Abstract
Natural killer (NK) cells are an important effector cell type for adoptive cancer immunotherapy. Similar to T cells, NK cells can be modified to express chimeric antigen receptors (CARs) to enhance antitumor activity, but experience with CAR-engineered NK cells and their clinical development is still limited. Here, we redirected continuously expanding and clinically usable established human NK-92 cells to the tumor-associated ErbB2 (HER2) antigen. Following GMP-compliant procedures, we generated a stable clonal cell line expressing a humanized CAR based on ErbB2-specific antibody FRP5 harboring CD28 and CD3ζ signaling domains (CAR 5.28.z). These NK-92/5.28.z cells efficiently lysed ErbB2-expressing tumor cells in vitro and exhibited serial target cell killing. Specific recognition of tumor cells and antitumor activity were retained in vivo, resulting in selective enrichment of NK-92/5.28.z cells in orthotopic breast carcinoma xenografts, and reduction of pulmonary metastasis in a renal cell carcinoma model, respectively. γ-irradiation as a potential safety measure for clinical application prevented NK cell replication, while antitumor activity was preserved. Our data demonstrate that it is feasible to engineer CAR-expressing NK cells as a clonal, molecularly and functionally well-defined and continuously expandable cell therapeutic agent, and suggest NK-92/5.28.z cells as a promising candidate for use in adoptive cancer immunotherapy.
Introduction
Successful application of chimeric antigen receptor (CAR)-modified T cells in patients with CD19-positive malignancies has demonstrated the potency of this approach for adoptive cancer immunotherapy,1,2,3,4 and CAR T cells targeting a variety of different tumor antigens are under active clinical development.5 CAR-mediated retargeting of natural killer (NK) cells has been attempted less frequently, and so far no clinical data for such an approach are available. NK cells play an important role in cancer immunosurveillance,6,7,8 and represent an important effector cell type for adoptive cancer immunotherapy.9,10,11 In contrast to T cells, they do not require prior sensitization and recognition of peptide antigens presented in complex with MHC molecules. Instead, their cytotoxicity can be triggered rapidly upon appropriate stimulation through germline-encoded cell surface receptors,12,13 that in part signal through CD3ζ. Hence, CD3ζ-containing CARs readily link to endogenous signaling pathways in NK cells and trigger cytolytic activity, as demonstrated for CARs specific for differentiation antigens expressed by hematologic malignancies,14,15,16,17 as well as antigens associated with solid tumors.18,19,20,21 Despite these advances, experience with CAR-engineered NK cells and their clinical development is still limited. Due to efficient antiviral defense mechanisms, gene transfer into NK cells with retro- and lentiviral vectors as well as physical transfection methods are less efficient than in T cells, complicating the generation of large numbers of CAR-expressing cells.16,22 This restriction can be overcome by employing clinically applicable NK cell lines such as NK-92, which allow isolation and expansion of CAR-expressing cells from a bulk of untransduced cells.18
Phase 1 studies in cancer patients demonstrated the safety of infusion of unmodified NK-92 cells, which were irradiated prior to application to prevent permanent engraftment. Clinical responses were achieved in a subset of patients.23,24 Similarly, CAR-engineered NK-92 cells may be developed as a targeted allogeneic cell therapeutic agent. Here, we describe the generation and the molecular and functional characterization of a clonal ErbB2-specific NK-92 cell line suitable for clinical applications. These NK-92/5.28.z cells were derived from a single cell clone after lentiviral transduction with a vector encoding a second generation CAR that targets the ErbB2 (HER2) receptor tyrosine kinase, a tumor-associated self-antigen expressed at elevated levels by many human cancers of epithelial origin.25 ErbB2-specific NK-92/5.28.z cells efficiently lysed ErbB2-expressing tumor cells in vitro that were resistant to parental NK-92 cells, and exhibited serial target cell killing. Importantly, specific recognition of ErbB2-positive tumor cells and antitumoral activity were retained in vivo, resulting in selective enrichment of NK-92/5.28.z cells in orthotopic breast carcinoma xenografts, and reduction of pulmonary metastasis of renal cell carcinoma cells in murine models.
Results
ErbB2-targeted NK-92 cells display antigen-specific cell killing activity
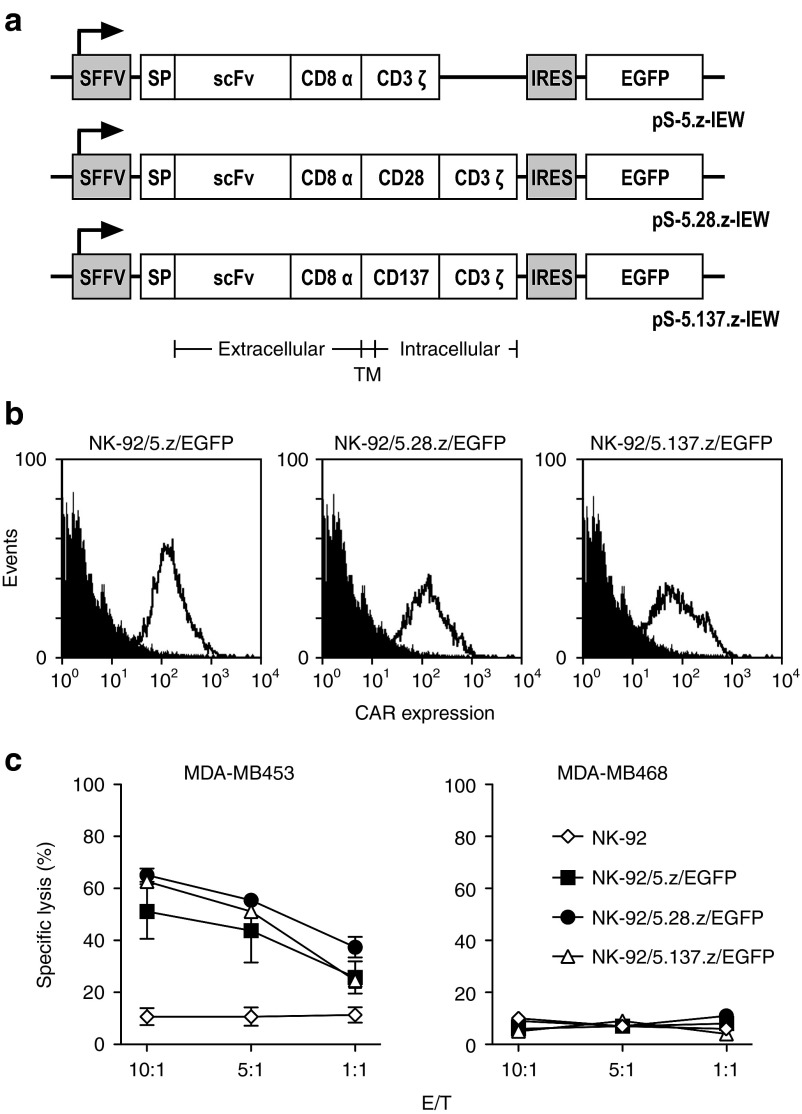
We first compared the activity of NK-92 cells carrying codon-optimized CARs which harbor the ErbB2-specific scFv(FRP5) antibody fragment26,27 linked to human CD3ζ, or composite CD28-CD3ζ or CD137-CD3ζ signaling domains via a CD8α hinge region (Figure 1a). In all constructs, an unpaired cysteine within the hinge region was replaced by serine, which increased overall CAR expression in NK-92 cells (Supplementary Figure S1). Upon lentiviral transduction and flow cytometric sorting of NK-92 cells, we found similar surface expression of CARs 5.z and 5.28.z, with CAR 5.137.z expressed at lower levels (Figure 1b). All three CARs mediated selective killing of ErbB2-expressing human MDA-MB453 breast carcinoma cells, while more prominent cytotoxicity was observed for NK-92 cells carrying the second generation CARs 5.28.z or 5.137.z (65, 62, and 51% specific lysis at an E/T ratio of 10:1 for NK-92 cells expressing CARs 5.28.z, 5.137.z or 5.z, respectively) (Figure 1c). ErbB2-negative MDA-MB468 cells were not lysed by any of the NK-92 derivatives.
