Abstract
Here, we report a simple and low-cost oral oligodeoxynucleotide (ODN) delivery system targeted to the gut Peyer's patches (PPs). This system requires only Dulbecco's modified eagle's medium, calcium chloride, ODNs, and basic laboratory equipment. ODN nanocapsules (ODNcaps) were directly delivered to the PPs through oral administration and were taken up by macrophages in the PPs, where they induced an immune response. Long-term continuous oral dosing with inhibitory/suppressive ODNcaps (iODNcaps, “iSG3caps” in this study) was evaluated using an atopic dermatitis mouse model to visually monitor disease course. Administration of iSG3caps improved skin lesions and decreased epidermal thickness. Underlying this effect is the ability of iSG3 to bind to and prevent phosphorylation of signal transducer and activator of transcription 6, thereby blocking the interleukin-4 signaling cascade mediated by binding of allergens to type 2 helper T cells. The results of our iSG3cap oral delivery experiments suggest that iSG3 may be useful for treating allergic diseases.
Introduction
Genomic DNA derived from pathogenic microorganisms can activate immune cells such as B cells.1 Chemosynthetic immuno-functional oligodeoxynucleotides (ODNs) such as cytosine nonmethylated CpG-ODNs are also useful as adjuvants for vaccines against infectious agents, cancer, allergies, and inflammatory disorders.2,3,4,5 A synthetic phosphorothioate (PS)-modified CpG-ODN was used in an in vivo/in vitro study involving various disease models.6 For medical purposes, ODNs have been administered in a variety of ways, including via intraperitoneal (i.p.),7 intravenous (i.v.),8 and subcutaneous (s.c.)9 routes. Although many reports have demonstrated that CpG-ODNs can be used at doses greater than 100 μg in mice, there are few reports of oral (i.e., intragastric (i.g.)) administration of ODNs as a drug or food material. This may be due to the significant challenges associated with oral delivery of nucleic acids. For instance, ODNs may be degraded by the acidic pH of the stomach or by nucleases, lipases, and peptidases in the gastrointestinal tract. Both mucin and the intestinal epithelium are largely impermeable to nucleic acid–based medicines. To overcome these problems, we developed ODN nanocapsules (ODNcaps) using carbonate-apatite nanoparticles.
We first examined the effectiveness of ODNcaps as devices for oral delivery. We then examined the efficacy of oral administration of CpG-ODNcaps (CpGcaps) or inhibitory/suppressive ODNcaps (iODNcaps) in preventing atopic dermatitis (AD) in mice. We previously reported the development of a class I (H154)/class II (A151) hybrid iODN (designated “iSG”) that includes the telomeric motif 5′-TTAGGG-3′.10 We also found that iSG3 suppresses type 2 helper T (Th2) immune responses in ovalbumin-treated spleen cells obtained from ovalbumin-allergic model mice.10 However, the pharmacologic properties of ODNcaps have not been characterized. We employed an AD mouse model to be able to observe disease states according to the appearance of symptoms. In the case of in vivo experiments, it is difficult to establish an endpoint. This is particularly true in studies involving mice or other animals, in which symptoms may occur within the body and thus may not be readily apparent.
In this study, we employed an AD mouse model to investigate the effect of long-term oral administration of ODNcaps. The results of the in vivo AD trial indicated that iSG3 significantly inhibits the development of AD skin lesions in mice, whereas the “B”-type CpG-ODN11 (also known as “K”-type ODN)12 accelerates development of AD skin lesions in mice. Oral administration of iSG3caps prevented the formation of AD lesions through control of intestinal mucosal immunity, at least in part by inhibiting interleukin (IL)-4/signal transducer and activator of transcription (STAT) 6 signaling. Should similar activity be observed in humans, iSG3caps may provide an inexpensive, safe, and effective means of preventing AD. The results of our study suggest that ODNcaps are potent immunomodulators and therefore may be effective as novel supplements or drugs.
Results
Synthesis and characterization of ODNcaps
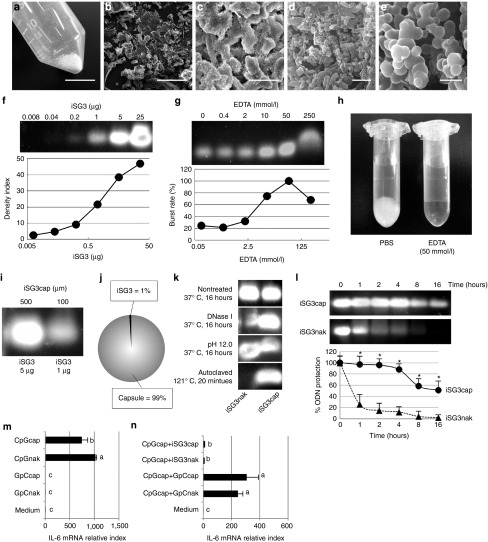
ODNcaps were synthesized under endotoxin-free conditions using a modification of the method of Chowdhury et al.13 The synthetic strategy is shown in Supplementary Figure S1. We used iSG3 as a representative ODN in the following experiments. The size and morphology of the iSG3caps were characterized using a digital camera (Figure 1a) and scanning electron microscopy (SEM) (Figure 1b–e). The iSG3caps were composed primarily of a large number of irregularly shaped nanoparticles that were about 100–200 nm in size and that readily aggregated. We examined the stability of naked iSG3 (iSG3nak) and iSG3caps by agarose gel electrophoresis (Figure 1f) and also determined the concentration of EDTA needed for calcium-disodium chelation to elute the iSG3caps (Figure 1g,h). Approximately 5 μg of iSG3 was incorporated into 500 μg of iSG3caps, corresponding to an ODN encapsulation rate of 1% (Figure 1i,j). Next, we investigated the extent of protection afforded the ODN by encapsulation. iSG3caps and iSG3nak were treated with DNase I, strong alkali (pH 12.0), and autoclaving, and the effects of these treatments were evaluated using agarose gel electrophoresis (Figure 1k). Strong nucleic acid signals were detected for iSG3caps as compared with iSG3nak following these treatments. We also exposed iSG3caps to simulated gastric juice (pH 1.71) for 16 hours at 37 °C (Figure 1l). iSG3caps were degraded by exposure to simulated gastric juice in a time-dependent manner, but encapsulation strongly protected the iSG3 for up to 4 hours. Exposure to simulated gastric juice rapidly digested the ODNnak (Figure 1l).
Figure 1.
Characterization of iSG3caps. (a) Digital camera photograph and (b–e) SEM photographs of iSG3caps. Scale bar equals (a) 10 mm, (b) 100 μm, (c) 10 μm, (d) 1 μm, and (e) 200 nm. (f) Optimum density of iSG3 for agarose gel electrophoresis. PS-iSG3 was diluted 1:5 serially, from 25 μg/well to 0.008 μg/well for 3% (w/v) agarose gel electrophoresis. Data are representative of at least three independent experiments. (g,h) Optimum concentration of EDTA for bursting capsules. EDTA was diluted 1:5 serially, from 250 to 0.4 mmol/l for analysis of capsule bursting. Data are representative of at least three independent experiments. (i,j) iSG3caps (500 μg) were synthesized containing 5 μg of iSG3. The encapsulation rate of ODN was calculated as ~1%. (k) Agarose gel electrophoresis analysis of ODN stability. iSG3nak and iSG3caps were incubated with DNase I at 37 °C for 16 hours; NaOH, pH 12.0, at 37 °C for 16 hours; or autoclaved at 121 °C for 20 minutes. Data are representative of at least three independent experiments. (l) Resistance of iSG3nak and iSG3caps to simulated gastric juice following incubation at 37 °C for 16 hours. The nontreated control was not incubated in simulated gastric juice (i.e., time zero). Data are shown as the mean + SD (n = 3) of one representative of three independent experiments with similar results. *P < 0.05. (m,n) RT-qPCR analysis of IL-6 mRNA expression in SP cells. (m) SP cells (2 × 106 cells/ml) were preincubated in medium for 3 hours prior to exposure to GpCnak, GpCcaps, CpGnaks, or CpGcaps for 6 hours. (n) CpGcaps plus GpCnak, GpCcaps, iSG3nak, or iSG3caps were added to examine the suppressive effect. Results are shown as the ratio of IL-6 mRNA levels (normalized to β-actin) in stimulated versus nontreated cells. Data are shown as the mean ± SD (n = 4) of one representative of three independent experiments with similar results. Values with different letters (i.e., a, b, and c) were significantly different (P < 0.01).
