Abstract
The current study investigated the genetic and environmental etiology of individual differences in salivary testosterone during adolescence, using data from 49 pairs of monozygotic twins and 68 pairs of dizygotic twins, ages 14–19 years (M = 16.0 years). Analyses tested for sex differences in genetic and environmental influences on testosterone and its relation to pubertal development. Among adolescent males, individual differences in testosterone were substantially heritable (55%), and significantly associated with self-reported pubertal status (controlling for age) via common genetic influences. In contrast, there was no heritable variation in testosterone for females, and testosterone in females was not significantly associated with pubertal status after controlling for age. Rather, environmental influences shared by twins raised together accounted for all of the familial similarity in female testosterone (53%). This study adds to a small but growing body of research that investigates genetic influences on individual differences in behaviorally-relevant hormones.
Keywords: Behavioral Genetics, Twins, Heritability, Adolescence, Testosterone
Testosterone, a steroid hormone, is an index of the hypothalamic-pituitary-gonadal axis, a primary neuroendocrine system involved in advancing puberty and regulating human behavior. Testosterone levels increase over the course of adolescence, particularly in males, and individual differences in testosterone are associated with socially dominant and status-seeking behavior (Eisenegger et al., 2011; Mazur & Booth, 1998) and with adolescent sexual behavior (Udry et al., 1986; Udry, 1988; Halpern et al., 1993; Halpern et al., 1998). Because of its relevance for social behavior, testosterone has been implicated as an individual differences variable that contributes to the biological basis of personality (Newman & Josephs, 2009; Sellers et al., 2007). From this perspective, testosterone is a possible endophenotype for personality variation: a biologically-based, intermediary phenotype that bridges genetic variation with a complex, higher-order behavioral phenotype, such as social dominance (Gottesman & Gould, 2003). The goal of the current study is to (a) evaluate the contribution of genes and environmental influences to individual differences in adolescent testosterone, (b) test whether such contributions differ by sex, and (c) test the extent to which differences in testosterone reflect genetically-influenced differences in pubertal development.
Sex Differences and Age Differences in Testosterone
As the principal hormone released by the gonads, testosterone increases dramatically in males from childhood to adulthood (Biro, Lucky, Huster, & Morrison, 1995; Granger, Schwartz, Booth, & Arentz, 1999). Indeed, increases in testosterone are necessary for male physical development, driving the emergence of secondary sex characteristics (e.g., height, muscle mass, voice register) that are characteristic of male puberty (Hiort, 2002). Testosterone also rises in females, but this increase is small compared to males (Granger et al., 1999; Legro, Lin, Demers, & Lloyd, 2000). Moreover, the relation between testosterone and the physical changes of puberty in females is less straightforward (Shirtcliff, Dahl, & Pollack, 2009).
There are also sex differences in the associations between testosterone and behavior (Mazur et al., 1997; Archer et al., 2005; Filaire & Lac, 2000; Eisenegger et al., 2011; Josephs et al., 2011). For instance, although some correlational studies have linked testosterone to aggressive behavior in both males and females (Dabbs, et al., 1995; Dabbs & Hargrove, 1997; Book et al., 2001; Archer et al., 2005), recent testosterone administration studies suggest a possible sex-specific effect of testosterone on reactive aggression, with testosterone increasing reactive aggression in men but not in women (Eisenegger et al., 2011; Josephs et al., 2011). Studies of situationally-driven changes in testosterone (e.g., in anticipation of and in response to competition) have indicated anticipatory release of testosterone in men but not women (Mazur et al., 1997; Filaire & Lac, 2000). However, these findings have not always been replicated (Bateup et al., 2002), and some research suggests gender differences in testosterone response are only apparent in the context of complex interactions with other personality and contextual variables (e.g., Denson, et al., 2012; Kivlighan et al., 2005). The extent to which specific environmental features differentially elicit state levels of testosterone has implications for broader environmental influences on basal levels of testosterone, as well, and further suggests the possibility that the etiology of testosterone differs between males and females.
