SUMMARY
The accumulation of misfolded proteins compromises cellular function and causes debilitating diseases. However, the cellular systems that detect and degrade these proteins remain poorly understood. Here we show that the tripartite motif protein PML/TRIM19 and the SUMO-dependent ubiquitin ligase RNF4 act together to promote the degradation of various misfolded proteins in mammalian cell nuclei. Mechanistically, PML selectively interacts with misfolded proteins through distinct substrate recognition sites, and conjugates these proteins with the small ubiquitin-like modifiers (SUMOs) through its SUMO ligase activity. SUMOylated misfolded proteins are subsequently recognized and ubiquitinated by RNF4 and targeted for proteasomal degradation. We further show that PML deficiency exacerbates polyglutamine (polyQ) disease in a mouse model of spinocerebellar ataxia 1 (SCA1). These findings reveal a system in mammalian cells that remove misfolded proteins through sequential PML-mediated SUMOylation and RNF4-mediated ubiquitination. They also suggest that the PML-RNF4 system may be a potential target for treating protein-misfolding diseases.
INTRODUCTION
Proteins are the most abundant macromolecules of the cell, and are critical to virtually all physiological processes. To perform their biological functions, the majority of proteins need to fold into and maintain their native conformations. Although the native conformation of a protein is determined by its amino acid sequence, the folding process is extraordinarily complex and highly prone to error, and its utility can be limited further in situations of genetic mutations, biogenetic inaccuracies, and post-translational damages (Dobson, 2003; Goldberg, 2003). Proteins that have adopted aberrant conformations, and the aggregates formed by them, pose a constant threat to cell viability and function. Failure to eliminate these proteins is closely linked to the pathogenesis of various debilitating human diseases (Selkoe, 2003; Taylor et al., 2002).
To protect against protein misfolding, cells employ two broad sets of protein quality control (PQC) systems: systems that assist proteins in achieving their native conformations, and systems that eliminate misfolded proteins once they are formed. The former consist mainly of a large number of molecular chaperones and their co-chaperones, which in an ATP-dependent manner protect proteins in their non-native state and reduce misfolding and aggregation. Notable examples include 1) heat shock protein 70 (Hsp70), which aids the folding of a wide range of proteins, 2) Hsp60/chaperonin, which forms a macromolecular cage to encapsulate relatively small unfolded proteins for uninterrupted folding, and 3) HSP90, which most commonly acts on proteins involved in cell signaling and transcription (Hartl et al., 2011).
In prokaryotes or lower eukaryotes, protein aggregates can be re-solubilized by Hsp100 proteins (e.g. ClpB in bacteria and Hsp104 in yeast), which function in concert with Hsp70 and its co-chaperone Hsp40 (Glover and Lindquist, 1998). Nevertheless, given that protein misfolding is inevitable and often cannot be reversed, cells ultimately rely on degradative systems to eliminate misfolded proteins. These systems are still poorly understood. Although the ubiquitin-proteasome pathway, along with autophagy, must be an important part of these systems, the critical issue of how they recognize misfolded proteins for degradation remains elusive (Goldberg, 2003; Tyedmers et al., 2010).
Furthermore, compared to the other cellular compartments such as the cytoplasm and the endoplasmic reticulum (Buchberger et al., 2010), the PQC systems in the nucleus are conspicuously unclear. Misfolded proteins in the nucleus can be particularly damaging to post-mitotic mammalian cells (e.g., neurons and cardiac myocytes), which are unable to remove these proteins through the breakdown of the nuclear envelope during mitosis. The importance of understanding PQC in this cellular compartment is underscored by the formation of neuronal intranuclear inclusions that are associated with various devastating and dominantly inherited neurodegenerative diseases, including Huntington’s disease (HD) and several types of spinocerebellar ataxias (SCAs). These diseases are caused by an expansion within the relevant genes of a CAG repeat, which encodes a polyQ stretch. They are manifested when the polyQ stretch exceeds a threshold length that is disease-specific, and become progressively more severe as its length increases (Orr and Zoghbi, 2007).
The promyelocytic leukemia protein (PML; also known as TRIM19) is a member of the tripartite motif (TRIM) family of proteins, which contain an N-terminal TRIM/BRCC region, consisting of a RING domain, one or two B-Boxes, and a coiled-coil (CC) motif, followed by a variable C-terminal region. PML is predominantly a nuclear protein and is the eponymous component of PML nuclear bodies (PML NBs). PML is implicated in a wide variety of cell processes, including DNA damage signaling, apoptosis, and transcription (Bernardi and Pandolfi, 2007). Of note, PML co-localizes with aggregates formed by polyQ proteins associated with SCAs (Skinner et al., 1997; Takahashi et al., 2003) and, upon overexpression, promotes degradation of at least one of them (mutant ataxin-7) (Janer et al., 2006). Despite the potential importance of these observations, the role of PML in the removal of misfolded proteins is not well understood. In particular, it is unclear whether PML plays a broad role in the removal of nuclear misfolded proteins. The critical issue of the molecular mechanisms by which PML removes misfolded proteins is unaddressed. Moreover, the physiological relevance of the effect of PML on misfolded proteins is not known. We set out to investigate these issues in the current study.
RESULTS
PML-mediated proteasomal degradation of pathogenic Ataxin-1 protein
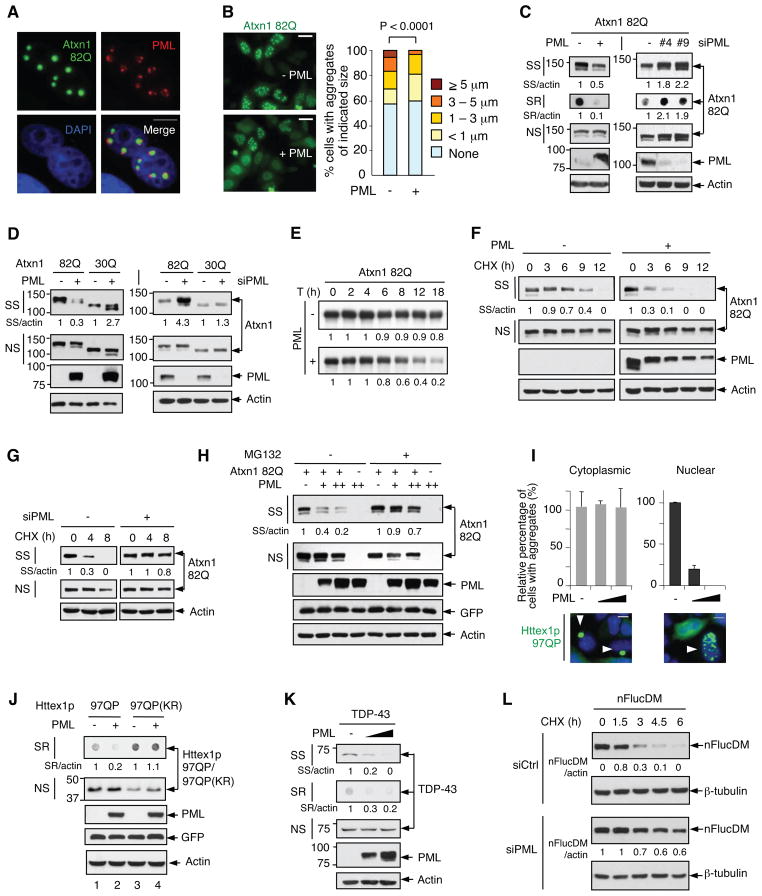
SCA1 is a fatal neurological disorder characterized by progressive ataxia and loss of neurons, especially cerebellar Purkinje cells. It is caused by the expansion of a polyQ stretch in the SCA1 gene product, Ataxin-1 (Atxn1) (Orr and Zoghbi, 2007). To investigate the role of PML in eliminating nuclear misfolded proteins, we generated a cell culture model in which a pathogenic Atxn1 protein with 82 contiguous glutamines that was C-terminally fused to the enhanced green fluorescent protein, Atxn1 82Q-GFP, was expressed in HeLa cells. Similar to pathogenic Atxn1 proteins in human SCA1 patients and mouse SCA1 transgenic models (Skinner et al., 1997), Atxn1 82Q-GFP was localized to the nucleus, exhibiting a diffuse localization pattern with markedly higher concentration in microscopically visible inclusions (Figures 1A and 1B). Atxn1 82Q-GFP also yielded both NP-40-soluble (soluble or NS) and NP-40-insoluble (aggregated) species in cell lysates. The latter could be further divided into SDS-soluble (SS) and SDS-resistant (SR) species (Figure 1C).
Figure 1. PML promotes the degradation of Atxn1 82Q and other nuclear misfolded proteins.
(A) HeLa cells transfected with Atxn1 82Q-GFP were stained with anti-PML antibody (red) and DAPI (blue). Individual and merged images are shown. Scale bar: 10 μm.
(B) Atxn1 82Q-GFP was expressed alone or together with PML in HeLa cells. Left: representative fluorescence images of cells. Scale bar: 20 μm. Right: quantification of cells based on sizes of Atxn1 82Q-GFP inclusions.
(C) Atxn1 82Q-GFP was expressed alone or together with PML in HeLa cells (left), or alone in HeLa cells that were previously treated with control (-) or PML siRNA. Cell lysate fractions (when indicated) and whole cell lysates (WCL) were analyzed by filter retardation assay (for SR fraction) or Western blot (WB; for the rest). Molecular weight standards (in kDa) and relative ratios of SS or SR Atxn1 versus actin are indicated.