To determine whether the immunological effects of ODN are retained following encapsulation, SP cells were stimulated in culture for 6 hours with control GpCnak, CpGnak, GpCcaps, or CpGcaps (Figure 1m). After stimulation, the level of IL-6 mRNA expression in the SP cells was assessed by real-time quantitative PCR (RT-qPCR). IL-6 mRNA is a convenient gene marker for measuring the activity of CpG-ODN. In in vitro splenocyte culture, a system for measuring IL-6 mRNA following CpG-ODN stimulation has already been established.10 Expression of IL-6 mRNA was enhanced in SP cells stimulated with both naked and encapsulated CpG-ODN as compared with cells stimulated with control GpC-ODN. We also examined the ability of iSG3caps to suppress IL-6 mRNA expression in SP cells (Figure 1n). Expression of IL-6 mRNA was significantly inhibited by iSG3nak and iSG3caps (Figure 1n). These results indicated that encapsulation does not diminish the immunological effects of ODN.
iSG3caps reach the Peyer's patches following oral administration
The effectiveness of in vivo oral administration of iSG3caps depends upon their behavior in the intestinal mucosa. Encapsulated iSG3 labeled with 6-carboxyfluorescein-aminohexyl amidite (6FAM) was used to determine whether iSG3caps administered orally arrive at enteric immune sites such as the Peyer's patches (PPs). A strong fluorescence signal derived from the feed the mice were provided impacted the determination of the optimal dosage of fluorescent ODN (data not shown). Mice were therefore reared for 4 weeks using iVid#2 alfalfa-free feed to reduce background fluorescence (Figure 2a). Background fluorescence in the intestinal tract could be held to an undetectable level using the iVid#2 feed. Unencapsulated 6FAM-iSG3nak was not absorbed in the intestinal tract, and therefore, no fluorescence was observed in the PPs (Figure 2b). In contrast, fluorescence associated with 6FAM-iSG3caps was clearly observed in the PPs of jejunal follicles (Figure 2b). These results demonstrated that iSG3caps resist the digestive actions of gastric acid, enabling them to reach intestinal PPs.
Figure 2.
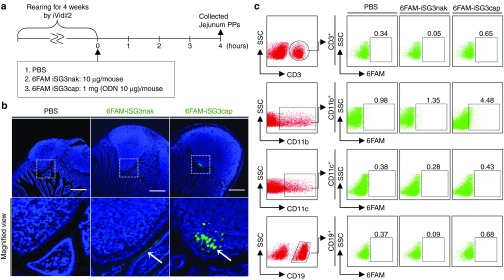
Assay of iSG3caps uptake following oral administration. (a) Schedule for experiments to determine the localization of orally administered 6FAM-iSG3caps in the intestinal mucosa. Effective uptake of iSG3caps by jejunal PPs. (b) Confocal laser microscopic images of PPs 4 hours after oral administration of PBS, 6FAM-conjugated iSG3nak (6FAM-iSG3nak), and 6FAM-conjugated iSG3caps (6FAM-iSG3caps). 6FAM-iSG3caps, but not 6FAM-iSG3nak, specifically reached the PPs (arrow). 6FAM-iSG3nak was attached to the apical surfaces of the follicle-associated epithelium (arrow). Scale bar equals 200 μm. Lower panels are high-magnification views. Data are representative of at least three independent experiments with similar results. (c) Assay of uptake of orally administered PBS, 6FAM-iSG3nak, or 6FAM-iSG3caps by PP cells. Flow cytometric analysis of PP cells stained with anti-CD3, CD11b, CD11c, or CD19 antibodies. The level of 6FAM-iSG3 or 6FAM-iSG3cap uptake by stained cells is shown as a percentage in the dot plots of gated cells. Analyses were carried out at least in triplicate with similar results, and representative results are presented.
Macrophages take up iSG3caps in PPs following oral administration
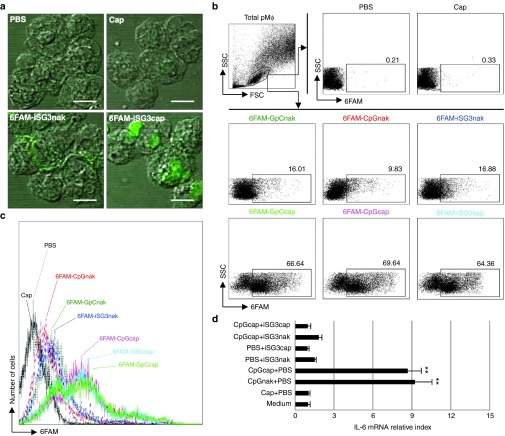
We prepared SP cells and performed an uptake assay using 6FAM-iSG3caps in vitro (Supplementary Figure S2). We used 6FAM-iSG3 as a representative ODN. SP cells were treated with PBS, nanocapsules alone (Cap), 6FAM-iSG3nak, or 6FAM-iSG3caps. SP cells were then stained for the following cell markers: T cells (CD3+), B cells (CD19+), macrophages (MΦs, CD11b+), and dendritic cells (DCs, CD11c+). The cells taking up 6FAM-iSG3caps were identified using flow cytometry. The results showed that all types of immune cells examined in vitro took up 6FAM-ODNcaps (Supplementary Figure S2). Previous studies showed that DNA complexes based on carbonate apatite and calcium phosphate can be carried across the cell membrane via ion channel–mediated endocytosis.14,15 Therefore, our results suggest that the observed uptake involved endocytosis induced by physical characteristics of the capsules.
We then examined the type of immune cells in PPs that take up iSG3caps following oral administration (Figure 2a,c). Interestingly, we found that a few percent of the CD11b+ cells (mainly MΦs) took up 6FAM-iSG3caps in PP cells following oral administration of iSG3. Uptake of 6FAM-iSG3caps was not observed in CD3+, CD19+, and CD11c+ cells. These results suggest that ODNcaps reach the intestinal PPs within several hours of oral administration and that a few percent of the MΦs residing in the PPs take up ODNcaps.
CpGcaps activate PP cells following oral administration
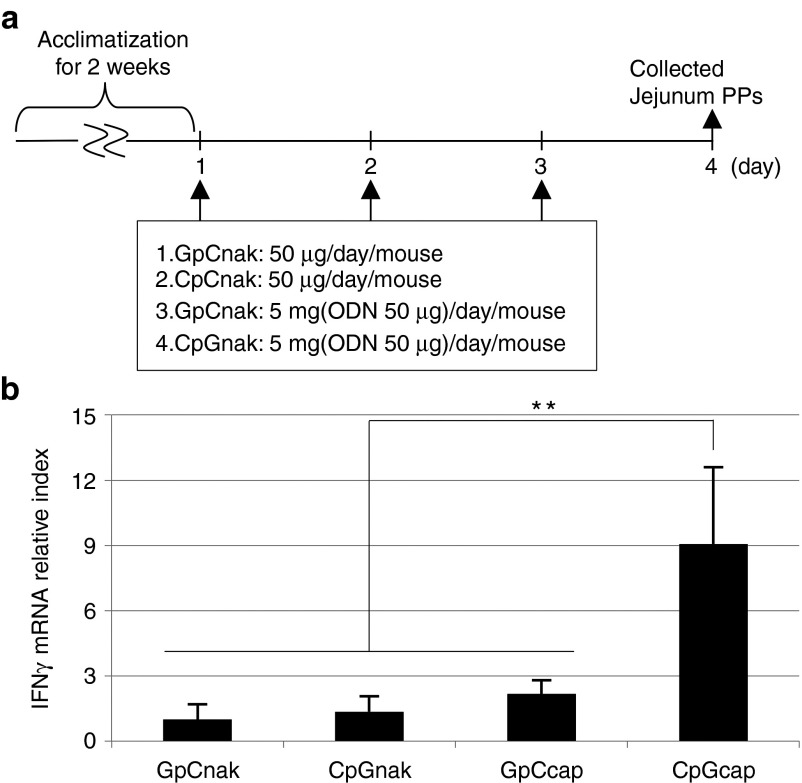
Next, mice were given CpGcaps by oral administration for 3 consecutive days, after which expression of interferon (IFN)-γ mRNA in jejunal PPs was examined (Figure 3a,b). With oral administration of CpGcaps, we found no significant difference with respect to IL-6 mRNA between the CpGcap and control groups. However, we found a significant difference with respect to IFN-γ mRNA. Expression of IFN-γ mRNA in jejunal PPs in mice orally administered CpGcaps was significantly higher than in mice given control GpCnak, GpCcaps, or CpGnak (Figure 3b). These results were in agreement with those of laser microscopic analyses. Although CpGnak could not be delivered to the intestinal PPs, CpGcaps did reach these important components of the intestinal immune system and induced expression of IFN-γ mRNA.
Figure 3.
CpGcaps activate PP cells following oral administration. (a) Schedule for experiments examining the immune activity of orally administered CpGcaps in jejunal PPs. After rearing for 2 weeks, mice were administered PBS, ODNnak (GpCnak or CpGnak), or ODNcaps (GpCcaps or CpGcaps) for 3 consecutive days. (b) RT-qPCR analysis of IFN-γ mRNA levels in jejunal PPs isolated from mice orally administered ODNnak or ODNcaps. Results are shown as the level of IFN-γ mRNA normalized to that of β-actin. Data are representative and shown as the mean ± SE for each animal; n = 5 mice/group. **P < 0.01.
Activation of MΦs following uptake of CpGcaps
We then examined the uptake and immunostimulatory activity of ODNcaps using peritoneal macrophages (pMΦs) as model PP MΦs (Figure 4). The uptake of 6FAM-ODNnak and 6FAM-ODNcaps by pMΦs was investigated using confocal laser scanning microscopy (Figure 4a). The pMΦs took up all of the 6FAM-ODNcaps (Figure 4b,c). This uptake involved endocytosis, as described above (Supplementary Figure S2). Cellular activation of pMΦs as a marker of IL-6 mRNA expression was linked to enhanced vesicular uptake of CpGcaps (Figure 4d). CpGcaps-induced IL-6 mRNA expression was inhibited by iSG3caps (Figure 4d).
