Abstract
Cerebral Dopamine Neurotrophic Factor (CDNF) and Mesencephalic Astrocyte-derived Neurotrophic factor (MANF) are members of a recently discovered family of neurotrophic factors (NTFs). Here, we used intranigral or intrastriatal lentiviral vector-mediated expression to evaluate their efficacy at protecting dopaminergic function in the 6-OHDA model of Parkinson's disease (PD). In contrast to the well-studied Glial-Derived Neurotrophic Factor (GDNF), no beneficial effects were demonstrated by striatal overexpression of either protein. Interestingly, nigral overexpression of CDNF decreased amphetamine-induced rotations and increased tyroxine hydroxylase (TH) striatal fiber density but had no effect on numbers of TH+ cells in the SN. Nigral MANF overexpression had no effect on amphetamine-induced rotations or TH striatal fiber density but resulted in a significant preservation of TH+ cells. Combined nigral overexpression of both factors led to a robust reduction in amphetamine-induced rotations, greater increase in striatal TH-fiber density and significant protection of TH+ cells in the SN. We conclude that nigral CDNF and MANF delivery is more efficacious than striatal delivery. This is also the first study to demonstrate that combined NTF can have synergistic effects that result in enhanced neuroprotection, suggesting that multiple NTF delivery may be more efficacious for the treatment of PD than the single NTF approaches attempted so far.
Introduction
Parkinson's disease (PD) is a progressive and debilitating age-associated neurodegenerative condition that is characterized by tremor, bradykinesia, rigidity, postural instability and also by nonmotor symptoms including cognitive disturbances. One of the main features of PD is the degeneration of dopaminergic neurons in the substantia nigra (SN) and the loss of dopaminergic neurotransmission in the corpus striatum, which underlies the motor symptoms. However, in advanced stages, the pathology also spreads towards cortical areas and causes cognitive decline and psychiatric symptoms.1
Neurotrophic factors (NTFs) are naturally occurring proteins that are essential to neuronal differentiation and maturation during development and adulthood. It is now well established that GDNF can protect dopamine neurons from several insults and restore function in animal models of PD (for review see ref. 1,2). More recently, another member of the GDNF family, Neurturin has also been shown to be neuroprotective in animal models of PD.3,4,5 Both GDNF6,7,8 and Neurturin9,10 have been tested in clinical trials but so far, the results are modest. Furthermore, GDNF, the prototypical NTF for dopaminergic neurons failed to prevent dopamine neuron degeneration in the rat α-synuclein model of PD11,12 suggesting that although extremely effective in toxin-based models, GDNF might not be applicable in models more relevant to the pathology of PD and that alternative NTFs are needed.
The newest candidate growth factor for dopamine neurons is Cerebral Dopamine Neurotrophic Factor (CDNF).13 It is a vertebrate specific paralogue of the recently-identified human Mesencephalic Astrocyte-derived Neurotrophic Factor (MANF).14 CDNF mRNA expression has been shown in the developing mouse brain and in various adult tissues by RT-PCR. CDNF mRNA transcripts were present both in the embryonic and adult midbrain and were also detected in the striatum.13 Similarly, MANF is also present in all stages of development and in a wide range of tissues. In the brain, MANF protein is found in neurons throughout the cortex, the cerebellum, hippocampus and midbrain where it colocalizes partially with TH.15 Interestingly, disruption of the MANF gene in Drosophila melanogaster, leads to a striking loss of TH+ neurites—but not dopaminergic cell bodies—and reduced dopamine levels.16
More importantly, like GDNF and Neurturin, CDNF and MANF are neurorestorative when delivered following an intra-striatal 6-OHDA injection in the rat.13,17 CDNF delivery into the striatum prior to 6-OHDA lesion was able to dose dependently prevent the loss of TH+ neurons in the SN.13 In addition, CDNF administered 4 weeks following a 6-OHDA lesion was able to increase the number of TH+ neurons in the SN by preventing the death of remaining neurons compared to vehicle treated controls, when measured 12 weeks after lesion.13 In this study, one single dose of the purified CDNF protein could mediate significant neuroprotection. Similarly, one single dose of MANF into the rat striatum was also neuroprotective as well as neurorestorative.18 Moreover, CDNF elicited significant neuroprotective and neurorestorative effects in the mouse MPTP model of PD19 and MANF promoted the survival of dopamine neurons in vitro.14 Taken together, these studies suggest the CDNF/MANF family may be beneficial for the treatment of PD.
In this study, we tested if CDNF and MANF are protective in the 6-OHDA rat model of PD, evaluating both striatal and nigral NTF-delivery using lentiviral vectors, which are suitable for long-term and stable transduction of neural cells (for review see ref. 20). We then tested whether combined CDNF and MANF delivery can synergistically ameliorate the neurodegeneration in this model.
Results
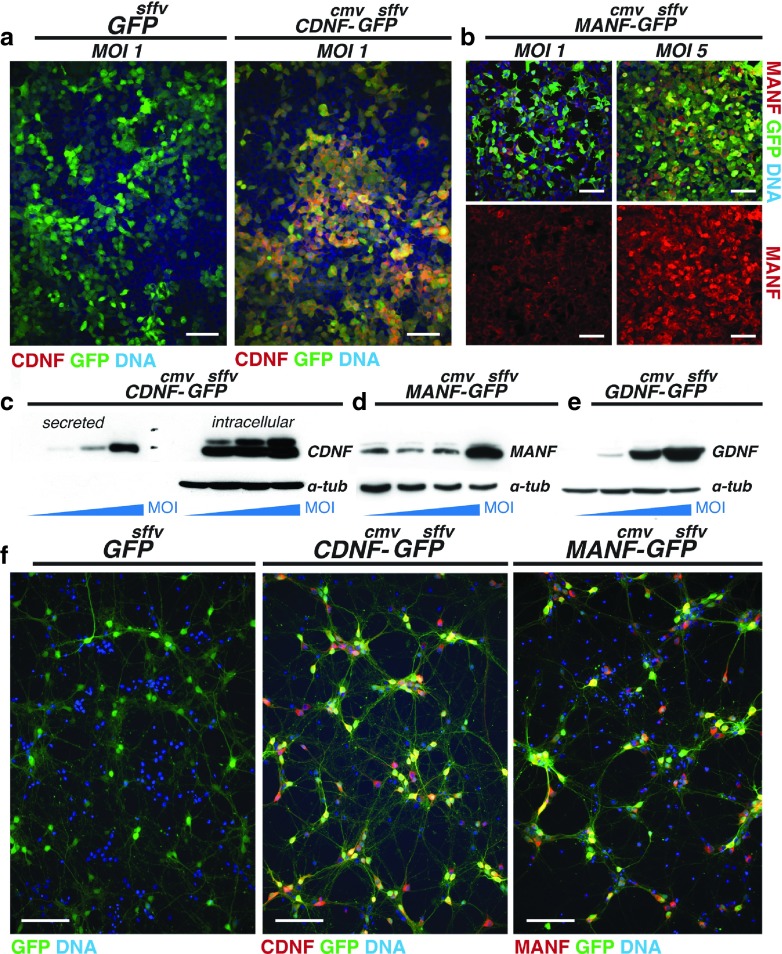
We generated four lentiviral vectors, namely Lenti.GFP, Lenti.CDNF-GFP, Lenti.MANF-GFP, and Lenti.GDNF-GFP (Figure 1) in order to evaluate the effects of CDNF, MANF, and GDNF overexpression in the 6-OHDA lesion model of Parkinson's disease. To ensure that the lentiviral vectors generated were able to infect target cells and induce the expression of the respective neurotrophic factors, concentrated lentiviral preparations were used to transduce HEK293T cells at increasing MOI (0, 0.1, 1, and 5). 72 hours after transduction, the culture medium and cell pellets were collected and analyzed by immunoblotting or the cells fixed and processed for immunocytochemistry. Abundant CDNF protein was produced and secreted by the transduced cells and production levels were correlated to increasing MOI of the vector (Figure 2a,c). CDNF appeared in two separated bands that correspond to different glycosylation forms21 and was not detected in control cells, suggesting that endogenous CDNF was not present in HEK293T cells. In contrast, HEK293T cells have endogenous MANF expression but robust MANF overexpression can be effectively achieved with Lenti.MANF-GFP at high MOIs (Figure 2b,d). No MANF expression could be detected in the conditioned medium from control or transduced cells, indicating that MANF is not secreted under these experimental conditions. Similarly, transduction with the Lenti.GDNF-GFP vector resulted in robust GDNF protein levels in HEK293T cells (Figure 2e). Lenti.CDNF-GFP and Lenti.MANF-GFP were then used to transduce E18 cortical neurons. Robust CDNF and MANF expression from the respective vectors was observed in the transduced neurons (Figure 2f). As functional validation, we confirmed the ability of Lenti.CDNF-GFP to promote neurite outgrowth from embryonic midbrain TH+ neurons (Supplementary Figure S3) and to transduce dopaminergic neurons in vivo following intranigral delivery (Supplementary Figure S4).
