calf mortality due to hemophilosis in a large feedlot. Can Vet J. 1990;31:821–825. [PMC free article] [PubMed] [Google Scholar]