Figure 4.
Activation of pMΦs following uptake of CpGcaps. (a–c) The pMΦs were treated with PBS, 1 μg of 6FAM-ODNnak, 100 μg of Caps, or 100 μg of 6FAM-ODNcaps for 1 hour at 37 °C. (a) Confocal laser microscopic analysis of 6FAM-iSG3nak and 6FAM-iSG3caps in pMΦs. Green fluorescence is indicative of 6FAM-iSG3. Scale bar equals 5 μm. (b) Representative FACS plots of analyses gated on pMΦs. (c) Histogram illustrating uptake of 6FAM-ODNnak or 6FAM-ODNcaps. (d) RT-qPCR analysis of IL-6 mRNA expression in mouse pMΦs. Results are shown as the level of IL-6 mRNA normalized to that of β-actin. Data are shown as the mean ± SD (n = 3) of one representative of three independent experiments with similar results. **P < 0.01.
Effect of oral administration of ODNcaps to AD mice
We measured the weight of all mice weekly during the study period (Figure 5a,b). No significant weight differences were observed between mice that were not administered (NA) and mice that were administered Caps, CpGcaps, or iSG3caps with picryl chloride (PiCl). A clear trend toward increased weight was observed in mice in the nontreated (NT; i.e., mice that did not receive any treatment) group without PiCl treatment as compared with the other groups after the sixth week.
Figure 5.
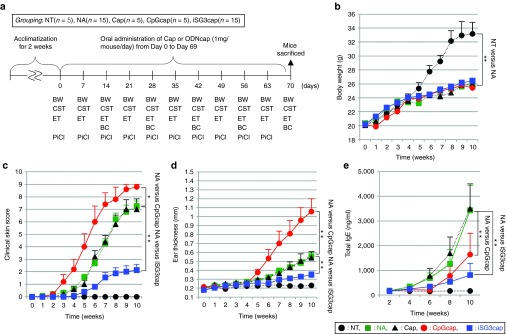
The effect of oral administration of ODNcaps to AD mice. (a) Schedule for the long-term in vivo trial. After rearing for 2 weeks, each mouse was administered 1 mg of Caps, CpGcaps, or iSG3caps for 10 weeks. The (b) body weight (BW), (c) clinical skin test results (CST; clinical skin sore, CSS), and (d) ear thickness (ET) of each mouse were monitored during the experiment. Blood was collected from the caudal vein every other week. After 10 weeks, all mice were sacrificed and their spleens were extracted for analysis of the immune response. BC, blood collection; PiCl, PiCl challenge. (b) Change in body weight of mice. (c) The dermatitis score was defined as the sum of scores for three clinical criteria. (d) The thickness of the right and left ear auricles was measured using a vernier micrometer once per week. (e) Total serum IgE levels were monitored for 10 weeks. Data are shown as the mean ± SE for each animal at each time point; n = 5–15 mice/group (b–e). The data for the NA and iSG3cap groups (n = 15) are pooled from three independent experiments (b–e). **P < 0.01; *P < 0.05 for indicated groups at the same time point.
We scored the severity of skin reactions in AD mice according to a modification of the method of Yamaguchi et al. (Supplementary Table S1).16 The severity of AD symptoms on the skin of the auricles and the back of each mouse was recorded digitally in a blinded test (Figure 5c). In the NA and Caps groups, AD symptoms appeared 4 weeks after the start of the study (Figure 5c,d). Aggravated allergic symptoms were observed in the CpGcaps group 3 weeks after the start of the study. In the iSG3caps group, the clinical skin score increased slowly relative to that of all other groups except the NT group. A significant difference in clinical skin score was observed between the NA and iSG3caps groups after the sixth week. No allergic symptoms appeared in the NT group. We also measured the thickness of the right and left auricles weekly during the study period using a vernier micrometer (Figure 5d). Thickening of the auricle was observed in the NA and Caps groups after the sixth week. Thickening of the auricles was observed in the CpGcaps group after the fifth week, and the degree of thickening was clearly greater than that observed in all other groups except the NT and iSG3caps groups. In the iSG3caps group, the degree of thickening was significantly less than that observed in all other groups except the NT group. A significant difference in auricle thickness (P < 0.05) was observed between the iSG3caps and NA groups in the eighth, ninth, and the tenth weeks. In the NT group, the auricle was relatively flat, with a thickness of 0.20–0.25 mm. We also performed a preliminary study of the effects of oral administration of low-concentration ODNnak (10 μg/mouse/dose) (Supplementary Figure S3). Oral administration of CpGnak and iSG3nak at 10 μg/mouse produced similar results to those obtained with the NA group. These results indicate that low concentrations of ODNnak do not arrive at the PPs via the oral route, as shown in Figures 2b and 3b.
Levels of total serum IgE were also measured (Figure 5e). In the NA and Caps groups, the total serum IgE level increased after the fourth week. In the CpGcaps and iSG3caps groups, total serum IgE levels increased after the sixth week. Serum IgE levels were significantly different in the NA and iSG3caps groups in the tenth week, suggesting that administration of iSG3caps inhibits IgE production.
Administration of iSG3caps improves the histologic symptoms of AD
Head photographs of mice showing the average clinical score for each group are shown in Figure 6a. Sections of the auricles were obtained at the end of the study period, embedded in paraffin wax, stained with hematoxylin and eosin (HE) (Figure 6b) and acidic toluidine blue (TB) (pH 4.0) (Figure 6c), and observed under a light microscope. Fields stained dark purple represent the epidermis. Epidermal thickness was measured at five random points in the auricle sections from five mice/group (Figure 6d). Epidermal thickness was significantly higher in the NA, Caps, and CpGcaps groups as compared with the NT group, and hypertrophy of the skin was also observed. However, the degree of epidermal thickness was significantly lower in the iSG3caps group as compared with the NA, Caps, and CpGcaps groups, indicating that hypertrophy with cutaneous inflammation was controlled in the iSG3caps mice. The number of mast cells was significantly lower in the iSG3caps group compared with the NA, Caps, and CpGcaps groups (Figure 6e). Photographs of the backs of mice in the NA and iSG3caps groups taken in the tenth week are shown in Supplementary Figure S4. Administration of iSG3caps had an inhibitory effect on AD development. Both histologic and macroscopic improvements in AD symptoms were achieved following oral administration of iSG3caps.
Figure 6.
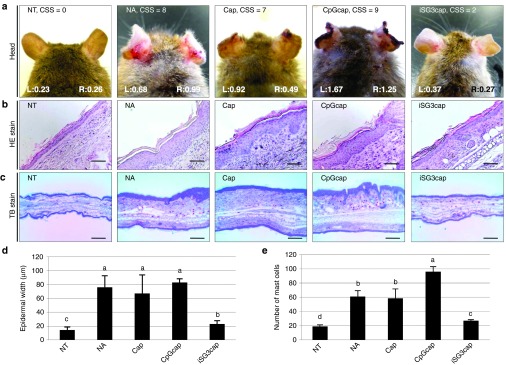
Histologic changes in ear skin observed on day 70. (a) Head, (b) HE, and (c) TB staining of thin ear sections of mice. The scores (left ear: L; right ear: R) shown in the photographs in a indicate ear thickness (mm). The average clinical skin score (CSS; by clinical skin test) is shown at the top of the panels in a. Thin sections (5 μm) were cut and stained with HE (b, scale bar equals 200 μm) or TB for mast cells (red-purple staining) (c, scale bar equals 100 μm). (d) The thickness of the epidermis in HE sections and (e) the number of mast cells were determined from five mice, and the results are presented as the mean ± SE (n = 5). Letters (i.e., a, b, c, and d) represent significant differences (P < 0.05).
