Abstract
Oncolytic immunotherapy with cytokine armed replication competent viruses is an emerging approach in cancer treatment. In a recent randomized trial, an increase in response rate was seen but the effect on overall survival is not known with any virus. To facilitate randomized trials, we performed a case–control study assessing the survival of 270 patients treated in an Advanced Therapy Access Program (ATAP), in comparison to matched concurrent controls from the same hospital. The overall survival of all virus treated patients was not increased over controls. However, when analysis was restricted to GMCSF-sensitive tumor types treated with GMSCF-coding viruses, a significant improvement in median survival was present (from 170 to 208 days, P = 0.0012, N = 148). An even larger difference was seen when analysis was restricted to good performance score patients (193 versus 292 days, P = 0.034, N = 90). The survival of ovarian cancer patients was especially promising as median survival nearly quadrupled (P = 0.0003, N = 37). These preliminary data lend support to initiation of randomized clinical trials with GMCSF-coding oncolytic adenoviruses.
Introduction
Following positive results in clinical trials, oncolytic viruses are nearing routine use in oncology. However, only one herpes virus has been studied in a randomized international phase 3 trial. Thus, although many different oncolytic viruses seem to have antitumor activity in cancer patients, their impact on survival remains mostly unknown. In oncology, it is widely accepted that reliable survival data can only be obtained in randomized trials. However, in the absence of such evidence, it might be of interest to attempt to estimate the magnitude of effect. Furthermore, preliminary survival data might facilitate development of randomized studies, assist in power calculations, and perhaps suggest tumor or patient types most likely to benefit. Therefore, we performed a case–control analysis on cancer patients treated with oncolytic adenoviruses in an Advanced Therapy Access Program (ATAP), and compared their survival to similar patients not treated with virus. Subgroup analyses were performed on patients treated with viruses coding for granulocyte-macrophage colony stimulating factor (GMCSF), good performance score patients, and patients with tumors we thought likely (based on previous patient series)1,2,3,4,5,6,7,8,9,10,11,12,13,14 to respond to virus coding for GMCSF. Exploratory analyses were performed in different tumor types.
Results
GMCSF-sensitivity of various cancer types
This case–control study consists of 270 cases and 186 controls from the same hospital and same time period, collected according to cancer type and disease phase (i.e., refractory to standard therapy). Based on previous analyses of ATAP patient series, we suspected that certain tumor types were more sensitive to treatment with GMCSF-coding viruses than others.1,2,3,4,5,6,7,8,9,10,11,12,13,14 To formally evaluate this, all 270 ATAP patients with survival information were grouped according to tumor types, and some of the more rare tumors were grouped together according to organ type (Table 1). Then, focusing on patients that had been treated with GMCSF-coding viruses, including the great majority, tumor types were ranked according to signs of treatment benefit in imaging analysis, defined as disease control rate, meaning stable disease or response in patients progressing prior to therapy. Tumor types featuring >30% disease control rate were categorized as GMCSF-sensitive while the remainder were classified as non-GMCSF-sensitive (Table 2). Of note, GMCSF-sensitivity was determined based on imaging, and then overall survival was studied as the study endpoint. In immunotherapy, there has been a disconnect between imaging results and survival data15,16 and therefore we did not take for granted that these aspects were linked. De facto, survival analysis was performed blinded with regard to imaging data thus constituting an independent prospective assay.
Table 1. Patient characteristics.
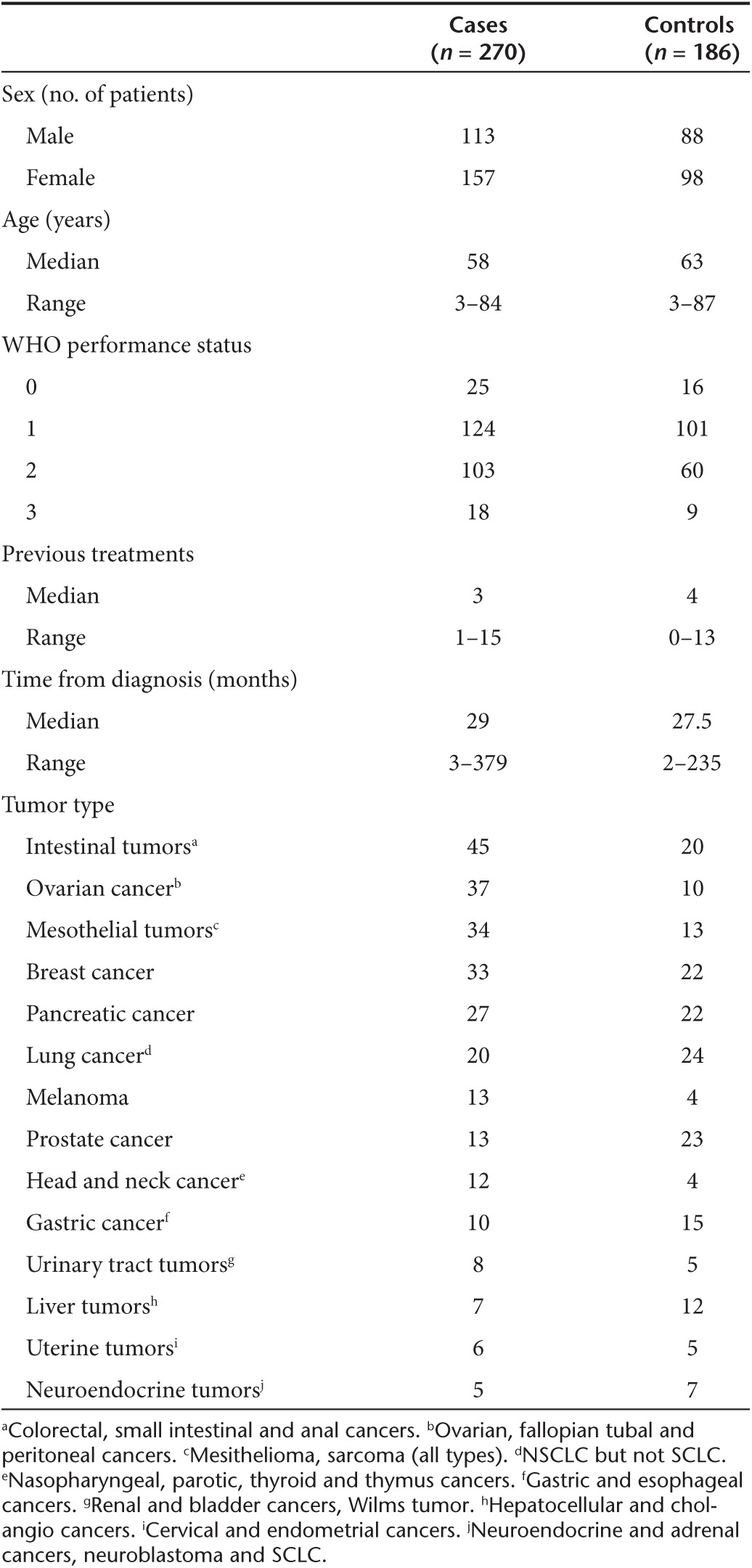
Table 2. GMCSF-sensitivity of various cancer types.
