Abstract
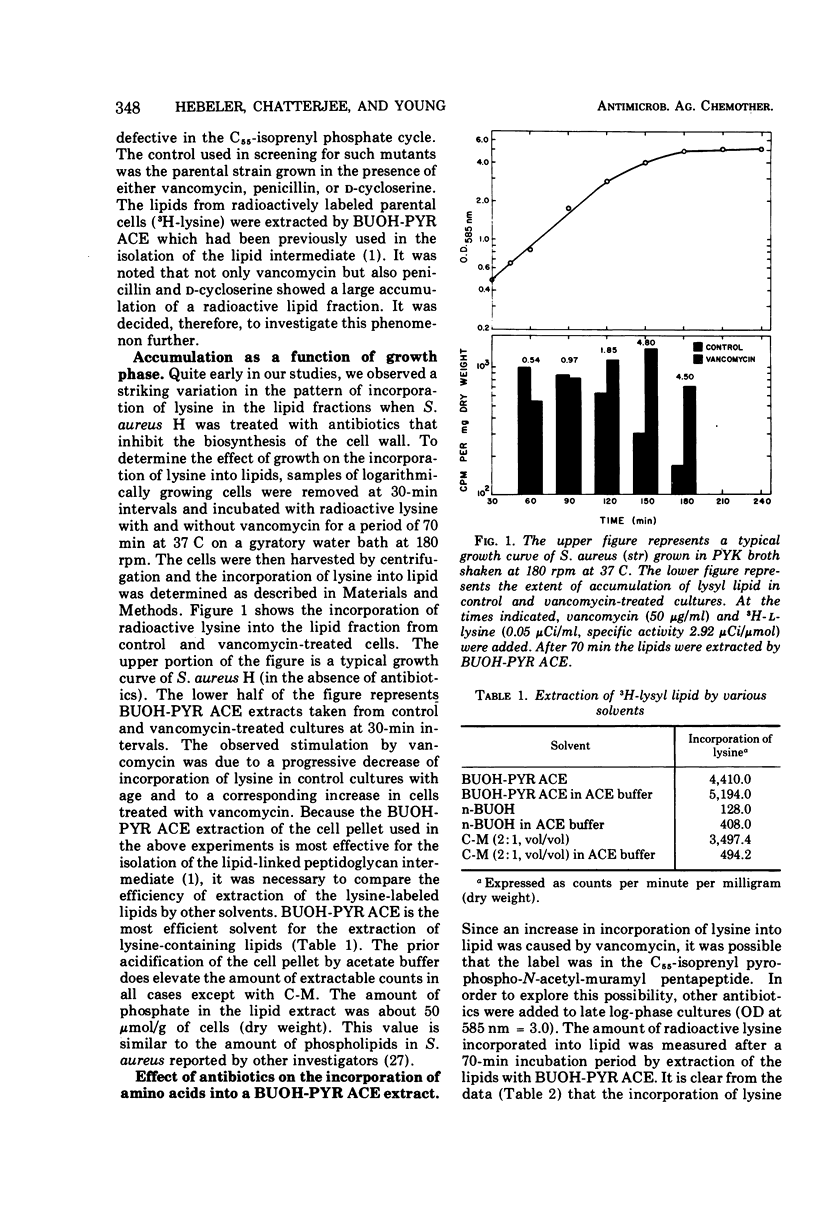
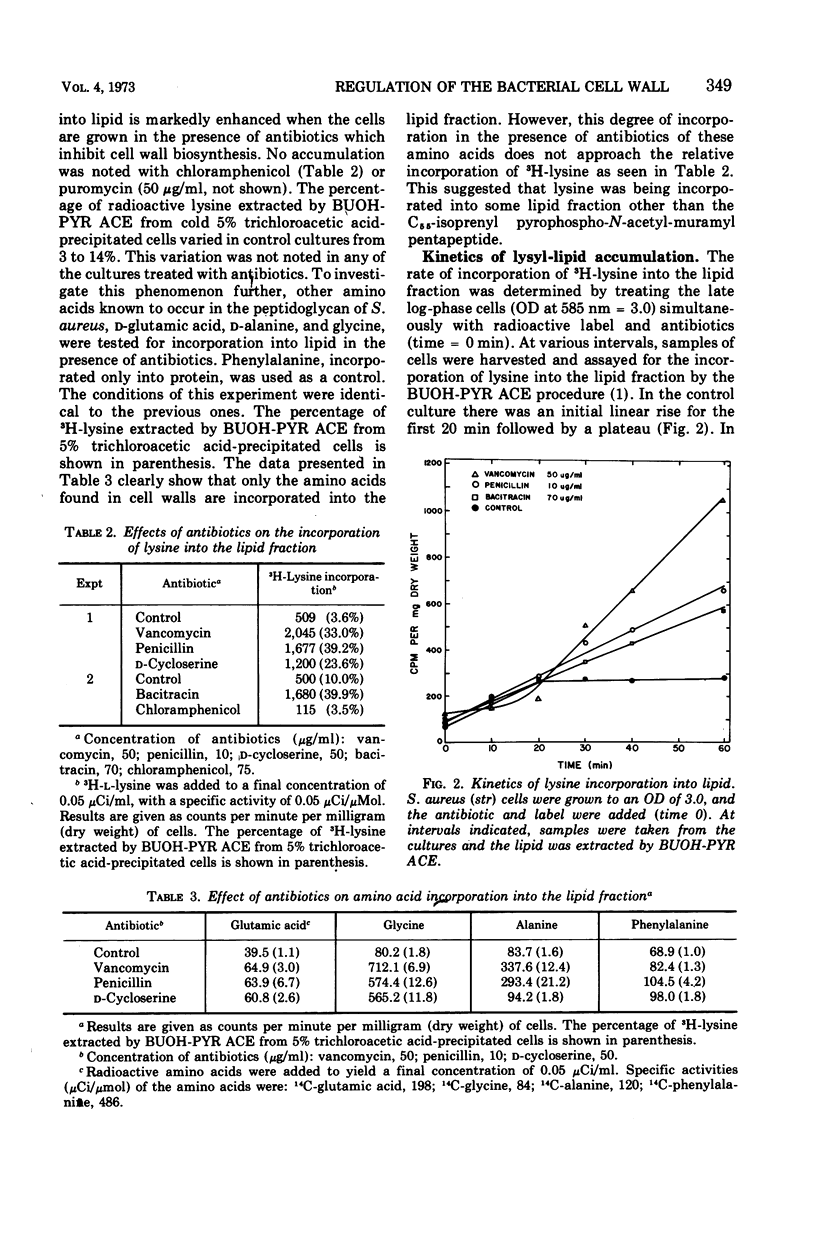
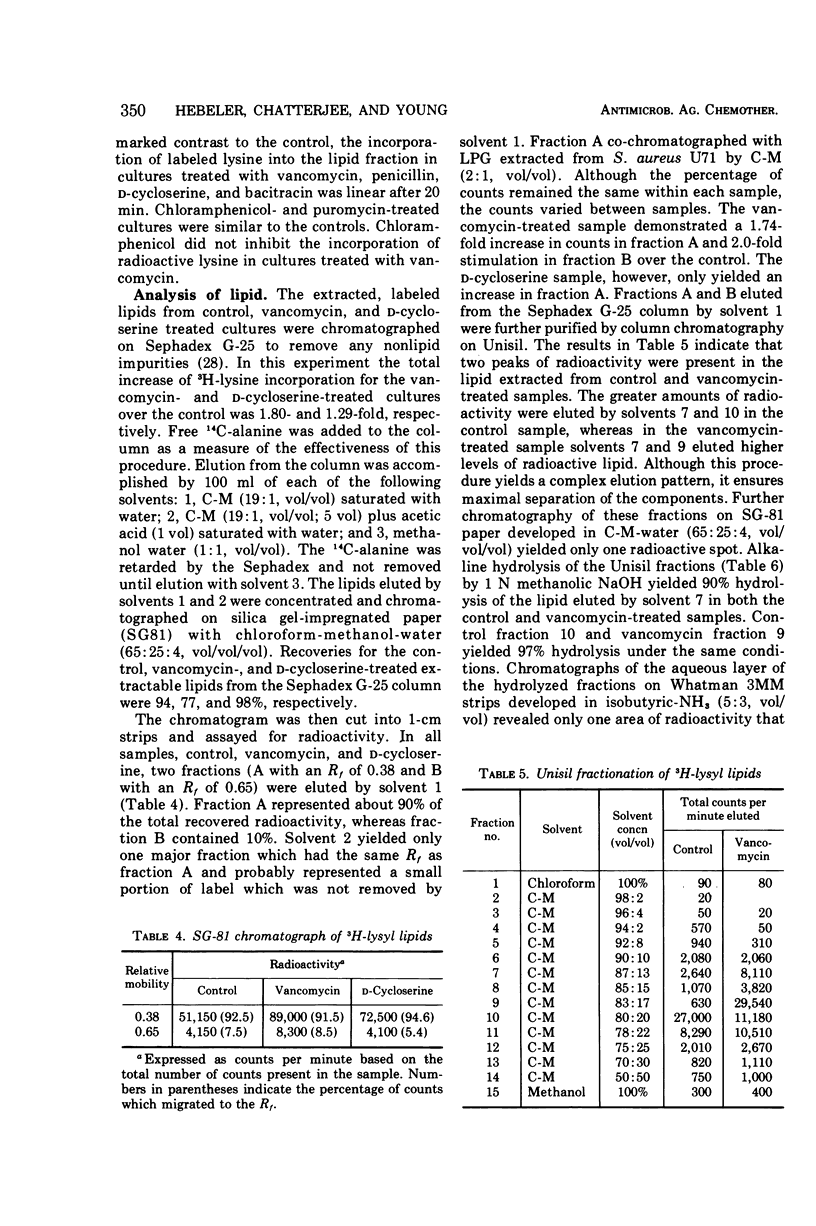
Antibiotics that inhibit the biosynthesis of the cell wall, such as vancomycin, penicillin, d-cycloserine, and bacitracin, stimulate the incorporation of lysine into lipids that are extractable with n-butanol-6 M pyridinium acetate. Approximately 93% of this lysine is in lysylphosphatidylglycerol (LPG). The remaining lysine is incorporated in another as yet uncharacterized lipid. Because the lysine in the latter lipid is released by mild alkaline hydrolysis, it is not the C55-isoprenyl-pyrophospho-N-acetyl-muramyl pentapeptide. Vancomycin and penicillin stimulate the incorporation of lysine into both LPG and the minor lipid fractions, whereas treatment with d-cycloserine results in an increase only in LPG. Antibiotics that inhibit protein synthesis do not influence the incorporation of lysine into the lipid fractions. Analysis of the extracted lipids indicate that the incorporation of radioactive lysine into LPG is due to an enhancement in synthesis of LPG from phospholipids in the cytoplasmic membrane.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. S., Matsuhashi M., Haskin M. A., Strominger J. L. Biosythesis of the peptidoglycan of bacterial cell walls. II. Phospholipid carriers in the reaction sequence. J Biol Chem. 1967 Jul 10;242(13):3180–3190. [PubMed] [Google Scholar]
- Chatterjee A. N. Use of bacteriophage-resistant mutants to study the nature of the bacteriophage receptor site of Staphylococcus aureus. J Bacteriol. 1969 May;98(2):519–527. doi: 10.1128/jb.98.2.519-527.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas L. J., Baddiley J. A lipid intermediate in the biosynthesis of a teichoic acid. FEBS Lett. 1968 Aug;1(2):114–116. doi: 10.1016/0014-5793(68)80034-1. [DOI] [PubMed] [Google Scholar]