(D) Steady-state levels of FLAG-Atxn1 82Q or 30Q when expressed alone or together with PML in HeLa cells (left), or when expressed alone in HeLa cells that were treated with control or PML siRNA (right), analyzed by WB.
(E) Effect of PML on the stability of total FLAG-Atxn1 82Q protein, analyzed by a pulse chase assay and autoradiography. The relative amounts of 35S-labeled Atxn1 82Q is indicated.
(F and G) Effect of PML overexpression (F) and knockdown (G) on the stability of Atxn1 82Q-GFP, analyzed by CHX treatment and WB.
(H) Effect of PML on Atxn1 82Q-GFP levels in the absence or presence of MG132.
(I) Top: Relative percentages of Httex1p 97QP-expressing cells with cytoplasmic (left) and nuclear (right) inclusions, in the absence or presence of PML (means + SD, n = 3). Bottom: Representative fluorescence images of transfected cells immunostained with an anti-Htt antibody. Arrowheads indicate Httex1p 97QP aggregates.
(J and K) Levels of HA-Httex1p 97QP and HA-Httex1p 97QP(KR) (J) and GFP-TDP-43 (K) in cells with and without PML over-expression. Virtually Htt aggregates were in the SR fraction.
(L) Stability of nFucDM-GFP in control and PML-depleted cells, analyzed by CHX treatment and WB.
See also Figure S1.
Concordant with previous reports (Skinner et al., 1997), endogenous PML co-localized with Atxn1 82Q-GFP inclusions, accumulating in bodies adjacent to them and also being distributed within (Figure 1A). PML is expressed as several isoforms (Nisole et al., 2013). We examined five major PML isoforms (I, II, III, IV, and VI) and found that all five co-localized with Atxn1-GFP inclusions (Figure S1A). For subsequent analyses, we chose the commonly used isoform IV (hereafter called PML).
When co-expressed with Atxn1 82Q, PML significantly decreased the size of Atxn1 82Q-GFP inclusions (Figure 1B). It also reduced the steady-state levels of the Atxn1 82Q-GFP protein, especially the aggregated SS and SR species (Figure 1C, left). To evaluate the effect of endogenous PML (all isoforms), we knocked it down using two independent small interfering RNAs (siRNAs). This noticeably raised the levels of Atxn1 82Q-GFP, especially aggregated species (Figure 1C, right). Silencing PML also increased the steady-state levels of a FLAG-tagged Atxn1 82Q protein (Figure S1B). The effect of PML siRNA on Atxn1 82Q could be reversed by an siRNA-resistant form of PML (Figure S1C), ruling out off-target effects of the siRNA.
To evaluate whether PML specifically reduces pathogenic Atxn1 proteins, we used a non-pathogenic ataxin-1 protein, Atxn1 30Q. Forced expression of PML did not reduce the abundance of Atxn1 30Q-GFP, while knockdown of PML did not significantly augment it either (Figure 1D), underscoring the selective effect of PML on pathogenic Atxn1 proteins.
PML did not inhibit the transcription of the Atxn1 82Q gene (Figure S1D). To determine whether PML promotes the degradation of the Atxn1 82Q protein, we performed a pulse-chase assay. In the absence of co-transfected PML, total [35S]-labeled Atxn1 82Q protein was rather stable, and its levels declined only ~20% in 18 h. By contrast, in the presence of PML, total [35S]Atxn1 82Q protein was destabilized, and its levels declined ~80% over the same period of time (Figure 1E).
To extend this analysis, we used cycloheximide (CHX) to block protein synthesis and examined the degradation of the pre-existing Atxn1 82Q protein. Forced expression of PML accelerated the degradation of aggregated Atxn1 82Q, reducing its half-life from ~8 h to ~2 h, while having a minimal effect on soluble Atxn1 82Q (Figure 1F). Conversely, silencing PML prolonged the half-life of aggregated Atxn1 82Q and, to a lesser extent, the half-life of soluble Atxn1 82Q (Figure 1G). The ability of PML to remove aggregated Atxn1 82Q was markedly diminished by the proteasome inhibitor MG132 (Figure 1H). In contrast to its effect on Atxn1 82Q, PML did not alter the half-life of Atxn1 30Q (Figure S1E). Collectively, these results indicate that PML targets pathogenic, but not normal, Atxn1 protein for proteasomal degradation.
A general role for PML in degrading nuclear misfolded proteins
To assess whether PML plays a broad role in degrading misfolded proteins in the nucleus, we tested two additional proteins linked to neurodegeneration: 1) a pathogenic fragment of huntingtin (Htt) encoded by the first exon of the HD gene, Httex1p 97QP (Steffan et al., 2004); and 2) TAR DNA-binding protein 43 (TDP-43), which is associated with both amyotrophic lateral sclerosis (ALS, also known as Lou Gehrig’s disease) and frontotemporal lobar degeneration with ubiquitinated inclusions (FTLD-U) (Chen-Plotkin et al., 2010). Httex1p 97QP formed microscopically visible inclusions in both the nucleus and the cytoplasm (Figure 1I). PML reduced the nuclear, but not the cytoplasmic, Httex1p 97QP inclusions (Figure 1I), and decreased the amount of aggregated Httex1p 97QP (Figure 1J, lanes 1 and 2). TDP-43, on the other hand, formed inclusions in a small percentage of transfected cells and mainly in the nucleus. PML lowered aggregated, but not soluble, forms of TDP-43 (Figure 1K).
To extend these analyses, we used a structurally-destabilized mutant of the model chaperone substrate firefly luciferase (FlucDM), which was developed as a probe for the capacity of cellular PQC systems (Gupta et al., 2011). Endogenous PML partially co-localized with inclusions formed by a nuclear form of FlucDM (nFlucDM-GFP), but not with the wild-type counterpart (nFlucWT-GFP) (Figure S1F). Silencing PML noticeably elevated the levels of aggregated nFlucDM-GFP (Figure S1G), and extended the half-life of total nFlucDM-GFP protein (Figure 1L). Taken together, these results indicate that PML facilitates the removal of multiple misfolded proteins in mammalian cell nuclei.
Recognition of misfolded proteins by distinct sites on PML
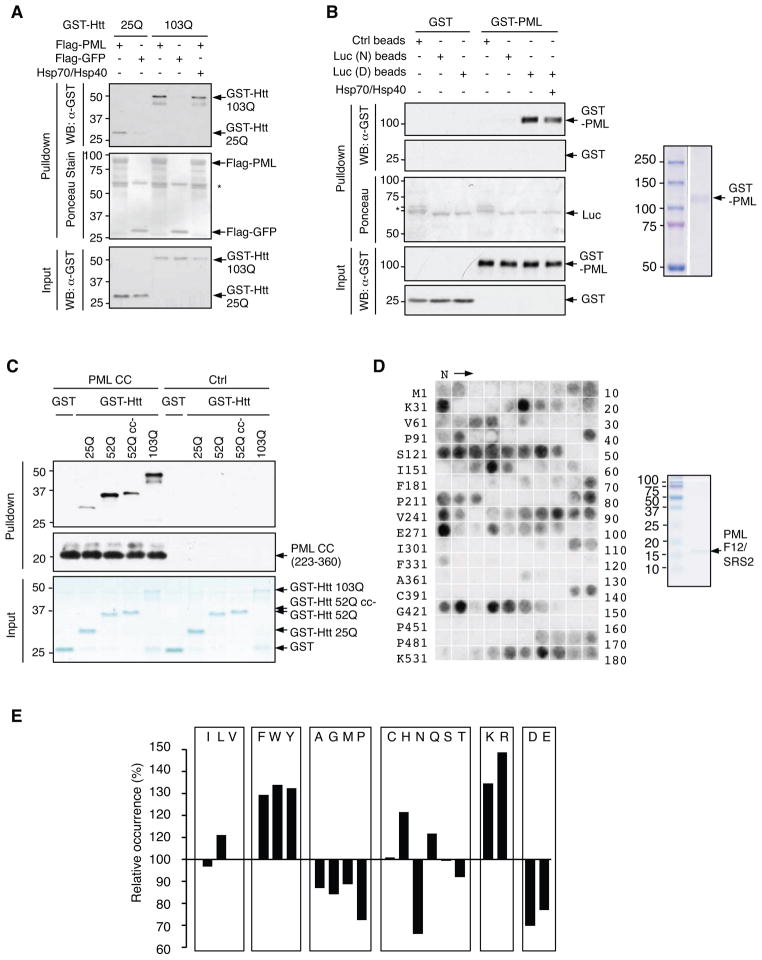
To investigate the mechanism by which PML degrades misfolded proteins, we first examined whether PML is able to directly recognize these proteins (Figures 1 and S1). We used a pathogenic (103Q) and a non-pathogenic (25Q) Htt fragment, each being fused to glutathione S-transferase (GST). In an in vitro assay with purified recombinant proteins, immobilized FLAG-PML, but not the control protein FLAG-GFP, pulled down GST-Htt 103Q (Figure 2A), indicating a specific and direct interaction between PML and Htt 103Q. FLAG-PML also pulled down GST-Htt 25Q; however, this interaction was substantially weaker than the PML-Htt 103Q interaction (Figure 2A). In a reciprocal experiment, immobilized GST-Htt 103Q proteins also interacted more strongly with FLAG-PML than immobilized GST-Htt 25Q did (Figure S2A). Hsp70 and Hsp40, which recognize a broad range of misfolded proteins, did not enhance the PML-Htt 103Q interaction (Figure 2A). These results suggest that PML directly associates with polyQ proteins and preferentially with the pathogenic form.
Figure 2. Recognition of misfolded proteins by PML.