Figure 1.
Specific cytotoxicity of CAR-engineered NK-92 cells against ErbB2-expressing cancer cells. (a) Schematic representation of lentiviral transfer plasmids encoding under the transcriptional control of the spleen focus forming virus promoter (SFFV) different chimeric antigen receptors, followed by an internal ribosome entry site (IRES) and enhanced green fluorescent protein (EGFP) cDNA as a marker. In pS-5.z-IEW, the CAR consists of an immunoglobulin heavy chain signal peptide (SP), the ErbB2-specific scFv(FRP5) antibody fragment (scFv), a modified CD8α hinge region (CD8α), followed by transmembrane and intracellular domains of CD3ζ (CAR 5.z). In pS-5.28.z-IEW, the hinge region is followed by transmembrane and intracellular domains of CD28 and the intracellular domain of CD3ζ (CAR 5.28.z), while in pS-5.137.z-IEW, the hinge region is followed by transmembrane and intracellular domains of CD137 (4-1BB) and the intracellular domain of CD3ζ (CAR 5.137.z). (b) CAR-expression by NK-92/5.z/EGFP, NK-92/5.28.z/EGFP and NK-92/5.137.z/EGFP cells generated by transduction of NK-92 cells with the lentiviral vectors shown in a was determined by flow cytometry with ErbB2-Fc fusion protein (open area). Parental NK-92 cells served as control (filled area). (c) Cell killing by NK-92/5.z/EGFP (filled squares), NK-92/5.28.z/EGFP (filled circles), and NK-92/5.137.z/EGFP cells (open triangles) was investigated in FACS-based cytotoxicity assays at different effector to target ratios (E/T) using human ErbB2-positive MDA-MB453 (left panel) and ErbB2-negative MDA-MB468 breast carcinoma cells (right panel) as targets. Parental NK-92 cells were included for comparison (open diamonds). Mean values ± SEM are shown; n = 3.
NK-92/5.28.z cells derived from a single cell clone retain ErbB2-specific cytotoxicity
Due to its stable surface expression and high cytotoxic activity, CAR 5.28.z was chosen as a candidate receptor to generate a clinically applicable ErbB2-specific NK-92 cell line. VSV-G pseudotyped lentiviral CAR vector particles were produced and NK-92 cells from a certified NK-92 master cell bank23 were transduced following GMP-compliant procedures. Single cell clones were derived by limiting dilution, and CAR-expressing cells were identified by flow cytometric analysis with ErbB2-Fc fusion protein. A total of 15 CAR-expressing single cell clones were functionally and molecularly characterized, which harbored between one and four vector copies. One cell clone termed NK-92/5.28.z which displayed high and stable CAR-expression during continuous culture in a setting reflecting large-scale expansion under GMP conditions was selected for further analysis (Figure 2a). Linear amplification-mediated PCR (LAM-PCR), DNA sequencing and fluorescence in situ hybridization revealed one vector integration each in an intergenic region on chromosome 2, and in the TRAF2 gene on chromosome 9 (Figure 2b).
Figure 2.
Molecular and functional characterization of clonal NK-92/5.28.z cells. (a) CAR-expression by the clonal NK-92/5.28.z cell line generated under GMP conditions by transduction with lentiviral vector S-5.28.z-W was determined by flow cytometry with ErbB2-Fc fusion protein (open area). Parental NK-92 cells served as control (gray area). (b) Three-color fluorescence in situ hybridization (FISH) was performed on metaphase spreads of the NK-92/5.28.z cell clone using the CAR-encoding fragment of pS-5.28.z-W as probe together with whole painting probes to specifically stain chromosomes 2 (wcp2) and 9 (wcp9). Probes were labeled using biotin-, digoxigenin-, and FITC-conjugated nucleotides, respectively. For biotin- and digoxigenin-labeled probes, immunological detection was performed using AMCA (blue) and Cy3 (red) fluorescent dyes. Integrated copies of CAR-encoding lentiviral vector S-5.28.z-W (red signals; indicated by white arrowheads) were found at the terminal regions of the long arms of one copy of chromosomes 2 (blue) and 9 (green), respectively. (c) Cell killing by NK-92/5.28.z cells (filled circles) was investigated in FACS-based cytotoxicity assays at different effector to target ratios (E/T) using human MDA-MB453 (ErbB2-positive) and MDA-MB468 breast carcinoma cells (ErbB2-negative) as targets. Parental NK-92 cells were included as a control (open circles). For comparison, MDA-MB468 cells which are EpCAM-positive were also treated with NK-92/31.28.z cells that express an EpCAM-specific CAR. Mean values ± SEM are shown; n = 3. (d) To confirm specificity of cell killing, similar experiments were performed with murine renal cell carcinoma cells as targets that stably express human ErbB2 (Renca-lacZ/ErbB2) or human EGFR (Renca-lacZ/EGFR). Mean values ± SEM are shown; n = 3. (e) Reactivity with normal tissues was investigated using primary human cardiomyocytes (CM), lung epithelial cells (LEC), lung fibroblasts (LF), and peripheral blood mononuclear cells (PBMC) as targets. Mean values ± SEM are shown; n ≥ 3.
Next, cytotoxic activity of the retargeted cells was evaluated. Clonal NK-92/5.28.z cells displayed high cytotoxicity towards ErbB2-expressing MDA-MB453 cells (86% specific lysis at an E/T ratio of 10:1), which were resistant to parental NK-92 (Figure 2c). As observed before, NK-92/5.28.z cells like parental NK-92 failed to lyse ErbB2-negative MDA-MB468 cells included as a control. Nevertheless, MDA-MB468 cells which express the pancarcinoma antigen EpCAM were readily killed by EpCAM-specific NK-92/31.28.z cells,21 demonstrating that enhanced activity of the CAR NK cells against otherwise NK-resistant tumor cells is strictly determined by CAR specificity. Likewise, Renca-lacZ/ErbB2 murine renal cell carcinoma cells stably expressing human ErbB2 were selectively killed by NK-92/5.28.z cells, while otherwise isogenic Renca-lacZ/EGFR cells expressing epidermal growth factor receptor displayed no enhanced sensitivity to the effector cells (Figure 2d). This indicates that cell killing was indeed mediated by interaction of CAR 5.28.z with its target antigen. In addition to breast carcinoma cells, NK-92/5.28.z also effectively lysed ErbB2-positive ovarian carcinoma and melanoma cells that were resistant to parental NK-92 (Supplementary Figure S2). Coculture of NK-92/5.28.z with ErbB2-positive targets induced secretion of IFN-γ, TNF-α, IL-10, and the chemokine MIP-1α, while no measurable amounts of IL-4 and IL-6 were produced by the NK cells (Supplementary Figure S3 and data not shown). Potential reactivity against normal tissues was investigated using primary cells derived from different human tissues as targets. At a relatively high E/T ratio of 10:1, we only observed minimal cytotoxicity of NK-92/5.28.z cells towards lung epithelial cells but no cytotoxicity above background values towards cardiomyocytes, lung fibroblasts, and peripheral blood mononuclear cells (Figure 2e).