Genetic and Environmental Influences on Testosterone
Quantitative genetic designs leverage differences in the degree of genetic relatedness between different types of family relationships (e.g. monozygotic twins compared to dizygotic twins) in order to estimate the magnitude of genetic influence on variation in a trait. Several quantitative genetic studies have examined plasma testosterone in male adults (e.g. ages 20–70) and have found evidence for heritable variation, with estimates ranging from 26% to 91% (Bogaert et al., 2008; Hong et al., 2001; Meikle et al., 1987; Ring et al., 2005, summarized in Caramaschi et al., 2012). In contrast, infant studies have found no genetic variation in testosterone assessed using cord blood (Sakai et al., 1991) or saliva (Caramaschi et al., 2012); rather, environmental factors accounted for all the variation in testosterone in infancy. Adolescence may be a critical developmental period when genetically-driven individual differences in testosterone emerge and possibly diverge between sexes, resulting in sex differences in behavioral organization (Sisk & Zehr, 2005).
Three previous studies have used quantitative genetic methods to examine the heritability of testosterone levels in periods surrounding adolescence, with results consistent with the emergence of a sex difference after early adolescence. Hoekstra, Bartels, and Boomsma (2006) found no sex differences in the magnitude of genetic influence on salivary testosterone in a sample of 183 pairs of 12-year-old twins: genes accounted for 52% of the variation in testosterone for both sexes. Harris, Vernon, and Boomsma (1998) detected sex differences in the heritability of total plasma testosterone in 160 pairs of adolescent and young adult twins (ages 14–21 years): 60% in males vs. 40% in females. Finally, Koenis et al. (2013) examined salivary testosterone levels in 112 pairs of 9-year old twins, with a sub-sample of 89 pairs re-assessed at age 12-years. In boys, the broad heritability of testosterone increased from age 9 to age 12 (64% to 78%), whereas heritability decreased for girls over that same period (70% to 51%). These previous studies have not specifically examined the extent to which there are genetic influences on testosterone that are independent of genetically-influenced individual differences in pubertal development. Moreover, all previous quantitative genetic studies of testosterone levels during adolescence used predominantly White samples from the Netherlands; this topic has not been investigated in a racially and ethnically diverse American sample. Previous literature regarding race/ethnic differences in testosterone has been mixed, with some studies finding differences between Caucasian and African-American men (e.g., Winters et al., 2001) but others failing to observe racial differences (e.g., Richards et al., 2002; Rohrmann et al., 2007). More generally, researchers in adolescent health have noted the need to understand hormonal differences and similarities across race/ethnic groups as a potential mechanism for health disparities (DeSantis et al., 2007).
The Current Study
The current study used a quantitative genetic approach to (a) estimate the magnitudes of genetic and environmental influences on salivary testosterone, (b) test whether such contributions differ by sex, and (c) test the extent to which differences in testosterone reflect genetically influenced differences in pubertal development. We used quantitative genetic structural equation modeling to assess genetic and/or environmental sources of individual differences in testosterone. Because the age of our sample encompassed mid-to-late adolescence, we predicted that a sex difference in the heritability of testosterone would be detected in this diverse adolescent sample, with stronger genetic influences on testosterone in males than in females. Additionally, because of its central role in pubertal development amongst males, we hypothesized that testosterone would be related to male pubertal status, even after controlling for age, and that this association would largely reflect common underlying genes.
Method
Participants
Adolescent twin pairs were recruited from a larger, on-going registry of school-age twins and their parents (The Texas Twin Project; Harden et al., 2012). Twins were identified from public school rosters in a large metropolitan area in the southwestern U.S., as well as through advertising at conventions for parents of multiples and referrals from other twin parents. All pairs of twins who were in the 9th to 12th grade and who lived within driving distance of the laboratory were eligible to participate. We present data on 234 adolescents from 117 twin pairs (49 MZ pairs [23 male, 26 female], and 68 DZ pairs [25 male, 15 female, and 28 opposite-sex], ages 13.6 to 19.9 years (M = 16.02, SD = 1.51 years). Fifty-nine percent (59%) of participants were non-Hispanic White, 25% were Hispanic / Latino, 9% were African-American, and the remaining 7% were another race/ethnicity (including Native American, East Asian, Southeast Asian, and “Other”). The sample was economically diverse, with 32% of twin parents reporting that they had received food stamps or another form of public assistance for low-income families.