(A) Binding of GST-Htt 25Q and GST-Htt 103Q to immobilized FLAG-PML and FLAG-GFP (negative control), analyzed by an in vitro pull down assay followed by WB (top and bottom) and Ponceau S staining (middle). *, IgG heavy chain.
(B) Binding of GST-PML (shown on the right) and the control GST protein to native (N) and urea-denatured (D) luciferase (luc) immobilized on Ni-NTA beads, analyzed as in (A). *, Non-specific proteins from BL21 bacterial lysates that bound to the control beads.
(C) Binding of indicated GST-Htt fusions to FLAG-PML CC conjugated on anti-Flag M2 beads or to control beads, analyzed by WB (top and middle) and Coomassie staining (bottom).
(D) Binding of PML F12/SRS2 (shown on the right) to peptide library derived from luciferase. The N-terminal amino acid of the first peptide and the number of the last peptide spotted in each row are indicated.
(E) The occurrence of each amino acid in PML SRS2-binding peptides relative to its occurrence in the luciferase peptide array (set at 100%).
See also Figures S2 and S3.
We also examined whether PML selectively binds to denatured luciferase. 6xHis-tagged luciferase immobilized on Ni-NTA beads were either denatured with urea or kept in the native form. Denatured, but not native, luciferase specifically interacted with GST-PML, and Hsp70/Hsp40 did not enhance this interaction (Figure 2B). Thus, PML can directly recognize misfolded, but not native, luciferase.
To understand the molecular basis for interaction of PML with misfolded proteins, we sought to identify the substrate recognition site(s) of PML, as well as the structural features on substrates that these SRSs discern. It was previously shown that, in a manner dependent on the length of the polyQ stretch, polyQ and the flanking regions form CC structures, which facilitate the assembly of polyQ proteins into an oligomeric or aggregated state and also mediate the interaction of polyQ proteins with CC-containing proteins (Fiumara et al., 2010). This led us to hypothesize that PML, via its CC region within the TRIM/RBCC motif, might interact with pathogenic polyQ proteins. We constructed a set of PML fragments (F1 to F5) that either contained or lacked the CC region (Figure S2B). A fragment containing the CC region, F1, interacted with Htt 103Q, while two fragments lacking this region, F2 and F3, did not (Figures S2B and S2C). Moreover, the CC region alone (F4) bound to Htt 103Q, while deleting this region from the entire PML protein (F5 or ΔCC) greatly diminished this binding. Thus, PML recognizes Htt 103Q almost exclusively through the CC region. Similar to the full-length PML, PML CC displayed a clear binding preference for the pathogenic Htt 103Q to the non-pathogenic Htt 25Q (Figure 2C). PML CC also strongly interacted with another pathogenic Htt construct, Htt 52Q (Figure 2C). Thus, PML CC is likely an substrate recognition site (called SRS1).
To test whether PML CC recognizes the homologous CC structure in Htt proteins, we mutated the residues in Htt 52Q that were predicted to be involved in the CC formation, yielding Htt 52Q cc- (Figure S2D). A similar mutation was previously shown to reduce the formation of the CC structure in Htt 72Q (Fiumara et al., 2010). Indeed, compared to Htt 52Q, Htt 52Q cc- displayed a noticeably reduced propensity to form aggregates (not shown) and a substantially weaker interaction with PML CC (Figure 2C). Therefore, PML CC/SRS1 likely interacts with the CC structure on pathogenic Htt proteins.
Given that PML also promotes the degradation of non-polyQ proteins such as luciferase and TDP-43 (Figure 1 and S1), we reasoned that PML might contain at least another substrate recognition site that could discern non-CC structural features on misfolded proteins. To test this possibility, we examined the panel of PML fragments for interaction with denatured luciferase. Although the CC region alone could interact with denatured luciferase, significant levels of interaction were also retained in two fragments (F2 and F5) that lacked this region but contained the C-terminus (aa 361 to 633) (Figure S2B and S3A). Using additional deletion constructs within the C-terminus (F6 to F18, Figures S2B and S3B), we found that a stretch of 63 amino acids (aa 571 to 633) was sufficient for binding to denatured luciferase. Further deletions in this region abolished the binding (Figures S3). Thus, the last 63 amino acids of PML likely constitute another substrate recognition site (called SRS2).
To investigate the linear sequences in luciferase that can be recognized by PML SRS2, we used purified PML SRS2 to screen cellulose-bound peptide scans that represented the complete sequence of luciferase. The scans consisted of 180 peptides, each containing thirteen amino acid residues that overlapped adjacent peptides by ten. Similar to chaperones such as Hsp70 and ClpB (Rudiger et al., 1997; Schlieker et al., 2004), PML SRS2 only bound to a subset of these peptides (Figure 2D), indicating its ability to distinguish peptides with different amino acid compositions. An analysis of the relative occurrence of all twenty amino acids in PML SRS2-interacting peptides versus all peptides in the scans showed that PML SRS2-interacting peptides strongly favor aromatic (Phe, Trp, and Tyr) and positively-charged (Arg and Lys) residues, and disfavored negatively-charged residues (Asp and Glu) (Figure 2E). This amino acid preference was similar to that of ClpB, except that SRS2 had an additional preference for Leu and His, which are disfavored by ClpB (Schlieker et al., 2004).
For comparison, we also tested the binding of PML CC/SRS1 to the peptide scans. Consistent with the notion that this region recognizes higher order structures instead of linear sequences, PML CC/SRS1 weakly bound to few peptides (Figure S2E). Based on these results, we conclude that PML contains at least two regions that can recognize misfolded proteins: the CC region within the TRIM/RBCC motif (SRS1) and the 63-amino acid stretch at its C-terminus (SRS2), which can discern CC structures and exposed peptides enriched in both aromatic and basic amino acids, respectively.
Involvement of SUMOylation in the degradation of Atxn1 82Q
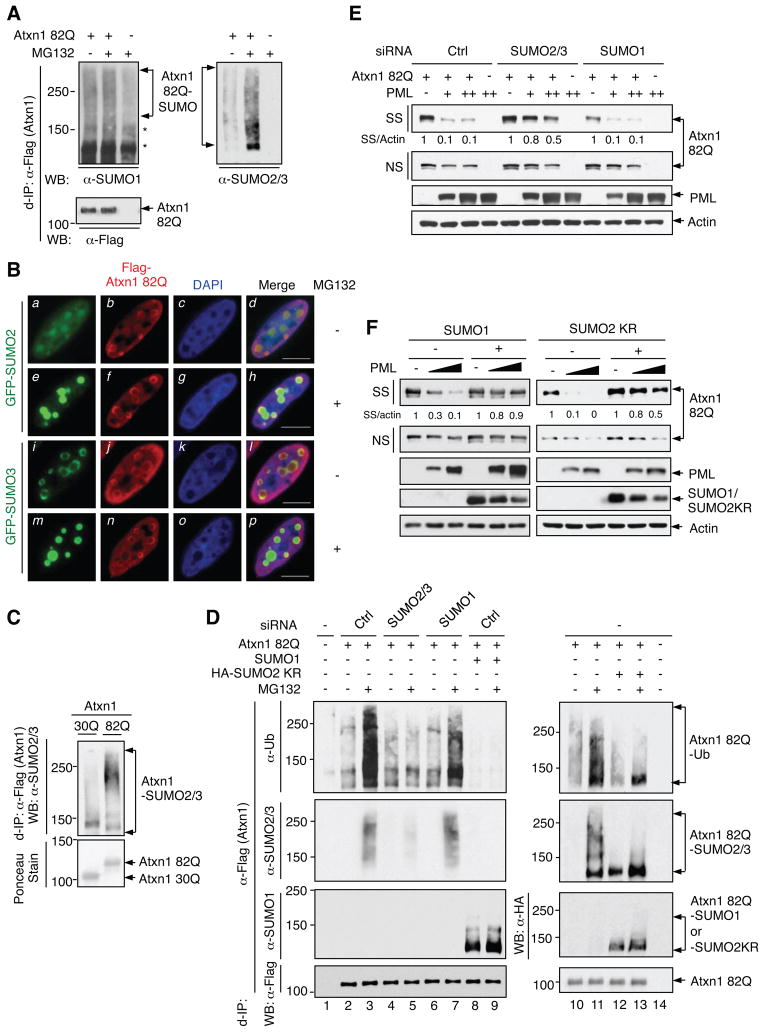
How might PML promote the degradation of misfolded proteins upon recognition? Misfolded proteins associated with neurodegeneration are frequently modified by SUMO, although its function remains unclear (Martin et al., 2007). We previously demonstrated that PML possesses SUMO E3 ligase activity (Chu and Yang, 2011). Mammalian cells express three major SUMO proteins, SUMO1-3. SUMO2 and SUMO3 are nearly identical to each other in their sequence (collectively called SUMO2/3), and are approximately 50% identical to SUMO1 (Wilkinson and Henley, 2010). We thus investigated the modification of Atxn1 82Q by these SUMO proteins and its role in Atxn1 82Q degradation.
Atxn1 82Q was modified by both exogenous (Riley et al., 2005) and endogenous (Figure 3A, left) SUMO1, and this modification was weaker than that of Atxn1 30Q (Riley et al., 2005). Atxn1 82Q was also modified by endogenous SUMO2/3 (Figure 3A, right), and co-localized with GFP-SUMO2/3 in the nucleus (Figure 3B). The sites in Atxn1 82Q that were conjugated with SUMO1 and SUMO2/3 might be different, because a mutant Atxn1 82Q with impaired SUMO1 conjugation, Atxn1 82Q (5KR) (Riley et al., 2005), showed no defect in SUMO2/3 conjugation (Figure S4A).