iSG3 inhibits Th2 signaling and CpG-ODN enhances IL-33 production
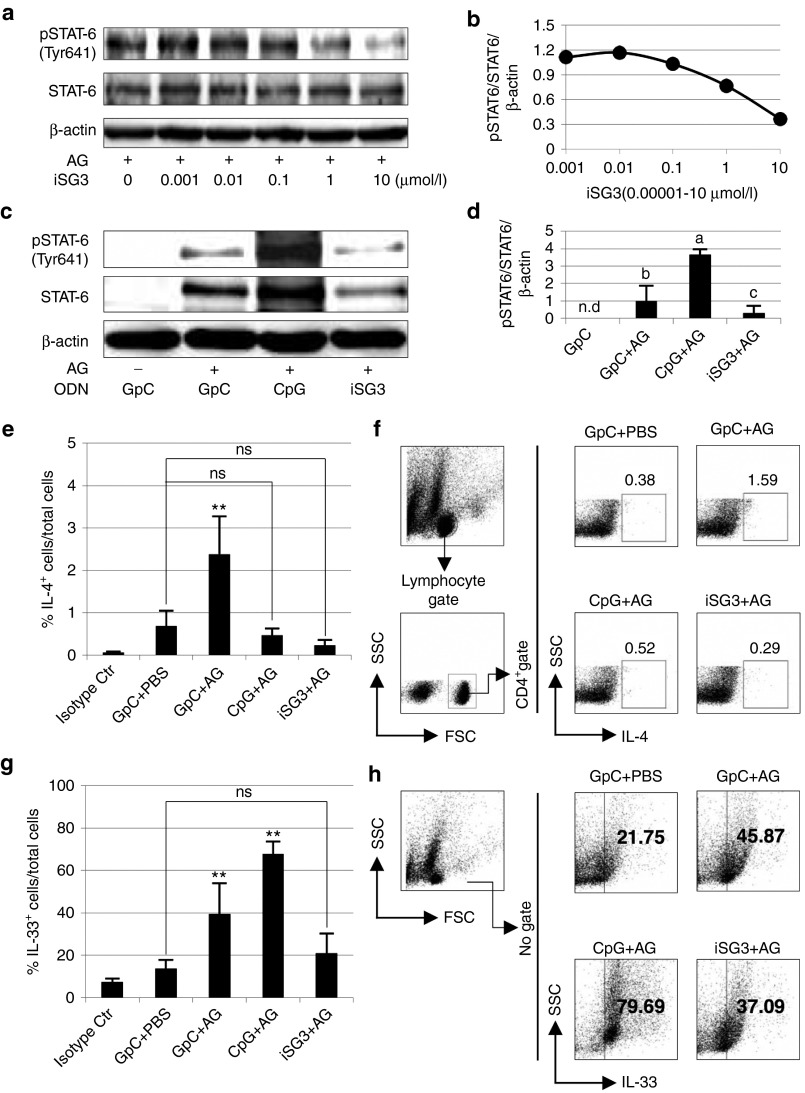
We analyzed the expression of STAT6 and phospho (p)-STAT6 in SP cells isolated from immunized mice treated with/without ODNs and antigen (AG). Expression of pSTAT6 was inhibited by iSG3 in a concentration-dependent manner (Figure 7a,b). We demonstrated that iSG3 can block tyrosine phosphorylation of STAT6 in immune cells from allergic model mice stimulated with allergen (Figure 7c,d). These results suggest that iSG3 inhibits Th2 differentiation by selectively blocking the phosphorylation of STAT6. In the FACS dot plots shown in Figure 7f,h, the vertical and horizontal axes indicate the intensity of IL-4-, CD4-, and IL-33-associated fluorescence. The proportions of various CD4+IL-4+ cells (i.e., Th2 cells) relative to the total cell count were as follows: (isotype control), 0.06 ± 0.02%; (GpC + PBS), 0.68 ± 0.37%; (GpC + AG), 2.38 ± 0.89%; (CpG + AG), 0.47 ± 0.16%; and (iSG3 + AG), 0.26 ± 0.06% (Figure 7e). These results demonstrate that differentiation of (iSG3 + AG) treated-cells into Th2 cells is significantly suppressed by iSG3. The proportions of various IL-33+ cells relative to the total cell count were as follows: (isotype control), 7.32 ± 1.63%; (GpC + PBS), 13.64 ± 4.09%; (GpC + AG), 39.44 ± 14.56%; (CpG + AG), 67.68 ± 6.02%; and (iSG3 + AG), 20.93 ± 9.35% (Figure 7g). These results demonstrate that differentiation of (CpG + AG)-treated cells into IL-33+ cells is significantly enhanced by CpG-ODN.
Figure 7.
Inhibition of Th2 signaling and IL-33 production by iSG3. (a–d) Effect of iSG3 on STAT6 phosphorylation in SP cells isolated from AG-immunized mice. (a,b) Concentration-dependent effect of iSG3 on STAT6 phosphorylation. Protein extracted from SP cells was treated for 60 minutes with different concentrations of iSG3 (0.001–10 μmol/l) or PBS and then blotted to detect STAT6 Tyr641 phosphorylation (pSTAT6). Concentration-response curve shows the result from a densitometric analysis. Data are representative of three independent experiments with similar results. (c,d) SP cells were incubated with/without 10 μg/ml of AG and 3 μmol/l ODN (GpC-ODN, CpG-ODN, or iSG3) for 60 minutes. The effect on STAT6 phosphorylation was determined by Western blot analysis of cell lysates. Data are representative of three independent experiments with similar results. (d) Densitometric analysis of three independent Western blots shows quantitation of pSTAT6/STAT6/β-actin levels. The results shown are the mean ± SD (n = 3) from three independent experiments. Values with different letters (i.e., a, b, and c) are significantly different (P < 0.01). (e–h) Flow cytometric analysis was performed to determine the proportions of CD4+IL-4+ cells and IL-33+ cells. SP cells isolated from AG-immunized mice were treated with ODN and/or AG for 72 hours. (f,h) Representative FACS plots gated on CD4+ IL-4+ cells and IL-33+ cells. (e,g) The results represent the mean ± SD (n = 3) from three independent experiments with similar results. **P < 0.01 for indicated group(s). ns, not significant.
Finally, we showed that oral administration of ODNcaps regulates systemic immunity. AG-immunized mice were orally administered Caps, CpGcaps, or iSG3caps for 28 days (Figure 8a). We measured the weight of the spleen (Figure 8b) and prepared SP cells from AG-immunized mice at day 28. The SP cells were resensitized with AG. The CpGcaps group showed a statistically significant increase in spleen weight compared with the NT and iSG3caps groups (Figure 8b). The spleen weight of the iSG3caps group was similar to that of the NT group (Figure 8b). AG administration (>10 μg/ml) is capable of inducing high levels of in vitro IL-33 mRNA expression in SP cells.17 We resensitized the SP cells with 50 μg/ml of AG and investigated the effect of oral administration of ODNcaps (CpGcaps and iSG3caps) on systemic immunity. Interestingly, oral administration of iSG3caps to mice significantly suppressed the expression of IL-33 mRNA (Figure 8c) and the proportion of IL-33+ cells (Figure 8d) among the resensitized SP cells. An allergic reaction was induced by i.p. AG administration and oral administration of CpGcaps, and the spleen became significantly enlarged. These results suggest that continual oral administration of ODNcaps can be used to regulate systemic immunity.
Figure 8.
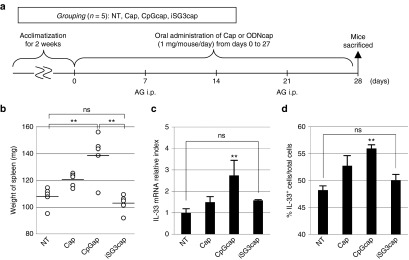
ODNcaps regulate systemic immunity following oral administration. (a) Schedule for experiments examining the systemic immune activity of orally administered Caps, CpGcaps, and iSG3caps in AG-immunized mice. After rearing for 2 weeks, mice were administered PBS, Caps, or ODNcaps (GpCcaps or CpGcaps) for 4 weeks (days 0–27). The mice were sensitized with i.p. injections of 100 μg of AG with adjuvant on days 7 and 21. After 4 weeks, all mice were sacrificed and their spleens were extracted and cultured with 50 μg/ml of AG for 72 hours. (b) Spleen weight (mg). (c) RT-qPCR analysis of IL-33 mRNA expression in SP cells derived from mice immunized with in vitro AG resensitization. Results are shown as the level of mRNA normalized to that of β-actin. Data are representative and shown as the mean ± SE of three independent experiments with n = 5 mice/group. (d) Flow cytometric analysis was performed to determine the proportion of IL-33+ cells. SP cells (2 × 106) isolated from AG-immunized mice were treated with ODN and/or AG for 72 hours. Each symbol represents an individual mouse, n = 5 mice/group. **P < 0.01 for NT versus CpGcaps. ns, not significant.
Discussion
CpG-ODN has attracted considerable attention for its potential to serve as an anti-allergic functional molecule. The anti-allergic activities of CpG-ODN have been examined in various in vivo trials. A number of reports have demonstrated the palliative effects of CpG-ODN in reducing allergic symptoms/bronchitis.18,19,20,21,22,23,24,25 In particular, Youn et al. reported improvement in symptoms following i.p. administration of CpG-ODN in an ovalbumin-allergy respiratory tract inflammation mouse model.25 They found that CpG-ODN significantly lowers expression of transforming growth factor (TGF)-β and thymus and activation-regulated chemokine and reduces the number of CD4+ cells in the airways.25 In addition, Takakura et al. reported the effects of CpG-ODN administration in AD mice.26 Although the authors of that study reported that i.p. administration of CpG-ODN had a prophylactic effect in some NC/Nga mice affected by AD, symptoms were worsened in others (10/26).26 In those mice in which AD worsened in that study, the number of Th1 cells in the spleen increased, and the level of total serum IgE decreased. Furthermore, Takakura et al. reported that AD is exacerbated by i.p. administration of recombinant IFN-γ. In both our study and that of Takakura et al., CpG-ODN strongly induced IFN-γ production and inhibited Th2-type immune responses. Although it is clear that CpG-ODN is responsible for inhibition of IgE production, we found that there is a risk of aggravating allergic symptoms. This effect appears to depend on the dose and dosing period; timing of the administration appears critical for preventing or reducing allergic symptoms. Our CpGcap experiments serve as a supplement to the study of Takakura et al. Our results show that CpGcaps are delivered to the intestinal mucosa following oral administration and that this presents a risk of worsening allergies through induction of IFN-γ production. Moreover, we showed that oral administration of CpGcaps up-regulates systemic IL-33 production.