Measures
Zygosity
Opposite-sex (male-female) twin pairs were classified as dizygotic (DZ). For same-sex twin pairs, zygosity was assessed on the basis of adolescents’ responses to nine items concerning their physical similarity to their co-twin (facial appearance, hair color, hair structure, eye color) and the frequency with which others mistake one twin for the other. Previous twin research has validated questionnaire assessments of zygosity with molecular genetic classifications, and found very high agreement between methods (> 90%; Rietveld et al., 2000).
For each pair, both twins’ responses to all zygosity items were summed, yielding a total score ranging from 18 to 44 (M = 32.1, SD = 7.79). These sum scores had a clear bimodal distribution, with no overlap in the mid-range of possible scores; thus, pairs scoring 18–31 were classified as DZ, while pairs scoring 34–44 were classified as MZ. These classifications were validated using a latent class analysis (LCA), a structural equation model that analyzes multivariate data to detect latent subgroups. Uncertainty in an LCA model is quantified using entropy, with values closer to 1.0 indicating less uncertainty regarding classifications. The two-class LCA of the self-report zygosity items had very little uncertainty (entropy = .999). Zygosity classifications based on the LCA model were identical to classifications based on a simple dichotomization of the sum score, as described above.
Testosterone
Saliva samples were collected using passive-drool. Whenever possible, female adolescents were assessed during the first fourteen days of their menstrual cycle, starting from and including the first day of menstruation.1 All samples were collected at one of three appointment times: 0900h–1000h (25% of participants), 1200–1300h (48% of participants), or 1600–1700h (27% of participants). Both members of a twin pair were assessed at the same time.2 Participants were instructed to avoid eating or drinking anything for two hours before the beginning of their laboratory session; to avoid smoking for four hours before the beginning of their session; and to avoid flossing the morning of their session. After 10–15 minutes of sedentary activities (e.g., completing consent forms), participants were asked to drool through a sanitary straw into a 2-ml vial. After collection, saliva samples were frozen at −40°C until they were shipped on dry ice to Dr. Clemens Kirschbaum’s laboratory at Technical University of Dresden for analyses of hormone levels. After thawing, saliva was centrifuged at 3,000 rpm for 5 min, which resulted in a clear supernatant of low viscosity. Salivary concentrations were measured using commercially available chemiluminescence-immunoassays with high sensitivity (IBL International, Hamburg, Germany).
One female participant had an abnormally high testosterone measurement (> 700 pg/mL); data from this participant was omitted from models. All testosterone measurements were then square-root transformed to better approximate a normal distribution prior to quantitative genetic analyses.
Pubertal status
Pubertal status was assessed using the Pubertal Development Scale (PDS; Petersen, Crockett, Richards, & Boxer, 1988). Males rated growth in height, growth of body hair, growth of facial hair, skin changes, and deepening of voice on a 4-point scale ranging from 1=Not Yet Begun to Change to 4=Finished Changing. Male pubertal status scores were obtained by averaging reports on the 5 items (range = 1 to 4, M = 2.86, SD = 0.62).
Females rated growth in height, growth of body hair, growth of breasts, and skin changes on the same 4-point scale; they also reported whether they had begun to menstruate (95% of female participants reported Yes), and if so, at what age (in years). The question regarding menstruation was recoded to be consistent with the response scale of the other items (No=1, Yes=4), and reports on the 5 items were averaged (range = 1.80 to 4, M = 3.47, SD = 0.43). Age at menarche ranged from 10 to 16 years (M = 12.29 years, SD = 1.08).