NK-92/5.28.z cells specifically recognize ErbB2-expressing targets in mixed cultures and are capable of serial target cell killing
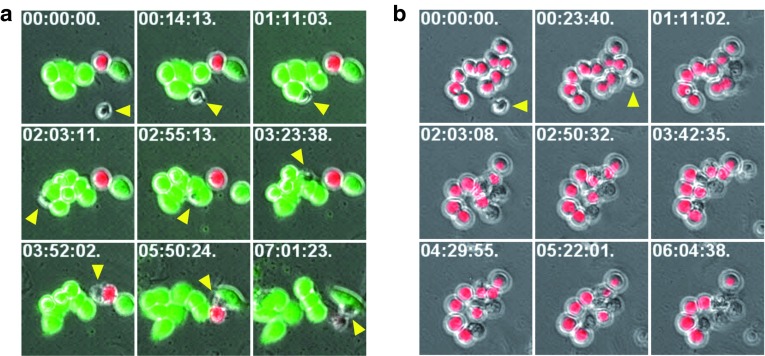
Next, we investigated selectivity of NK-92/5.28.z cells and kinetics of target cell killing in more detail. Mixtures of tdTOMATO-expressing ErbB2-positive MDA-MB453 and EGFP-expressing ErbB2-negative MDA-MB468 breast carcinoma cells were incubated with NK-92/5.28.z cells. Cultures were followed by live cell imaging for ~7 hours, with phase-contrast and fluorescent images taken every 4 minutes 45 seconds. Evaluation of serial images of individual microscopic fields revealed multiple brief contacts of single NK-92/5.28.z cells with MDA-MB468 cells (green cells), which remained unaffected by the NK cells and continued to replicate (Figure 3a and Supplementary Video S1). In contrast, NK-92/5.28.z cells made prolonged contacts with MDA-MB453 cells (red cells) interspersed with the ErbB2-negative targets, followed by cell lysis. Thereby, single NK-92/5.28.z cells sequentially attacked and killed multiple ErbB2-positive targets, with cell death indicated by massive membrane blebbing, the appearance of apoptotic bodies and loss of the marker gene signal typically occurring between 1 and 3 hours after initial contact (Figure 3b and Supplementary Video S2).
Figure 3.
Kinetics of target cell killing by NK-92/5.28.z cells. (a) To investigate selectivity and kinetics of target cell killing, live cell imaging experiments were performed with cocultures of clonal NK-92/5.28.z cells and mixtures of tdTOMATO-expressing MDA-MB453 and EGFP-expressing MDA-MB468 breast carcinoma cells. Serial images of a microscopic field with a single NK-92/5.28.z cell (yellow arrowhead), a single MDA-MB453 cell (red fluorescence), and initially five MDA-MB468 cells (green fluorescence) are shown. The first contact between the NK cell and tumor cells occurred 14 minutes after beginning the observation (second image of the series). MDA-MB468 cells were not affected in their growth despite multiple contacts with the NK cell and continued to divide during the observation time. Merged phase-contrast and fluorescence microscopy images are shown. The time stamp indicates hours:minutes:seconds from the beginning of observation. (b) Serial images of a microscopic field with a single NK-92/5.28.z cell (yellow arrowhead) and 10 MDA-MB453 cells (red fluorescence). The first contact between the NK cell and tumor cells occurred 23 minutes after beginning the observation (second image of the series). Serial killing of five MDA-MB453 target cells by the single NK-92/5.28.z cell was completed ~5 hours and 40 minutes after initial contact (last image of the series).
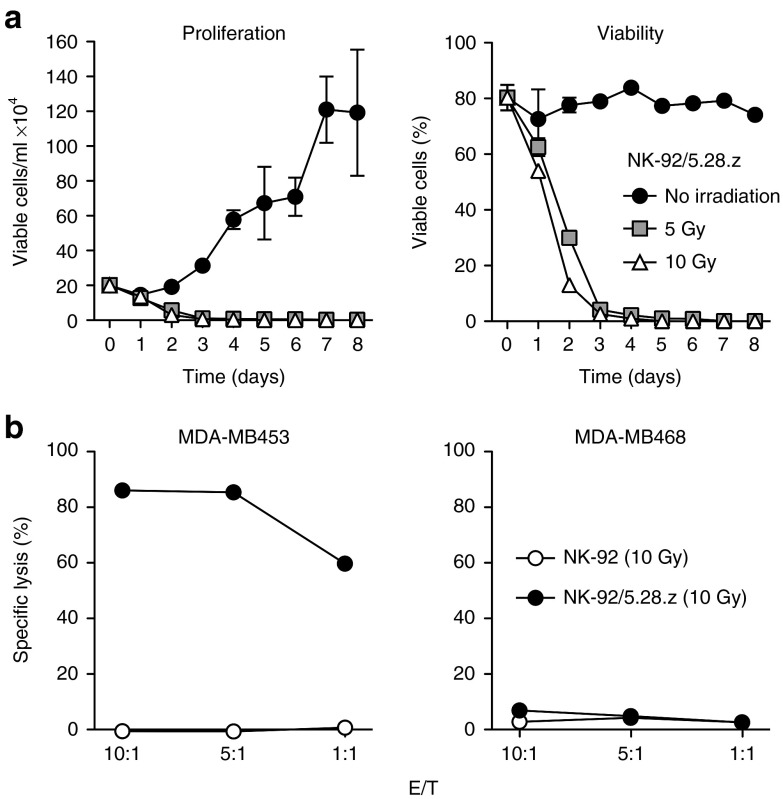
NK-92/5.28.z cells maintain specific target cell killing upon irradiation
In phase 1 clinical trials with untargeted NK-92, irradiation of cells with 10 Gy prior to infusion had been included as a safety measure to prevent permanent engraftment.23,24 Similar safety measures may be important for clinical use of retargeted NK-92 cells. Hence, we tested the effects of γ-irradiation on growth and cytotoxic activity of clonal NK-92/5.28.z cells. After exposure to 5 or 10 Gy, further replication was prevented and the number of viable NK-92/5.28.z cells declined gradually, with living cells no longer detectable at day 5 (10 Gy) and day 7 (5 Gy), respectively (Figure 4a). To assess effects on cytotoxic activity, NK-92/5.28.z cells irradiated with 10 Gy were cultured for 24 hours and then coincubated for 2 hours with target cells. Similar to untreated NK cells, irradiated NK-92/5.28.z retained high and specific cytotoxicity towards ErbB2-expressing MDA-MB453 targets (86% specific lysis at an E/T ratio of 10:1) (Figure 4b).
Figure 4.
Growth and cytotoxic activity of NK-92/5.28.z cells upon γ-irradiation. (a) To investigate the effect on viability, NK-92/5.28.z cells were irradiated with 5 or 10 Gy and cultured for up to 8 days. Proliferation (left panel) and percentage of viable cells (right panel) were analyzed by counting viable cells at the indicated time points using trypan blue exclusion. Mean values ± SEM are shown; n = 3. (b) Cytotoxic activity of NK-92/5.28.z cells 24 hours after irradiation with 10 Gy against ErbB2-positive MDA-MB453 and ErbB2-negative MDA-MB468 breast carcinoma cells was determined in FACS-based cytotoxicity assays at different effector to target ratios (E/T) as indicated (filled circles). Parental NK-92 cells 24 hours after irradiation were included for comparison (open circles). Mean values ± SEM are shown; n = 3.
