Abstract
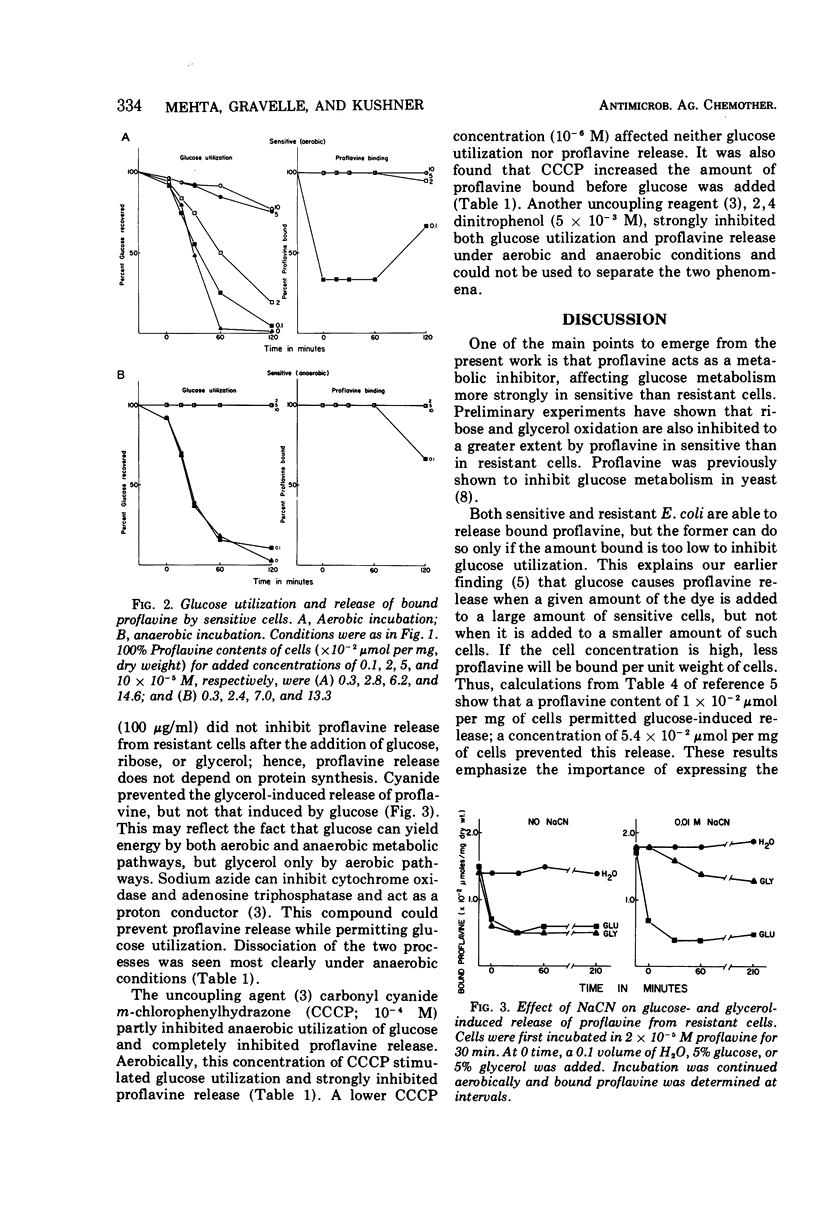
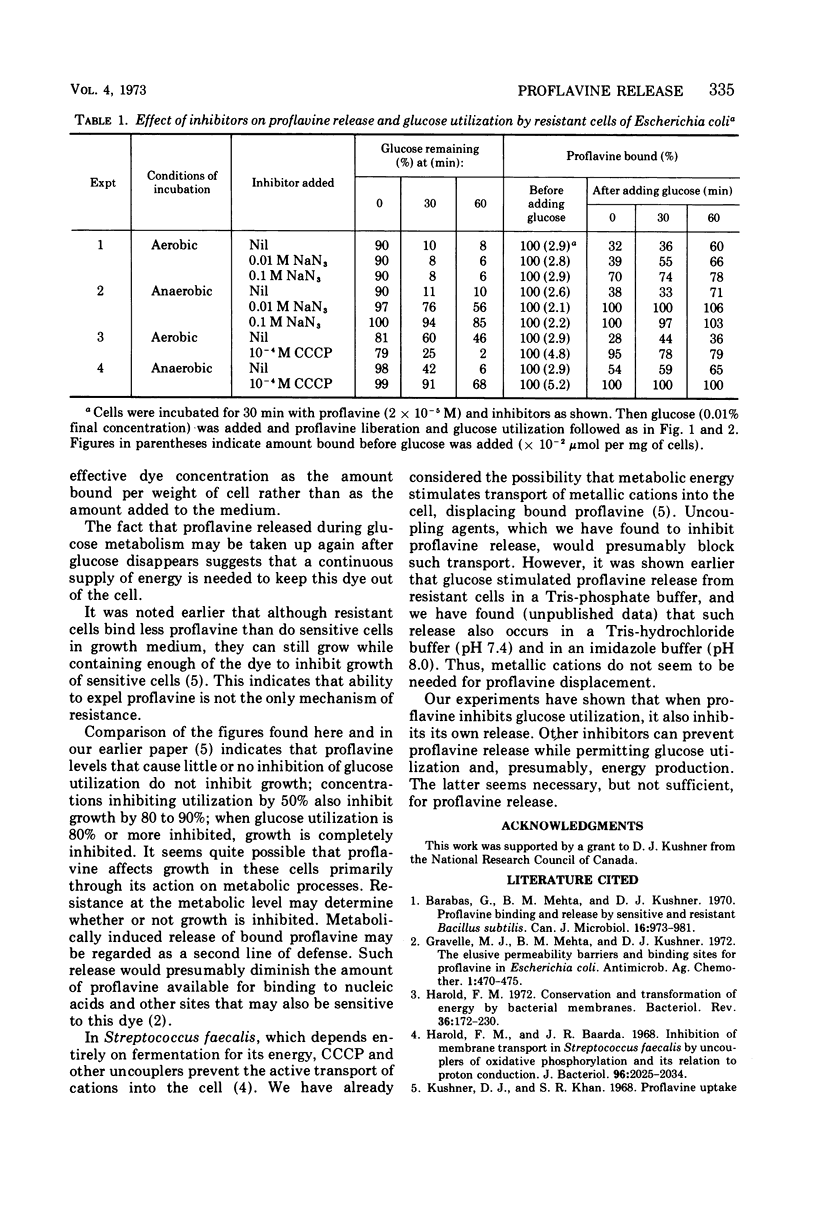
Proflavine inhibited the aerobic and anaerobic utilization of glucose by sensitive but not by resistant Escherichia coli. In resistant cells that had bound proflavine, glucose utilization was accompanied by release of the dye. After glucose was used up, the cells could again take up proflavine. If the amount of proflavine bound to sensitive cells was too low to inhibit glucose utilization, adding glucose to these cells caused them to release the dye. With higher proflavine concentrations, inhibitory to glucose utilization, the dye remained cell bound. Thus, metabolic energy causes the release of proflavine by both sensitive and resistant cells. In the former, energy production is inhibited by proflavine, and thus the dye prevents its own release. Chloramphenicol did not interfere with metabolically induced release of proflavine from resistant cells. Cyanide inhibited the glycerol-induced loss of proflavine, but not the glucose-induced loss. Azide and carbonyl cyanide m-chlorophenylhydrazone could prevent proflavine release without inhibiting glucose utilization.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barabas G., Mehta B. M., Kushner D. J. Proflavine binding and release by sensitive and resistant Bacillus subtilis. Can J Microbiol. 1970 Oct;16(10):973–981. doi: 10.1139/m70-166. [DOI] [PubMed] [Google Scholar]
- Gravelle M. J., Mehta B. M., Kushner D. J. The elusive permeability barriers and binding sites for proflavine in Escherichia coli. Antimicrob Agents Chemother. 1972 Jun;1(6):470–475. doi: 10.1128/aac.1.6.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R. Inhibition of membrane transport in Streptococcus faecalis by uncouplers of oxidative phosphorylation and its relationship to proton conduction. J Bacteriol. 1968 Dec;96(6):2025–2034. doi: 10.1128/jb.96.6.2025-2034.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972 Jun;36(2):172–230. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D. L. Quantitative Determination of Carbohydrates With Dreywood's Anthrone Reagent. Science. 1948 Mar 5;107(2775):254–255. doi: 10.1126/science.107.2775.254. [DOI] [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]