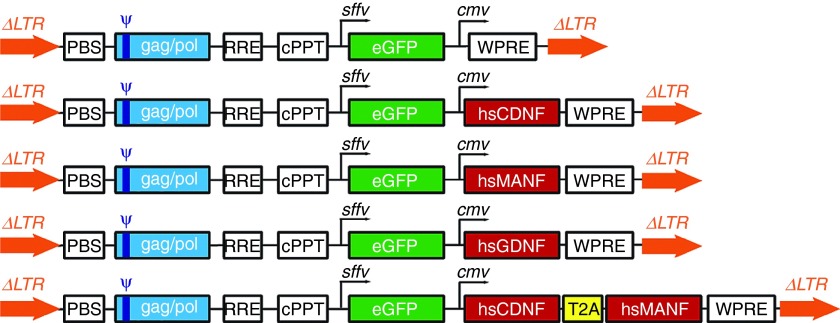
Figure 1.
Lentiviral vector design. All vectors contain GFP as reporter gene under the spleen focus-forming virus (sffv) promoter, the gene of interest under the cmv promoter. cPPT, central poly-purine tract; PBS, primer binding site; RRE, rev response element; WPRE, woodchuck hepatitis virus posttranscriptional regulatory element; ΔLTR, truncated long terminal repeats; ψ: packaging signal.
Figure 2.
Lentiviral vector validation in vitro. (a) Transduction with Lenti.CDNF-GFP induces CDNF overexpression in HEK293T cells; (b) Transduction with Lenti.MANF-GFP induces MANF overexpression. Scale bars = 100 µm. Immunoblotting showing; (c) increased CDNF expression in culture supernatants and cell pellets with higher Lenti.CDNF-GFP MOI; (d) Increased MANF expression in cell pellets transduced with Lenti.MANF-GFP (MOI 5); (e) Increased GDNF expression with higher Lenti.GDNF-GFP MOI. α-tubulin was used as an endogenous loading control; (f) Immunocytochemistry on transduced E18 rat cortical neurons (MOI 5); left, control virus Lenti.GFP; middle, transduction with Lenti.CDNF-GFP virus stained for CDNF (red) and GFP (green); right, transduction with Lenti.MANF-GFP virus stained for MANF (red) and GFP (green). All sections were counterstained with Hoescht to label cell nuclei (blue). Scale bars = 50 µm.
We next wanted to test the ability of lentiviral-mediated delivery of NTFs to protect the nigro-striatal dopaminergic system in the 6-OHDA model of PD. We delivered the lentiviral vectors at the time of lesion (Supplementary Figure S5) which allows expression at phase 1 (0–7 days) of the 6-OHDA-induced neurodegeneration—a period of axonal loss and atrophy of dopamine neuron cell bodies—and also at phase 2 (1–4 weeks) when TH-downregulation and rapid dopaminergic cell death occurs.22 In addition, this timeframe falls within the therapeutic window reported for CDNF and MANF.13,18,23 To assess the functional integrity of the nigro-striatal dopaminergic system, animals were injected with the dopamine agonist apomorphine, which induces contralateral rotations to the lesioned hemisphere or amphetamine, which induces ipsilateral rotations. Apomorphine-induced rotations were analyzed at 2, 4, 6, and 8 weeks following lesion; animals were monitored for 70 minutes and the cumulative turns recorded in 5 minutes intervals. Amphetamine-induced rotations were analyzed at 8 weeks following lesion; animals were monitored for 90 minutes and the cumulative turns recorded in 5 minutes intervals. A summary of all the results discussed below can be found in Supplementary Table S4.
Intrastriatal delivery of CDNF or MANF does not improve apomorphine or amphetamine-induced rotational behavior following 6-OHDA lesion
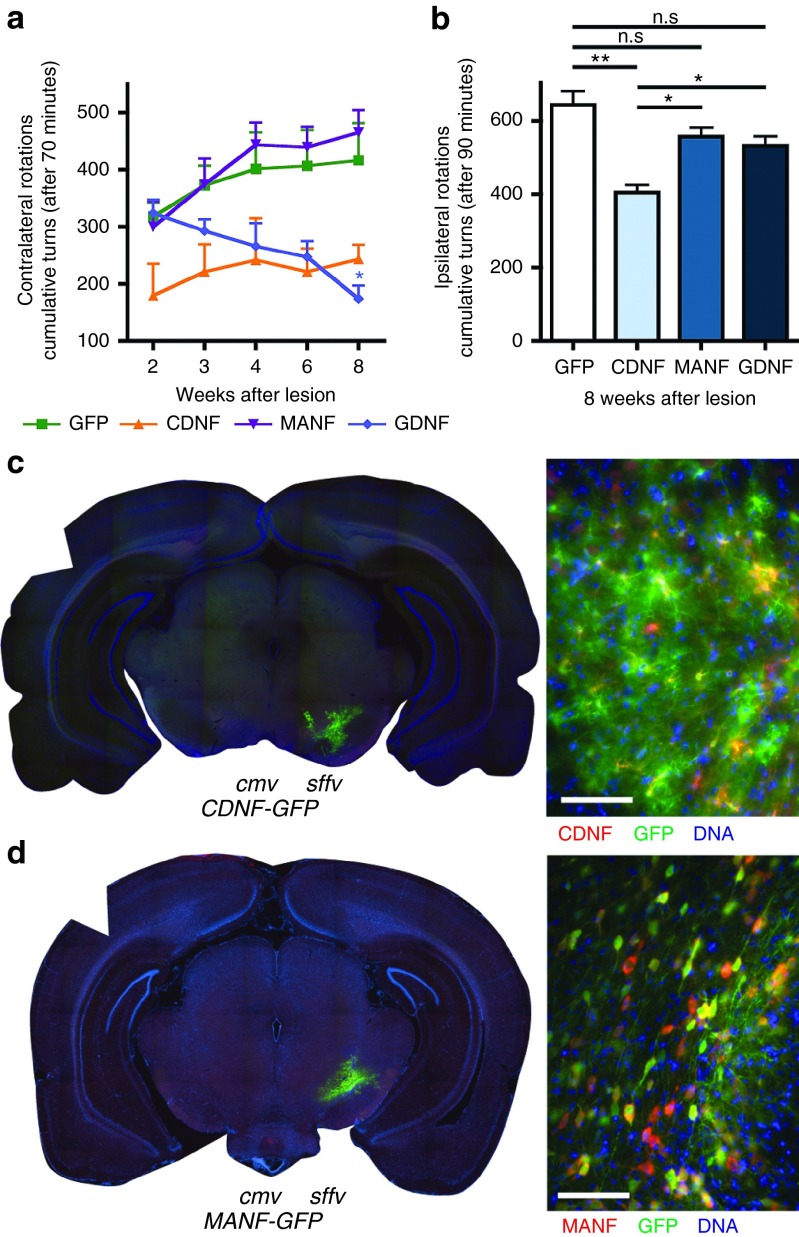
The lentiviral vectors expressing the respective NTFs were injected into the striatum at the time of 6-OHDA lesion and monitored using apomorphine- or amphetamine-induced rotational behaviors. Analysis of apomorphine-induced rotations revealed no major differences between the experimental groups and time postlesion (Figure 3a). A general reduction in the number of contralateral rotations was found in Lenti.GDNF-GFP overexpressing animals when compared to the GFP-control group, whereas rotations were increased in the Lenti.MANF-GFP group (stMANF 735.17 ± 56.81 turns/70 minutes versus stGFP 492.0 ± 56.78, Newman–Keuls post hoc test, P < 0.05, n = 6). A similar effect of MANF-overexpression in contralateral rotations was consistently observed throughout this study (see below). Furthermore, this increase in apomorphine-induced turns did not correlate with the lesion severity in MANF injected animals, indicating that MANF overexpression is directly enhancing the striatal responsiveness to DA and DA-agonists. No differences in the number of amphetamine-induced rotations were found either for Lenti.CDNF-GFP (529 ± 93.36 turns/90 minutes) or Lenti.MANF-GFP (573.83 ± 165.68 turns/90 minutes) overexpressing animals when compared to the GFP-control animals (667.83 ± 137.93 turns/90 minutes; Figure 3b). In contrast, lentiviral vector mediated GDNF overexpression in the striatum (Supplementary Figure S6) resulted in a marked decrease in the number of ipsilateral turns compared to controls (stGDNF 181.2 ± 66.67 turns/90 minutes versus stGFP 667.83 ± 137.93 turns/90 minutes; Newman–Keuls post hoc test, P < 0.05; n = 6; Figure 3b).
Figure 3.
Striatal delivery of lentiviral vectors; rotational behavior. (a) Apomorphine induced rotations at 2, 4, 6, and 8 weeks after lesion; (b) Amphetamine induced rotations at 8 weeks after lesion. Newman–Keuls post hoc test, *P < 0.05, n ≥ 6 per group. Immuno-fluorescence on 40 µm coronal sections at 8 weeks following unilateral preterminal lesion with 6-OHDA. Lentiviral transduction in the cortex striatum following striatal delivery of (c) Lenti.CDNF-GFP or (d) Lenti.MANF-GFP. Sections were stained for CDNF or MANF (red), GFP (green), and DNA (blue). Scale bars = 100 µm.