Analyses
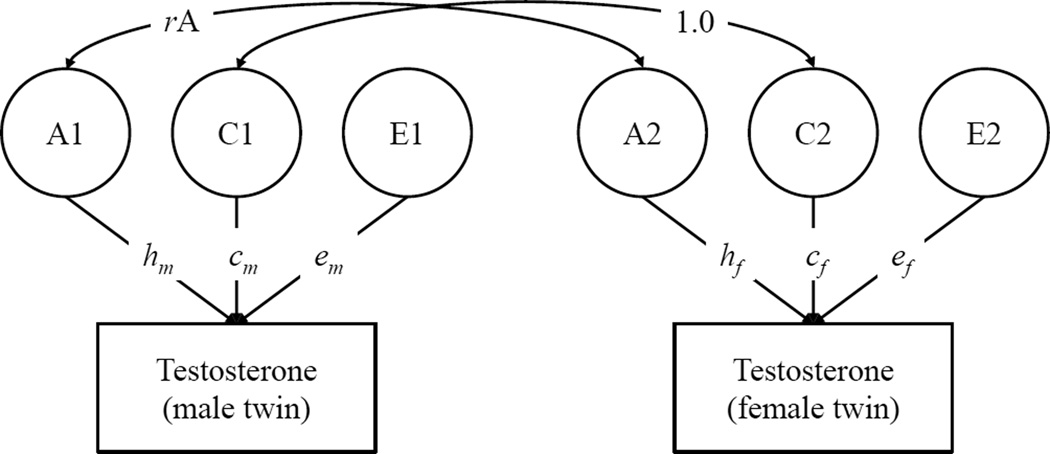
Quantitative genetic models were fit to data on testosterone from both male and female adolescents, controlling for age and age-squared, but not controlling for individual differences in pubertal status.3 A quantitative genetic model for twins raised together is shown in Figure 1. This model partitions variation in testosterone into three latent (i.e., unobserved) components: additive genetic influences (A), environmental influences that are shared by twins and make them more similar (the shared environment, or C), and environmental influences that are unique to each twin, plus measurement error (the non-shared environment, or E). Each of the ACE components is standardized, and the paths from the ACE components are estimated. Consistent with genetic theory, the correlation between the A components in the first and second member of each twin pair is fixed to 1.0 for MZ pairs and 0.5 for same-sex DZ pairs. (The A correlation for opposite-sex DZ pairs depends on the model tested; this is described in more detail below.) By definition, the shared environment (C) is common to both twins, and the correlation between C components in the first and second member of each twin pair is fixed to 1.0 for both MZ and DZ pairs. Finally, the correlation between E components is fixed to 0 for both MZ and DZ pairs. The square of the standardized path from the A component is the heritability coefficient, i.e., the proportion of variance in testosterone that is due to genetic differences between people.
Figure 1.
Quantitative Genetic Model of Testosterone in Adolescent Male and Female Twins
Note. A = Additive Genetic; C = Shared Environmental; E = Non-Shared Environmental. A, C, and E factors are standardized (M = 0, SD = 1). m = male; f = female. Model for opposite-sex DZ pairs is shown; rA was freely estimated in the qualitative sex-differences model, and fixed to equal 0.5 for all other models. For MZ pairs and same-sex DZ pairs (not shown), rA was fixed to equal 1.0 and 0.5, respectively. The main effect of sex and the linear and quadratic effects of age in years were controlled in all models.
Figure 1 illustrates a qualitative sex differences model. In this model, the magnitudes of the paths from the ACE components to testosterone are allowed to differ between males and females. In addition, for opposite-sex DZ pairs, the correlation between the A component for the male twin and the A component for the female twin (rA) is freely estimated (rather than fixed to 0.5). This model tests whether (a) genetic and/or environmental factors account for more of the variance in testosterone in one sex versus the other, and (b) whether the same set of genetic factors influence testosterone in both males and females. The full qualitative sex differences model was compared to progressively simpler models. First, we fit a quantitative sex-differences model, in which the rA for opposite-sex DZ twin pairs is fixed to 0.5 rather than freely estimated. This model specifies that the same set of genes influence testosterone in both males and females, but the magnitude of genetic (and environmental influences) is allowed to differ across sexes. The most constrained model (no sex-differences) fixed all parameters to be equal across males and females. Once the best-fitting sex-differences model was selected, this model can be further trimmed by fixing non-significant parameters to zero and testing whether this results in significant decrements in fit. The final trimmed model, then, is considered the most parsimonious representation of the data.