Comparability of patients and controls
To evaluate if there was imbalance between cases and controls potentially affecting survival data, we analyzed the key baseline variables possibly relevant in the context of overall survival (Supplementary Table S2). The comparison was done both according to cancer type and also for all cancer types combined. Furthermore, patients treated with GMCSF-expressing viruses were analyzed separately (Supplementary Table S3). In general, most baseline variables were evenly distributed between cases and controls. However, controls were slightly older while cases had received more previous therapy (Table 1, Supplementary Table S2). If the distribution was uneven, the effect of the variable on overall survival was further analyzed with log rank-test (data not shown). While there were some baseline variables which were unevenly distributed, as expected due to chance, the only combination of baseline variable with tumor type with significant impact on survival was in neuroendocrine cancer type (log rank P = 0.039). Here, overall survival was in favor of older patients and as the control group for this tumor type consisted of older patients (Supplementary Table S2), any imbalance would favor controls. Furthermore, there were only five cases and seven controls in this category. Therefore, this finding was considered unlikely to result in a bias in favor of cases.
Cox model hazard ratio analysis (HR)
With regard to baseline variables in the overall population (cases+controls), the only significant findings were that females survived longer than males in hazard ratio (HR) analysis (P = 0.043) and that performance score and tumor type affected survival (P < 0.0001). Time from diagnosis was also significant statistically but not clinically (HR = 0.96 i.e., 10 days).
In the overall population, there was no significant difference in survival between cases and controls (unadjusted HR = 1.01). However, since there were subsignificant differences in the patient versus control populations, including a higher proportion of WHO 2 performance score in cases, and some unevenness in the distribution of tumor types as would be expected due to chance (Table 1), it was appropriate to adjust for baseline factors and significant interactions (performance score, tumor type and age) with group (cases/controls). Adjustment resulted in a rather striking HR of 0.38, which was nevertheless not significant (P = 0.088, Table 3), suggesting that the overall population is heterogeneous, containing patients that benefit from treatment and those that do not.
Table 3. Hazard ratio (HR) calculations for overall survival, with confidence intervals (CI), adjusted for baseline factors and significant interactions with group (cases/controls).
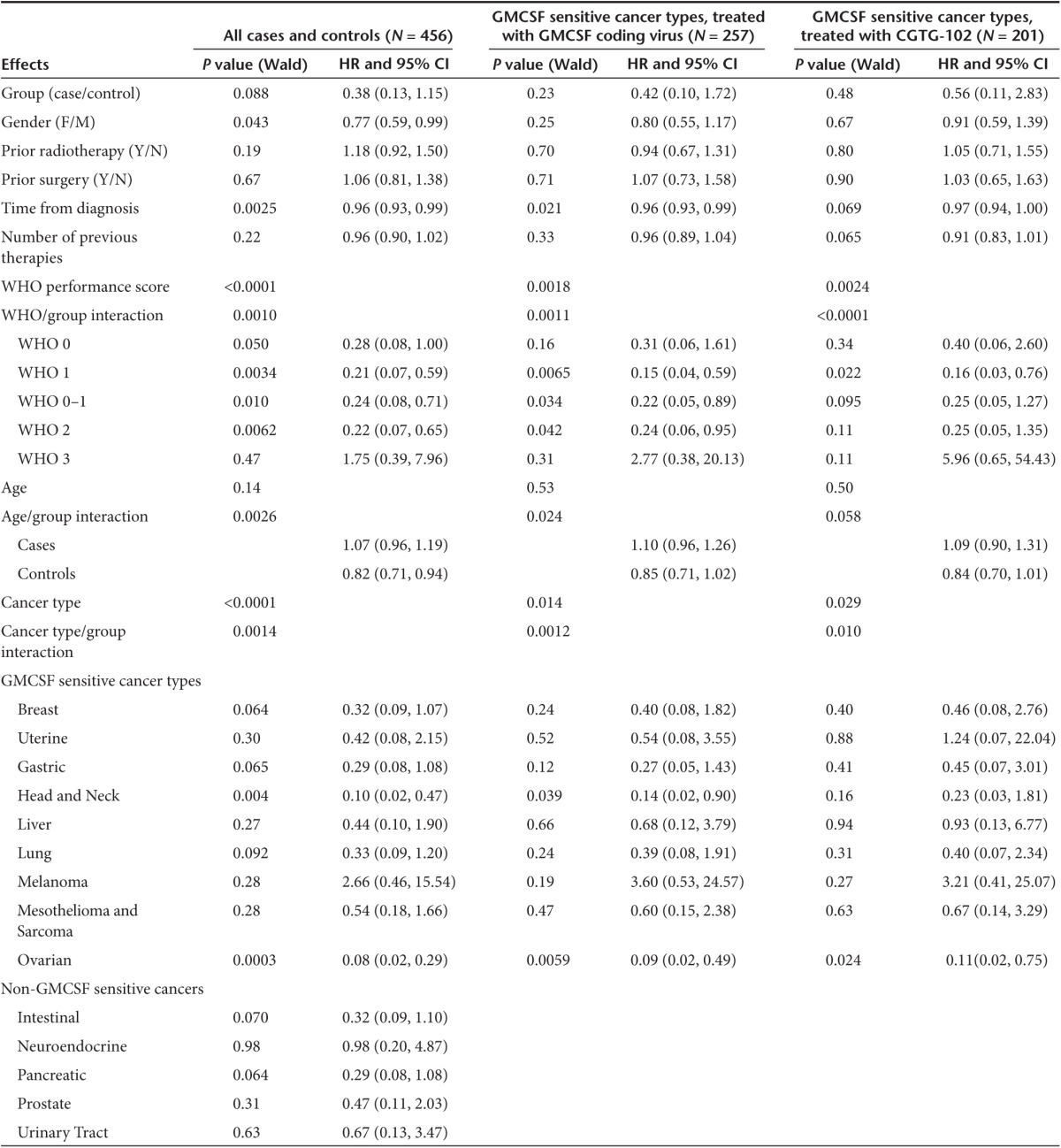
Clinical immunotherapy trials typically enroll patients with a good performance score. In our case–control study, performance score was associated with survival, and extended survival was seen in good performance score (WHO 0–1) patients treated with oncolytic adenoviruses (P = 0.010), but also in patients with more advanced disease (WHO 2) already affecting performance (P = 0.0062). No survival advantage was seen in WHO 3 patients. Age correlated with survival only in controls, but not in cases, suggesting that patients of all ages can benefit from oncolytic immunotherapy.