Previously, we reported that imiquimod and CpG-ODN strongly induce IL-33 expression in mouse SP cells via Toll-like receptors (TLRs) 7, 8, and 9, an effect that is not observed with TLR2, TLR3, and TLR4.11 We also found that CpG-ODN strongly induces production of IL-33 in AG-stimulated SP cells derived from AG-immunized mice. Here, we also found that orally administered CpGcaps were delivered to the gut PPs of AG-immunized mice, where they induced systemic IL-33 expression. IL-33 is a member of the IL-1 family of cytokines and shares many characteristics with IL-1α, IL-1β, and IL-18.27,28 IL-33 plays important roles in inflammatory disorders as well as infectious and autoimmune diseases. IL-33 binds to the IL-1-related receptor protein ST2 and induces the production of inflammatory and Th2 cytokines in mast cells, Th2 cells, basophils, and eosinophils.29 ProIL-33 (31 kDa), which is found in high levels in endothelial cells, binds to the ST2 receptor on the surface of Th2 cells when released from injured cells, inducing a Th2 type immune response. A Japanese group recently published the results of a study of the association between IL-33 and allergic diseases (e.g., AD, allergic asthma); in an allergic mouse model, excessive expression of IL-33 was shown to worsen allergic symptoms.30,31
In this study, we expected that in vivo oral administration of CpG-ODN would exhibit anti-allergic effects through induction of IFN-γ production. Unexpectedly, a worsening of allergic symptoms was observed. Our results showed that CpG-ODN is not suitable for use in balancing the Th1/Th2 response in patients with allergies.26 An important finding in this study was that oral dosing with CpGcaps in a state of allergen sensitization presents a risk of aggravating symptoms. Strong induction of IFN-γ and IL-33 production following oral administration of CpGcaps was shown to potentially worsen AD. However, CpGcaps may be effective for posttreatment in the absence of allergen. CpGcaps may be effective for use as a vaccine adjuvant and may also prove useful for preventing infections and tumors in vivo if the focus of their use is on enhancing overall immunity.
In this study, we used iODN, which has an opposite effect on immune function compared with CpG-ODN, as evidenced by exacerbation of allergic symptoms induced by administration of CpGcaps. In 2013, our research group designed a class I/II hybrid-type iODN, designated iSG3, which strongly inhibits both Th1 and Th2 immune responses.10 In a study of the class II iODN A151, Klinman et al. showed that A151 inhibits IFN-γ production and signaling associated with the production of Th1 cytokines such as IL-12 by inhibiting phosphorylation of STAT1, STAT3, and STAT4.32,33,34,35 The antagonism of Th1 immunity by A151 results in a shift in the immune response to a Th2 type.35 Prior to the development of iSG3,10 Th2 suppression by iODNs had not been reported. Therefore, in this study, we investigated the anti-allergic effects of iSG3 in vivo. Long-term continuous oral dosing with iSG3caps was evaluated using an AD mouse model. This in vivo study of the effects of oral administration of iSG3caps followed our previous in vitro study by Ito et al.10 Administration of iSG3caps to AG-immunized mice resulted in inhibition of Th2 immune responses and IL-33 production. Interestingly, long-term oral administration of iSG3caps regulated systemic immune responses and exhibited strong anti-allergic activity. It will be necessary to investigate the role of IL-33 further relative to the differences in symptoms in mice administered iSG3caps versus those administered CpGcaps.
It will also be important to investigate how the oral administration of ODNcaps may alter the gut microbiome. Numerous reports have established that the intestinal microbial community plays a fundamental role in human physiology and disease.36,37 Particularly, Pickard et al. showed that systemic exposure to TLR ligands such as lipopolysaccharide (TLR4 ligand), B type CpG ODN (TLR9 ligand), and the TLR2 agonist Pam3CSK4 cause α(1,2)-fucosylation of small intestine epithelial cells in mice.38 Further precise studies will be necessary to confirm whether alterations in the gut microbiome are the mechanism through which oral administration of ODNcaps regulates systemic immunity.
Finally, we discuss the system for oral delivery of ODNs. Mucosal immunological responses develop primarily in gut-associated lymphoid tissues. There are a number of reports in the literature describing methods for oral delivery of pharmacologic agents to the intestinal tract, in addition to our calcium-based nanoparticle carrier for ODN.39,40,41,42,43 Mucosal immunological responses have been employed in the successful development of oral vaccines.44 It is anticipated that in the future, immuno-functional ODNs will prove to be excellent adjuvants. However, because nucleic acids lack stability, oral delivery of intact molecules to the intestinal mucosa is difficult. As mentioned above, ODNs have been administered by i.p., i.v., and s.c. routes in vivo. In 2012, Zhu et al. reported an oral delivery system using TLR ligands (such as CpG-ODN) that target the large intestine using pH-dependent PLGA (poly-D,L-lactide-co-glycolide).45 The authors reported that the PLGA complexes pass through the upper gastrointestinal tract (stomach–small intestine) and can act as a vaccine in the large intestine.45 Although Zhu et al. used CpG-ODN as a vaccine adjuvant, their study demonstrated the feasibility of delivering functional nucleic acid molecules to the intestinal mucosa.45 Based on the work of Chowdhury et al.,13,46 we developed ODNcaps that are resistant to simulated gastric juice. ODNcaps are capable of targeting PPs. We demonstrated that ODNcaps pass through the follicle-associated epithelium (FAE) in the PPs and are taken up by MΦs.
The procedure of ODNcap synthesis is simple and requires only Dulbecco's modified eagle's medium (DMEM), calcium chloride (CaCl2), an ODN, and basic laboratory equipment. Furthermore, our calcium-based nanoparticle carrier is less expensive than previously proposed nucleic acid carriers. However, further improvement in the ODN encapsulation efficiency is needed.
Reducing the cost of synthesizing PS-modified ODNs is also necessary for clinical applications. It has been reported that phosphodiester (PO)-bonded CpG-ODN, which does not require PS, exhibits strong immune activity due to the characteristics of its structure.47 We therefore anticipate the development of PS-independent ODNs in the near future. The ability to compound capsules containing various ODN sequences opens the possibility of using ODNcaps in in vivo studies employing a variety of mouse disease models. More basic data are needed to confirm that immuno-functional ODNs are useful food and drug materials.
Materials and Methods
ODNs. Endotoxin-free desalted PS-ODNs and 6FAM-labeled PS-ODNs were synthesized by Integrated DNA Technologies (Coralville, IA). The PS-ODNs were reconstituted in endotoxin-free water and passed through a 0.22-μm pore microfilter (Nihon Millipore K.K., Tokyo, Japan). ODN sequences were as follows: B type CpG ODN, MsST derived from the Streptococcus thermophilus lacZ gene (5′-CAGGACGTTGTATCACTGAA-3′),11 class I/II hybrid iODN, iSG3 (5′-CCTCATTAGGGTGAGGG-3′),10 control GpC ODN (5′-GCTAGAGCTTAGGCT-3′).48
Preparation of ODNcaps. Carbonate apatite–based ODNcaps were prepared using a modification of the method of Fukuda et al.46 A 4-ml volume of 1 mol/l CaCl2 was mixed with 150 μg of ODN in 26 ml of fresh serum-free HCO3−-buffered DMEM high-glucose medium (C11995500BT, pH 7.5, Invitrogen, Carlsbad, CA) and then incubated for 30 minutes at 37 °C for complete generation of DNA/carbonate apatite particles (Supplementary Figure S1). For SEM analysis, ODNcap powder was affixed to an aluminum holder and the particles were coated with osmium tetroxide (OsO4) (Neoc-AN; Meiwafosis, Tokyo, Japan). The resulting ODNcaps were examined under a field emission scanning electron microscope (JSM-6700F; Jeol, Tokyo, Japan) at an accelerating voltage of 15 kV.
The stability of iSG3caps was also examined by incubation at pH 12 for 16 hours at 37 °C and by treatment or no treatment (i.e., time zero) involving incubation in simulated gastric juice for 1–16 hours at 37 °C.
Simulated gastric juice was prepared by dissolving 0.2 g of sodium chloride and 0.32 g of pepsin (#24–0940, Sigma-Aldrich, St Louis, MO) in 0.7 ml of hydrochloric acid and sufficient water to a final volume of 100 ml.49 The solution had a pH of 1.71 as measured using a pH meter.49 After treatment, the ODNcaps were dissolved in 50 mmol/l EDTA/PBS for calcium-disodium chelation. Gels were prepared with 3% (w/v) agarose (Nippon Gene, Toyama, Japan) in Tris-acetate–EDTA buffer (pH 8.3). Gel electrophoresis was performed at a constant voltage of 100 V for 15 minutes. ODN was visualized using SYBR Gold staining for single-stranded ODN, and gel images were obtained using a Gel Doc EZ System (Bio-Rad Laboratories, Philadelphia, PA).