Intrastriatal delivery of CDNF or MANF does not protect striatal TH-fibers or TH positive neurons in the Substantia nigra against 6-OHDA toxicity
To determine the effects of striatal CDNF or MANF overexpression on the nigro-striatal system, TH immunoreactivity was examined in the striatum and SN. Despite extensive viral transduction in the striatum (Figure 3c,d), the levels of TH immunoreactivity in the Lenti.CDNF-GFP and Lenti.MANF-GFP groups were not significantly different from the control group (stCDNF 32.84 ± 2.83% or stMANF 29.31 ± 5.76% versus stGFP 33.16 ± 1.65%), suggesting that CDNF and MANF did not protect striatal dopaminergic fibers against 6-OHDA toxicity (Figure 4a,b). In contrast, TH density was significantly higher in the Lenti.GDNF-GFP group compared to GFP-control animals (stGDNF 47.66 ± 1.45% versus stGFP 33.16 ± 1.65%; P < 0.01, Newman–Keuls post hoc test; n = 6), indicating that striatal GDNF but not CDNF or MANF reduced the dopaminergic deafferentation in this model. We also investigated whether the loss of dopaminergic innervation in the striatum was accompanied by the loss of dopaminergic cell bodies in the SN. In control animals, 6-OHDA administration results in a dramatic reduction in the number of TH+ nigral dopaminergic neurons, with almost 90% loss of TH immunoreactivity in the lesioned hemisphere (Figure 4c,d). This could not be prevented by CDNF (stCDNF 7.78 ± 1.89% TH+ neurons) or MANF (stMANF 8.7 ± 0.6% TH+ neurons) but was notably circumvented by striatal GDNF (stGDNF 56.52 ± 5.39% versus stGFP 8.07 ± 1.15% TH+ neurons; Newman–Keuls post hoc test, P < 0.001; n = 6; Figure 4c,d).
Figure 4.
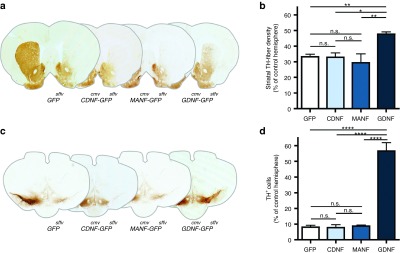
Striatal delivery, TH immunohistochemistry. (a) Striatal TH-fiber density at 8 weeks after viral transduction using TH immunohistochemistry on coronal sections of the corpus striatum (Bregma +0.6), representative sections of Lenti.GFP, Lenti.CDNF-GFP, Lenti.MANF-GFP, and Lenti.GDNF-GFP. (b) Intrastriatal delivery of CDNF or MANF fails to prevent the loss of striatal TH innervation caused by 6-OHDA but GDNF is protective compared to GFP-control animals. (c) Immunohistochemistry of DAB-TH on coronal sections of the substantia nigra. (d) Intrastriatal delivery of CDNF or MANF fails to protect nigral dopamine neurons against 6-OHDA toxicity but GDNF is protective compared to GFP-control animals. Newman–Keuls post hoc test, *P < 0.05; **P < 0.01; ****P < 0.0001, n ≥ 6 per group.
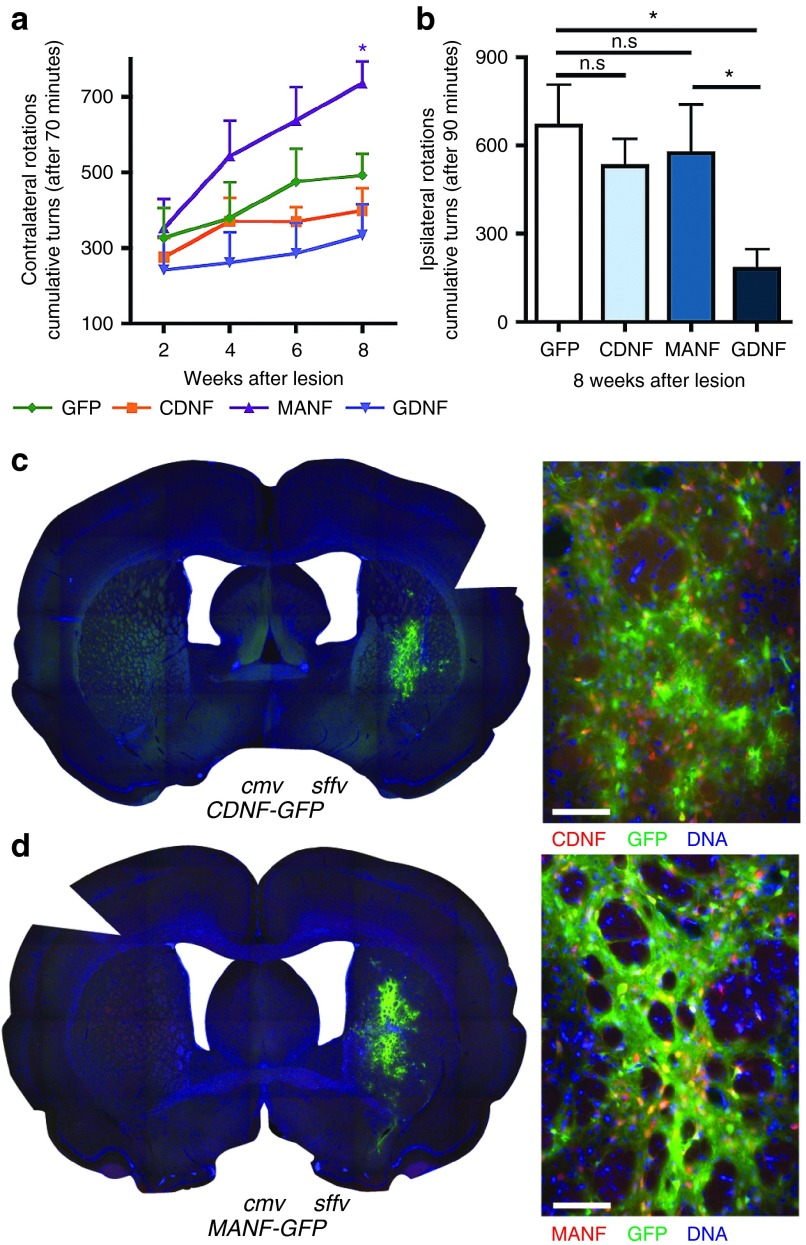
Intranigral delivery of CDNF but not MANF reduces amphetamine-induced rotational behavior
We next wanted to test the efficacy of the lentiviral vectors in the SN using the same lesion paradigm as described above. Delivery of CDNF or MANF did not significantly reduce the number of apomorphine-induced rotations in the experimental groups compared to controls, although apomorphine-induced rotations were decreased in the Lenti.GDNF-GFP animals (niGDNF 173.67 ± 23.13, niCDNF 243.33 ± 25.41, versus niGFP 416.60 ± 65.21 turns/70 minutes at 8 weeks after lesion, P < 0.05, Bonferroni post hoc test n = 6) and increased in Lenti.MANF-GFP animals (Figure 5a). In contrast, lentiviral vector-mediated CDNF overexpression in the SN resulted in a marked decrease in the number of amphetamine-induced ipsilateral turns compared to controls (niCDNF 404.33 ± 35.57 turns/90 minutes versus niGFP 643.40 ± 82.73 turns/90 minutes; Newman–Keuls post hoc test, P < 0.05; n = 6; Figure 5b). Lentiviral-vector mediated delivery of MANF or GDNF had no effect on amphetamine-induced rotations.
Figure 5.
Nigral delivery of lentiviral vectors; rotational behavior. (a) Apomorphine induced rotations at 2, 4, 6, and 8 weeks after lesion; (b) Amphetamine induced rotations at 8 weeks after lesion. Newman–Keuls post hoc test, *P < 0.05; **P < 0.01, n ≥ 6 per group. Immuno-fluorescence on 40 µm coronal sections at 8 weeks following unilateral preterminal lesion with 6-OHDA. Lentiviral transduction in the substantia nigra following intranigral delivery of (c) Lenti.CDNF-GFP or (d) Lenti.MANF-GFP. Sections were stained for CDNF or MANF (Red), GFP (Green) and DNA (Blue). Scale bars = 100 µm.
CDNF and MANF have complementary neuroprotective effects on the nigro-striatal system
After confirming the successful intranigral delivery of Lenti.CDNF-GFP (Figure 5c) and Lenti.MANF-GFP (Figure 5d), TH immunoreactivity was examined in order to determine the effects of intranigral CDNF or MANF overexpression in the degenerating nigro-striatal system. Intranigral overexpression of CDNF significantly reduced the loss of TH-innervation to the striatum when compared to GFP overexpressing animals (niCDNF 45.05 ± 4.49%; versus niGFP 35.31 ± 1.02%; P < 0.01, Newman–Keuls post hoc test; n = 6; Figure 6a,b). In contrast, no differences in striatal TH-innervation were found in Lenti.GDNF-GFP (niGDNF 31.19 ± 3.3%) or Lenti.MANF-GFP (niMANF 30.36 ± 6.48%) overexpressing animals, indicating that only CDNF was able to reduce the loss of striatal dopaminergic afferents when overexpressed from the SN (Figure 6a,b).
Figure 6.