Targeted NK-92 cells accumulate in ErbB2-positive breast carcinomas
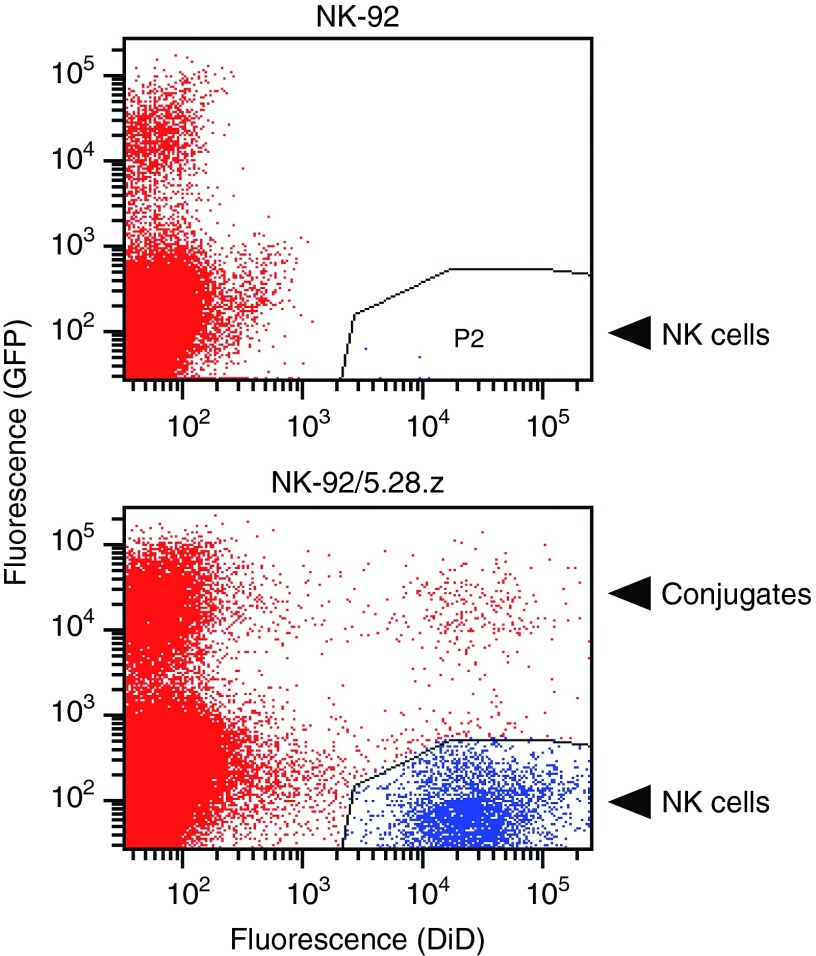
The potential of NK-92/5.28.z cells to reach established tumors was investigated in an orthotopic breast carcinoma model. MDA-MB453 cells transduced with an EGFP-encoding lentiviral vector were implanted into the mammary fat pad of female NSG mice, and allowed to grow until tumors were palpable. Then, NK-92/5.28.z and unmodified parental NK-92 cells were labeled with fluorescent DiD labeling reagent, and intravenously injected into the tumor-bearing animals. Twenty-four hours later, tumors were excised, single cell suspensions were prepared, and analyzed for the presence of EGFP-expressing tumor cells and DiD-labeled NK cells. In mice injected with untargeted NK-92, only a few of the NK cells were found in the tumors (Figure 5, upper panel). In contrast, NK-92/5.28.z cells were strongly enriched in MDA-MB453/EGFP xenografts (Figure 5, lower panel). Importantly, we also found conjugates of NK-92/5.28.z and MDA-MB453/EGFP cells in the cell suspensions prepared from the tumors. These data demonstrate that NK-92/5.28.z cells retain target cell specificity in vivo, and are capable of penetrating tissues and reach distant tumor sites.
Figure 5.
Accumulation of NK-92/5.28.z cells in ErbB2-positive breast carcinomas in vivo. Parental NK-92 (upper panel) or ErbB2-specific NK-92/5.28.z cells (lower panel) were labeled with fluorescent DiD labeling reagent and intravenously injected into NSG mice carrying established orthotopic MDA-MB453/EGFP breast carcinoma xenografts. Twenty-four hours after injection, tumors were excised, single cell suspensions were prepared, and analyzed for the presence of EGFP-expressing and DiD-labeled cells. DiD-positive NK cells are indicated in blue (lower right quadrants). EGFP-positive breast carcinoma cells (upper left quadrants) and double-negative murine stromal cells (lower left quadrants) are indicated in red. Double-positive events (upper right quadrant) represent conjugates of CAR-expressing NK-92/5.28.z and MDA-MB453/EGFP target cells. Representative flow cytometric data from one animal of each group are shown (n = 3).
NK-92/5.28.z cells exhibit specific antitumor activity in vivo
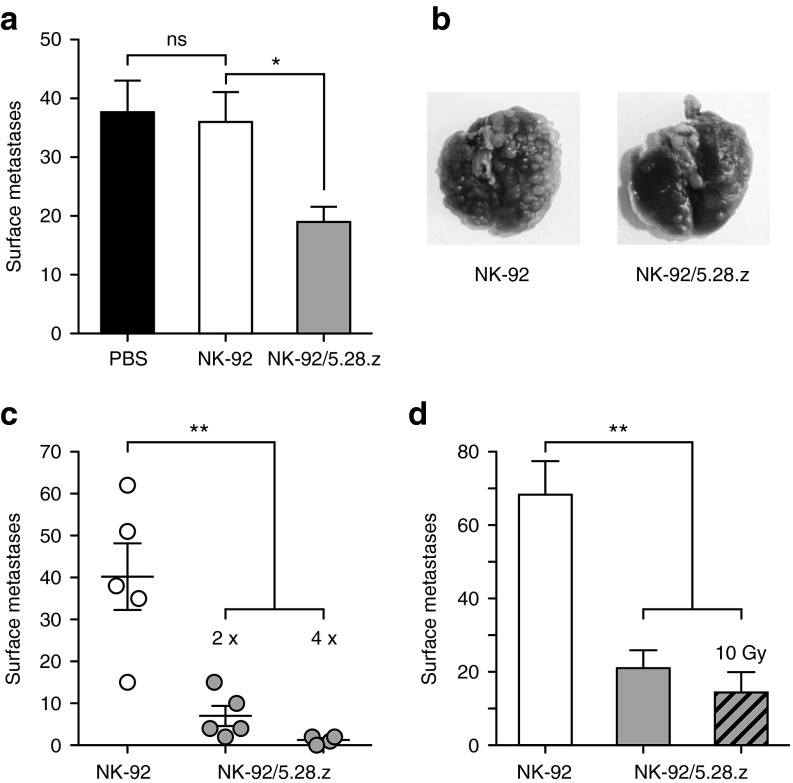
For evaluation of in vivo antitumor activity, we chose an experimental lung metastasis model. NSG mice received intravenous injections of Renca-lacZ/ErbB2 cells, followed by i.v. injections of parental NK-92 or retargeted NK-92/5.28.z cells at days 1 and 3 after tumor cell inoculation. Control mice received PBS. Four weeks after tumor challenge, lungs were excised and tumor nodules on the lung surface were counted. While treatment with parental NK-92 cells did not affect metastasis formation in comparison to PBS-treated controls, retargeted NK-92/5.28.z cells reduced the number of pulmonary tumor nodules in this experiment by ~50% (mean number of lung surface metastases: PBS: 37.7 ± 5.4; NK-92: 36 ± 5.1; NK-92/5.28.z: 19 ± 2.6; P < 0.05) (Figure 6a,b). We did not observe distress, weight loss or other signs of adverse effects in these animals (Supplementary Figure S4). When mice were treated repeatedly with NK-92/5.28.z cells at days 1, 3, 6, and 8 after tumor challenge, efficacy was further enhanced with one out of four animals in this group not showing any tumor nodules on the lung surface when the experiment was terminated at day 28 (Figure 6c). To assess whether NK-92/5.28.z cells retain in vivo antitumor activity after γ-irradiation, a similar experiment was performed employing NK-92/5.28.z cells that were irradiated with 10 Gy prior to injection. Control animals were treated with nonirradiated NK-92/5.28.z or nonirradiated parental NK-92 cells. In comparison to treatment with unmodified NK-92 cells, both, nonirradiated and irradiated NK-92/5.28.z cells markedly reduced the number of pulmonary tumor nodules (mean number of lung surface metastases: NK-92: 68.3 ± 9.1; NK-92/5.28.z: 21 ± 4.9; irradiated NK-92/5.28.z: 14.4 ± 5.5; P < 0.01) (Figure 6d). These data demonstrate specific antitumor activity of systemically applied NK-92/5.28.z cells against ErbB2-expressing tumor cells in a model reflecting disseminated disease. Importantly, viability and functionality of NK-92/5.28.z cells were transiently preserved after γ-irradiation at a dose that prevents further effector cell replication, permitting target cell recognition and killing in vivo.