Although the overall ATAP patient population is of reasonable size for hypothesis generating case–control comparisons (N = 270), the caveat in looking at individual tumor types is that patient numbers become small and thus statistical power is lost. Nevertheless, as such preliminary analyses might be useful for the purpose of identifying candidate trial populations, preplanned exploratory examination of different tumor types was performed. In Cox model analysis of survival, ovarian cancer (P = 0.0003) and squamous cell cancer of the head and neck (P = 0.004) stood out with HRs of 0.08 and 0.10 (Table 3). The number of cases was low in the latter group and thus chance could have played a role but there were 37 ovarian cancer patients rendering this into a tantalizing finding. In GMCSF virus treated patients, HRs were also low for these two tumor types (HR = 0.09 for ovarian, P = 0.0059; HR = 0.14, P = 0.039 for head and neck). With CGTG-102, only ovarian was significant (P = 0.024, HR = 0.11).
Kaplan–Meier analysis
Since cases and controls were rather well balanced overall (Table 1, Supplementary Table S2), we proceeded to Kaplan–Meier survival analysis which has the benefit of yielding median survival figures, useful for estimating clinical relevance in addition to statistical significance. The comparison of overall survival was done first for all patients (N = 270) versus all controls (N = 186) and as in unadjusted HR analysis, there was no significant difference (Figure 1a, Breslow's test P = 0.793). Next, we looked at all patients (N = 197) that had received GMCSF-expressing viruses and although now median survival was 25 days longer in cases than controls, the difference was not significantly different suggesting that in an unselected population GMSCF-armed viruses have limited or no effect on overall survival (P = 0.128, Figure 1b). However, when we focused on patients with GMCSF-sensitive cancer types (Table 2), treated with GMCSF-coding viruses (N = 148), the median survival benefit was 38 days (22%) and statistically significant (Breslow's P = 0.034, Figure 1c).
Figure 1.
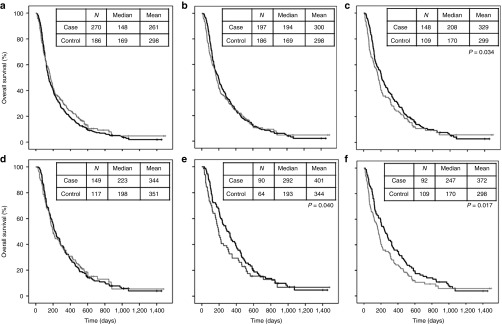
Cumulative survival data. Virus treated patients = black line, control patients = gray line. (a) All viral treatments pooled. (b) All patients treated with GMCSF-coding viruses. (c) Patient with GMCSF-sensitive cancer types and treatment with GMCSF-coding viruses. (d) Patients with WHO performance status 0 or 1. (e) Patients with good performance score (WHO 0 or 1), GMCSF-sensitive tumor types and treatment with a GMCSF-coding virus. (f) Subpopulation of patients with GMCSF-sensitive cancer types and treatment with CGTG-102 virus.
Next, we focused on a typical trial population featuring good performance score (WHO 0–1), GMCSF-sensitive tumor types and treatment with a GMCSF-coding virus (N = 90). In these patients, median survival was 99 days longer in virus treated patients (Breslow's P = 0.040, Figure 1e), constituting a 51% increase in median overall survival, which is significant not only statistically but also clinically.