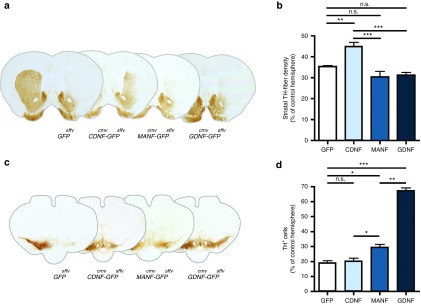
Nigral delivery, TH immunohistochemistry. (a) Striatal TH-fiber density at 8 weeks after viral transduction using TH immunohistochemistry on coronal sections of the corpus striatum (Bregma +0.6), representative sections of Lenti.GFP, Lenti.CDNF-GFP, Lenti.MANF-GFP, and Lenti.GDNF-GFP. (b) Intranigral delivery of CDNF but not MANF or GDNF can prevent the loss of striatal TH innervation caused by 6-OHDA compared to GFP-control animals. (c) Immunohistochemistry of TH on coronal sections of the substantia nigra; (d) Intranigral delivery of CDNF fails to protect nigral dopamine neurons against 6-OHDA toxicity but both MANF and GDNF are protective compared to GFP-control animals. Newman–Keuls post hoc test *P < 0.05; **P < 0.01; ***P < 0.001, n ≥ 6 per group.
However, nigral overexpression of CDNF (niCDNF 20.42 ± 4.87%) did not prevent the loss of TH+ neurons in the SN, despite extensive nigral transduction. In contrast, nigral GDNF-overexpression resulted in a robust protection of dopaminergic cell bodies (niGDNF 66.80 ± 4.13% versus niGFP 19.06 ± 3.37; Newman–Keuls post hoc test, P < 0.001; n = 6). This is in agreement with Kirik et al. who have shown that although nigral GDNF can prevent the dopaminergic cell loss caused by 6-OHDA, only striatal delivery provides functional improvement and preservation of dopaminergic terminals.24 In addition, nigral overexpression of MANF showed significant protection of TH+ cell bodies (niMANF 28.93 ± 5.69% versus niGFP 19.06 ± 3.37; Newman–Keuls post hoc test, P < 0.05; n = 5) but to a much lesser extent than niGDNF (Figure 6c,d).
Combined nigral delivery of CDNF and MANF improves the functionality of the nigro-striatal system, prevents the loss of striatal TH innervation after 6-OHDA lesion and protects TH positive neurons in the substantia nigra
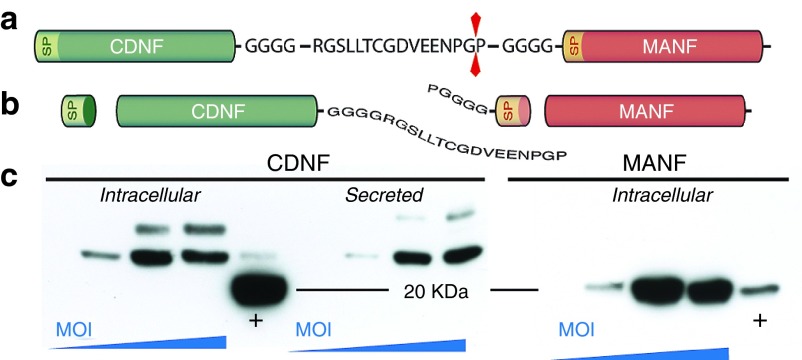
In the knowledge that MANF had a modest but significant protective effect on dopamine neurons in the SN and CDNF protected striatal fibers when injected into the SN, we constructed a vector that allowed the production of both NTFs from a single lentiviral vector, Lenti.CDNF-T2A-MANF-GFP (Lenti.CtM-GFP, Figure 7a,b). Lenti.CtM-GFP effectively produces both CDNF (intracellular and secreted) and MANF (intracellular) in HEK293T cells following transduction (Figure 7c). It should be noticed that CDNF-T2A had a higher molecular weight than native CDNF (+ lane) whereas T2A-MANF and native MANF (+ lane) had identical molecular weights. These sizes correspond to the theoretical fragments illustrated in (Figure 7b). With our design, the hsCDNF-T2A-hsMANF gene is translated as one single polypeptide chain that is then cleaved into two fragments by the autocatalytic properties of T2A.25 Both MANF and CDNF contain signal peptides at the amino-terminal region that are removed by endogenous ER signal peptidases. The final CDNF mature protein is 19 amino acids longer that native CDNF as a small fragment of the t2a peptide remains attached. This explains the difference in size observed in Figure 7c. In contrast, after removal of the signal peptide, the final MANF protein is indistinguishable from endogenous MANF. Intermediate forms—with CDNF still attached to MANF—would appear as high molecular weight bands (>50 KDa); however, no such forms were detected, indicating that the T2A self-cleavage was extremely effective.
Figure 7.
Processing of CDNF-T2A-MANF. (a) Translation of the CDNF-T2A-MANF gene as one single polypeptide; (b) removal of signal peptides (SP) and T2A-induced self-cleaving yields four peptidic fragments, including CDNF+19 aminoacids and native MANF; (c) increased intracellular CDNF and MANF as well as secreted CDNF with higher Lenti.CDNF-T2A-MANF-GFP MOI (+ = positive control).
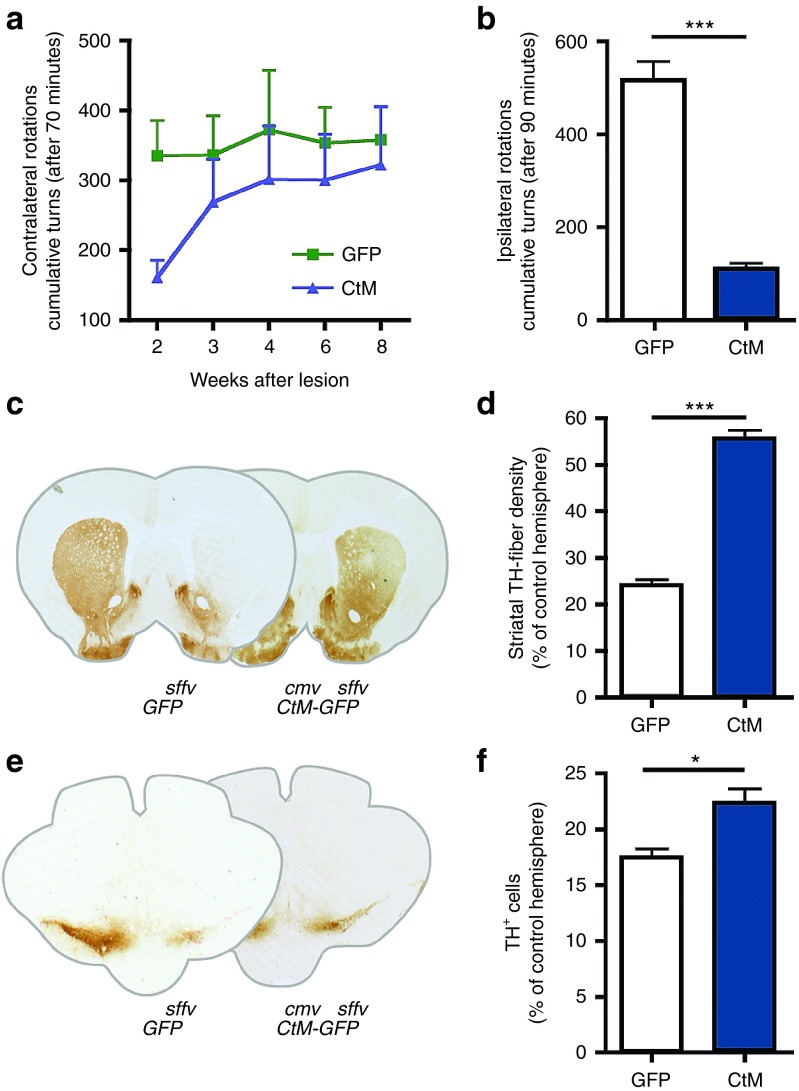
We next injected the Lenti.CtM-GFP vector into the SN using the same lesion paradigm as described above (Supplementary Figure S4). Results show that Lenti.CtM-GFP had a complex effect on apomorphine-induced rotations (Figure 8a) where animals behaved like the CDNF group in the initial time points, with reduced rotations compared to GFP, but resemble MANF injected animals at later time points - with contralateral turns progressively increasing. This did not correlate with amphetamine-induced rotations as Lenti.CtM-GFP led to a remarkable reduction of ipsilateral turns when tested at 8 weeks (niCtM 110.5 ± 34.80 versus niGFP 516.4 ± 99.01 turns/90 minutes; P < 0.001, T-test, n = 6; Figure 8b). This indicates that CtM is both enhancing the striatal responsiveness to DA-agonists (given by apomorphine) and the functional integrity of the nigro-striatal system (given by amphetamine-induced dopamine release). In addition, Lenti.CtM-GFP showed a robust preservation of striatal fibers (niCtM 55.57 ± 5.49% versus niGFP 24.41 ± 1.81%, P < 0.001, T-test, n = 6; Figure 8c,d) and also resulted in significant protection of TH+ cell bodies in the SN (niCtM 22.12 ± 4.43% versus 17.41 ± 1.83%, P = 0.03, T-test, n = 6; Figure 8e,f).
Figure 8.
Nigral delivery of Lenti.CDNF-T2A-MANF-GFP (Lenti.CtM) vector. Intranigral Lenti.CtM-GFP reduces the number of ipsilateral rotations, increases the TH-striatal innervation and increases the number of surviving neurons at weeks after lesion. (a) Apomorphine induced rotations at 2, 4, 6, and 8 weeks after lesion; (b) Amphetamine induced rotations at 8 weeks after lesion; (c) Striatal TH-fiber density at 8 weeks after viral transduction; TH immunohistochemistry on coronal sections of the corpus striatum (Bregma +0.6), representative sections of Lenti.GFP and Lenti.CtM-GFP delivered into the nigra; (d) Quantification of TH-fiber density by optical densitometry; (e) Immunohistochemistry of TH on coronal sections of the substantia nigra; (f) Intranigral delivery of CtM protects nigral dopamine neurons against 6-OHDA toxicity compared to GFP-control animals. T-test; *P < 0.05; **P < 0.01; ***P < 0.001, n ≥ 6 per group.