Cells and cell culture. SP cells10 and pMΦs11 were prepared using standard methods. Cells were cultured in triplicate-quadruplicate wells of a 24-well plate (Nalge Nunc International K.K., Tokyo, Japan) at a final concentration of 2 × 106 cells/well (total 1 ml/well) in complete RPMI 1640 medium (Sigma-Aldrich) supplemented with 10% fetal calf serum (Sigma-Aldrich), 100 U/ml of penicillin, 100 mg/ml of streptomycin, 25 mmol/l HEPES, 1.0 mmol/l sodium pyruvate, nonessential amino acids, and 0.0035% 2-ME.
RT-qPCR analysis. RT-qPCR analyses were performed with SYBR Premix Ex Taq (TaKaRa Bio) using specific primers, as previously described.10 Primers for β-actin, IL-6, IL-33, and IFN-γ were purchased from TaKaRa Bio. As a control, poly(A)+ RNA samples were used as templates to check for the presence of contaminating genomic DNA. The sensitivity of the reaction and amplification of contaminating products, such as self-annealed primers, were evaluated by amplifying serial dilutions of cDNA. For cross-sample comparison of results obtained following various treatments, levels of cytokine mRNA were first normalized to those of β-actin mRNA. Data are shown as the mean ± SD of one representative of three independent experiments with similar results.
Localization analysis and assay of uptake by PPs of orally administered 6FAM-iSG3caps. After weaning (4 weeks of age), mice were fed a nonfluorescent feed “iVid#2” (Oriental Yeast, Tokyo, Japan) ad libitum for 4 weeks (Figure 2a). Localization of orally administered PBS, 6FAM-iSG3nak, or 6FAM-iSG3caps was investigated. We used iSG3 as a representative ODN. Each mouse was orally administered 200 μl of PBS, 10 μg of 6FAM-iSG3nak in 200 μl of PBS, or 1 mg of 6FAM-iSG3caps in 200 μl of PBS. Four hours after administration, the mice were euthanized and jejunul PPs were isolated.
For the localization assay, PPs of the jejunum were embedded in Tissue-Tek O.C.T. compound (Sakura Finetek, Tokyo, Japan) and frozen in liquid nitrogen. The frozen samples were sectioned longitudinally at a thickness of 10 μm, mounted on silane-coated glass slides, and the nuclei were stained with 4′, 6-diamidino-2-phenylindole (DAPI). The localization of 6FAM-iSG3nak and 6FAM-iSG3caps was determined using a confocal laser scanning microscope (FV1000D-IX81; Olympus, Tokyo, Japan) equipped with selective DAPI and fluorescein isothiocyanate (FITC) filters. Image analysis was performed using Fluoview software (Olympus).
For the uptake assay, PP cells were isolated from jejunal and ileal PPs. Briefly, PPs were gently pressed through a nylon mesh and washed three times in complete RPMI 1640 medium (Sigma-Aldrich) supplemented with 10% fetal calf serum (Sigma-Aldrich), 100 U/ml of penicillin, 100 mg/ml of streptomycin, 25 mmol/l HEPES, 1.0 mmol/l sodium pyruvate, nonessential amino acids, and 0.0035% 2-ME. Cells were fixed in 4% paraformaldehyde (PFA) for 15 minutes at room temperature. Cells were first stained with biotin-labeled anti-mouse CD3, CD19, CD11b, or CD11c antibody (Biolegend, San Diego, CA) for 60 minutes on ice. The cells were then washed, incubated with PE-labeled streptavidin, and washed again. The number of cells that had taken up 6FAM-ODN (6FAM+ cells) was determined using a FACSCalibur (BD Biosciences, Mountain View, CA). Data were acquired and analyzed using BD CellQuest software (BD Biosciences).
Immune activity of orally administered CpG-ODNcaps in PPs. GpCnak, CpGnak, GpCcaps, or CpGcaps were administered orally to mice at 6 weeks of age consecutively for 3 days (Figure 3a). Food was withheld from mice overnight on the last day of administration, and the jejunal PPs were then resected. We measured IFN-γ mRNA expression by RT-qPCR.
Flow cytometric assay of 6FAM-ODNnak and 6FAM-ODNcap uptake. The pMΦs were treated with PBS, 6FAM-ODNnak (GpCnak, CpGnak, iSG3nak; 10 μg/ml), or 6FAM-ODNcaps (GpCcaps, CpGcaps, iSG3caps; 1 mg/ml) for 1 hour at 37 °C. Cells were fixed in 4% PFA for 15 minutes at room temperature. Cells were then washed and the uptake of ODN (6FAM+ cells) was determined using a FACSCalibur (BD Biosciences). Data were acquired and analyzed using BD CellQuest software (BD Biosciences).
Confocal laser scanning microscopic assay of 6FAM-iSG3nak and 6FAM-iSG3cap uptake by pMΦs. Freshly isolated pMΦs were treated with PBS, 6FAM-iSG3nak (5 μg/ml), or 6FAM-iSG3caps (500 μg/ml, containing 5 μg/ml iSG3) for 1 hour at 37 °C. We used iSG3 as a representative ODN. The pMΦs were then fixed in 4% PFA for 15 minutes at room temperature. Uptake of 6FAM-iSG3nak and 6FAM-iSG3caps was determined using a confocal laser scanning microscope (FV1000D-IX81, Olympus) equipped with selective FITC filters. Image analysis was performed using Fluoview software (Olympus).
Protocol for inducing AD and method for oral administration of test materials. Pathogen-free male NC/Nga mice (4 weeks of age) were purchased from Japan SLC (Shizuoka, Japan) and maintained for at least 2 weeks before use in a filtered laminar-flow enclosure in a bioclean room. AD was induced by repeated topical application of 2,4,6-trinitrochlorobenzene (picryl chloride; PiCl; Tokyo Kasei Kogyo, Tokyo, Japan). First, 150 μl of 5% PiCl dissolved in 99.5% ethanol and acetone mixture (4:1) was applied to the abdomen, breast, and sole of the feet of mice. Four days after the first application, 25 μl of 1% PiCl dissolved in olive oil was applied to each side of the ear auricles at 1-week intervals for a total of 10 challenges. At the same time, the back of each mouse was also painted with 200 μl of 1% PiCl with a brush. Each mouse was orally administered 1 mg of ODNcaps or Caps in 200 μl of PBS for 70 consecutive days (i.e., days 0–69).
Evaluation of skin reaction severity. The severity of AD-like auricle skin lesions was assessed once per week using the following clinical skin test. AD mice were scored by two persons blinded to the treatment protocol. Before auricle skin conditions were scored, the head/ears and shoulders of each mouse were photographed, and the thickness of the right and left ears was measured using a digital vernier micrometer. The total clinical severity of AD-like lesions was defined as the sum of the individual scores, graded as 0 (none), 1 (mild), 2 (moderate), and 3 (severe) for each of three signs and symptoms (erythema/hemorrhage, ear thickness/edema, dorsal excoriation/erosion) (Supplementary Table S1).
Dermal histology. NC/Nga mice were sacrificed and the auricles were fixed overnight in cold 4% PFA/PBS and then embedded in paraffin wax according to standard procedures after washing several times with phosphate buffer. Paraffin sections were obtained from the mice showing the average clinical score for each group, cut at a thickness of 5 μm, and mounted on silane-coated glass slides. The thin sections were stained with HE or acidic TB (pH 4.0) and examined by light microscopy for histologic changes. The thickness of the epidermis in HE sections was expressed as the total from counts of five fields. The mast cells between the epithelium and panniculus carnosus were counted as previously described50 and the data expressed as the total number of mast cells in five fields.
Immunization. Pathogen-free male BALB/c mice (4 weeks of age) were purchased from Japan SLC and kept under temperature- and light-controlled conditions. Mice were given a standard diet of Labo MR Breeder feed (Nihon Nosan, Kanagawa, Japan) and sterile water ad libitum. After a preliminary period of 2 weeks, mice (6 weeks of age) were sensitized once weekly for 3 weeks with i.p. injections of 100 μg of AG (buckwheat, Fagopyrum esculentum, lyophilized form, GREER Laboratories, Lenoir, NC) and alum adjuvant (allergen to adjuvant ratio of 1:20). Mice were used for the study at 8 weeks of age. All experimental procedures were carried out in accordance with the Regulations for Animal Experimentation of Shinshu University, and the animal protocol was approved by the Committee for Animal Experiments of Shinshu University. Based on national regulations and guidelines according to Law No. 105 and Notification No. 6, all experimental procedures were reviewed by the Committee for Animal Experiments and finally approved as No. 230070 by the president of Shinshu University.
Immunoglobulin enzyme-linked immunosorbent assay. Serum IgE levels were quantified using a commercially available ELISA kit (Mouse IgE ELISA Quantitation Kit; Bethyl Laboratories, Montgomery, TX) according to the manufacturer's instructions.