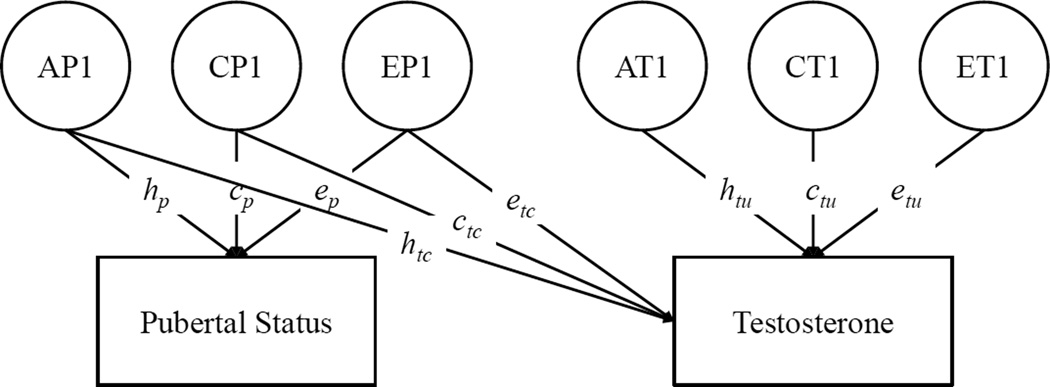
After evaluating sex-differences in the genetic and environmental etiology of testosterone, we next fit an extension of the quantitative genetic model that estimated the extent to which heritable variation in testosterone was associated with individual differences in pubertal status relative to age. This model is illustrated in Figure 2: Variation in pubertal status and testosterone (controlling for age and age-squared) is divided into ACE components, and testosterone is regressed on the ACE components of pubertal status. This bivariate Cholesky decomposition tests the extent to which genetic and environmental influences on testosterone are shared with versus independent from pubertal status relative to age.
Figure 2.
Quantitative Genetic Model of Testosterone and Pubertal Status Relative to Age
Note. Only one twin per pair is shown. A = Additive Genetic; C = Shared Environmental; E = Non-Shared Environmental. A, C, and E factors are standardized (M = 0, SD = 1). p = pubertal status; tc = common between testosterone and pubertal status; tu = unique to testosterone. The linear and quadratic effects of age in years on both pubertal status and testosterone were controlled.
Models were estimated in the software program Mplus (Muthén & Muthén, 1998–2010). Model fit was evaluated using the root mean square error of approximation (RMSEA; Steiger, 1990), Akaike information criterion (AIC; Akaike, 1974) and χ2 values. Based on the guidelines of Hu and Bentler (1999), the RMSEA cut-off value for acceptable model fit was RMSEA ≤ 0.06. AIC is used to compare the relative fit of different models, with lower values indicating a more parsimonious representation of the data.