Figure 6.
In vivo antitumor activity of NK-92/5.28.z cells. (a) To investigate antitumor activity, NSG mice were intravenously injected with Renca-lacZ/ErbB2 renal cell carcinoma cells. Then, animals were treated twice by i.v. injection of parental NK-92 or clonal NK-92/5.28.z cells at days 1 and 3 after tumor cell injection. Control mice received PBS. Four weeks after tumor challenge, lungs were excised and tumor nodules on the lung surface were counted. Mean values ± SEM are shown; n = 5. ns, P > 0.05; *P < 0.05. (b) Representative images of lungs at day 28 from animals treated with NK-92 or NK-92/5.28.z cells. (c) To evaluate the effect of multiple treatments, NSG mice injected with Renca-lacZ/ErbB2 cells were treated with NK-92 or NK-92/5.28.z cells twice as described in panel a (n = 5), or four times at days 1, 3, 6, and 8 (n = 4). Data points for each animal and mean values ± SEM are shown. **P < 0.01. (d) In a separate experiment, NSG mice injected with Renca-lacZ/ErbB2 cells were treated twice as described in panel a with nonirradiated NK-92 or NK-92/5.28.z cells, or NK-92/5.28.z cells irradiated with 10 Gy as indicated. Mean values ± SEM are shown; n = 5. **P < 0.01.
Discussion
Our data demonstrate successful targeting of human NK-92 cells to the tumor-associated cell surface antigen ErbB2 by expression of a humanized and codon-optimized second-generation CAR. Following GMP-compliant procedures, we established from a molecularly defined single cell clone a continuously expanding CAR-modified NK cell line suitable for clinical development. These NK-92/5.28.z cells display stable CAR expression upon prolonged culture and target-antigen-specific cytotoxicity in vitro and in vivo. The possibility to fully characterize this cell clone at the molecular and cellular levels adds an important degree of safety for the clinical application of the clone, in contrast to the heterogeneous composition of CAR-modified primary NK and T cells, in which no exhaustive molecular analysis is possible. Live cell imaging experiments with mixed cultures of ErbB2-positive and ErbB2-negative breast carcinoma cells confirmed target selectivity of NK-92/5.28.z cells. We observed serial killing of up to five ErbB2-expressing targets by a single NK-92/5.28.z cell within 6 hours, indicating that the cells can perform attacks on multiple targets without apparent reduction in activity or viability. Previously, it was shown that serial killing of tumor cells by CD16-positive primary NK cells was increased twofold in the presence of rituximab as a tumor-specific antibody.28 CAR 5.28.z appears to function in a similar fashion in the CD16-negative NK-92 cells, facilitating ADCC-like activity and enhanced serial killing. Exposure of NK-92/5.28.z cells to ErbB2-positive targets induced secretion of cytokines such as IFN-γ, TNF-α, and the chemokine MIP-1α (CCL3) that are typical for activated NK cells and could in principle result in recruitment and maturation of antigen presenting cells and enhancement of endogenous antitumor immune responses in an immunocompetent host. This, however, may to some extent be balanced by the immunoregulatory cytokine IL-10 which was also produced by NK-92/5.28.z cells at high levels.29
In mice, fluorochrome-labeled NK-92/5.28.z cells selectively enriched in ErbB2-positive orthotopic breast carcinoma xenografts within 24 hours after intravenous injection, while untargeted parental NK-92 cells failed to accumulate in tumors. This demonstrates that NK-92/5.28.z cells retain target cell specificity in vivo, and are capable of penetrating tissues and homing to distant tumor sites. In an experimental metastasis model based on ErbB2-expressing renal cell carcinoma cells, intravenous injection of NK-92/5.28.z cells markedly reduced metastasis formation, while untargeted NK-92 cells failed to affect outgrowth of pulmonary tumor nodules. Importantly, in vivo antitumor activity of NK-92/5.28.z cells irradiated with 10 Gy was the same as that of nonirradiated cells. This may be relevant for future clinical application of NK-92/5.28.z, where irradiation of cells may be included as a safety measure as previously done in phase 1 clinical trials with untargeted NK-92 cells.23,24
Immune cells in tumor patients are often functionally compromised due to the immunosuppressive activity of the cancer. Hence, for adoptive cancer immunotherapy with NK cells, donor-derived allogeneic cells are being preferred since they do not recognize tumor cells as “self,” thereby bypassing inhibitory signals.10,11 This advantage may be extended to CAR-engineered NK-92 cells. NK-92 lack expression of most of the inhibitory KIRs and phenotypically resemble activated NK cells.30 Moreover, the fact that CAR NK cells do not carry an endogenous TCR of unknown specificity may be considered a safety feature limiting possible graft-versus-host activities when compared to CAR T cells, which would require alloanergization in an MHC-mismatched situation.31 Existing clinical experience with allogeneic NK cells and their short in vivo half-life in comparison to T cells make NK cells an attractive cell type for genetic modification with CAR constructs and other immunomodulatory functions, in particular in cases where tumor-associated antigens are targeted that are present at lower levels in normal tissues.32,33 Nevertheless, also in the case of CAR NK cells potential CAR reactivity with a self-antigen like ErbB2 expressed at low or moderate levels by normal epithelial tissues may result in unwanted toxicities.34
In a clinical trial conducted at the National Cancer Institute, a fatal adverse event occurred after infusion of autologous T cells modified to express an ErbB2-specific third generation CAR that employed a trastuzumab-derived scFv antibody fragment. Presumably, massive T-cell activation and respiratory failure immediately after infusion were triggered by ErbB2 expressed on normal lung epithelium.35 This possibility needs to be considered for other ErbB2-targeted adoptive cell therapies. Apparently, the location of the CAR binding epitope within the target antigen can have a decisive effect on effector cell activation. In the case of CAR T cells, CARs directed to membrane-distal epitopes were shown to be superior in binding but less potent in mediating activation than CARs directed to membrane-proximal epitopes of the same antigen.36,37 While the trastuzumab antibody fragment used by Morgan et al. binds to the juxtamembrane region of the target receptor,38 antibody FRP5 employed for the generation of CAR 5.28.z recognizes a discontinuous epitope within residues 11–169 of mature human ErbB2 protein facing away from the cell surface.39,40 Hence, FRP5-based CARs may be less likely than trastuzumab-based CARs to get activated by ErbB2 expressed at moderate levels by normal epithelial tissues. In our in vitro experiments, at the highest E/T ratio applied we only observed minimal cytotoxicity of NK-92/5.28.z cells towards lung epithelial cells but no cytotoxicity above background values against other normal tissues including cardiomyocytes. Importantly, so far no on-target/off-tumor toxicities have been observed upon clinical application of other experimental therapeutics utilizing FRP5 for targeting to ErbB2. In a clinical trial investigating safety of an intravenously infused scFv(FRP5)-Pseudomonas exotoxin A fusion protein neither cardiac toxicity, as associated with trastuzumab treatment,41 nor lung toxicity, as observed by Morgan et al.,35 were found in any of the patients.42
Early phase clinical trials in cancer patients showed safety of infusion of irradiated NK-92 cells at doses up to 1010 cells/m2, with clinical responses achieved in a subset of patients.23,24 Our data demonstrate that it is feasible to develop CAR-engineered NK-92 cells in a similar manner as a clonal, molecularly and functionally well defined and continuously expandable off-the-shelf cell therapeutic agent with selective and markedly enhanced antitumor activity in vitro and in vivo. Such cells may become clinically useful for the treatment of various ErbB2-positive malignancies. Thereby, the potent antitumor activity, the immediate availability as a fully characterizable cell product, and the lack of obvious risks of manufacturing failures suggest these cells as a valid and cost-effective alternative to CAR-modified patient T cells.