Western blotting. Cells were cultured with 10 μg/ml of buckwheat and 3 μmol/l ODN for 1 hour and then lysed in cell lysis buffer (CelLytic M, Sigma-Aldrich) containing protease inhibitor cocktail (Sigma-Aldrich). Protein concentrations were determined using a BCA Protein Assay kit (Thermo Fisher Scientific, Waltham, MA). Samples of whole cell extracts (10 μg each) were incubated for 30 minutes in SDS sample buffer at 70 °C and resolved by 15% (v/v) SDS-PAGE. Proteins were then transferred from the gel onto a Hybond-P PVDF membrane (GE Healthcare Japan, Tokyo, Japan). Immunoblots were probed with antibodies specific to STAT6 and pSTAT6 (pTyr641) (Sigma-Aldrich), followed by horseradish peroxidase–conjugated secondary antibody (Sigma-Aldrich). Signals were visualized using ECL Prime Western Blotting Detection Reagent (GE Healthcare, Japan). Blots were probed with anti-β-actin antibody (Sigma-Aldrich) to normalize for protein loading.
Intracellular staining. SP cells (2 × 106 cells/well) were stimulated with 50 μg/ml AG and 3 μmol/l ODN for 72 hours. After stimulation, cells were cultured at 37 °C in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 U/ml of penicillin, 100 μg/ml of streptomycin, 20 μg/ml of brefeldin A, 2 μg/ml of ionomycin, and 20 ng/ml of phorbol 12-myristate 13-acetate for 5 hours. The stimulated SP cells were first stained with FITC-labeled anti-mouse CD4 antibody (Biolegend) for 15 minutes at 4 °C. For intracellular staining, cells were fixed in 4% PFA for 15 minutes at room temperature, washed, permeabilized in 0.5% Triton X-100/PBS with 1% BSA for 15 minutes on ice, and then further incubated with PE-labeled anti-mouse IL-4 antibody (Biolegend). SP cells were first stained with anti-mouse IL-33 antibody (Medical & Biological Laboratories, Tokyo, Japan) for 60 minutes on ice and then washed and further incubated with FITC-labeled anti-mouse IgG (Biolegend). Cells were washed again and the percentages of CD4+IL-4+ and IL-33+ cells were determined using a FACSCalibur (BD Biosciences). Data were acquired and analyzed using BD CellQuest software (BD Biosciences). All analyses were carried out at least in triplicate, and representative results are presented.
Oral administration of ODNcaps to AG-immunized mice. After a 1-week preliminary period, mice (5 weeks of age) were orally administered 1 mg of Caps, CpGcaps, or iSG3caps for 4 weeks (i.e., days 0–27). Mice were sensitized with i.p. injections of 100 μg of AG and alum adjuvant (allergen to adjuvant ratio of 1:20) on days 7 and 21. After 4 weeks (day 28), all mice were sacrificed, their spleens were extracted and weighed, and the cells were cultured with 50 μg/ml of AG for 72 hours. After stimulation, cells were activated for 5 hours, fixed, permeabilized, and stained with antibodies. The stained cells were washed and the percentages of CD4+IL-4+ and IL-33+ cells were determined using a FACSCalibur (BD Biosciences). Data were acquired and analyzed using BD CellQuest software (BD Biosciences).
Statistical analysis. ANOVA and post hoc tests were performed using a statistical software package (ystat2004.xls, Igakutosho Shuppan, Tokyo, Japan). One-way ANOVA with post hoc Student–Newman–Keuls test was used to determine the significance of differences in in vitro experiments. The significance of differences with respect to body weight, clinical skin score, ear thickness, serum IgE levels, and the proportion of IL-33+ cells was determined using two-sided Student's t-tests. Differences were considered significant at P < 0.05. Values for in vivo experiments are expressed as the mean ± standard error (SE). Other values are expressed as the mean ± standard deviation (SD).
SUPPLEMENTARY MATERIAL Figure S1. Procedure for synthesis of ODNcaps. Figure S2. Assay of iSG3caps uptake in vitro. Figure S3. Oral administration of ODNnak to AD mice. Figure S4. Photographs of the backs of mice in the NA and iSG3caps groups. Table S1. Clinical skin score.
Acknowledgments
We thank Nao Akiba and Yuto Sakamoto (Faculty of Agriculture, Shinshu University) for excellent animal care and technical support. The study was supported by a Grant-in-Aid for Scientific Research I (No. 24580388) from the Japan Society for the Promotion of Science (JSPS) and by a grant from the Mishima Kaiun Memorial Foundation (Heisei 24, No. 8) to T.S. The authors declare no conflict of interest.
Supplementary Material
Procedure for synthesis of ODNcaps.
Assay of iSG3caps uptake in vitro.
Oral administration of ODNnak to AD mice.
Photographs of the backs of mice in the NA and iSG3caps groups.
Clinical skin score.
References
- Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R.et al. (1995CpG motifs in bacterial DNA trigger direct B-cell activation Nature 374546–549. [DOI] [PubMed] [Google Scholar]
- Costa LB, Noronha FJ, Roche JK, Sevilleja JE, Warren CA, Oriá R.et al. (2012Novel in vitro and in vivo models and potential new therapeutics to break the vicious cycle of Cryptosporidium infection and malnutrition J Infect Dis 2051464–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbach J, Neumann A, Wahle C, Brand K, Gnjatic S, Jäger E. Therapeutic administration of a synthetic CpG oligodeoxynucleotide triggers formation of anti-CpG antibodies. Cancer Res. 2012;72:4304–4310. doi: 10.1158/0008-5472.CAN-12-0257. [DOI] [PubMed] [Google Scholar]
- Klinman DM. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat Rev Immunol. 2004;4:249–258. doi: 10.1038/nri1329. [DOI] [PubMed] [Google Scholar]
- Pico de Coana Y, Carnes J, Gallego MT, Alonso C, Parody N. Modulation of the humoral response to Dermatophagoides pteronyssinus allergens in BALB/c mice by extract modification and adjuvant use. Int Arch Allergy Immunol. 2012;157:331–338. doi: 10.1159/000329636. [DOI] [PubMed] [Google Scholar]
- Sester DP, Naik S, Beasley SJ, Hume DA, Stacey KJ. Phosphorothioate backbone modification modulates macrophage activation by CpG DNA. J Immunol. 2000;165:4165–4173. doi: 10.4049/jimmunol.165.8.4165. [DOI] [PubMed] [Google Scholar]
- Klaschik S, Tross D, Shirota H, Klinman DM. Short- and long-term changes in gene expression mediated by the activation of TLR9. Mol Immunol. 2010;47:1317–1324. doi: 10.1016/j.molimm.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma H, Zamri NB, Sekine S, Fukuyama Y, Tokuhara D, Gilbert RS.et al. (2012A novel combined adjuvant for nasal delivery elicits mucosal immunity to influenza in aging Vaccine 30803–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng WK, Wee K, Kollmann TR, Dutz JP. Topical CpG adjuvantation of a protein-based vaccine induces protective immunity to Listeria monocytogenes. Clin Vaccine Immunol. 2014;21:329–339. doi: 10.1128/CVI.00734-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y, Shigemori S, Sato T, Shimazu T, Hatano K, Otani H.et al. (2013Class I/II hybrid inhibitory oligodeoxynucleotide exerts Th1 and Th2 double immunosuppression FEBS Open Bio 341–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimosato T, Fujimoto M, Tohno M, Sato T, Tateo M, Otani H.et al. (2010CpG oligodeoxynucleotides induce strong up-regulation of interleukin 33 via Toll-like receptor 9 Biochem Biophys Res Commun 39481–86. [DOI] [PubMed] [Google Scholar]
- Gürsel M, Verthelyi D, Gürsel I, Ishii KJ, Klinman DM. Differential and competitive activation of human immune cells by distinct classes of CpG oligodeoxynucleotide. J Leukoc Biol. 2002;71:813–820. [PubMed] [Google Scholar]
- Chowdhury EH. Fluoride enhances transfection activity of carbonate apatite by increasing cytoplasmic stability of plasmid DNA. Biochem Biophys Res Commun. 2011;409:745–747. doi: 10.1016/j.bbrc.2011.05.079. [DOI] [PubMed] [Google Scholar]
- Kutsuzawa K, Akaike T, Chowdhury EH. The influence of the cell-adhesive proteins E-cadherin and fibronectin embedded in carbonate-apatite DNA carrier on transgene delivery and expression in a mouse embryonic stem cell line. Biomaterials. 2008;29:370–376. doi: 10.1016/j.biomaterials.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Luo D, Saltzman WM. Synthetic DNA delivery systems. Nat Biotechnol. 2000;18:33–37. doi: 10.1038/71889. [DOI] [PubMed] [Google Scholar]
- Yamaguchi J, Nakamura F, Aihara M, Yamashita N, Usui H, Hida T.et al. (2008Semaphorin3A alleviates skin lesions and scratching behavior in NC/Nga mice, an atopic dermatitis model J Invest Dermatol 1282842–2849. [DOI] [PubMed] [Google Scholar]