Discussion
Neurotrophic factors (NTFs) remain a potential neuroprotective/neurorestorative therapy for Parkinson's patients. Indeed numerous publications have documented the trophic actions of factors such as GDNF and Neurturin on dopaminergic neurons both in vitro and in vivo but the outcome from the small number of clinical trials to date could be best described as modest (for review see ref. 26). Thus, in addition to gaining a better understanding of the NTFs currently in clinical trials, it is also important to focus research on newer putative neurotrophic factors such as CDNF and MANF.
CDNF and MANF are members of a novel family of NTFs for dopaminergic neurons.13,14 Both CDNF and MANF are expressed in the developing and adult brain.13,15 MANF has been shown to be essential for the survival of dopaminergic fibers in D. Melanogaster16 and to protect rat embryonic dopaminergic neurons in vitro.14 A number of studies have demonstrated that CDNF and MANF protein infusion, either prior to or after lesion, can protect the nigro-striatal dopaminergic system and restore dopaminergic function in 6-OHDA models.13,18,23 In these studies, it was remarkable that neuroprotection was achieved with one single injection (3–10 µg) of the purified protein, leading us to hypothesize that prolonged treatment with these NTFs could enhance their efficacy. This goal can be achieved by lentiviral vectors, which have successfully been used for long-term overexpression of target genes in the central nervous system (CNS) with minimal immune reaction.20 We designed lentiviral vectors that allowed the expression of human CDNF, human MANF or combined expression of both as one single polypeptide chain separated by a self-cleaving peptide. We also included a human GDNF overexpressing lentiviral vector as a positive control given its well-established efficacy in this model (for review see ref. 27) and a GFP-only lentiviral vector as a negative control. Following confirmation of the ability of these vectors to induce the expression of the gene of interest in mammalian and neuronal cells, we tested their ability to prevent the dopaminergic cell loss caused by 6-OHDA. In our study, we performed a unilateral preterminal lesion by injecting 10 µg of 6-OHDA in two sites in the striatum. Kirik et al. have shown that this leads to around 70% loss in dopaminergic neurons and 40–60% reduction in striatal innervation,22 sparing a portion of the nigro-striatal system which can restore functional recovery. We chose to deliver our vectors at the same time of the lesion as this falls within the therapeutic window of opportunity for CDNF and MANF.13,18
Striatal overexpression of CDNF or MANF did not improve drug-induced rotational behavior or protect dopaminergic innervation in the striatum or cell bodies in the SN from the 6-OHDA neurotoxin. In contrast and as expected, striatal GDNF significantly reduced the number of amphetamine-induced rotations and resulted in a marked preservation of dopaminergic neurons and their terminals.24,28,29 The lack of effect of CDNF and MANF was a surprising finding and contradicts previous reports.13,18 However, there are possible explanations for this discrepancy: the 6-OHDA model used in the initial studies consisted of one single 6-OHDA injection (20 µg) into the striatum which leads to a 30–35% loss of TH+ neurons, whereas our model results in a much harsher lesion (75–90% TH+ cell loss). Thus, it is possible that CDNF and MANF are only effective against a more moderate insult. Indeed, in a more severe lesion model, the effects of CDNF and MANF were far less clear.23 Voutilainen et al. showed that striatal CDNF but not GDNF or MANF reduced cumulative amphetamine-induced rotations and this was only with one of three CDNF doses tested. Surprisingly, CDNF but not GDNF protected dopaminergic neurons against 6-OHDA and no protective effect of MANF was found in any of the parameters analyzed.23 This suggests that CDNF or MANF may not be as robust as initially reported and that their beneficial effects may depend on lesion severity. In addition, CDNF appears to be retrogradely transported from the striatum to the SN less readily than GDNF23 and only high amounts of CDNF (over 3 µg) afforded significant protection.13 It is possible that in our study, the lentiviral vector-mediated expression of CDNF in the striatum did not reach this protective threshold and that local CDNF concentrations in the SN were even lower due to a combination of poor retrograde transport and fiber deafferentation in a severe lesion model.
Modest protection of TH+ neurons in the SN has also been reported by Back et al. following adeno-associated virus 2 (AAV2)-mediated delivery of CDNF in the striatum.30 In contrast to Back et al., Ren et al. reported significant neurorestoration of TH+ cells when AAV2-CDNF was given 6 weeks after lesion in the striatum.17 It is noteworthy that the only two published studies to date using AAV2-CDNF report very different outcomes despite the fact that both studies used similar viral particles per millilitre, transgene expression was driven by the CMV promoter in both cases and similar lesion models were employed. However, there are differences in the timing of the lesion in relation to the AAV2-CDNF delivery, with Back et al. administering AAV2-CDNF prior to a 6-OHDA lesion and Ren et al. allowing the lesion to develop for 6 weeks prior to vector delivery. Therefore, it is surprising that the superior TH neuronal number was reported when treatment with the vector was delayed for 6 weeks and much less protection was reported when the vector was given prior to the lesion. Interestingly, Ren et al. did not see a protective effect of AAV2-GDNF, which is in contrast to our study. Although lentiviral vectors and AAVs are both highly efficient vehicles for gene transfer into the brain, it is difficult to compare their efficacy across studies.
Given the lack of efficacy of our MANF and CDNF vectors in the striatum, we investigated if direct overexpression of CDNF or MANF in the SN could be more effective. Indeed, MANF overexpression in the SN showed a modest but significant protection of TH+ neurons in the SN but failed to prevent the loss of striatal afferents. Accordingly, this did not result in any behavioral recovery. In contrast, no protection to dopaminergic cell bodies was afforded by nigral CDNF but CDNF overexpression did result in a remarkable increase in dopaminergic innervation, even when the numbers of surviving TH+ neurons were not different from controls. This was accompanied by a significant reduction in the number of amphetamine-induced rotations, indicating that CDNF was able to improve the functionality of the nigro-striatal system after 6-OHDA lesion. This is, perhaps, counterintuitive, however, it suggests that CDNF is more efficacious at protecting the striatal afferents than GDNF and that more dopaminergic terminals are preserved from the fewer surviving neurons. Interestingly, CDNF overexpression has been shown to increase axonal regeneration following sciatic nerve injury31 and Bäck et al. have reported a “denser meshwork of TH-reactive fibers close to the CDNF infusion tract” but no obvious signs of abnormal sprouting were observed—unlike that caused by GDNF.30 Furthermore, programmed cell death and axonal degeneration appear to be governed by separated and distinct mechanisms.32 Indeed, intranigral overexpression of an active form of Rheb GTPase results in reinnervation of the deafferated striatum without any effect on the number of surviving TH+ neurons, when delivered 3 weeks after 6-OHDA lesion.33 Therefore, it is possible that nigral CDNF is able to induce sprouting and reinnervation of the deafferented striatum from surviving neurons. However, CDNF overexpression had no effect on TH innervation in the intact striatum17 suggesting the protective effects of CDNF are circumscribed to the degenerating nigro-striatal system.
Given the ability of MANF to protect TH+ neurons and the surprising preservation of dopaminergic fibers afforded by CDNF, we hypothesized that combined delivery of CDNF and MANF could result in enhanced neuroprotection. Indeed, nigral delivery of our CDNF-T2A-MANF lentiviral vector resulted in a significant increase in the number of TH+ cell bodies in the SN as well as a strong preservation of the striatal TH+-terminals. Interestingly, the preservation of TH+-terminals with CDNF-T2A-MANF was higher than with CDNF alone and this was accompanied by a greater reduction in the number of amphetamine-induced rotations, suggesting a synergistic effect of the two proteins. It should be noted that CDNF overexpressed from the CDNF-T2A-MANF gene is not identical to the native CDNF13 and contains 19 additional amino acids. It is conceivable that these additional residues could affect the properties of the mature CDNF, perhaps increasing its stability, diffusion or receptor binding thus accounting in part for the enhanced neuroprotection observed with CDNF-T2A-MANF. Nonetheless, the synergistic effects of CDNF and MANF on neurochemical and functional recovery of the lesioned nigro-striatal system suggests that although CDNF and MANF belong to the same NTF family, they may have functional differences and mediate protective effects through different mechanisms.34 In this respect, lentiviral vectors have an advantage over AAV vectors in terms of a larger cloning capacity to accommodate multiple NTFs to be delivered by a single vector. Additionally, integration-deficient lentiviral vectors, which become episomal molecules and hence have a much-reduced risk of causing insertional mutagenesis,35,36 can efficiently deliver hsGDNF and have a neuroprotective effect in the 6-OHDA model of PD.37 Therefore, it may be possible to achieve multiple/combined NTF delivery in a variety of experimental paradigms using safer therapeutic vectors.