- GALE E. F., FOLKES J. P. THE INCORPORATION OF GLYCEROL AND LYSINE INTO THE LIPID FRACTION OF STAPHYLOCOCCUS AUREUS. Biochem J. 1965 Feb;94:390–400. doi: 10.1042/bj0940390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould R. M., Lennarz W. J. Metabolism of Phosphatidylglycerol and Lysyl Phosphatidylglycerol in Staphylococcus aureus. J Bacteriol. 1970 Dec;104(3):1135–1144. doi: 10.1128/jb.104.3.1135-1144.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENDLER R. W. Passage of radioactive amino acids through nonprotein fractions of hen oviduct during incorporation into protein. J Biol Chem. 1959 Jun;234(6):1466–1473. [PubMed] [Google Scholar]
- HOUTSMULLER U. M., VAN DEENENL ON THE ACCUMULATION OF AMINO ACID DERIVATIVES OF PHOSPHATIDYLGLYCEROL IN BACTERIA. Biochim Biophys Acta. 1964 Feb 24;84:96–98. doi: 10.1016/0926-6542(64)90106-4. [DOI] [PubMed] [Google Scholar]
- HOUTSMULLER U. M., van DEENEN L. Identification of a bacterial phospholipid as an O-ornithine ester of phosphatidyl glycerol. Biochim Biophys Acta. 1963 Apr 23;70:211–213. doi: 10.1016/0006-3002(63)90743-1. [DOI] [PubMed] [Google Scholar]
- HUNTER G. D., GOODSALL R. A. Lipo-amino acid complexes from Bacillus megaterium and their possible role in protein synthesis. Biochem J. 1961 Mar;78:564–570. doi: 10.1042/bj0780564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haest C. W., de Gier J., den Kamp JA O. P., Bartels P., van Deenen L. L. Chages in permeability of Staphylococcus aureus and derived liposomes with varying lipid composition. Biochim Biophys Acta. 1972 Mar 17;255(3):720–733. doi: 10.1016/0005-2736(72)90385-9. [DOI] [PubMed] [Google Scholar]
- Higashi Y., Strominger J. L., Sweeley C. C. Structure of a lipid intermediate in cell wall peptidoglycan synthesis: a derivative of a C55 isoprenoid alcohol. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1878–1884. doi: 10.1073/pnas.57.6.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtsmuller U. M., van Deenen L. L. On the amino acid esters of phosphatidyl glycerol from bacteria. Biochim Biophys Acta. 1965 Dec 2;106(3):564–576. doi: 10.1016/0005-2760(65)90072-x. [DOI] [PubMed] [Google Scholar]
- Katz W., Matsuhashi M., Dietrich C. P., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. IV. Incorporation of glycine in Micrococcus lysodeikticus. J Biol Chem. 1967 Jul 10;242(13):3207–3217. [PubMed] [Google Scholar]