In order to avoid confounding due to different viruses, we performed an analysis restricted to a single virus, CGTG-102, and median survival of patients with GMCSF-sensitive tumor types (N = 92) was 247 days versus 170 days in controls, constituting a 77 days (45%) increase in median overall survival (Breslow's P = 0.017, Figure 1f). Finally, to focus on an anticipated trial population, CGTG-102 treated patients with good performance score (WHO 0–1) and GMCSF sensitive tumors (N = 60) featured a median survival of 326 days, a 69% improvement over 193 days in controls (133 days, Breslow's P = 0.022, graph not shown).
Recognizing the risk that with small case and control numbers chance can impact survival comparisons, we nevertheless proceeded with preplanned evaluation of tumor types, as this might guide in trial design. Focusing on cancers where at least 10 cases and controls were available, the only group with a significant difference in overall survival was ovarian cancer patients. Cases (N = 37) survived 180 days longer than controls, constituting a promising 240% increase in survival (Breslow's P < 0.001, Figure 2a).
Figure 2.
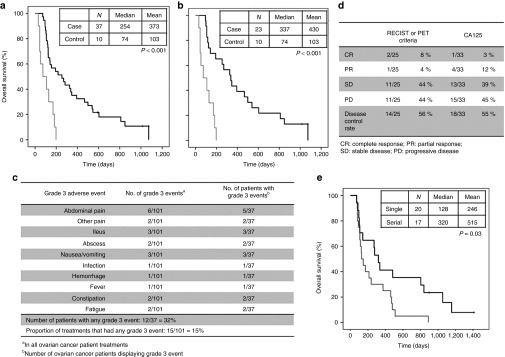
Patients with ovarian, fallopian tubal and primary peritoneal carcinomas. (a) Cumulative survival data of all ovarian cancer patients analyzed. Virus treated patients = black line, control patients = gray line. (b) Subgroup of ovarian cancer patients treated with CGTG-102 virus. (c) Grade 3 adverse events in ovarian cancer patients treated with oncolytic adenoviruses. (d) Clinical responses in ovarian cancer patients after viral treatments. (e) Overall survival of ovarian cancer patients with single or serial viral treatments. Serial treatment = black line, single treatment = gray line.
With ovarian cancer emerging as an interesting patient group, we further studied the cases in more detail. The 37 cases consisted of 28 ovarian, 6 fallopian tubal, and 3 primary peritoneal carcinomas. Treatment was generally well tolerated, and no mortality occurred, although 12 patients did experience grade 3 adverse events, of which intestinal problems were the most common (Figure 2c). There were no grade 4 or 5 adverse events. The overall disease control rates were 56 and 55% with imaging (RECIST1.1 or PET criteria) and CA125 tumor marker analysis, respectively (Figure 2d). The median survival for all ovarian cancer patients was 254 days, which compares favorably not only to the controls of this study, but also to historical data with similarly heavily pretreated patient populations.17,18
Focusing on ovarian cancer patients treated with CGTG-102, median overall survival was 337 days in patients (N = 23) and 74 days in controls, constituting a 355% improvement in overall survival (Breslow's P < 0.001, Figure 2b). Furthermore, as previous data suggests that multiple treatment may be advantageous over a single virus injection12 this was assessed also in these patients. There was a statistically significant difference in favor of multiple injections of virus (P = 0.030, Figure 2e).
Discussion
Oncolytic viruses armed with immunostimulatory transgenes are an emerging treatment approach for tumors incurable with available modalities. However, only one randomized phase 3 trial has been completed, featuring a herpes virus armed with GMCSF (T-Vec). This trial met its primary endpoint of durable response rate and also progression free survival was dramatically increased, but most importantly, the overall survival was ca. 4.5 months in favor of T-Vec (P = 0.051).19 Perhaps of relevance, as presented at ASCO 2014, a final overall survival analysis, with mature data, is pending in late 2014 or early 2015.19
However, because the T-Vec trial is the only available phase 3 where overall survival was assessed, albeit as a secondary endpoint in a 436 patient trial not powered for survival comparisons, it is interesting to compare their results to ours, keeping in mind that the T-Vec trial was randomized while our study is nonrandomized. In analysis of the overall population, their HR was 0.79 (P = 0.051) while our adjusted HR was 0.38 (P = 0.088). In subgroup analysis, T-Vec improved survival significantly when it was given as a first line therapy (HR = 0.50, P < 0.001) in Stage IIIB/C and IVM1a melanoma (HR = 0.57, P < 0.001), but not in more bulky Stage IVM1b/c disease (HR = 1.07).19 Seemingly in parallel, in our analysis significant differences in survival were only seen when the population was restricted to tumor types sensitive to GMCSF or to good performance score patients.
There is no doubt that well-designed large randomized phase 3 trials are needed to reliably demonstrate a survival advantage. However, in the absence of such trials, it may be of interest to attempt to obtain preliminary data using an observational approach such as the case–control design. Case–control studies have traditionally been employed to gain preliminary information on efficacy of treatments, estimate the magnitude of benefits if present, and to form hypotheses on patient populations likely to benefit.
The well-known caveats of case–control series relate to the difficulty of selecting appropriate controls. This, however, can be assessed by comparing the distribution of baseline characteristics of the study groups. In our study, the cases and the controls were well matched. The controls were somewhat older but, on the other hand, the cases were more heavily pretreated which might coincide with more advanced disease. At individual sites, the effect of age was only noted for neuroendocrine and ovarian tumors, both of borderline significance. Five-year net survival is highest in the youngest adults for nearly all cancers, with survival generally decreasing with increasing age.20 On one hand, older patients may respond less well to immunotherapy, but on the other it is known that cancer arising in younger individuals can behave in a particularly aggressive manner.21,22 The same uncertainty exists for number of previous treatments received prior to oncolytic virus. While more pretreatment may mean that the tumor is more refractory, more evolved, and may be further advanced and thus more aggressive than less treated tumors, one could also argue that heavily pretreated tumors may have been initially less aggressive than tumors that couldn't be heavily treated because the patient expired rapidly due to tumor progression.