- Shigemori S, Yonekura S, Sato T, Otani H, Shimosato T. Expression of the immunoreactive buckwheat major allergenic storage protein in Lactococcus lactis. Appl Microbiol Biotechnol. 2013;97:3603–3611. doi: 10.1007/s00253-012-4608-9. [DOI] [PubMed] [Google Scholar]
- Choudhury BK, Wild JS, Alam R, Klinman DM, Boldogh I, Dharajiya N.et al. (2002In vivo role of p38 mitogen-activated protein kinase in mediating the anti-inflammatory effects of CpG oligodeoxynucleotide in murine asthma J Immunol 1695955–5961. [DOI] [PubMed] [Google Scholar]
- Hattori K, Nishikawa M, Watcharanurak K, Ikoma A, Kabashima K, Toyota H.et al. (2010Sustained exogenous expression of therapeutic levels of IFN-gamma ameliorates atopic dermatitis in NC/Nga mice via Th1 polarization J Immunol 1842729–2735. [DOI] [PubMed] [Google Scholar]
- Inoue J, Aramaki Y. Suppression of skin lesions by transdermal application of CpG-oligodeoxynucleotides in NC/Nga mice, a model of human atopic dermatitis. J Immunol. 2007;178:584–591. doi: 10.4049/jimmunol.178.1.584. [DOI] [PubMed] [Google Scholar]
- Lee SY, Cho JY, Miller M, McElwain K, McElwain S, Sriramarao P.et al. (2006Immunostimulatory DNA inhibits allergen-induced peribronchial angiogenesis in mice J Allergy Clin Immunol 117597–603. [DOI] [PubMed] [Google Scholar]
- Park Y, Chang YS, Lee SW, Cho SY, Kim YK, Min KU.et al. (2001The enhanced effect of a hexameric deoxyriboguanosine run conjugation to CpG oligodeoxynucleotides on protection against allergic asthma J Allergy Clin Immunol 108570–576. [DOI] [PubMed] [Google Scholar]
- Sugai T, Mori M, Nakazawa M, Ichino M, Naruto T, Kobayashi N.et al. (2005A CpG-containing oligodeoxynucleotide as an efficient adjuvant counterbalancing the Th1/Th2 immune response in diphtheria-tetanus-pertussis vaccine Vaccine 235450–5456. [DOI] [PubMed] [Google Scholar]
- Volpi C, Fallarino F, Pallotta MT, Bianchi R, Vacca C, Belladonna ML.et al. (2013High doses of CpG oligodeoxynucleotides stimulate a tolerogenic TLR9-TRIF pathway Nat Commun 41852. [DOI] [PubMed] [Google Scholar]
- Youn CJ, Miller M, Baek KJ, Han JW, Nayar J, Lee SY.et al. (2004Immunostimulatory DNA reverses established allergen-induced airway remodeling J Immunol 1737556–7564. [DOI] [PubMed] [Google Scholar]
- Takakura M, Takeshita F, Aihara M, Xin KQ, Ichino M, Okuda K.et al. (2005Hyperproduction of IFN-gamma by CpG oligodeoxynucleotide-induced exacerbation of atopic dermatitis-like skin lesion in some NC/Nga mice J Invest Dermatol 1251156–1162. [DOI] [PubMed] [Google Scholar]
- Kakkar R, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov. 2008;7:827–840. doi: 10.1038/nrd2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK.et al. (2005IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines Immunity 23479–490. [DOI] [PubMed] [Google Scholar]
- Xiong Z, Thangavel R, Kempuraj D, Yang E, Zaheer S, Zaheer A. Alzheimer's disease: evidence for the expression of interleukin-33 and its receptor ST2 in the brain. J Alzheimers Dis. 2014;40:297–308. doi: 10.3233/JAD-132081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenuki Y, Matsushita K, Futatsugi-Yumikura S, Ishii KJ, Kawagoe T, Imoto Y.et al. (2012A critical role of IL-33 in experimental allergic rhinitis J Allergy Clin Immunol 130184–94.e11. [DOI] [PubMed] [Google Scholar]
- Imai Y, Yasuda K, Sakaguchi Y, Haneda T, Mizutani H, Yoshimoto T.et al. (2013Skin-specific expression of IL-33 activates group 2 innate lymphoid cells and elicits atopic dermatitis-like inflammation in mice Proc Natl Acad Sci USA 11013921–13926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gursel I, Gursel M, Yamada H, Ishii KJ, Takeshita F, Klinman DM. Repetitive elements in mammalian telomeres suppress bacterial DNA-induced immune activation. J Immunol. 2003;171:1393–1400. doi: 10.4049/jimmunol.171.3.1393. [DOI] [PubMed] [Google Scholar]
- Klinman DM, Gursel I, Klaschik S, Dong L, Currie D, Shirota H. Therapeutic potential of oligonucleotides expressing immunosuppressive TTAGGG motifs. Ann N Y Acad Sci. 2005;1058:87–95. doi: 10.1196/annals.1359.015. [DOI] [PubMed] [Google Scholar]
- Shirota H, Gursel I, Gursel M, Klinman DM. Suppressive oligodeoxynucleotides protect mice from lethal endotoxic shock. J Immunol. 2005;174:4579–4583. doi: 10.4049/jimmunol.174.8.4579. [DOI] [PubMed] [Google Scholar]
- Shirota H, Gursel M, Klinman DM. Suppressive oligodeoxynucleotides inhibit Th1 differentiation by blocking IFN-gamma- and IL-12-mediated signaling. J Immunol. 2004;173:5002–5007. doi: 10.4049/jimmunol.173.8.5002. [DOI] [PubMed] [Google Scholar]
- Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA.et al. (2013Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment Science 342967–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, Mazmanian SK. Has the microbiota played a critical role in the evolution of the adaptive immune system. Science. 2010;330:1768–1773. doi: 10.1126/science.1195568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard JM, Maurice CF, Kinnebrew MA, Abt MC, Schenten D, Golovkina TV.et al. (2014Rapid fucosylation of intestinal epithelium sustains host-commensal symbiosis in sickness Nature 514638–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballarín-González B, Dagnaes-Hansen F, Fenton RA, Gao S, Hein S, Dong M.et al. (2013Protection and Systemic Translocation of siRNA Following Oral Administration of Chitosan/siRNA Nanoparticles Mol Ther Nucleic Acids 2e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenschläger C, Schmidt C, Lehr CM, Fischer D, Stallmach A. PEG-functionalized microparticles selectively target inflamed mucosa in inflammatory bowel disease. Eur J Pharm Biopharm. 2013;85 3 Pt A:578–586. doi: 10.1016/j.ejpb.2013.09.016. [DOI] [PubMed] [Google Scholar]
- Mamaeva V, Rosenholm JM, Bate-Eya LT, Bergman L, Peuhu E, Duchanoy A.et al. (2011Mesoporous silica nanoparticles as drug delivery systems for targeted inhibition of Notch signaling in cancer Mol Ther 191538–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitragotri S, Burke PA, Langer R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat Rev Drug Discov. 2014;13:655–672. doi: 10.1038/nrd4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Zhuang X, Deng ZB, Jiang H, Mu J, Wang Q.et al. (2014Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit Mol Ther 22522–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunisawa J, Kurashima Y, Kiyono H. Gut-associated lymphoid tissues for the development of oral vaccines. Adv Drug Deliv Rev. 2012;64:523–530. doi: 10.1016/j.addr.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Talton J, Zhang G, Cunningham T, Wang Z, Waters RC.et al. (2012Large intestine-targeted, nanoparticle-releasing oral vaccine to control genitorectal viral infection Nat Med 181291–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K, Kutsuzawa K, Maruyama K, Akiyama Y, Chowdhury EH. Synergistic effect of PKC activation and actin filament disruption on carbonate apatite-facilitated lymphocyte transfection. Biochem Biophys Res Commun. 2012;419:482–484. doi: 10.1016/j.bbrc.2012.02.023. [DOI] [PubMed] [Google Scholar]
- Maeyama J, Takatsuka H, Suzuki F, Kubota A, Horiguchi S, Komiya T.et al. (2014A palindromic CpG-containing phosphodiester oligodeoxynucleotide as a mucosal adjuvant stimulates plasmacytoid dendritic cell-mediated T(H)1 immunity PLoS ONE 9e88846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Ishii KJ, Ihata A, Klinman DM. Contribution of nitric oxide to CpG-mediated protection against Listeria monocytogenes. Infect Immun. 2005;73:3803–3805. doi: 10.1128/IAI.73.6.3803-3805.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cengiz S, Sarac S, Özcan M. Effects of simulated gastric juice on color stability, surface roughness and microhardness of laboratory-processed composites. Dent Mater J. 2014;33:343–348. doi: 10.4012/dmj.2013-265. [DOI] [PubMed] [Google Scholar]
- Hamasaka A, Abe R, Koyama Y, Yoshioka N, Fujita Y, Hoshina D.et al. (2009DNA vaccination against macrophage migration inhibitory factor improves atopic dermatitis in murine models J Allergy Clin Immunol 12490–99. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Procedure for synthesis of ODNcaps.
Assay of iSG3caps uptake in vitro.
Oral administration of ODNnak to AD mice.
Photographs of the backs of mice in the NA and iSG3caps groups.
Clinical skin score.