Materials and Methods
Cells and culture conditions. Human MDA-MB453, MDA-MB468, and SK-BR-3 breast carcinoma cells, SK-OV-3 ovarian carcinoma cells (all ATCC, Manassas, VA), MDA-MB435 (ATCC) and SK-MEL-23 (ref. 43) melanoma cells, and HEK 293T cells (ATCC) were cultured in DMEM (Lonza, Köln, Germany). All media were supplemented with 10% heat-inactivated FBS, 2 mmol/l L-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin. Human NK-92 cells (kindly provided by Conkwest, Cardiff-by-the-Sea, CA) were propagated in X-VIVO 10 medium (Lonza) supplemented with 5% heat-inactivated human plasma (German Red Cross Blood Donation Service Baden-Württemberg–Hessen, Frankfurt, Germany) and 100 IU/ml IL-2 (Proleukin; Novartis Pharma, Nürnberg, Germany). Murine Renca-lacZ/ErbB2 and Renca-lacZ/EGFR renal cell carcinoma cells expressing human ErbB2 or EGFR were cultured in RPMI-1640 medium (Lonza) supplemented with 10% FBS, 2 mmol/l L-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, 0.25 mg/ml zeocin, and 0.48 mg/ml G418.44 Primary human cardiomyocytes (kindly provided by Stefanie Dimmeler and Reinier Boon, Institute of Cardiovascular Regeneration, Goethe University, Frankfurt am Main, Germany) and primary human lung epithelial cells (PromoCell, Heidelberg, Germany) were cultured in Myocyte Growth Medium and Small Airway Epithelial Cell Growth Medium (both PromoCell), respectively. Human primary lung fibroblasts (Lonza) were grown in Fibroblast Growth Medium (Lonza).
Generation of CAR-expressing NK-92 cells. CAR sequences 5.z, 5.28.z, and 5.137.z were designed by in silico assembly of an immunoglobulin heavy chain signal peptide, ErbB2-specific scFv(FRP5) antibody fragment,26 and a modified CD8α hinge region, followed by CD3ζ transmembrane and intracellular domains (CAR 5.z), CD28 transmembrane and intracellular domains and CD3ζ intracellular domain (CAR 5.28.z), or CD137 (4-1BB) transmembrane and intracellular domains and CD3ζ intracellular domain (CAR 5.137.z). Codon-optimized fusion genes were synthesized (GeneArt, Regensburg, Germany) and inserted into lentiviral transfer plasmid pHR'SIN-cPPT-SIEW (pSIEW)45 upstream of IRES and EGFP sequences, resulting in constructs pS-5.z-IEW, pS-5.28.z-IEW, and pS-5.137.z-IEW. The CAR 5.28.z expression cassette was in addition inserted into the similar pHR'SIN-cPPT-WPREmut vector lacking IRES-EGFP,46 resulting in lentiviral transfer plasmid pS-5.28.z-W. VSV-G pseudotyped vector particles were generated and NK-92 cells were transduced as described previously.21
CAR-expressing NK-92 cells were derived by flow cytometric cell sorting with a FACSAria fluorescence-activated cell sorter (BD Biosciences, Heidelberg, Germany) using ErbB2-Fc fusion protein (R&D Systems, Wiesbaden-Nordenstadt, Germany) followed by APC-coupled secondary antibody (Dianova, Hamburg, Germany). CAR expression by sorted cells was confirmed as described above with a FACSCanto II flow cytometer (BD Biosciences). Data were analyzed with FACSDiva software (BD Biosciences). Alternatively, production of lentiviral vector pS-5.28.z-W and transduction of NK-92 cells were carried out following good manufacturing practice procedures (EU-GMP) (EUFETS GmbH, Idar-Oberstein, Germany). Single cell clones were generated by limiting dilution, and CAR-expressing cells were identified as described above. In selected cell clones, number and position of vector integrations were determined by linear amplification-mediated PCR (LAM-PCR) and DNA sequencing as described.47 For the NK-92/5.28.z cell clone chosen for further analysis, vector integrations were visualized by fluorescence in situ hybridization following standard protocols. Microscopic images were captured using a DM-RXA epifluorescence microscope controlled by Q-FISH software (Leica).
Cytotoxicity assays. Cytotoxicity of NK-92 cells towards tumor target cells and primary human cardiomyocytes and lung epithelial cells was analyzed in FACS-based assays as described.21 Briefly, target cells were labeled with calcein violet AM (Molecular Probes, Invitrogen, Karlsruhe, Germany) and cocultured with effector cells at various effector to target (E/T) ratios for 2 hours at 37 °C. After coculture, 250 µl of a 1 µg/ml propidium iodide (PI) solution were added to each sample 5 minutes before flow cytometric analysis in a FACSCanto II flow cytometer. Data were analyzed using FACSDiva software. To calculate specific cytotoxicity, the number of spontaneously lysed target cells in the absence of effector cells was subtracted from the number of dead target cells determined as calcein violet AM and PI double positive in the measured sample. Cytotoxicity assays with primary human lung fibroblasts and NK-depleted PBMCs were carried out for 4 hours as described.48
Time-lapse imaging. MDA-MB453 and MDA-MB468 breast carcinoma cells as targets were transduced with lentiviral vectors encoding tdTOMATO-hImportin or EGFP, respectively. Target cells were seeded in 24-well culture plates equipped with a silicon culture insert (IBIDI, Martinsried, Germany) at 10% confluency. NK-92/5.28.z cells were added and time-lapse imaging was carried out with a Cell Observer system (Carl Zeiss, Göttingen, Germany) with temperature and gas control. Phase-contrast and fluorescent images of each position were taken every 4 minutes 45 seconds with a 10× Neofluar objective (Carl Zeiss) and an AxioCamHRm camera (at 1,388 × 1,040 pixel resolution) with Carl Zeiss AxioVision 4.8 software. The HXP illumination system was used for fluorescence illumination. Movies were assembled using QuickTime 7.6 software (Apple).
Irradiation of NK-92 cells. NK-92/5.28.z and parental NK-92 cells were collected by centrifugation, counted, washed, resuspended in fresh growth medium and irradiated with 5 or 10 Gy using a Biobeam 2000 device (Gamma Service Medical, Leipzig, Germany). For in vitro proliferation, viability and cytotoxicity assays, irradiated cells were washed, resuspended in fresh growth medium and cultured for up to 8 days. Proliferation and viability were analyzed by counting viable cells at different time points using trypan blue exclusion. For in vivo experiments, cells were irradiated with 10 Gy and applied directly.