- LEA C. H., RHODES D. N., STOLL R. D. Phospholipids. 3. On the chromatographic separation of glycerophospholipids. Biochem J. 1955 Jul;60(3):353–363. doi: 10.1042/bj0600353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennarz W. J., Nesbitt J. A., 3rd, Reiss J. The participation of sRNA in the enzymatic synthesis of O-L-lysyl phosphatidylgylcerol in Staphylococcus aureus. Proc Natl Acad Sci U S A. 1966 Apr;55(4):934–941. doi: 10.1073/pnas.55.4.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillich T. T., White D. C. Phospholipid metabolism in the absence of net phospholipid synthesis in a glycerol-requiring mutant of Bacillus subtilis. J Bacteriol. 1971 Sep;107(3):790–797. doi: 10.1128/jb.107.3.790-797.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore L. D., Kocun F. J., Umbreit W. W. Cell-free protein synthesis: effects of age and state of ribosomal aggregation. Science. 1966 Dec 9;154(3754):1350–1353. doi: 10.1126/science.154.3754.1350. [DOI] [PubMed] [Google Scholar]
- PARK J. T., HANCOCK R. A fractionation procedure for studies of the synthesis of cell-wall mucopeptide and of other polymers in cells of Staphylococcus aureus. J Gen Microbiol. 1960 Feb;22:249–258. doi: 10.1099/00221287-22-1-249. [DOI] [PubMed] [Google Scholar]
- Short S. A., White D. C. Metabolism of phosphatidylglycerol, lysylphosphatidylglycerol, and cardiolipin of Staphylococcus aureus. J Bacteriol. 1971 Oct;108(1):219–226. doi: 10.1128/jb.108.1.219-226.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siakotos A. N. Analytical separation of nonlipid water soluble substances and gangliosides from other lipids by dextran gel column chromatography. J Am Oil Chem Soc. 1965 Nov;42(11):913–919. doi: 10.1007/BF02632444. [DOI] [PubMed] [Google Scholar]