Very few publications have tested the efficacy of the CDNF/MANF family of neurotrophic factors in PD models; these publications have followed the GDNF paradigm in which targeting the striatal afferents seems to afford optimal neuroprotection. To our knowledge, ours is the first study to compare alternative delivery sites. Remarkably, we have shown that, unlike GDNF, intranigral CDNF and MANF delivery appears to be far more efficacious than striatal delivery. This suggests that GDNF and the CDNF/MANF families have different modes of action depending on where they are delivered and may have implications for future therapeutic interventions. This is also the first study to demonstrate that combined delivery of CDNF and MANF can have synergistic effects that result in enhanced neuroprotection. Only DmMANF is found in invertebrates but the family duplicated in vertebrates with the acquisition of CDNF,38 perhaps suggesting that the development of a larger and more complex nervous system was accompanied by a diversification of neurotrophic support. Our data indicate that CDNF and MANF have differential modes of action, thus this duplication event was not simply an increase in gene dosage but the acquisition of specialized functions. In our view, gene therapy approaches aiming to restore the neurotrophic support to the degenerating nigro-striatal system would be more successful if we exploited this NTF diversity. Accordingly, combined infusion of GDNF and TGF-β1 in the 6-OHDA model results in enhanced neuroprotection compared to either factor alone.39 However, Sun et al. saw no beneficial effect of BDNF and GDNF coexpression in this model.40 Indicating that these neuroprotective pairs must be considered carefully. Nevertheless, multiple/combined NTF delivery may prove more efficacious for the treatment of PD than the single NTF approaches attempted so far and testing these strategies in other models of PD or neurodegeneration—such as the α-synuclein model of PD11,12—is a pressing need.
Materials and Methods
Generation of lentiviral vector constructs. cDNA clones for human CDNF (hsCDNF), human MANF (hsMANF) and human GDNF (hsGDNF) were purchased from Gene Service (Source BioScience, Oxford, UK). The genes of interest were amplified using PfuUltra high fidelity DNApol (Agilent, Stockport, UK) using primers (Sigma, Gillingham, UK) and PCR conditions detailed in (Supplementary Figure S1) and (Supplementary Table S1) respectively. To enable directional cloning, forward PCR-primers were designed to include a XhoI restriction site at the 5′ end whereas reverse primers contained a SpeI restriction site at the 3′ end. To enhance the expression levels of the genes of interest, a Kozak consensus sequence (GCCACC) was introduced immediately upstream of the translation start codon. All DNA inserts (Supplementary Figure S2) were cloned into the pRRL-sffv-eGFP-cmv backbone to generate pRRL-sffv-eGFP-cmv-hsCDNF (GeneBank: KJ697750), pRRL-sffv-eGFP-cmv-hsMANF (GeneBank: KJ697751), pRRL-sffv-eGFP-cmv-hsGDNF (GeneBank: KJ697753), and pRRL-sffv-eGFP-cmv-hsCDNF-T2A-hsMANF (GeneBank: KJ697752). Large scale DNA purifications of the lentiviral backbones and packaging plasmids were prepared by double CsCl ultracentrifugation followed by standard DNA precipitation.
Generation of concentrated lentiviral particles. Lentiviral vectors pseudotyped with the VSVg coat were produced using the four plasmid transient transfection protocol as previously described.41 Briefly, HEK293T cells were cotransfected with the lentiviral backbone containing the gene of interest, pMDLg/pRRE gag/pol, pMD2-env-VSVG, and pRSV-Rev using calcium phosphate mediated transfection and the cell supernatants were harvested 24 and 48 hours after transfection. The supernatant was filtered through a 0.45 μm Nalgene filter unit (Fisher Scientific, Loughborough, UK), concentrated 2000-fold by ultracentrifugation and the resultant viral pellet was resuspended in TSSM buffer. Titration of the lentiviral vectors was performed by flow cytometry using pRRL-CMV-GFP as a reference. 105 HEK293T cells were transduced in a dilution series with the individual lentiviral vectors, 72 hours after transduction, cells were fixed with 4% paraformaldehyde (PFA) and the number of GFP+ cells was determined using FACScalibur flow cytometer (BD Biosciences, Oxford, UK). The titers of the lentiviral vectors were as follows: Lenti.CDNF-GFP (3·108 tu/ml), Lenti.MANF-GFP (8·108 tu/ml), Lenti.GDNF-GFP (4·108 tu/ml), Lenti.CtM-GFP (6·108 tu/ml), and Lenti.GFP (9·108 tu/ml).
Lentiviral-vector validation in HEK293T cells. HEK293T cells were seeded at a density of 7.5 × 104 cells per well in a 12-well plate in DMEM with 4.5 g/l D-glucose, supplemented with 10% fetal calf serum (FCS), 100 U/ml penicillin, 0.1 g/l streptomycin, 2 mmol/l Glutamax, and 1% nonessential amino acids (Life Tech). The following day, cells were transduced at multiplicities of infection (MOI) of 0, 0.1, 1, and 5. After 72 hours, the cells were processed for immunocytochemistry or collected for Western blot analysis.
Lentiviral-vector validation in primary rat E18 cortical neurons. All procedures were approved by the local veterinarian and ethical committees and carried out according to UK Home Office regulations. Pregnant Wistar rats at gestation day E18 (University of Bristol, Bristol, UK) were anesthetized by isoflurane inhalation and sacrificed by cervical dislocation. The embryos were harvested and cortices were collected in Hanks Balanced Salt Solution (HBSS, Life Tech, Paisley, UK) containing 0.05% trypsin-EDTA (Sigma) and incubated for 15 minutes at 37 °C. The cortices were washed three times in HBSS and triturated with a flame-polished Pasteur pipette in 1 ml of plating medium (Neurobasal medium (Life Tech); 2% B-27 supplement (Life Tech); 0.5 mmol/l L-glutamine (Sigma); 25 µmol/l L-glutamate (Life Tech); 100 U/ml penicillin; and 0.1 g/l streptomycin). 7.5 × 104 cells were carefully placed into the centre of poly-D-lysine (Sigma) coated coverslips. Neurons were maintained in feeding medium (Neurobasal medium; 2% B-27 supplement, 100 U/ml penicillin, and 0.1 g/l streptomycin) and transduced after 5 days at an MOI of 5. Five days after transduction, they were fixed with 4% PFA for immunohistochemistry.
Neurite outgrowth assay in rat MB cultures. The ventral mesencephalon was dissected from E14 Wistar embryos, incubated in accutase (Sigma) for 15 minutes at 37 °C and washed twice with DMEM. Dissociated cells were plated at a density of 5 × 104 in DMEM/F12 (7:3) 100 U/ml penicillin, 0.1 g/l streptomycin and supplemented with 2% B27. Neurons were maintained in this medium and transduced at after 3 days at an MOI of 2 or 5. Five days after transduction, they were fixed with 4% PFA for immunohistochemistry.
Immunofluorescence on cell monolayers. The cells were permeabilized with ice-cold methanol at −20 °C for 20 minutes, followed by two washes with PBS. They were blocked in PBS containing 10% normal goat serum (NGS, Vector Labs, Peterborough, UK) at 4 °C for at least 3 hours. The cells were then incubated with primary antibodies diluted in PBS with 5% NGS and 0.01% NaN3 (Sigma) at 4 °C overnight. This was followed by three PBS washes before incubating with the appropriate secondary antibodies in PBS containing 5% NGS, at room temperature for 90 minutes. Following washing with PBS and an optional step of counterstaining cell nuclei with 1 mg/l Hoechst (Sigma) for 10 minutes at room temperature, coverslips were mounted in Fluorsave (Merck Millipore, Watford, UK) and allowed to dry before imaging. The antibodies used are detailed on (Supplementary Table S3)
Western blotting. Cells were washed once with ice-cold PBS and lysed in cold RIPA buffer (1X PBS, 1% Igepal CA-630 (Sigma); 0.5% sodium deoxycholate (Sigma); 0.1%, SDS (Melford, Ipswich, UK) containing complete Mini EDTA-free protease inhibitor (Roche, West Sussex, UK). Samples were homogenized and centrifuged at 12,000 RCF for 10 minutes at 4 °C. Conditioned medium (without RIPA) was centrifuged at 1,200 RCF to eliminate any cell debris and snap-frozen on dry ice. All protein samples were kept at −80 °C.
A 25 µg of cell extracts or 40 µl of culture medium were electrophoresed on a 10% polyacrylamide gel and transferred onto a PVDF membrane. Membranes were then blocked in 10% milk in TBS-0.1% Tween20 (Sigma), probed with primary antibodies, washed and incubated with the appropriate HRP-conjugated antibody. Signals were detected using the Supersignal West Pico chemiluminescent substrate (Fisher Scientific). Autoradiographic films were developed using a Kodak autoprocessor. The antibodies used are detailed on (Supplementary Table S3).