Figure 3. SUMO2/3 is involved in the ubiquitination and PML-mediated degradation of Atxn1 82Q.
(A) SUMO1- and SUMO2/3-modification of Atxn1 82Q by in HeLa cells treated without or with MG132. For better comparison of modified Atxn1 82Q, IP products with a similar level of unmodified Atxn1 82Q were analyzed by WB. *: non-specific bands.
(B) Localization of Atxn1 82Q (detected by anti-FLAG antibody, red) in GFP-SUMO2- or GFP-SUMO3-expressing U2OS cells treated with or without MG132. Scale bar: 10 μm.
(C) SUMO2/3 modification of Atxn1 82Q and 30Q in HeLa cells, analyzed by d-IP followed by WB (top) and Ponceau S staining (bottom).
(D) Atxn1 82Q was expressed alone or together with SUMO1 or HA-SUMO2 KR in HeLa cells that were previously transfected with the indicated siRNA or un-transfected (-). Cells were treated with or without MG132. SUMOylation and ubiquitination of FLAG-Atxn1 82Q was analyzed by d-IP and WB.
(E) Levels of Atxn1 82Q-GFP expressed alone or together with increasing amounts of PML in HeLa cells pre-treated with the indicated siRNA.
(F) Effect of PML on Atxn1 82Q-GFP levels in the presence or absence of SUMO1 or SUMO2 KR.
See also Figure S4.
Of note, SUMO2/3-modification of Atxn1 82Q was substantially stronger compared to that of Atxn1 30Q (Figure 3C), correlating with the different responses of these Atxn1 proteins to PML-mediated degradation (Figures 1 and S1). Similarly, TDP-43 was modified by endogenous SUMO2/3 (Figure S4B). So was FlucDM, as well as another structurally-destabilized luciferase mutant, FlucSM (Gupta et al., 2011); this modification was stronger than that of wild-type luciferase (Figure S4C).
Moreover, proteasome inhibition resulted in a markedly increase in SUMO2/3-modified Atxn1 82Q, concurrently with ubiquitinated Atxn1 82Q, but not in SUMO1-modified Atxn1 82Q (Figure 3A and Figure 3D, lanes 2 and 3). It also enhanced the co-localization of Atxn1 82Q with GFP-SUMO2/3 (Figure 3B). Likewise, proteasome inhibition enhanced modification of TDP-43 and luciferase mutants by SUMO2/3 (Figures S4B and S4C).
To assess the role of SUMO proteins in the ubiquitination and proteasomal degradation of Atxn1 82Q, we silenced SUMO2/3 and SUMO1 separately using siRNA. Silencing SUMO2/3, but not SUMO1, effectively reduced Atxn1 82Q ubiquitination (Figure 3D, lanes 4-7). Silencing SUMO2/3 also raised the levels of Atxn1 82Q, especially the aggregated form, but not the levels of the control protein GFP (Figures S4D-S4F), and diminished the ability of PML to remove aggregated Atxn1 82Q (Figure 3E).
SUMO2 and SUMO3 contain an internal SUMOylation consensus site that enables the formation of poly-chains. SUMO1 does not contain this site; when conjugated to the SUMO2/3 chain, SUMO1 can terminate the chain elongation (Wilkinson and Henley, 2010). Therefore, we used two additional strategies to reduce the modification of Atxn1 82Q by SUMO2/3. First, we overexpressed SUMO1. This strongly reduced Atxn1 82Q conjugation to SUMO2/3. This also strongly impaired Atxn1 82Q conjugation to ubiquitin (Figure 3D, lanes 8 and 9) and its degradation by PML (Figure 3F, left). Second, we used a SUMO2 mutant that was deficient in chain formation, SUMO2 KR. Forced expression of SUMO2 KR effectively reduced the amount of SUMO2/3-modified Atxn1 82Q species, especially those of high molecular weights. It also reduced ubiquitin-modified Atxn1 82Q species (Figure 3D, lanes 12 and 13) and blunted the ability of PML to degrade Atxn1 82Q (Figure 3F, right). Moreover, we used a SUMOylation-defective Htt mutant, Httex1p 97QP(KR) (Steffan et al., 2004) and found that it was resistant to the PML-mediated degradation (Figure 1J). Taken together, these results show that ubiquitination and degradation of Atxn1 82Q and likely other misfolded proteins is dependent on their modification by SUMO2/3.
PML as a SUMO E3 ligase of Atxn1 82Q
When co-expressed with Atxn1 82Q in cells, PML strongly increased SUMO2/3-modification of Atxn1 82Q, both in the absence and in the presence of the proteasome inhibitor MG132 (Figure 4A). Conversely, silencing PML markedly reduced SUMO2/3-modified Atxn1 82Q under these conditions (Figure 4B). Similarly, silencing PML also reduced SUMO2/3-modification of nFlucDM (Figure 4C).
Figure 4. PML promotes SUMOylation of Atxn1 82Q.
(A) SUMOylation of FLAG-Atxn1 82Q in HeLa cells in the absence or presence of HA-PML cells, and without or with MG132 treatment. The amount of Atxn1 82Q DNA used for transfected were adjusted to yield comparable levels of the unmodified protein.
(B and C) SUMOylation of FLAG-Atxn1 82Q (B) and FLAG-nFlucDM-GFP (C) in control and PML siRNA-transfected HeLa cells treated without or with MG132.
(D and E) SUMOylation of purified HA-Atxn1 82Q-FLAG was performed in the presence of recombinant FLAG-PML, FLAG-PML M6, and SUMO2 as indicated. In (D), the amounts of d-IP products were adjusted to yield a similar level of unmodified Atxn1 82Q (middle panel).
(F) Levels of Atxn1 82Q-GFP in HeLa cells in the absence or presence of increasing amounts of PML or PML M6.
See also Figure S4.
To confirm the SUMO E3 activity of PML towards Atxn1 82Q, we performed in vitro SUMOylation assays with purified recombinant proteins. In the absence of PML, Atxn1 82Q was weakly modified by SUMO2 (Figures 4D and 4E), consistent with previous observations that SUMOylation can proceed in vitro without a SUMO E3 ligase (Wilkinson and Henley, 2010). PML augmented Atxn1 82Q SUMOylation in a dose-dependent manner (Figures 4D and 4E). In contrast, a SUMO E3-defective mutant, PML M6 (Chu and Yang, 2011), failed to do so (Figures 4E and S4G). PML M6 was also ineffective at reducing aggregated Atxn1 82Q (Figure 4F). These results suggest that PML is a SUMO E3 ligase of Atxn1 82Q, and that this activity is involved in Atxn1 82Q degradation.
A role for RNF4 in degrading misfolded proteins
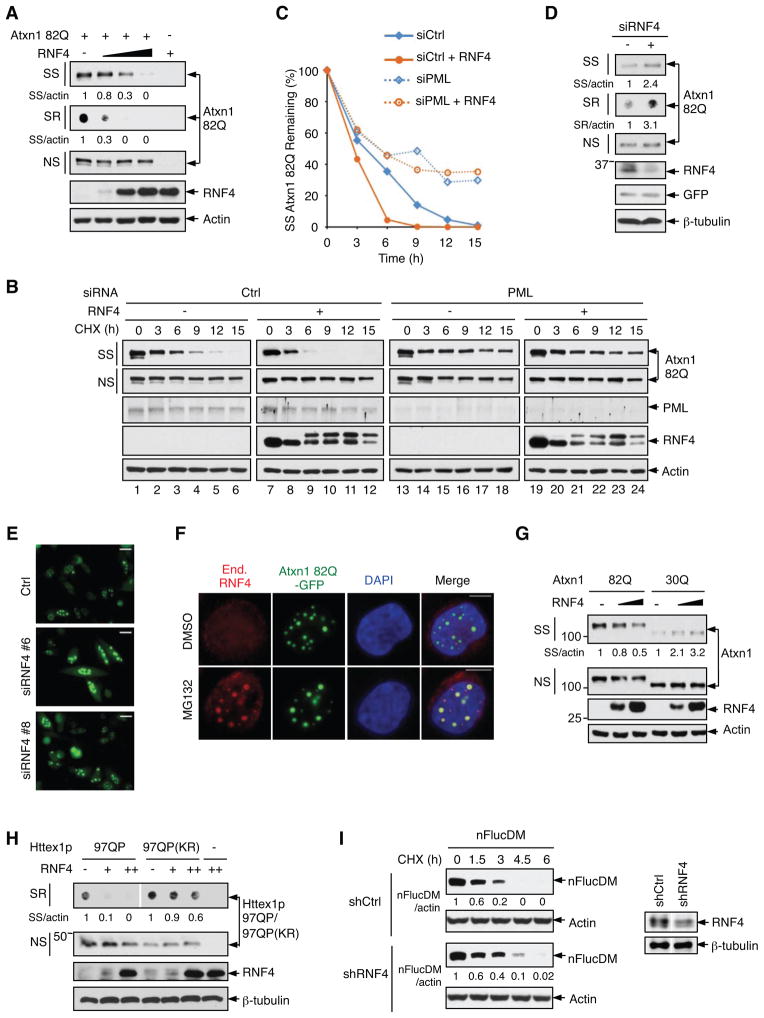
Proteins conjugated with a poly-SUMO2/3 chain can be recognized and ubiquitinated by RNF4, a RING domain ubiquitin ligase with four tandem SUMO-interacting motifs (SIMs) (Sun et al., 2007). However, the role of RNF4 in degrading misfolded proteins remains unknown. We found that forced RNF4 expression strongly reduced the steady-state levels of aggregated Atxn1 82Q in cell lysates (Figure 5A), as well as the number of Atxn1 82Q inclusions in the nucleus (Figure S5A). RNF4 shortened the half-life of aggregated, but not soluble, Atxn1 82Q (Figures 5B, lanes 1-12; 5C; and S5B). Conversely, knocking down endogenous RNF4 with three siRNAs, individually or in combination, increased total and aggregated Atxn1 82Q protein in cell lysates (Figures 5D, S5C, and S5D), as well as Atxn1 82Q inclusions in the nucleus (Figure 5E). An siRNA-resistant form of RNF4 could reverse the effect of RNF4 knockdown (Figure S5C), indicative of the specificity of the siRNA.