Accumulation of NK-92 cells in established tumors. EGFP-expressing MDA-MB453 breast carcinoma cells were derived by transduction of MDA-MB453 cells with an EGFP-encoding lentiviral vector and enrichment by flow cytometric cell sorting. Orthotopic breast carcinoma xenografts were induced in 4- to 6-week-old, female NOD-SCID IL2R γnull (NSG) mice (Charles River, Sulzfeld, Germany) by injection of 5 × 106 MDA-MB453/EGFP cells suspended in Matrigel (BD Biosciences) into the mammary fat pad. When tumors were palpable, NK-92/5.28.z or parental NK-92 cells were labeled with DiD (1,1′-dioctadecyl-3,3,3′,3′ tetramethylindodicarbocyanine) labeling reagent (Molecular Probes/Life Technologies, Darmstadt, Germany) as described,49 and injected into the lateral tail vein of the tumor bearing mice (1 × 107 cells/animal; three animals per group). Twenty-four hours after injection, mice were sacrificed, tumors were excised, single cell suspensions were prepared, and analyzed for the presence of EGFP-expressing and DiD-labeled cells in a FACSCanto II flow cytometer.
In vivo antitumor activity. Four- to 6-week-old, female NSG mice were injected with 1 × 105 Renca-lacZ/ErbB2 cells into the lateral tail vein at day 0. Then animals were treated by i.v. injection of 1 × 107 NK-92/5.28.z or parental NK-92 cells at days 1 and 3 (five mice/group), or at days 1, 3, 6, and 8 after tumor cell injection (four mice/group). Control mice received PBS. In separate experiments, NSG mice injected with Renca-lacZ/ErbB2 cells were also treated with irradiated NK-92/5.28.z cells (10 Gy), or nonirradiated NK-92/5.28.z and parental NK-92 cells as controls (five mice/group). Four weeks after tumor challenge, all animals were sacrificed, lungs were excised, and tumor nodules on the lung surface were counted as described.44
Ethics statement. All animal experiments were approved by the review board of the Georg-Speyer-Haus and the responsible government committee, and were conducted according to the applicable guidelines and regulations.
Statistical analysis. Data are presented as means ± SEM as stated in the figure legends. Results were analyzed by two-tailed unpaired Student's t-test. P values <0.05 were considered significant. Statistical calculations were performed using Prism 5 software (GraphPad Software, La Jolla, CA).
SUPPLEMENTARY MATERIAL Figure S1. Effect of the CD8α hinge region on CAR expression in NK-92 cells. Figure S2. Cytotoxic activity of NK-92/5.28.z cells against human tumor cells. Figure S3. Cytokine secretion upon activation of NK-92/5.28.z by target cells. Figure S4. Body weight development in mice treated with NK-92/5.28.z cells. Video S1. Specific recognition of ErbB2-expressing targets by NK-92/5.28.z cells. The video represents serial images of the experiment shown in Figure 3a assembled into a QuickTime movie. Video S2. Serial target cell killing by NK-92/5.28.z cells. The video represents serial images of the experiment shown in Figure 3b assembled into a QuickTime movie.
Acknowledgments
This work was supported in part by grants from the German Federal Ministry of Education and Research (BMBF) (FKZ 01GU0805 and FKZ 131A009A, Ci3), the Deutsche Forschungsgemeinschaft (DFG) (GRK1172), the LOEWE Center for Cell and Gene Therapy Frankfurt (CGT), and institutional funds of the Georg-Speyer-Haus. The Georg-Speyer-Haus is funded jointly by the German Federal Ministry of Health (BMG) and the Ministry of Higher Education, Research and the Arts of the State of Hessen (HMWK). The LOEWE Center for Cell and Gene Therapy Frankfurt is funded by HMWK, reference number: III L 5–518/17.004 (2013). We thank Barry J. Simon, Conkwest, Inc., for providing NK-92 cells from Conkwest's proprietary master cell bank, Stefanie Dimmeler and Reinier Boon, Institute of Cardiovascular Regeneration, Goethe University, Frankfurt am Main, for primary human cardiomyocytes, Stefan Stein and Tefik Merovci, Georg-Speyer-Haus, for help with flow cytometric cell sorting, Petra Schön, Pediatric Pulmonology, Allergy and Cystic Fibrosis, University Hospital Frankfurt am Main, and Sarah Oelsner, Georg-Speyer-Haus, for help with cytokine analysis, and Annemarie Schimpf, Barbara Uherek and Claudia Jourdan, Georg-Speyer-Haus, and Mareike Alef, German Red Cross Blood Donation Service Baden-Württemberg–Hessen, for technical assistance. H.G.K. is a cofounder and employee of Conkwest, Inc., an entity commercially developing NK-92 cells. The other authors declare no conflict of interest.
Supplementary Material
References
- Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A.et al. (2011T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia Sci Transl Med 395ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR.et al. (2013Chimeric antigen receptor-modified T cells for acute lymphoid leukemia N Engl J Med 3681509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG.et al. (2013CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia Sci Transl Med 5177ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochenderfer JN, Dudley ME, Carpenter RO, Kassim SH, Rose JJ, Telford WG.et al. (2013Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation Blood 1224129–4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalos M, June CH. Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology. Immunity. 2013;39:49–60. doi: 10.1016/j.immuni.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. 2000;356:1795–1799. doi: 10.1016/S0140-6736(00)03231-1. [DOI] [PubMed] [Google Scholar]
- Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–271. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- Vivier E, Ugolini S, Blaise D, Chabannon C, Brossay L. Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol. 2012;12:239–252. doi: 10.1038/nri3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suck G. Novel approaches using natural killer cells in cancer therapy. Semin Cancer Biol. 2006;16:412–418. doi: 10.1016/j.semcancer.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Geller MA, Miller JS. Use of allogeneic NK cells for cancer immunotherapy. Immunotherapy. 2011;3:1445–1459. doi: 10.2217/imt.11.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingemann HG. Cellular therapy of cancer with natural killer cells-where do we stand. Cytotherapy. 2013;15:1185–1194. doi: 10.1016/j.jcyt.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Orange JS. Formation and function of the lytic NK-cell immunological synapse. Nat Rev Immunol. 2008;8:713–725. doi: 10.1038/nri2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch J, Steinle A, Watzl C, Mandelboim O. Activating natural cytotoxicity receptors of natural killer cells in cancer and infection. Trends Immunol. 2013;34:182–191. doi: 10.1016/j.it.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106:376–383. doi: 10.1182/blood-2004-12-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller T, Uherek C, Maki G, Chow KU, Schimpf A, Klingemann HG.et al. (2008Expression of a CD20-specific chimeric antigen receptor enhances cytotoxic activity of NK cells and overcomes NK-resistance of lymphoma and leukemia cells Cancer Immunol Immunother 57411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissel L, Betancur M, Wels WS, Tuncer H, Klingemann H. Transfection with mRNA for CD19 specific chimeric antigen receptor restores NK cell mediated killing of CLL cells. Leuk Res. 2009;33:1255–1259. doi: 10.1016/j.leukres.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J, Deng Y, Benson DM, Jr, He S, Hughes T, Zhang J.et al. (2013CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo anti-tumor activity against human multiple myeloma Leukemia 28917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uherek C, Tonn T, Uherek B, Becker S, Schnierle B, Klingemann HG.et al. (2002Retargeting of natural killer-cell cytolytic activity to ErbB2-expressing cancer cells results in efficient and selective tumor cell destruction Blood 1001265–1273. [PubMed] [Google Scholar]