Stereotactic surgery. All procedures were approved by the local veterinarian and ethical committees and carried out according to UK Home Office regulations. All animals were housed under standard lighting (12 hours light/dark cycle) and temperature (21–22 °C) conditions with food and water available ad libitum. Three animals were housed per cage. Adult Wistar male rats were obtained from Charles Rivers (Margate, UK) and all animals were between 280 and 310 g at the time of surgery. A minimum of six animals was used per experimental group. Animals were deeply anaesthetized with a mixture of 0.25 mg/Kg medetomidine (Dormitor, Pfizer, Cambridge, UK) and 60 mg/Kg ketamine (Vetalar, Pfizer) intraperitoneally. Animals were mounted on a stereotactic frame and craniotomies were made at coordinates relative to bregma and dura, according to the brain atlas of Paxinos and Watson (1998). Using a Hamilton syringe fitted with a 33G needle, 2 µl of concentrated lentiviral vector preparations were injected unilaterally at a rate of 0.2 µl/min into the Substantia Nigra (AP −5.3, ML −2.2, DV −7.2) or the cortex striatum (AP −0.6, ML −3.3, DV −5). 6-OHDA was injected unilaterally in two sites of the cortex striatum (AP 0, ML −2.6, DV −5 and AP −1.2, ML −3.9, DV −5) at a rate of 0.4 µl/minute and the syringe was left in place for a further 2 minutes. A total of 10 µg of 6-OHDA were given per site on the same day as lentiviral vectors. At the end of the procedure, the wound was closed using reabsorbable sutures and the animal recovered by subcutaneous administration of 0.2 mg/Kg atipamezole (Antisedan, Pfizer). Animals were euthanized 8 weeks after the surgical procedure.
Behavioral analysis. The integrity of the nigro-striatal dopaminergic system was assessed using drug-induced rotation. Animals were injected subcutaneously with 0.25 mg/Kg R-apomorphine hydrochloride hemihydrate (Sigma) at 2, 4, 6, and 8 weeks after lesion. In addition, animals were injected with 2.5 mg/Kg D-amphetamine sulphate (Sigma) intraperitoneally at 8 weeks after lesion. Ipsilateral and contralateral rotations were recorded every 5 minutes using in-house software. Animals were monitored for up to 70 or 90 minutes following administration of apomorphine or amphetamine respectively.
Brain processing. Eight weeks after 6-OHDA injection, animals were terminally anaesthetized with 150 mg/Kg pentobarbital (Euthatal, Merial, Harlow, UK) and transcardially perfused with 200 ml of PBS-Heparin (1 U/ml, Sigma), followed by perfusion with 200 ml of 4% PFA at 40 ml/min. The brains were postfixed in 4% PFA overnight and transferred into a 30% sucrose (Melford, Ipswich, UK) solution. Sucrose-equilibrated brains were embedded in OCT-matrix (Fisher Scientific), frozen and sectioned at −20 °C using a Leica CM1900 cryostat (Leica, Milton Keynes, UK). The brains were sectioned coronally at 40 µm.
Immunofluorescence on free-floating sections. Free-floating 40 µm-brain sections were stained as previously described.42 Briefly, sections were blocked with 1 ml of PBS-0.1%Tx100 (PBS; 0.1% Triton-X100, Sigma) containing 10% NGS, 2% BSA (Sigma) and 0.01% NaN3 at 4 °C overnight, after which they were incubated with primary antibodies diluted in 500 µl of PBS-0.1%Tx100 containing 5% NGS, 1% BSA and 0.01% NaN3 at 4 °C overnight. The sections were washed with PBS-0.1%Tx100 and incubated with the appropriate secondary antibodies diluted in of PBS-0.1%Tx100 containing 5% serum, 1% BSA at 4 °C overnight. Following washing with PBS-0.1%Tx100 and an optional step of counterstaining cell nuclei with 1 mg/l Hoechst (Sigma) for 20 minutes at room temperature, sections were mounted in Fluorsave and kept at 4 °C. The antibodies used are detailed on (Supplementary Table S3)
DAB-immunostaining on free-floating sections. 3-3′-diaminobenzidine (DAB) staining on free-floating sections was performed as previously described.42 Sections were blocked and incubated with primary antibodies as for immunofluorescence in a TBS-based buffer. Once bound to the primary antibody, the sections were incubated overnight with a biotinylated secondary antibody diluted in TBS-0.1%Tx100 containing 5% serum and 1% BSA. The following day, sections were treated with HRP-conjugated avidin (Vectastain Elite ABC-KIT, Vector Labs, Peterborough, UK), washed and incubated with DAB following manufacturer's instructions (DAB peroxidase substrate kit, Vector Labs). Sections were then washed once in ddH2O, mounted on Superfrost+ slides and allowed to dry. Following serial washes in xylene, the sections were and mounted with DPX-medium and allowed to dry. The antibodies used are detailed on (Supplementary Table S3).
Image acquisition and analysis. Image acquisition was performed with a Leitz DMRD microscope attached to a Leica DC500-12MP-color digital camera using the Leica IM50 4.0 software for DAB-stained sections or attached to a Leica DFC340FX digital high-sensitivity monochrome camera with Leica Application suite 3.3.1 for immunofluorescence. Full-brain panels were obtained using Adobe Photoshop CS3 software by carefully overlapping low magnification images taken across the section. For neuronal counts, images were taken from at least four sections (20× magnification) from each animal and quantified using ImageJ (http://rsbweb.nih.gov/ij/) and the cell counting plugin from Kurt De Vos at the University of Sheffield (http://rsb.info.nih.gov/ij/plugins/cell-counter.html). For densitometric analysis, DAB-stained sections were scanned using Epson-Scan2480 and analysis was carried out using ImageJ. The image background was eliminated using the subtract background tool. Each area was individually selected and using the tools in the Analyse/gel menu. Once all areas were highlighted, a histogram was generated using the tool Analyse/gel/plot/lanes. Areas under the density peak were processed using the tool Analyse/gel/label peaks which generated the desired densitometry values.
Statistical analysis. In the striatal and nigral delivery groups, amphetamine induced rotations, TH-densitometry and TH-neuronal counts were screened by one-way analysis of variance followed by Newman–Keuls multiple comparisons post hoc test. Apomorphine data at different time points were screened by two-way analysis of variance followed by Bonferroni post hoc test. For the Lenti.CtM-GFP only group, data were compared using T-tests. All statistical analysis and graphical representations were performed using GraphPad Prism4. A minimum of six animals per experimental group were used.
SUPPLEMENTARY MATERIAL Figure S1. Primer sequences. Figure S2. Insert sequences. Figure S3. Neurite outgrowth. Figure S4. Transduction of dopaminergic neurons in vivo. Figure S5. Experimental design. Figure S6. Striatal GDNF-GFP delivery. Table S1. Pfu Ultra II-High fidelity PCR. Table S2. Overlap-extension hfPCR, cycle parameters. Table S3. Antibodies used. Table S4. Result summary.
Acknowledgments
This study was funded by Parkinson's UK (grant numbers K-1104, G-0915) and by the Elizabeth Blackwell Institute, University of Bristol (MAC). BCH was supported by an MRC Industrial CASE Studentship. RJYM received financial support from the 7th EU Framework Programme (Neugene; Grant Agreement No. 222925). The project was conceived and directed by LFW and MAC. All surgical procedures, behavioural analysis, lentiviral vector production and validation were performed by OCL and BCH. Immunohistochemical analysis and stereological counts were performed by OCL, BCH and FR. HT carried out the VM cultures and neurite outgrowth assays. RJYM and JBU contributed with novel reagents and their lentiviral vector expertise. The manuscript was written by OCL, LFW and MAC. All authors contributed to the edition and revision of the final article.
Supplementary Material
Primer sequences.
Insert sequences.
Neurite outgrowth.
Transduction of dopaminergic neurons in vivo.
Experimental design.
Striatal GDNF-GFP delivery.