However, since the differences were small in this study, we feel that neither aspect impacts the analyses performed. Importantly, the most important prognostic factors were well balanced: performance score, sex, tumor types, and time from diagnosis. The lack of imbalance between groups seems to be supported by the fact that there was no difference in survival of the overall case and control populations. In fact, the median survival of cases was slightly less than controls suggesting that if anything, the baseline characteristics were “in favor” of the controls, perhaps due to more WHO 2 patients among cases. This was confirmed with hazard ratio analysis where improved survival was seen in performance score classes WHO 0 (borderline), WHO 1, and WHO 2.
Interestingly, when we focused on tumor types possibly sensitive to viruses armed with GMCSF, a significant survival advantage was seen for patients treated with GMSCF-coding viruses. Conversely, this implies that not all tumors are sensitive to GMCSF-mediated oncolytic immunotherapy. Like all cytokines, GMCSF is a double edged sword immunologically. In classic studies, it was initially reported as the most potent stimulator of anti-tumor immunity23 and therefore it has become popular in various cancer vaccination and immunotherapy approaches. Although we had a suspicion that some tumor types are more sensitive to GMCSF-mediated immunotherapy than others, the grouping into “GMSCF-sensitive” or not, was somewhat exploratory. However, it seemed to be validated by the data presented in this manuscript. Specifically, when patients were grouped by imaging response (Table 2), subsequent prospective (and blinded) analysis of survival seemed to be in accord with the notion of some tumor types being more “GMCSF sensitive” than others. While considering this hypothesis, which will require randomized trials for proof, it is noteworthy that imaging and survival are not automatically linked in proinflammatory immunotherapy, to the same degree as with classic tumor therapies.15,16,24
GMCSF has effects on antigen presenting cells and natural killer cells. However, GMCSF stimulates also myeloid derived suppressor cells (MDSC).25 One could speculate that the reason why some tumor types seem sensitive to GMCSF and some do not, could relate to the relative importance of stimulation of antigen presentation and the counterproductive effects of MDSC recruitment. However, much further studies are required in this regard. Also, within tumor types there could be significant variation between individuals. These studies are incredibly challenging as many different cell types are involved. In intact organisms, immune cells are constantly morphing from suppressive to immunostimulatory subtypes and local effects probably do not correlate with effects seen in blood, which is just “the highway” for cells whose activity is local.12 Biopsies reflect a snapshot of a complex, constantly changing phenomena occurring over weeks, months and years. Also, biopsies can only reveal information from the biopsy location.
Nevertheless, our understanding of MDSC and the immune system in general is developing rapidly, yet we may not know enough of the relevant cell types to fully understand why GMCSF-based immunotherapy works in some animal models or patients while not in others. Our data presented here suggests that some tumor types are more likely to respond to GMCSF-based immunotherapy. Some preclinical and clinical evidence suggest that GMCSF expression in pancreatic cancer might have a dismal role.26,27 Corresponding features were seen in our study with pancreatic cancer having only 20% (3/15) response rate. Nevertheless, it seems clear that advantageous or disadvantageous immunological profiles may not be tumor type specific. Instead, it seems likely that each tumor will be an individual in this regard. One could speculate that Amgen's T-Vec result is in accord with this notion; nonbenefiting patients had bulkier disease19 which could also mean more MDSC.28 However, while the molecular details of the GMCSF-sensitive phenotype are being worked out, the clinical evaluation presented here can be helpful in focusing the first generation of trials. With regard to GMCSF mediated immunotherapy, it would be of critical importance to understand better the possible links between MDSC induction and tumor progression, and the differences, if any, between different tumor types.
Thus, while mouse studies are critically important for hypothesis generation and testing in oncology, immunology and immunotherapy, the human data obtainable from patient biopsies is of critical importance. Clinical trials can be prohibitively expensive to initiate. Thus, any data that can be helpful in designing a successful trial is most welcome. In this regard, we feel the case–control approach can have merit. While case–control comparisons cannot prove anything, they may lend support to patient selection and provide general enthusiasm (“reasons to believe”) for performing a randomized trial.
To summarize our key findings, we did not see improvement in median survival when all 270 patients were compared to the matched controls. However, when analysis was restricted to GMCSF-sensitive tumor types treated with GMCSF-coding viruses, a significant survival advantage was seen. When only good performance score patients were included, the survival advantage increased further. When focusing on a single virus, CGTG-102, which is currently being studied in clinical trials by Oncos Therapeutics, a survival advantage was seen in GMCSF-sensitive tumor types, and the magnitude of the benefit was greatest in WHO 0–1 patients. In hazard ratio analysis, which takes into account even nonsignificant imbalances between groups, treatment benefits were evident in WHO classes 0–2.
There is no doubt that a 69% improvement in median overall survival, from 193 to 326 days, is significant not only statistically but also clinically. As reported previously, three rounds of treatment were more effective than a single injection. By extension, and taking into account that most patients treated in the ATAP progressed while off therapy, it would be interesting to see if efficacy would be further improved with longer treatment.