Figure 5. A role of RNF4 in the degradation of Atxn1 82Q.
(A) Levels of Atxn1 82Q-GFP in HeLa cells without and with RNF4 overexpression.
(B and C) Effect of RNF4 overexpression on Atxn1 82Q-GFP stability in control and PML-depleted HeLa cells, analyzed by CHX treatment and WB. Relative SS Atxn1 82Q/actin ratios are shown in (C).
(D) Levels of FLAG-Atxn1 82Q in HeLa cells pretreated with a control siRNA (-) or a combination of three RNF4 siRNAs (D).
(E) Representative fluorescent images of Atxn1 82Q-GFP in control and RNF4-knock down HeLa cells. Scale bar: 20 μm
(F) Localization of Atxn1 82Q-GFP and endogenous RNF4 in HeLa cells treated with vehicle (DMSO) or MG132. Scale bar: 10 μm.
(G and H) Levels of FLAG-Atxn1 82Q and FLAG-Atxn1 30Q (G) or HA-Httex1p 97QP and HA-Httex1p 97QP(KR) (H) in HeLa cells without and with RNF4 overexpression.
(I) Stability of nFlucDM-GFP in HeLa cells stably expressing shCtrl and shRNF4 (left) and the levels of RNF4 in these cells (right).
See also Figure S5.
Moreover, both endogenous and exogenous RNF4 proteins normally displayed a diffuse nuclear distribution pattern with minimal or moderate co-localization with Atxn1 82Q inclusions. But they became highly enriched in Atxn1 82Q inclusions on proteasome blockage (Figures 5F and S5E), likely reflecting a stalled attempt in clearing Atxn1 82Q. In contrast to its effect on Atxn1 82Q, RNF4 did not reduce the levels of Atxn1 30Q (Figure 5G). Collectively, these results demonstrate a role for RNF4 in eliminating pathogenic Atxn1 proteins.
To assess a general effect of RNF4 on misfolded proteins, we tested it on Httex1p 97QP, TDP-43, and nFlucDM. Forced expression of RNF4 markedly reduced Httex1p 97QP, especially the aggregated form, while having a much weaker effect on Httex1p 97QP(KR) (Figure 5H). Forced expression of RNF4 also decreased the levels of TDP-43 (Figure S5F), whereas silencing RNF4 augmented the percentage of TDP-43-expressing cells with nuclear inclusions (Figures S5G and S5H). Upon proteasome inhibition, endogenous RNF4 became highly enriched in TDP-43 inclusions (Figure S5I), similar to its accumulation in Atxn1 82Q inclusions under the same conditions (Figure 5F). Moreover, silencing RNF4 prolonged the half-life of nFlucDM (Figure 5I). Collectively, these observations suggest that RNF4 plays a critical role in the degradation of misfolded proteins.
Ubiquitinates and degradation of SUMO2/3-modified Atxn1 82Q by RNF4
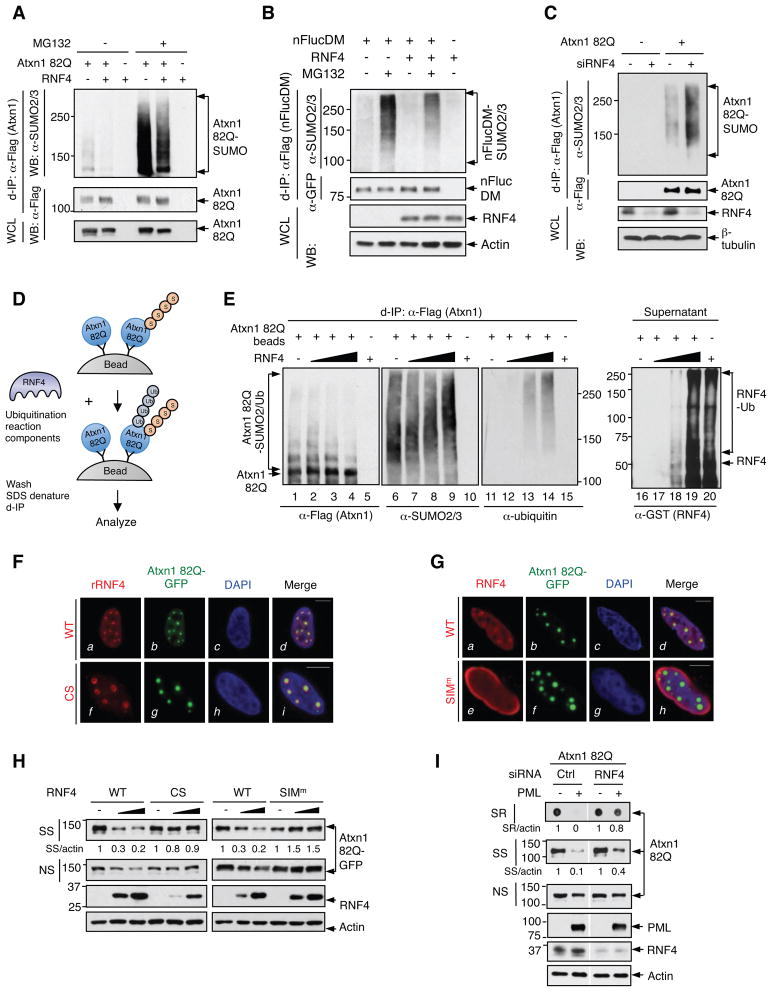
Similar to PML, the ability of RNF4 to eliminate misfolded proteins was dependent on SUMO2/3, as this ability was compromised in cells devoid of SUMO2/3, but not of SUMO1 (Figure S6A). Forced expression of RNF4 preferentially reduced SUMO2/3-modified Atxn1 82Q and nFlucDM over the unmodified proteins (Figures 6A and 6B). Conversely, silencing RNF4 increased SUMO2/3-modified Atxn1 82Q (Figure 6C) and enhanced the co-localization of Atxn1 82Q with GFP-SUMO2 (Figure S6B). These results show that RNF4 targets SUMO2/3-modified misfolded proteins for degradation.
Figure 6. RNF4 promotes the ubiquitination and degradation of SUMO2/3-modified Atxn1 82Q.
(A and B) Levels of SUMOylated FLAG-Atxn1 82Q (A) and FLAG-nFlucDM-GFP (B), in the absence or presence of RNF4, in HeLa cells treated with or without MG132. d-IP products with similar levels of unmodified proteins and WCL were analyzed.
(C) Levels of SUMOylated FLAG-Atxn1 82Q in HeLa cells that were pre-treated with a control siRNA or a combination of RNF4 siRNAs, analyzed as in (A).
(D and E) Unmodified and SUMO2-modified FLAG-Atxn1 82Q proteins conjugated on M2 beads (+), or control M2 beads (-), were incubated with ubiquitination reaction mixture, in the absence or presence of GST-RNF4. (D) A schematic diagram of the experimental design. (E) WB analysis of FLAG-Atxn1 82Q (left) and GST-RNF4 (right).
(F and G) Localization of Atxn1 82Q-GFP and RNF4 proteins (detected by anti-FLAG antibody) in HeLa. Scale bar: 10 μm.
(H) Effect of the indicated RNF4 proteins on Atxn1 82Q-GFP levels in HeLa cells.
(I) Effect of PML overexpression on Atxn1 82Q-GFP levels in HeLa cells that were pre-treated with control or RNF4 siRNA.
See also Figure S6.
To confirm that RNF4 ubiquitinates SUMO2/3-conjugated misfolded proteins, we performed an in vitro ubiquitination assay using a mixture of unmodified and SUMO2-modified Atxn1 82Q proteins (Figure 6D). In the presence of increasing doses of RNF4, the unmodified Atxn1 82Q protein was not ubiquitinated (lanes 1-4). In contrast, the SUMO2-modified Atxn1 82Q proteins, which were of relatively low molecular weight (Figure 6E, lanes 1, 6, and 9), were progressively converted to higher molecular weight species that were also modified by ubiquitin (lanes 2-4, 7-9, and 12-14). Therefore, RNF4 is a ubiquitin ligase for SUMO2/3-modified, but not unmodified, Atxn1 82Q protein.
RNF4 possesses both ubiquitin ligase and SUMO-binding activities (Sun et al., 2007). To ascertain the involvement of these activities in degrading Atxn1 82Q, we generated RNF4 mutants defective in either ubiquitin ligase (CS and CS1) or SUMO-binding (SIMm) activity (Figure S6C). CS and CS1, albeit losing their ubiquitin E3 activity (Figure S6D), were still able to co-localize with Atxn1 82Q inclusions (Figure 6F). SIMm, on the other hand, retained a substantial level of ubiquitin ligase activity (Figure S6E), but failed to co-localize with Atxn1 82Q inclusions (Figure 6G). Neither mutants were capable of removing aggregated Atxn1 82Q (Figure 6H). Collectively, these results show that RNF4 likely binds to SUMO2/3-modified misfolded proteins via its SIM region and ubiquitinates these proteins via its ligase activity for degradation.