- Siewert G., Strominger J. L. Bacitracin: an inhibitor of the dephosphorylation of lipid pyrophosphate, an intermediate in the biosynthesis of the peptidoglycan of bacterial cell walls. Proc Natl Acad Sci U S A. 1967 Mar;57(3):767–773. doi: 10.1073/pnas.57.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöholm I., Ekenäs A. K., Sjöquist J. Protein A from Staphylococcus aureus. Acetylation of protein A with acetylimidazole. Eur J Biochem. 1972 Sep 25;29(3):455–460. doi: 10.1111/j.1432-1033.1972.tb02009.x. [DOI] [PubMed] [Google Scholar]
- Strominger J. L., Izaki K., Matsuhashi M., Tipper D. J. Peptidoglycan transpeptidase and D-alanine carboxypeptidase: penicillin-sensitive enzymatic reactions. Fed Proc. 1967 Jan-Feb;26(1):9–22. [PubMed] [Google Scholar]
- Tipper D. J., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. XII. Inhibition of cross-linking by penicillins and cephalosporins: studies in Staphylococcus aureus in vivo. J Biol Chem. 1968 Jun 10;243(11):3169–3179. [PubMed] [Google Scholar]
- Tipper D. J., Strominger J. L. Mechanism of action of penicillins: a proposal based on their structural similarity to acyl-D-alanyl-D-alanine. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1133–1141. doi: 10.1073/pnas.54.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbreit J. N., Strominger J. L. Complex lipid requirements for detergent-solubilized phosphoacetylmuramyl-pentapeptide translocase from Micrococcus luteus. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1972–1974. doi: 10.1073/pnas.69.7.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise E. M., Jr, Park J. T. Penicillin: its basic site of action as an inhibitor of a peptide cross-linking reaction in cell wall mucopeptide synthesis. Proc Natl Acad Sci U S A. 1965 Jul;54(1):75–81. doi: 10.1073/pnas.54.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A., Dankert M., Fennessey P., Robbins P. W. Characterization of a polyisoprenoid compound functional in O-antigen biosynthesis. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1798–1803. doi: 10.1073/pnas.57.6.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Kamp JA O. P., Houtsmuller U. M., van Deenen L. L. On the phospholipids of Bacillus megaterium. Biochim Biophys Acta. 1965 Oct 4;106(2):438–441. doi: 10.1016/0005-2760(65)90059-7. [DOI] [PubMed] [Google Scholar]