With regard to individual tumor types, we remain cautious of interpreting the data based on small patient numbers. Thus, although there seem to be some parallels between our “GMCSF sensitive” tumor types and the number of mutations reported for different tumors (which has been proposed to as a partial explanation for sensitivity to immunotherapy),29 the case numbers are too small to draw any conclusions. However, preplanned exploratory analyses highlighted ovarian cancer as an interesting choice for inclusion in clinical trials. Nevertheless, it may be of relevance that grade 3 abdominal adverse events were frequently seen in these patients. As ileus and abdominal pain are common in patients with peritoneally disseminated cancer, caused both by the adhesive effects of carcinomatosis and the after-effects of often multiple operations, the association of these events with oncolytic adenovirus treatment deserves further study. If there is association, it is interesting to study if the same mechanisms that contribute to adverse events also play a role in efficacy. For example, T cells and NK cells kill tumor cells by releasing perforin and other and granzymes and proteases.30 Given the intimate association of ovarian carcinomatosis with the nerves covering intestines, the aforementioned molecules might affect also normal tissues and might explain the adverse events experienced. Also, transient swelling of these gut-lining tumor lesions after immunotherapy might result in physical distension.
It does not come as a surprise that ovarian cancer patients might benefit from oncolytic immunotherapy. Although this tumor type has traditionally not been among the classic “immune sensitive” cancers, there is evidence of anti-tumor immune responses in patients, as exemplified by the frequent presence of tumor infiltrating lymphocytes.31,32 A practical advantage is that virus can be applied directly into the peritoneal cavity, increasing local versus systemic concentrations. Also, the peritoneal cavity can be seen as a giant immunological organ with easy access by immune cells, complement and antibodies through the peritoneal lining.33,34 Moreover, given frequent presentation as cavity-lining carcinomatosis, instead of large immunosuppressive tumor masses, suppressive mechanisms could be less daunting than with some other tumors.
In conclusion, the case–control comparison presented here suggests that good performance score patients with certain tumor types survived longer subsequent to oncolytic immunotherapy with GMCSF-coding oncolytic adenoviruses. These results lend support to randomized clinical trials with GMCSF-coding oncolytic viruses.
Materials and Methods
Treatments. Treatments were given in the context of ATAP in Docrates Hospital, Helsinki, Finland, between 2007–2011.1,2,3,4,5,6,7,8,9,10,11,12,13,14 ATAP is a personalized therapy approach, not a clinical trial, and the treatments are based on Article 37 (previously Article 35 and initially Article 32) of World Medical Association Declaration of Helsinki. The general inclusion criteria for treatment in ATAP were: solid tumors refractory to conventional therapies, progressive disease, WHO performance score ≤3 and no major organ function deficiencies. General exclusion criteria were: organ transplant, HIV or other major immunosuppression, known brain metastasis, elevated bilirubin, ALT or AST elevated ×3 upper limit of normal, severe thrombocytopenia and other severe disease or organ malfunction. All patients signed written informed consent. A total of 290 patients were treated in ATAP, 270 of which had survival information available, and could thus be included in this study (Table 1). Survival information was collected from medical records and updated through the Finnish Population Registry for Finnish patients.
The oncolytic viruses administered to patients in ATAP were regulated by Regulation (EC) No 1394/2007 on advanced therapy medicinal products, amending Directive 2001/83/EC and Regulation (EC) No 726/2004. According to EC/1394/2007, manufacturing of advanced therapy medicinal products shall be authorized by the competent authority of the Member State. The competent authority authorizing the manufacturing of the products administered in ATAP is Finnish Medical Agency (FIMEA). FIMEA also requires reporting of adverse reactions. The Helsinki University Central Hospital Operative Ethics committee has rendered a favorable opinion on this case–control study (62/13/03/02/2013).
Individually tailored treatments were performed intratumorally in ultrasound or CT guidance, intravenously, or as an intraperitoneal or intrapleural injection as published.1,2,3,4,5,6,7,8,9,10,11,12,13,14 Patients were monitored for 24 hours in the hospital and for 4 weeks as outpatients. Viruses used for treatments have been published previously1,2,3,4,5,6,7,8,9,10,11,12,13,14 and are listed in Supplementary Table S1. Of note, GMCSF and GM-CSF indicate the same molecule. The definition for serial treatment is three rounds of oncolytic adenovirus within 10 weeks; treatments not fulfilling this criterion were evaluated as single treatments.12 The decision of the number of injections was done on a patient-by-patient basis. The length of survival or low WHO classification did not automatically increase it.
Analysis of treatment efficacy. RECIST 1.1 criteria were applied to overall disease status including injected and noninjected tumors. In some cases, PET-CT was used instead of conventional CT, using published criteria,24 in which the five most active lesions, maximum two lesions per organ, were evaluated for SUVmax and the values were summed. Progressive metabolic disease indicates more than 30% increase in summed SUVmax or ≥2 cm PET positive new lesion. Stable metabolic disease indicates −29 to +29% change, partial metabolic response notes more than 30% decline in summed SUVmax. Complete metabolic response = disappearance of all metabolically active tumor. Tumor markers (e.g., CA125) were measured from serum when elevated at baseline. Overall survival was calculated from the initial oncologist appointment until death.