Although forced expression of PML led to effective clearance of aggregated Atxn1 82Q in control cells, this ability was greatly diminished in RNF4-depleted cells (Figure 6I). Reciprocally, forced expression of RNF4, although highly effective in accelerating Atxn1 82Q degradation in control cells, failed to do so in PML-depleted cells (Figure 5B, lanes 19-24 vs. 7-12; and Figure 5C). Moreover, in PML-depleted cells, which displayed high Atxn1 82Q levels, silencing RNF4 did not further elevate the levels of Atxn1 82Q (Figure S6F). These results indicate mutual dependence of PML and RNF4 in the degradation of Atxn1 82Q.
PML deficiency exacerbates behavioral and pathological phenotypes in a mouse model of SCA1
The results described above revealed a PQC system that degrades Atxn1 82Q and likely other nuclear misfolded proteins through sequential PML-mediated SUMOylation and RNF4-mediated ubiquitination. To investigate the physiological role of this system, we used a mouse model of SCA1 (B05), which expresses the Atxn1 82Q transgene (Atxn1tg/−) in the cerebellar Purkinje cells. Resembling human SCA1 patients, B05 mice develop ataxia and neurological abnormalities with increasing age (Burright et al., 1995). The loss of RNF4 in mice results in embryonic lethality (Hu et al., 2010), precluding the analysis of its effect on B05 mice. However, PML-knockout (PML−/−) mice are viable and appear to develop normally (Wang et al., 1998). We crossbred B05 mice with PML−/− and PML-wild-type (PML+/+) mice, and compared the littermates of all genotypes PML+/+, PML+/−, and PML−/−, PML+/+:Atxn1tg/−, PML+/−:Atxn1tg/−, and PML−/−:Atxn1tg/− for both motor performance and neuropathology.
Motor performance including balance, coordination, and endurance was evaluated using a Rotarod apparatus with accelerating speed. To determine whether any potential behavioral defects were due to a progressively diminished capacity, as opposed to a developmental impairment, we examined mice at different ages. To rule out the influence of the long-term motor memory, only naïve animals were used, each being tested for four consecutive days.
At 7 weeks of age, all mice performed similarly on the Rotarod (Figure 7A). Although some differences were observed among mice of distinct genotypes, they were not statistically significant (ANOVA p = 0.53), suggesting that PML−/− mice did not have pre-existing impairments in their motor functions. At 11 weeks of age, all mice lacking the Atxn1 82Q transgene (PML+/+, PML+/−, and PML−/−) still showed no statistical difference in their performance (ANOVA p = 0.33) (Figure 7B), and PML+/+ and PML+/+:Atxn1tg/− also performed similarly. These observations suggest that either PML deficiency or Atxn1 82Q transgene expression alone was insufficient to cause motor defects at this age. Interestingly, PML−/−:Atxn1tg/− showed severe impairments in Rotarod performance compared to either PML+/+:Atxn1tg/− or PML−/− mice. Although these three groups of animals were comparable at the beginning of the four consecutive testing days, unlike the other two groups, PML−/−:Atxn1tg/− mice showed minimal improvement over time. The lack of improvement of PML−/−:Atxn1tg/− mice on the Rotarod was reminiscent of Atxn1tg/− mice at advanced stages (Clark et al., 1997). The PML heterozygous counterparts (PML+/−:Atxn1tg/− mice) displayed an intermediate impairment on the Rotarod (ANOVA p = 0.0004 for the three Atxn1tg/− groups) (Figure 7B). Thus, PML deficiency aggravates motor defects of the Atxn1tg/− mice.
Figure 7. PML deficiency exacerbates behavioral and pathological phenotypes of SCA1 mouse model.
(A and B) Retention times (average + standard error of the means or SEM) on accelerating Rotarod at 7 (A) and 11 (B) weeks of age, with the number of animals indicated in parenthesis.
(C and D) Cerebellar sections of 12-week-old animals were stained with hematoxylin. (C) Quantification of molecular layer thickness (means + SEM, n = 3 mice/genotype). (D) Representative images of the staining. Scale bar: 200 μm.
(E and F) Cerebellar sections of 1-yr-old animals were stained with an anti-calbindin antibody. (E) Quantitation of Purkinje cells, graphed as the average number of soma per 1 mm length (means + SEM, n = 4 mice/genotype). (F) Representative images of the staining. Scale bar: 200 μm.
(G and H) Sections of the cerebellar cortex from 12-week-old mice were stained with an anti-ubiquitin antibody and counterstained with hematoxylin. (G) Percentage of Purkinje cells with aggregates (means + SEM, n = 3 mice/genotype). (H) Representative images of the immunohistochemistry staining. Arrowheads indicate the ubiquitin positive aggregates in Purkinje cell bodies. Scale bar: 50 μm. No ubiquitin positive aggregates were observed in Purkinje cells in mice without Atxn1tg/− (Figure S7D).
(I) A model for PQC by the PML-RNF4 system. PML recognizes misfolded proteins and conjugates them with poly-SUMO2/3 chain through its SUMO E3 ligase activity (a). RNF4 ubiquitinates SUMOylated-misfolded proteins (b) and targets them for proteasomal degradation (c).
See also Figure S7.
The major neuropathological phenotype of the Atxn1tg/− mice is the degeneration of Purkinjie cells, a main constituent of the top layer (the molecular layer) of the cerebellar cortex. This degeneration is manifested initially in the shrinkage of the molecular layer and the atrophy of Purkinje cell dendrites, and later in the loss of Purkinje cell bodies (Burright et al., 1995; Clark et al., 1997). At 12 weeks of age, PML+/− and PML−/− mice showed only a slight and statistically insignificant shrinkage in the molecular layers, while PML+/+:Atxn1tg/− mice exhibited a discernible shrinkage, compared to PML+/+ mice (Figures 7C and 7D). Because PML+/+:Atxn1tg/− mice performed similarly on the Rotarod to PML+/+ mice (Figure 7B), neurodegeneration in PML+/+:Atxn1tg/− mice might not have reached a critical threshold. This non-linear correlation between behavioral and pathological phenotypes of the SCA1 transgenic model has been previously observed (Gehrking et al., 2011). Importantly, compared to PML+/+:Atxn1tg/− mice, PML+/−:Atxn1tg/− and PML−/−:Atxn1tg/− mice displayed a moderate and a strong further reduction, respectively, in the thickness of molecular layer (Figures 7C and 7D). This correlated with the increasingly worse performance of these animals on the Rotarod (Figure 7B). Thus, PML deficiency aggravates the shrinkage of the molecular layer in Atxn1tg/− mice.
We also examined dendritic arborization of Purkinje cells by immunofluorescence staining with an antibody against the Purkinje cell-specific protein calbindin. At 12 weeks of age, the fluorescence intensity of Purkinje cell dendrites in all groups containing the Atxn1 82Q transgene was reduced to very low levels that precluded precise comparison (Figures S7A and S7B). Interestingly, compared to PML+/+ littermates, PML−/− mice already showed a strong reduction in dendritic arborization of Purkinje cells, while PML+/− mice showed an intermediate reduction (Figures S7A and S7B). These results indicate that PML itself has a role in protecting against neurodegeneration.
Despite the thinning of the molecular layer and the loss of Purkinje cell dendrites that were associated with PML deficiency, no significant difference in Purkinje cell population was observed among 12-week-old animals of different genotypes (Figure S7C). At one year of age, PML−/− mice displayed only a mild (11.0%) and statistically insignificant (p = 0.107) reduction in the number of Purkinje cells compared to PML+/+ mice, while PML+/+:Atxn1tg/− mice displayed a noticeable reduction (Figures 7E and 7F). Of note, PML−/−:Atxn1tg/− mice showed a significant further reduction in Purkinje cell density compared to PML+/+:Atxn1tg/− mice (~24%, p = 0.0023), and PML+/−:Atxn1tg/− mice showed an intermediate cell loss (Figures 7E and 7F). Again, these results demonstrate that PML deficiency worsens the neuropathological defects caused by the Atxn1 82Q transgene.
Neurodegeneration of B05 mice is accompanied by the formation of ubiquitin-positive Atxn1 82Q inclusions in Purkinje cells (Clark et al., 1997). To determine the effect of PML on Atxn1 82Q nuclear inclusions, we quantified Purkinje cells with these inclusions in mice at 12 weeks of age. PML deficiency alone did not result in the formation of aggregates (Figure S7D), but it significantly increased the number of aggregate-containing Purkinje cells in Atxn1 82Q transgenic mice (Figures 7G and 7H). Collectively, these results suggest that endogenous PML plays a role in preventing accumulation of misfolded proteins in SCA1 animals and suppressing the progression of this neurodegenerative disease.
DISCUSSION
Here we present evidence for a PQC system that degrades misfolded proteins in mammalian cell nuclei. This system consists of a recognition branch, PML, which selectively binds to misfolded proteins and marks these proteins with poly-SUMO2/3 chains, and an effector branch, RNF4, which ubiquitinates SUMOylated misfolded proteins and targets them proteasomal degradation. This relay system provides a link between misfolded proteins and the proteasome in mammalian cells, and it likely plays an important role in the protection against neurodegeneration and other proteopathies (Figure 7I).