References
- Maetzler W, Liepelt I, Berg D. Progression of Parkinson's disease in the clinical phase: potential markers. Lancet Neurol. 2009;8:1158–1171. doi: 10.1016/S1474-4422(09)70291-1. [DOI] [PubMed] [Google Scholar]
- Evans JR, Barker RA. Neurotrophic factors as a therapeutic target for Parkinson's disease. Expert Opin Ther Targets. 2008;12:437–447. doi: 10.1517/14728222.12.4.437. [DOI] [PubMed] [Google Scholar]
- Li H, He Z, Su T, Ma Y, Lu S, Dai C.et al. (2003Protective action of recombinant neurturin on dopaminergic neurons in substantia nigra in a rhesus monkey model of Parkinson's disease Neurol Res 25263–267. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Herzog CD, Dass B, Bakay RA, Stansell J, 3rd, Gasmi M.et al. (2006Delivery of neurturin by AAV2 (CERE-120)-mediated gene transfer provides structural and functional neuroprotection and neurorestoration in MPTP-treated monkeys Ann Neurol 60706–715. [DOI] [PubMed] [Google Scholar]
- Gasmi M, Brandon EP, Herzog CD, Wilson A, Bishop KM, Hofer EK.et al. (2007AAV2-mediated delivery of human neurturin to the rat nigrostriatal system: long-term efficacy and tolerability of CERE-120 for Parkinson's disease Neurobiol Dis 2767–76. [DOI] [PubMed] [Google Scholar]
- Gill SS, Patel NK, Hotton GR, O'Sullivan K, McCarter R, Bunnage M.et al. (2003Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease Nat Med 9589–595. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Burchiel KJ, Comella CL, Jankovic J, Lang AE, Laws ER., Jret al.; ICV GDNF Study Group. Implanted intracerebroventricular. Glial cell line-derived neurotrophic factor 2003Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD Neurology 6069–73. [DOI] [PubMed] [Google Scholar]
- Lang AE, Gill S, Patel NK, Lozano A, Nutt JG, Penn R.et al. (2006Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease Ann Neurol 59459–466. [DOI] [PubMed] [Google Scholar]
- Marks WJ, Jr, Bartus RT, Siffert J, Davis CS, Lozano A, Boulis N.et al. (2010Gene delivery of AAV2-neurturin for Parkinson's disease: a double-blind, randomised, controlled trial Lancet Neurol 91164–1172. [DOI] [PubMed] [Google Scholar]
- Marks WJ, Jr, Ostrem JL, Verhagen L, Starr PA, Larson PS, Bakay RA.et al. (2008Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson's disease: an open-label, phase I trial Lancet Neurol 7400–408. [DOI] [PubMed] [Google Scholar]
- Decressac M, Ulusoy A, Mattsson B, Georgievska B, Romero-Ramos M, Kirik D.et al. (2011GDNF fails to exert neuroprotection in a rat a-synuclein model of Parkinson's disease Brain 134Pt 82302–2311. [DOI] [PubMed] [Google Scholar]
- Lo Bianco C, Déglon N, Pralong W, Aebischer P. Lentiviral nigral delivery of GDNF does not prevent neurodegeneration in a genetic rat model of Parkinson's disease. Neurobiol Dis. 2004;17:283–289. doi: 10.1016/j.nbd.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Lindholm P, Voutilainen MH, Laurén J, Peränen J, Leppänen VM, Andressoo JO.et al. (2007Novel neurotrophic factor CDNF protects and rescues midbrain dopamine neurons in vivo Nature 44873–77. [DOI] [PubMed] [Google Scholar]
- Petrova P, Raibekas A, Pevsner J, Vigo N, Anafi M, Moore MK.et al. (2003MANF: a new mesencephalic, astrocyte-derived neurotrophic factor with selectivity for dopaminergic neurons J Mol Neurosci 20173–188. [DOI] [PubMed] [Google Scholar]
- Lindholm P, Peränen J, Andressoo JO, Kalkkinen N, Kokaia Z, Lindvall O.et al. (2008MANF is widely expressed in mammalian tissues and differently regulated after ischemic and epileptic insults in rodent brain Mol Cell Neurosci 39356–371. [DOI] [PubMed] [Google Scholar]
- Palgi M, Lindström R, Peränen J, Piepponen TP, Saarma M, Heino TI. Evidence that DmMANF is an invertebrate neurotrophic factor supporting dopaminergic neurons. Proc Natl Acad Sci USA. 2009;106:2429–2434. doi: 10.1073/pnas.0810996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Zhang T, Gong X, Hu G, Ding W, Wang X. AAV2-mediated striatum delivery of human CDNF prevents the deterioration of midbrain dopamine neurons in a 6-hydroxydopamine induced parkinsonian rat model. Exp Neurol. 2013;248:148–156. doi: 10.1016/j.expneurol.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Voutilainen MH, Bäck S, Pörsti E, Toppinen L, Lindgren L, Lindholm P.et al. (2009Mesencephalic astrocyte-derived neurotrophic factor is neurorestorative in rat model of Parkinson's disease J Neurosci 299651–9659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airavaara M, Harvey BK, Voutilainen MH, Shen H, Chou J, Lindholm P.et al. (2012CDNF protects the nigrostriatal dopamine system and promotes recovery after MPTP treatment in mice Cell Transplant 211213–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg C, Björklund T, Carlsson T, Jakobsson J, Hantraye P, Déglon N.et al. (2008Applications of lentiviral vectors for biology and gene therapy of neurological disorders Curr Gene Ther 8461–473. [DOI] [PubMed] [Google Scholar]
- Sun ZP, Gong L, Huang SH, Geng Z, Cheng L, Chen ZY. Intracellular trafficking and secretion of cerebral dopamine neurotrophic factor in neurosecretory cells. J Neurochem. 2011;117:121–132. doi: 10.1111/j.1471-4159.2011.07179.x. [DOI] [PubMed] [Google Scholar]
- Kirik D, Rosenblad C, Björklund A. Characterization of behavioral and neurodegenerative changes following partial lesions of the nigrostriatal dopamine system induced by intrastriatal 6-hydroxydopamine in the rat. Exp Neurol. 1998;152:259–277. doi: 10.1006/exnr.1998.6848. [DOI] [PubMed] [Google Scholar]
- Voutilainen MH, Bäck S, Peränen J, Lindholm P, Raasmaja A, Männistö PT.et al. (2011Chronic infusion of CDNF prevents 6-OHDA-induced deficits in a rat model of Parkinson's disease Exp Neurol 22899–108. [DOI] [PubMed] [Google Scholar]
- Kirik D, Rosenblad C, Bjorklund A, Mandel RJ. Long-term rAAV-mediated gene transfer of GDNF in the rat Parkinson's model: intrastriatal but not intranigral transduction promotes functional regeneration in the lesioned nigrostriatal system. J Neurosci. 2000;20:4686–4700. doi: 10.1523/JNEUROSCI.20-12-04686.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymczak AL, Workman CJ, Wang Y, Vignali KM, Dilioglou S, Vanin EF.et al. (2004Correction of multi-gene deficiency in vivo using a single ‘self-cleaving' 2A peptide-based retroviral vector Nat Biotechnol 22589–594. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Bjorklund A. Trophic factor gene therapy for Parkinson's disease. Mov Disord. 2013;28:96–109. doi: 10.1002/mds.25344. [DOI] [PubMed] [Google Scholar]
- Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- Kirik D, Georgievska B, Björklund A. Localized striatal delivery of GDNF as a treatment for Parkinson disease. Nat Neurosci. 2004;7:105–110. doi: 10.1038/nn1175. [DOI] [PubMed] [Google Scholar]
- Georgievska B, Kirik D, Rosenblad C, Lundberg C, Björklund A. Neuroprotection in the rat Parkinson model by intrastriatal GDNF gene transfer using a lentiviral vector. Neuroreport. 2002;13:75–82. doi: 10.1097/00001756-200201210-00019. [DOI] [PubMed] [Google Scholar]
- Bäck S, Peränen J, Galli E, Pulkkila P, Lonka-Nevalaita L, Tamminen T.et al. (2013Gene therapy with AAV2-CDNF provides functional benefits in a rat model of Parkinson's disease Brain Behav 375–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Liu Y, Zhao H, Zhang W, Guo YJ, Nie L. Lentiviral-mediated transfer of CDNF promotes nerve regeneration and functional recovery after sciatic nerve injury in adult rats. Biochem Biophys Res Commun. 2013;440:330–335. doi: 10.1016/j.bbrc.2013.09.084. [DOI] [PubMed] [Google Scholar]
- Cheng HC, Ulane CM, Burke RE. Clinical progression in Parkinson disease and the neurobiology of axons. Ann Neurol. 2010;67:715–725. doi: 10.1002/ana.21995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SR, Chen X, Oo TF, Kareva T, Yarygina O, Wang C.et al. (2011Dopaminergic pathway reconstruction by Akt/Rheb-induced axon regeneration Ann Neurol 70110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindström R, Lindholm P, Kallijärvi J, Yu LY, Piepponen TP, Arumäe U.et al. (2013Characterization of the structural and functional determinants of MANF/CDNF in Drosophila in vivo model PLoS ONE 8e73928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yáñez-Muñoz RJ, Balaggan KS, MacNeil A, Howe SJ, Schmidt M, Smith AJ.et al. (2006Effective gene therapy with nonintegrating lentiviral vectors Nat Med 12348–353. [DOI] [PubMed] [Google Scholar]
- Wanisch K, Yáñez-Muñoz RJ. Integration-deficient lentiviral vectors: a slow coming of age. Mol Ther. 2009;17:1316–1332. doi: 10.1038/mt.2009.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu-Nguyen NB, Broadstock M, Schliesser MG, Bartholomae CC, von Kalle C, Schmidt M.et al. (2014Transgenic expression of human glial cell line-derived neurotrophic factor from integration-deficient lentiviral vectors is neuroprotective in a rodent model of Parkinson's disease Hum Gene Ther 25631–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm P, Saarma M. Novel CDNF/MANF family of neurotrophic factors. Dev Neurobiol. 2010;70:360–371. doi: 10.1002/dneu.20760. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Aparicio R, Flores JA, Fernandez-Espejo E. Antiparkinsonian trophic action of glial cell line-derived neurotrophic factor and transforming growth factor ß1 is enhanced after co-infusion in rats. Exp Neurol. 2010;226:136–147. doi: 10.1016/j.expneurol.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Sun M, Kong L, Wang X, Lu XG, Gao Q, Geller AI. Comparison of the capability of GDNF, BDNF, or both, to protect nigrostriatal neurons in a rat model of Parkinson's disease. Brain Res. 2005;1052:119–129. doi: 10.1016/j.brainres.2005.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrophanous K, Yoon S, Rohll J, Patil D, Wilkes F, Kim V.et al. (1999Stable gene transfer to the nervous system using a non-primate lentiviral vector Gene Ther 61808–1818. [DOI] [PubMed] [Google Scholar]
- Cordero-Llana O, Rinaldi F, Brennan PA, Wynick D, Caldwell MA. Galanin promotes neuronal differentiation from neural progenitor cells in vitro and contributes to the generation of new olfactory neurons in the adult mouse brain. Exp Neurol. 2014;256:93–104. doi: 10.1016/j.expneurol.2014.04.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primer sequences.
Insert sequences.
Neurite outgrowth.
Transduction of dopaminergic neurons in vivo.
Experimental design.
Striatal GDNF-GFP delivery.