Control patients. Matched controls were selected from all patients treated at the same hospital during the same time interval (2007–2011). Some grouping by organ was performed to allow analysis of also rarer tumor types (Table 1), followed by group matching by cancer type and phase of the disease, the latter indicating that these patients were refractory to routinely used standard therapies as was the case for ATAP patients. Controls were selected first and survival data was collected afterwards. 186 control patients fulfilling these criteria were found. Refractory disease was defined as follows for different tumor types:
- Ovarian: relapse within 6 months from first line chemotherapy or progression after second line chemotherapy
- Metastatic soft tissue sarcoma, melanoma, pancreatic, gastric, esophageal, head and neck, anal, cervical, hepatocellular, bladder, mesothelioma, neuroendocrine tumors progressing after first line chemotherapy
- Metastatic colorectal cancer progressing subsequent to irinotecan and oxaliplatin therapy
- Renal: progressing after second line chemotherapy
- Metastatic breast cancer progressing after chemotherapy with anthracyclines and taxanes
- Lung cancer: progressing or persistent after first line chemotherapy
- Advanced or metastatic prostate cancer progressing after treatment with taxanes
- Cholangiocarcinoma and “other” tumors: progressing or persistent after treatment with evidence-based oncological therapy
Statistical analysis. Statistics were done with SPSS v15.0 (SSPS, Chicago, IL). First, baseline variables that might have had an impact on overall survival were evaluated. Continuous and ordinal variables (WHO performance score, age, time from initial diagnosis and number of previous regimen) were analyzed with Mann–Whitney U-test, while for categorical variables (sex, prior radiation therapy and surgery) Fisher's exact test was used. For both tests, a significance value of P < 0.05 was interpreted as a statistically significant difference. In the case of unevenly distributed variables, log rank-test was used. Hazard ratios (HRs) were calculated according to the Cox proportional hazards model. Survival data was also processed with Kaplan–Meier analysis and Breslow's (generalized Wilcoxon) test.
Cox proportional hazard model was applied with focus on comparison between cases and controls but adjusting for baseline factors and their possible interactions with the grouping term (cases and controls). The final model contained all baseline factors (gender, previous radiotherapy, time from diagnosis, number of previous therapies, WHO performance score, age, and cancer type) and statistically significant interactions with group (WHO performance score, age, and cancer type). The same model was repeated with a subgroup of GMCSF sensitive cancer types, and with CGTG-102 treated patients. The assumption of proportionality was assessed by inclusion of time dependent covariates in the model. Cox model calculations were performed with SAS version 9.3.
SUPPLEMENTARY MATERIAL Table S1. List of viruses used. Table S2. Log-rank P-values for comparison of survival in cases and controls: all patients Table S3. Log-rank P-values for comparison of survival in cases and controls: GMCSF treated patients.
Acknowledgments
We thank Arja Vilkko for expert assistance. This study was supported by Oncos Therapeutics Ltd, HUCH Research Funds (EVO), Sigrid Juselius Foundation, Academy of Finland, Biocentrum Helsinki, Biocenter Finland, Finnish Cancer Organizations and the University of Helsinki. O.H. and A.H. are shareholders in Oncos Therapeutics, Ltd. O.H., K.H., and A.H. are shareholders in TILT Biotherapeutics Ltd. A.H. is an employee of TILT Biotherapeutics Ltd. The work has been done in Helsinki, Finland. The other authors declared no conflict of interest.
Supplementary Material
References
- Pesonen S, Helin H, Nokisalmi P, Escutenaire S, Ribacka C, Sarkioja M.et al. (2010Oncolytic adenovirus treatment of a patient with refractory neuroblastoma Acta Oncol 49117–119. [DOI] [PubMed] [Google Scholar]
- Pesonen S, Nokisalmi P, Escutenaire S, Särkioja M, Raki M, Cerullo V.et al. (2010Prolonged systemic circulation of chimeric oncolytic adenovirus Ad5/3-Cox2L-D24 in patients with metastatic and refractory solid tumors Gene Ther 17892–904. [DOI] [PubMed] [Google Scholar]
- Cerullo V, Pesonen S, Diaconu I, Escutenaire S, Arstila PT, Ugolini M.et al. (2010Oncolytic adenovirus coding for granulocyte macrophage colony-stimulating factor induces antitumoral immunity in cancer patients Cancer Res 704297–4309. [DOI] [PubMed] [Google Scholar]
- Nokisalmi P, Pesonen S, Escutenaire S, Särkioja M, Raki M, Cerullo V.et al. (2010Oncolytic adenovirus ICOVIR-7 in patients with advanced and refractory solid tumors Clin Cancer Res 163035–3043. [DOI] [PubMed] [Google Scholar]
- Koski A, Kangasniemi L, Escutenaire S, Pesonen S, Cerullo V, Diaconu I.et al. (2010Treatment of cancer patients with a serotype 5/3 chimeric oncolytic adenovirus expressing GMCSF Mol Ther 181874–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajecki M, Raki M, Escutenaire S, Pesonen S, Cerullo V, Helminen A.et al. (2011Safety of glucocorticoids in cancer patients treated with oncolytic adenoviruses Mol Pharm 893–103. [DOI] [PubMed] [Google Scholar]
- Cerullo V, Diaconu I, Kangasniemi L, Rajecki M, Escutenaire S, Koski A.et al. (2011Immunological effects of low-dose cyclophosphamide in cancer patients treated with oncolytic adenovirus Mol Ther 191737–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesonen S, Diaconu I, Cerullo V, Escutenaire S, Raki M, Kangasniemi L.et al. (2012Integrin targeted oncolytic adenoviruses Ad5-D24-RGD and Ad5-RGD-D24-GMCSF for treatment of patients with advanced chemotherapy refractory solid tumors Int J Cancer 1301937–1947. [DOI] [PubMed] [Google Scholar]