Selective recognition of misfolded proteins by PML
The exquisite selectivity of this system resides in PML, which contains at least two SRSs. SRS1, consisting of the CC region within the TRIM/RBCC motif, favors CC structures on pathogenic polyQ proteins and perhaps other misfolded proteins. SRS2, consisting of the C-terminal 63 amino acids, recognizes short peptides enriched in both aromatic (Phe, Trp, and Tyr) and positively-charged (Arg and Lys) amino acids. SRS2 is similar to the bacterial Hsp100 ClpB (Schlieker et al., 2004), except that it also favors peptides containing Leu, a residue that is often exposed in misfolded proteins and is engaged by Hsp70 (Rudiger et al., 1997). Thus, SRS2 displays a hybrid substrate specificity of ClpB and Hsp70. These observations indicate that PML can recognize structures or regions that are commonly found in misfolded proteins.
A role for SUMOylation in degrading misfolded proteins
Conjugation to SUMO is a major post-translational modification. It occurs on numerous proteins and is vital to most eukaryotic life. Yet, beyond the generalization that it alters protein-protein interactions, the physiological function of SUMOylation remains elusive (Wilkinson and Henley, 2010). A prominent feature of the PML-RNF4 system is the involvement of SUMO2/3 modification prior to ubiquitination (Figures 3, 4, and S4). It was observed previously that conjugation of SUMO2/3 to cellular proteins is markedly enhanced by protein-denaturing stresses (Saitoh and Hinchey, 2000). The evidence presented in the current study provides an explanation for this observation, and suggests that a principal physiological function of SUMO2/3-modification is likely to facilitate the degradation of misfolded proteins, acting in concert with ubiquitination.
SUMO conjugation enhances protein solubility (Panavas et al., 2009). Because aggregated proteins cannot be effectively degraded by the proteasome (Verhoef et al., 2002), enhancing protein solubility may be a beneficial effect conferred by PML. Moreover, the extent of SUMOylation may enable the “triage decision” as to whether a given misfolded protein is selected for refolding or degradation. Conjugation to a single SUMO appears to be sufficient to enhance protein solubility (Panavas et al., 2009), and thus may facilitate refolding. In contrast, conjugation to SUMO2/3-chains is needed for effective recognition by the four tandem SIMs on RNF4 (Tatham et al., 2008). Such chains may form after unsuccessful refolding attempts.
SUMOylation of misfolded proteins has been reported to either promote or inhibit neurodegenerative diseases (Martin et al., 2007). These seemingly contradictory observations may be reconciled by the distinct functions of SUMO1 and SUMO2/3 in the removal of misfolded proteins (Figure 3), and by the dichotomy between the functions of SUMOylation: enhancing solubility of an abnormal protein (which may enhance its toxicity) and promoting its degradation. Thus, the outcome of elevated SUMOylation may depend on whether it can be matched by the cellular degradative capacity.
A potential major PQC system
Proteins in the nucleus may be mutated or sustain acute and chronic damages, as proteins elsewhere do. The highly crowded environment of the nucleus likely makes it especially challenging to maintain protein quality. The ubiquitin-proteasome pathway is expected to be the main degradative system in the nucleus, where autophagy is not known to operate. Previous studies have implicated a few ubiquitin ligases, such as yeast San1 and Doa10 and mammalian UHRF-2 and E6-AP, in the degradation of nuclear misfolded proteins (Cummings et al., 1999; Deng and Hochstrasser, 2006; Gardner et al., 2005; Iwata et al., 2009). The predominantly nuclear localization of PML, along with the potent effect of PML and RNF4 on diverse misfolded nuclear proteins (Figures 1 and S1), suggests that the PML-RNF4 system is likely a major PQC system in mammalian cell nuclei.
The TRIM family of proteins is shared among metazoans, from approximately 20 members in C. elegans to over 70 in mice and humans (Ozato et al., 2008). We previously demonstrated that at least several other TRIM proteins also possess SUMO E3 activity (Chu and Yang, 2011). Given their localization to the cytoplasm in addition to the nucleus (Ozato et al., 2008), we speculate that TRIM proteins also participate in PQC in the cytoplasm. The rapid expansion of the TRIM proteins during evolution may in part be a response to the increasing complexity of managing protein quality in cells of longer-living animals.
RNF4 is conserved among vertebrates (Sun et al., 2007). SUMO-dependent ubiquitin-ligases are also present in low eukaryotic species (Sun et al., 2007), and are involved in the degradation of at least one mutant yeast transcription factor (Wang and Prelich, 2009). Thus, it is also possible that systems analogous to the PML-RNF4 system may play a role in maintaining protein quality in these organisms.
The PML-RNF4 system and neurodegeneration
PML−/− mice have been extensively characterized for a variety of phenotypes including tumorigenesis (Wang et al., 1998). The present study indicates a heretofore-unrecognized role for PML in protection from neurodegeneration (Figures 7 and S7). Neurodegenerative disorders including SCAs and HD are usually late-onset diseases. Accumulating evidence suggests a progressive decline in PQC during aging (Balch et al., 2008). The strong effect of the PML-RNF4 on pathogenic proteins associated with SCA1, HD, and ALS and of PML deficiency on the progression of the SCA1 mouse model, along with the accumulation of PML in neuronal inclusions in patients with various neurodegenerative diseases (Skinner et al., 1997; Takahashi et al., 2003), suggest that insufficiency or dysfunction of the PML-RNF4 system may have a role in these diseases. We speculate that the PML-RNF4 system and analogous ones would be valuable targets in their treatment.
EXPERIMENTAL PROCEDURES
Cell lysate fractionation and filter retardation assay
Cell lysates were made in NP-40-containing buffer and fractionated into supernatant (NS) and pellet by centrifugation. Both fractions were boiled in buffer containing 2% SDS and analyzed by Western blot. A portion of the pellet was analyzed by a filter retardation assay for SR species.
Assays of protein half-life
Cells were pulse labeled in Met- and Cys-free DMEM medium supplemented with [35S]Met and [35S]Cys, and then cultured in regular DMEM. Alternatively, cells were treated with CHX. Immunoprecipitated [35S]Atxn1 82Q or unlabeled Atxn1 82Q in cell lysate were analyzed by autoradiography or Western blot.
Screening of cellulose-bound peptides for binding to PML domains
A peptide library (13-mers overlapping by ten amino acids) for firefly luciferase was prepared by automated spot synthesis (JPT peptide Technologies). The peptide array membrane was probed with purified PML SRS1 and SRS2 fragments.
SUMOylation and ubiquitination analysis
Cells or in vitro reaction mixtures were boiled in buffer containing 2% SDS and then diluted in buffer without SDS or passed through a Bio-Spin chromatography column to reduce the SDS concentration. Proteins were immunoprecipitated (denaturing IP or d-IP) and analyzed by Western blot.
Mouse breeding and behavioral analysis
The heterozygous B05 transgenic mice Atxn1tg/− (on FVB background) (Burright et al., 1995) were mated with PML−/− (on 129Sv background) (Wang et al., 1998). PML+/−:Atxn1tg/− mice from the F1 generation were mated with PML−/− or PML+/+ to generate mice used for Rotarod tests and pathology.
Immunostaining and pathological analysis of mouse cerebellum
Paraffin embedded cerebellar midsagital sections were stained with indicated antibodies and visualized using a Leica SP5 II laser scanning confocal microscope, or stained with hematoxylin and visualized using an Olympus BX51 microscope.
Supplementary Material
HIGHLIGHTS.
PML reduces various misfolded proteins in the mammalian cell nucleus
PML binds to misfolded proteins via substrate recognition sites and SUMOylates them
SUMOylated misfolded proteins are ubiquitinated by RNF4 for proteasomal degradation
PML deficiency exacerbates phenotypes of a mouse neurodegeneration model
Acknowledgments
We thank H.T. Orr for providing B05 mice and P.P. Pandolfi for providing PML knockout mice. We thank Z. Yu, M.P. Heenan, S. Slattery, and E.F. Fischer for technical assistance; N. Bonini, M.S. Marks, Y. Argon, E. Brown, and V. M.-Y. Lee for advice; A. Stonestrom, T. Agrawal, K. Wang, and C. O’Neill for help with manuscript preparation; C.-X. Yuan and UPenn Abramson Cancer Center Proteomic Core for mass spectrometry analysis. We also thank the following scientists for the gifts of reagents: S.D. Barr, K.S. Chang, Y. Chen, M. Dasso, P. Goloubinoff, R.T. Hay, A.-M. Herr, S. Lindquist, J.L. Marsh, H.T. Orr, J.J. Palvimo, and S. Raychaudhuri. This work was supported, in part, by grants from NIH (CA088868 and GM060911) and pilot grants from the Penn Medicine Neuroscience Center and the Penn Center for AIDS Research to X.Y.