- Altvater B, Landmeier S, Pscherer S, Temme J, Schweer K, Kailayangiri S.et al. (20092B4 (CD244) signaling by recombinant antigen-specific chimeric receptors costimulates natural killer cell activation to leukemia and neuroblastoma cells Clin Cancer Res 154857–4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser R, Müller T, Stefes D, Kloess S, Seidel D, Gillies SD.et al. (2012NK cells engineered to express a GD2 -specific antigen receptor display built-in ADCC-like activity against tumour cells of neuroectodermal origin J Cell Mol Med 16569–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahm C, Schönfeld K, Wels WS. Expression of IL-15 in NK cells results in rapid enrichment and selective cytotoxicity of gene-modified effectors that carry a tumor-specific antigen receptor. Cancer Immunol Immunother. 2012;61:1451–1461. doi: 10.1007/s00262-012-1212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutlu T, Nyström S, Gilljam M, Stellan B, Applequist SE, Alici E. Inhibition of intracellular antiviral defense mechanisms augments lentiviral transduction of human natural killer cells: implications for gene therapy. Hum Gene Ther. 2012;23:1090–1100. doi: 10.1089/hum.2012.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai S, Meagher R, Swearingen M, Myint H, Rich E, Martinson J.et al. (2008Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: a phase I trial Cytotherapy 10625–632. [DOI] [PubMed] [Google Scholar]
- Tonn T, Schwabe D, Klingemann HG, Becker S, Esser R, Koehl U.et al. (2013Treatment of patients with advanced cancer with the natural killer cell line NK-92 Cytotherapy 151563–1570. [DOI] [PubMed] [Google Scholar]
- Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- Wels W, Harwerth IM, Zwickl M, Hardman N, Groner B, Hynes NE. Construction, bacterial expression and characterization of a bifunctional single-chain antibody-phosphatase fusion protein targeted to the human erbB-2 receptor. Biotechnology (NY) 1992;10:1128–1132. doi: 10.1038/nbt1092-1128. [DOI] [PubMed] [Google Scholar]
- Moritz D, Wels W, Mattern J, Groner B. Cytotoxic T lymphocytes with a grafted recognition specificity for ERBB2-expressing tumor cells. Proc Natl Acad Sci USA. 1994;91:4318–4322. doi: 10.1073/pnas.91.10.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat R, Watzl C. Serial killing of tumor cells by human natural killer cells–enhancement by therapeutic antibodies. PLoS ONE. 2007;2:e326. doi: 10.1371/journal.pone.0000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol. 2008;180:5771–5777. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- Maki G, Klingemann HG, Martinson JA, Tam YK. Factors regulating the cytotoxic activity of the human natural killer cell line, NK-92. J Hematother Stem Cell Res. 2001;10:369–383. doi: 10.1089/152581601750288975. [DOI] [PubMed] [Google Scholar]
- Davies JK, Singh H, Huls H, Yuk D, Lee DA, Kebriaei P.et al. (2010Combining CD19 redirection and alloanergization to generate tumor-specific human T cells for allogeneic cell therapy of B-cell malignancies Cancer Res 703915–3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberoi P, Wels WS. Arming NK cells with enhanced antitumor activity: CARs and beyond. Oncoimmunology. 2013;2:e25220. doi: 10.4161/onci.25220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingemann H. Are natural killer cells superior CAR drivers. Oncoimmunology. 2014;3:e28147. doi: 10.4161/onci.28147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press MF, Cordon-Cardo C, Slamon DJ. Expression of the HER-2/neu proto-oncogene in normal human adult and fetal tissues. Oncogene. 1990;5:953–962. [PubMed] [Google Scholar]
- Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombach AA, Schildgen V, Heuser C, Finnern R, Gilham DE, Abken H. T cell activation by antibody-like immunoreceptors: the position of the binding epitope within the target molecule determines the efficiency of activation of redirected T cells. J Immunol. 2007;178:4650–4657. doi: 10.4049/jimmunol.178.7.4650. [DOI] [PubMed] [Google Scholar]
- James SE, Greenberg PD, Jensen MC, Lin Y, Wang J, Till BG.et al. (2008Antigen sensitivity of CD22-specific chimeric TCR is modulated by target epitope distance from the cell membrane J Immunol 1807028–7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HS, Mason K, Ramyar KX, Stanley AM, Gabelli SB, Denney DW., Jret al. (2003Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab Nature 421756–760. [DOI] [PubMed] [Google Scholar]
- Gerstmayer B, Altenschmidt U, Hoffmann M, Wels W. Costimulation of T cell proliferation by a chimeric B7-2 antibody fusion protein specifically targeted to cells expressing the erbB2 proto-oncogene. J Immunol. 1997;158:4584–4590. [PubMed] [Google Scholar]
- Grada Z, Hegde M, Byrd T, Shaffer DR, Ghazi A, Brawley VS.et al. (2013TanCAR: A Novel Bispecific Chimeric Antigen Receptor for Cancer Immunotherapy Mol Ther Nucleic Acids 2e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cosimo S. Heart to heart with trastuzumab: a review on cardiac toxicity. Target Oncol. 2011;6:189–195. doi: 10.1007/s11523-011-0203-8. [DOI] [PubMed] [Google Scholar]
- von Minckwitz G, Harder S, Hövelmann S, Jäger E, Al-Batran SE, Loibl S.et al. (2005Phase I clinical study of the recombinant antibody toxin scFv(FRP5)-ETA specific for the ErbB2/HER2 receptor in patients with advanced solid malignomas Breast Cancer Res 7R617–R626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton AN, Real FX, Davis LJ, Cordon-Cardo C, Old LJ. Phenotypic heterogeneity of melanoma. Relation to the differentiation program of melanoma cells. J Exp Med. 1987;165:812–829. doi: 10.1084/jem.165.3.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer-Gebhard M, Schmidt M, Azemar M, Altenschmidt U, Stöcklin E, Wels W.et al. (1998Systemic treatment with a recombinant erbB-2 receptor-specific tumor toxin efficiently reduces pulmonary metastases in mice injected with genetically modified carcinoma cells Cancer Res 582661–2666. [PubMed] [Google Scholar]
- Demaison C, Parsley K, Brouns G, Scherr M, Battmer K, Kinnon C.et al. (2002High-level transduction and gene expression in hematopoietic repopulating cells using a human immunodeficiency [correction of imunodeficiency] virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter Hum Gene Ther 13803–813. [DOI] [PubMed] [Google Scholar]
- Schambach A, Bohne J, Baum C, Hermann FG, Egerer L, von Laer D.et al. (2006Woodchuck hepatitis virus post-transcriptional regulatory element deleted from X protein and promoter sequences enhances retroviral vector titer and expression Gene Ther 13641–645. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Carbonaro DA, Speckmann C, Wissler M, Bohnsack J, Elder M.et al. (2003Clonality analysis after retroviral-mediated gene transfer to CD34+ cells from the cord blood of ADA-deficient SCID neonates Nat Med 9463–468. [DOI] [PubMed] [Google Scholar]
- Klöss S, Bochennek K, Huenecke S, Zimmermann SY, Kuçi S, Müller T.et al. (2007A novel five-colour flow cytometric assay to determine NK cell cytotoxicity against neuroblastoma and other adherent tumour cells J Immunol Methods 325140–147. [DOI] [PubMed] [Google Scholar]
- Tavri S, Jha P, Meier R, Henning TD, Müller T, Hostetter D.et al. (2009Optical imaging of cellular immunotherapy against prostate cancer Mol Imaging 815–26. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.