- Koski A, Raki M, Nokisalmi P, Liikanen I, Kangasniemi L, Joensuu T.et al. (2012Verapamil results in increased blood levels of oncolytic adenovirus in treatment of patients with advanced cancer Mol Ther 20221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesonen S, Diaconu I, Kangasniemi L, Ranki T, Kanerva A, Pesonen SK.et al. (2012Oncolytic immunotherapy of advanced solid tumors with a CD40L-expressing replicating adenovirus: assessment of safety and immunologic responses in patients Cancer Res 721621–1631. [DOI] [PubMed] [Google Scholar]
- Hemminki O, Diaconu I, Cerullo V, Pesonen SK, Kanerva A, Joensuu T.et al. (2012Ad3-hTERT-E1A, a fully serotype 3 oncolytic adenovirus, in patients with chemotherapy refractory cancer Mol Ther 201821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanerva A, Nokisalmi P, Diaconu I, Koski A, Cerullo V, Liikanen I.et al. (2013Antiviral and antitumor T-cell immunity in patients treated with GM-CSF-coding oncolytic adenovirus Clin Cancer Res 192734–2744. [DOI] [PubMed] [Google Scholar]
- Liikanen I, Ahtiainen L, Hirvinen ML, Bramante S, Cerullo V, Nokisalmi P.et al. (2013Oncolytic adenovirus with temozolomide induces autophagy and antitumor immune responses in cancer patients Mol Ther 211212–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramante S, Koski A, Kipar A, Diaconu I, Liikanen I, Hemminki O.et al. (2014Serotype chimeric oncolytic adenovirus coding for GM-CSF for treatment of sarcoma in rodents and humans Int J Cancer 135720–730. [DOI] [PubMed] [Google Scholar]
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB.et al. (2010Improved survival with ipilimumab in patients with metastatic melanoma N Engl J Med 363711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF.et al.; IMPACT Study Investigators 2010Sipuleucel-T immunotherapy for castration-resistant prostate cancer N Engl J Med 363411–422. [DOI] [PubMed] [Google Scholar]
- Markman M, Webster K, Zanotti K, Peterson G, Kulp B, Belinson J. Survival following the documentation of platinum and taxane resistance in ovarian cancer: a single institution experience involving multiple phase 2 clinical trials. Gynecol Oncol. 2004;93:699–701. doi: 10.1016/j.ygyno.2004.03.023. [DOI] [PubMed] [Google Scholar]
- Peled Y, Levavi H, Krissi H, Weill Y, Sabah G, Eitan R. The use of Fluorouracil (5-FU) and leucovorin in women with heavily pretreated advanced ovarian carcinoma. Am J Clin Oncol. 2013;36:472–474. doi: 10.1097/COC.0b013e3182549399. [DOI] [PubMed] [Google Scholar]
- Kaufman HL, Andtbacka RHI, Collichio FA, Amatruda T, Senzer NN, Chesney J.et al. (2014Primary overall survival (OS) from OPTiM, a randomized phase III trial of talimogene laherparepvec (T-VEC) versus subcutaneous (SC) granulocyte-macrophage colony-stimulating factor (GM-CSF) for the treatment (tx) of unresected stage IIIB/C and IV melanomaASCO Meeting Abstracts. J Clin Oncol 329008a [Google Scholar]
- UKCCCR Elderly Cancer Patients in Clinical Trials Working Group Position paper by the UKCCCR elderly cancer patients in clinical trials working group. Br J Cancer. 2000;82:1–3. doi: 10.1054/bjoc.1999.0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon AJ, Neuberg D, Hayes DF, Gelman R, Connolly JL, Schnitt S.et al. (1994Relationship of patient age to pathologic features of the tumor and prognosis for patients with stage I or II breast cancer J Clin Oncol 12888–894. [DOI] [PubMed] [Google Scholar]
- Xiong Q, Valero V, Kau V, Kau SW, Taylor S, Smith TL.et al. (2001Female patients with breast carcinoma age 30 years and younger have a poor prognosis: the M.D. Anderson Cancer Center experience Cancer 922523–2528. [DOI] [PubMed] [Google Scholar]
- Dranoff G. GM-CSF-based cancer vaccines. Immunol Rev. 2002;188:147–154. doi: 10.1034/j.1600-065x.2002.18813.x. [DOI] [PubMed] [Google Scholar]
- Koski A, Ahtinen H, Liljenback H, Roivainen A, Koskela A, Oksanen M.et al. (2013[(18)F]-fluorodeoxyglucose positron emission tomography and computed tomography in response evaluation of oncolytic adenovirus treatments of patients with advanced cancer Hum Gene Ther 241029–1041. [DOI] [PubMed] [Google Scholar]
- Kohanbash G, McKaveney K, Sakaki M, Ueda R, Mintz AH, Amankulor N.et al. (2013GM-CSF promotes the immunosuppressive activity of glioma-infiltrating myeloid cells through interleukin-4 receptor-a Cancer Res 736413–6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ.et al. (2012Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and t cell immunity in pancreatic cancer Cancer Cell 21822–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell. 2012;21:836–847. doi: 10.1016/j.ccr.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umansky V, Sevko A. Tumor microenvironment and myeloid-derived suppressor cells. Cancer Microenviron. 2013;6:169–177. doi: 10.1007/s12307-012-0126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside TL. Immune modulation of T-cell and NK (natural killer) cell activities by TEXs (tumour-derived exosomes) Biochem Soc Trans. 2013;41:245–251. doi: 10.1042/BST20120265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F.et al. (2005Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer Proc Natl Acad Sci USA 10218538–18543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayyoub M, Pignon P, Classe JM, Odunsi K, Valmori D. CD4+ T effectors specific for the tumor antigen NY-ESO-1 are highly enriched at ovarian cancer sites and coexist with, but are distinct from, tumor-associated Treg. Cancer Immunol Res. 2013;1:303–308. doi: 10.1158/2326-6066.CIR-13-0062-T. [DOI] [PubMed] [Google Scholar]
- Melichar B, Freedman RS. Immunology of the peritoneal cavity: relevance for host-tumor relation. Int J Gynecol Cancer. 2002;12:3–17. doi: 10.1046/j.1525-1438.2002.01093.x. [DOI] [PubMed] [Google Scholar]
- Wertel I, Nowicka A, Rogala E, Kotarski J. Peritoneal immune system in patients with advance epithelial ovarian cancer. Int Rev Immunol. 2011;30:87–101. doi: 10.3109/08830185.2011.569902. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