Footnotes
Please see the supplementary information for the Extended Experimental Procedures and any associated references.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- Bernardi R, Pandolfi PP. Structure, dynamics and functions of promyelocytic leukaemia nuclear bodies. Nat Rev Mol Cell Biol. 2007;8:1006–1016. doi: 10.1038/nrm2277. [DOI] [PubMed] [Google Scholar]
- Buchberger A, Bukau B, Sommer T. Protein quality control in the cytosol and the endoplasmic reticulum: brothers in arms. Mol Cell. 2010;40:238–252. doi: 10.1016/j.molcel.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Burright EN, Clark HB, Servadio A, Matilla T, Feddersen RM, Yunis WS, Duvick LA, Zoghbi HY, Orr HT. SCA1 transgenic mice: a model for neurodegeneration caused by an expanded CAG trinucleotide repeat. Cell. 1995;82:937–948. doi: 10.1016/0092-8674(95)90273-2. [DOI] [PubMed] [Google Scholar]
- Chen-Plotkin AS, Lee VM, Trojanowski JQ. TAR DNA-binding protein 43 in neurodegenerative disease. Nat Rev Neurol. 2010;6:211–220. doi: 10.1038/nrneurol.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y, Yang X. SUMO E3 ligase activity of TRIM proteins. Oncogene. 2011;30:1108–1116. doi: 10.1038/onc.2010.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark HB, Burright EN, Yunis WS, Larson S, Wilcox C, Hartman B, Matilla A, Zoghbi HY, Orr HT. Purkinje cell expression of a mutant allele of SCA1 in transgenic mice leads to disparate effects on motor behaviors, followed by a progressive cerebellar dysfunction and histological alterations. J Neurosci. 1997;17:7385–7395. doi: 10.1523/JNEUROSCI.17-19-07385.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings CJ, Reinstein E, Sun Y, Antalffy B, Jiang Y, Ciechanover A, Orr HT, Beaudet AL, Zoghbi HY. Mutation of the E6-AP ubiquitin ligase reduces nuclear inclusion frequency while accelerating polyglutamine-induced pathology in SCA1 mice. Neuron. 1999;24:879–892. doi: 10.1016/s0896-6273(00)81035-1. [DOI] [PubMed] [Google Scholar]
- Deng M, Hochstrasser M. Spatially regulated ubiquitin ligation by an ER/nuclear membrane ligase. Nature. 2006;443:827–831. doi: 10.1038/nature05170. [DOI] [PubMed] [Google Scholar]
- Dobson CM. Protein folding and misfolding. Nature. 2003;426:884–890. doi: 10.1038/nature02261. [DOI] [PubMed] [Google Scholar]
- Fiumara F, Fioriti L, Kandel ER, Hendrickson WA. Essential role of coiled coils for aggregation and activity of Q/N-rich prions and PolyQ proteins. Cell. 2010;143:1121–1135. doi: 10.1016/j.cell.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RG, Nelson ZW, Gottschling DE. Degradation-mediated protein quality control in the nucleus. Cell. 2005;120:803–815. doi: 10.1016/j.cell.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Gehrking KM, Andresen JM, Duvick L, Lough J, Zoghbi HY, Orr HT. Partial loss of Tip60 slows mid-stage neurodegeneration in a spinocerebellar ataxia type 1 (SCA1) mouse model. Hum Mol Genet. 2011;20:2204–2212. doi: 10.1093/hmg/ddr108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover JR, Lindquist S. Hsp104, Hsp70, and Hsp40: a novel chaperone system that rescues previously aggregated proteins. Cell. 1998;94:73–82. doi: 10.1016/s0092-8674(00)81223-4. [DOI] [PubMed] [Google Scholar]
- Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–899. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- Gupta R, Kasturi P, Bracher A, Loew C, Zheng M, Villella A, Garza D, Hartl FU, Raychaudhuri S. Firefly luciferase mutants as sensors of proteome stress. Nat Methods. 2011;8:879–884. doi: 10.1038/nmeth.1697. [DOI] [PubMed] [Google Scholar]
- Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475:324–332. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- Hu XV, Rodrigues TM, Tao H, Baker RK, Miraglia L, Orth AP, Lyons GE, Schultz PG, Wu X. Identification of RING finger protein 4 (RNF4) as a modulator of DNA demethylation through a functional genomics screen. Proc Natl Acad Sci U S A. 2010;107:15087–15092. doi: 10.1073/pnas.1009025107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata A, Nagashima Y, Matsumoto L, Suzuki T, Yamanaka T, Date H, Deoka K, Nukina N, Tsuji S. Intranuclear degradation of polyglutamine aggregates by the ubiquitin-proteasome system. J Biol Chem. 2009;284:9796–9803. doi: 10.1074/jbc.M809739200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janer A, Martin E, Muriel MP, Latouche M, Fujigasaki H, Ruberg M, Brice A, Trottier Y, Sittler A. PML clastosomes prevent nuclear accumulation of mutant ataxin-7 and other polyglutamine proteins. J Cell Biol. 2006;174:65–76. doi: 10.1083/jcb.200511045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S, Wilkinson KA, Nishimune A, Henley JM. Emerging extranuclear roles of protein SUMOylation in neuronal function and dysfunction. Nat Rev Neurosci. 2007;8:948–959. doi: 10.1038/nrn2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisole S, Maroui MA, Mascle XH, Aubry M, Chelbi-Alix MK. Differential Roles of PML Isoforms. Front Oncol. 2013;3:125. doi: 10.3389/fonc.2013.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr HT, Zoghbi HY. Trinucleotide repeat disorders. Annu Rev Neurosci. 2007;30:575–621. doi: 10.1146/annurev.neuro.29.051605.113042. [DOI] [PubMed] [Google Scholar]
- Ozato K, Shin DM, Chang TH, Morse HC., 3rd TRIM family proteins and their emerging roles in innate immunity. Nat Rev Immunol. 2008;8:849–860. doi: 10.1038/nri2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panavas T, Sanders C, Butt TR. SUMO fusion technology for enhanced protein production in prokaryotic and eukaryotic expression systems. Methods Mol Biol. 2009;497:303–317. doi: 10.1007/978-1-59745-566-4_20. [DOI] [PubMed] [Google Scholar]
- Riley BE, Zoghbi HY, Orr HT. SUMOylation of the polyglutamine repeat protein, ataxin-1, is dependent on a functional nuclear localization signal. J Biol Chem. 2005;280:21942–21948. doi: 10.1074/jbc.M501677200. [DOI] [PubMed] [Google Scholar]
- Rudiger S, Germeroth L, Schneider-Mergener J, Bukau B. Substrate specificity of the DnaK chaperone determined by screening cellulose-bound peptide libraries. EMBO J. 1997;16:1501–1507. doi: 10.1093/emboj/16.7.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh H, Hinchey J. Functional heterogeneity of small ubiquitin-related protein modifiers SUMO-1 versus SUMO-2/3. J Biol Chem. 2000;275:6252–6258. doi: 10.1074/jbc.275.9.6252. [DOI] [PubMed] [Google Scholar]
- Schlieker C, Weibezahn J, Patzelt H, Tessarz P, Strub C, Zeth K, Erbse A, Schneider-Mergener J, Chin JW, Schultz PG, et al. Substrate recognition by the AAA+ chaperone ClpB. Nat Struct Mol Biol. 2004;11:607–615. doi: 10.1038/nsmb787. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Folding proteins in fatal ways. Nature. 2003;426:900–904. doi: 10.1038/nature02264. [DOI] [PubMed] [Google Scholar]
- Skinner PJ, Koshy BT, Cummings CJ, Klement IA, Helin K, Servadio A, Zoghbi HY, Orr HT. Ataxin-1 with an expanded glutamine tract alters nuclear matrix-associated structures. Nature. 1997;389:971–974. doi: 10.1038/40153. [DOI] [PubMed] [Google Scholar]
- Steffan JS, Agrawal N, Pallos J, Rockabrand E, Trotman LC, Slepko N, Illes K, Lukacsovich T, Zhu YZ, Cattaneo E, et al. SUMO modification of Huntingtin and Huntington’s disease pathology. Science. 2004;304:100–104. doi: 10.1126/science.1092194. [DOI] [PubMed] [Google Scholar]
- Sun H, Leverson JD, Hunter T. Conserved function of RNF4 family proteins in eukaryotes: targeting a ubiquitin ligase to SUMOylated proteins. EMBO J. 2007;26:4102–4112. doi: 10.1038/sj.emboj.7601839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi J, Fujigasaki H, Iwabuchi K, Bruni AC, Uchihara T, El Hachimi KH, Stevanin G, Durr A, Lebre AS, Trottier Y, et al. PML nuclear bodies and neuronal intranuclear inclusion in polyglutamine diseases. Neurobiol Dis. 2003;13:230–237. doi: 10.1016/s0969-9961(03)00080-9. [DOI] [PubMed] [Google Scholar]
- Tatham MH, Geoffroy MC, Shen L, Plechanovova A, Hattersley N, Jaffray EG, Palvimo JJ, Hay RT. RNF4 is a poly-SUMO-specific E3 ubiquitin ligase required for arsenic-induced PML degradation. Nat Cell Biol. 2008;10:538–546. doi: 10.1038/ncb1716. [DOI] [PubMed] [Google Scholar]
- Taylor JP, Hardy J, Fischbeck KH. Toxic proteins in neurodegenerative disease. Science. 2002;296:1991–1995. doi: 10.1126/science.1067122. [DOI] [PubMed] [Google Scholar]
- Tyedmers J, Mogk A, Bukau B. Cellular strategies for controlling protein aggregation. Nat Rev Mol Cell Biol. 2010;11:777–788. doi: 10.1038/nrm2993. [DOI] [PubMed] [Google Scholar]
- Verhoef LG, Lindsten K, Masucci MG, Dantuma NP. Aggregate formation inhibits proteasomal degradation of polyglutamine proteins. Hum Mol Genet. 2002;11:2689–2700. doi: 10.1093/hmg/11.22.2689. [DOI] [PubMed] [Google Scholar]
- Wang Z, Prelich G. Quality control of a transcriptional regulator by SUMO-targeted degradation. Mol Cell Biol. 2009;29:1694–1706. doi: 10.1128/MCB.01470-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZG, Delva L, Gaboli M, Rivi R, Giorgio M, Cordon-Cardo C, Grosveld F, Pandolfi PP. Role of PML in cell growth and the retinoic acid pathway. Science. 1998;279:1547–1551. doi: 10.1126/science.279.5356.1547. [DOI] [PubMed] [Google Scholar]
- Wilkinson KA, Henley JM. Mechanisms, regulation and consequences of protein SUMOylation. Biochem J. 2010;428:133–145. doi: 10.1042/BJ20100158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.