Results
Descriptive Statistics
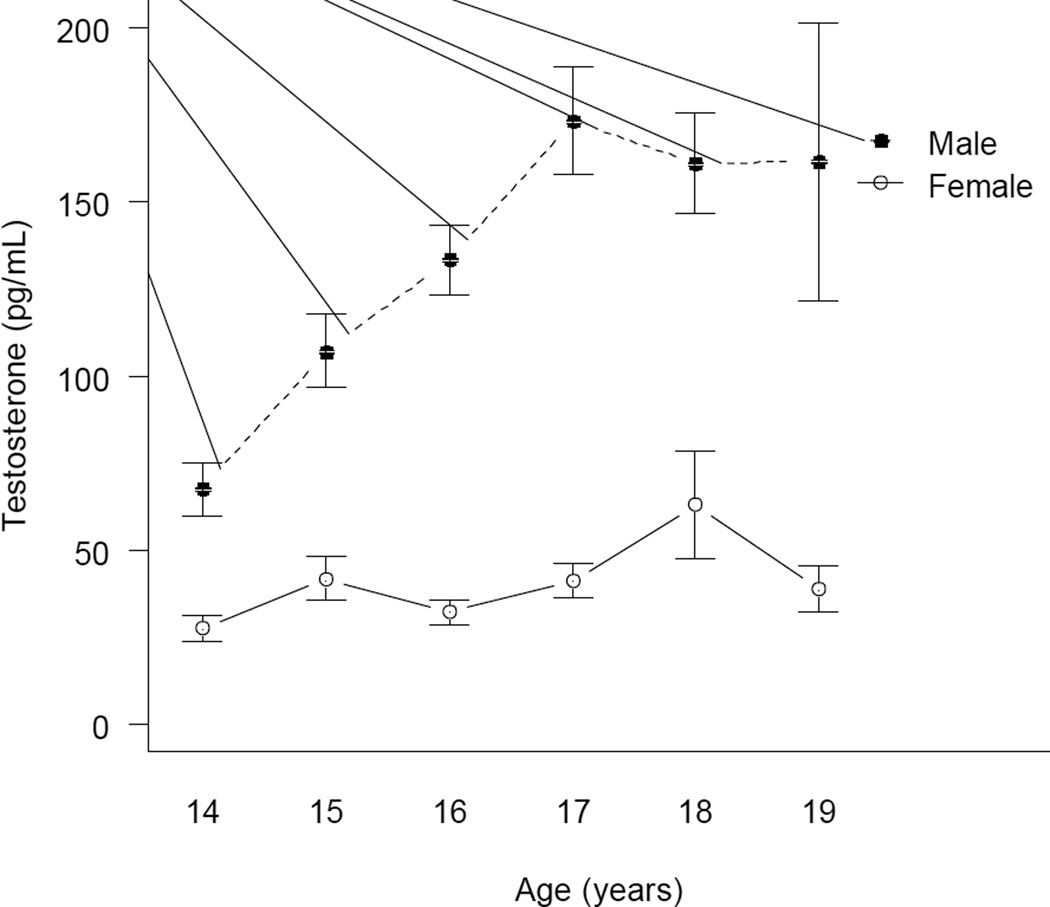
Males had significantly higher levels of testosterone (M = 121.67 pg/mL, SD = 66.39) than females (M = 39.71 pg/mL, SD = 28. 44; t (206) =13.34, P < .001). Sex differences and age trends in testosterone are illustrated in Figure 3. For males, there were significant linear (b = 2.51, SE = 0.55, P < .001) and quadratic (b = −0.31, SE = 0.12, P = .01) effects of age on testosterone, accounting for 30.6% of the variance. (Regression coefficients are unstandardized effects of age in years on square-root transformed testosterone levels.) In contrast, there were no significant linear (b = 0.63, SE = .41, P = .12) or quadratic (b = −.08, SE = .08, P = .31) effects of age for females. There were no significant differences in testosterone by race/ethnicity in either males [F = 1.31 (3, 110), P = 0.28] or females [F = 2.05 (3, 99), P = 0.11].
Figure 3.
Age Trends in Testosterone for Adolescent Males and Females
The correlation between pubertal status and age was 0.55 in males and 0.56 for females (ps < .001), and the correlation between pubertal status and testosterone was 0.51 (p<.001) in males and 0.17 (p = .07) in females. For females, the partial correlation between pubertal status and testosterone, controlling for age, was not significantly different from zero (r = .09, p = .36). Neither was testosterone significantly associated with age at menarche (r = −.04, p = .65). In contrast, the partial correlation between testosterone and pubertal status in males, controlling for age, was moderate and significant (r = 0.31, p < .001), indicating that males with higher testosterone reported greater pubertal status relative to age.
For males, the MZ correlation for testosterone (.78; 95% confidence interval = .54–.90) was nearly double the DZ correlation (.41; 95% CI = .01 – .69). For females, in contrast, the MZ correlation (.54; 95% CI = .19–.77) and DZ correlation (.71; 95% CI = .31–.90) were similar. The opposite-sex DZ correlation was negative (−.48, 95% CI = −.13, −.72). As indicated by the wide confidence intervals, there was considerable uncertainty surrounding each point estimate.
Quantitative Genetic Models of Testosterone
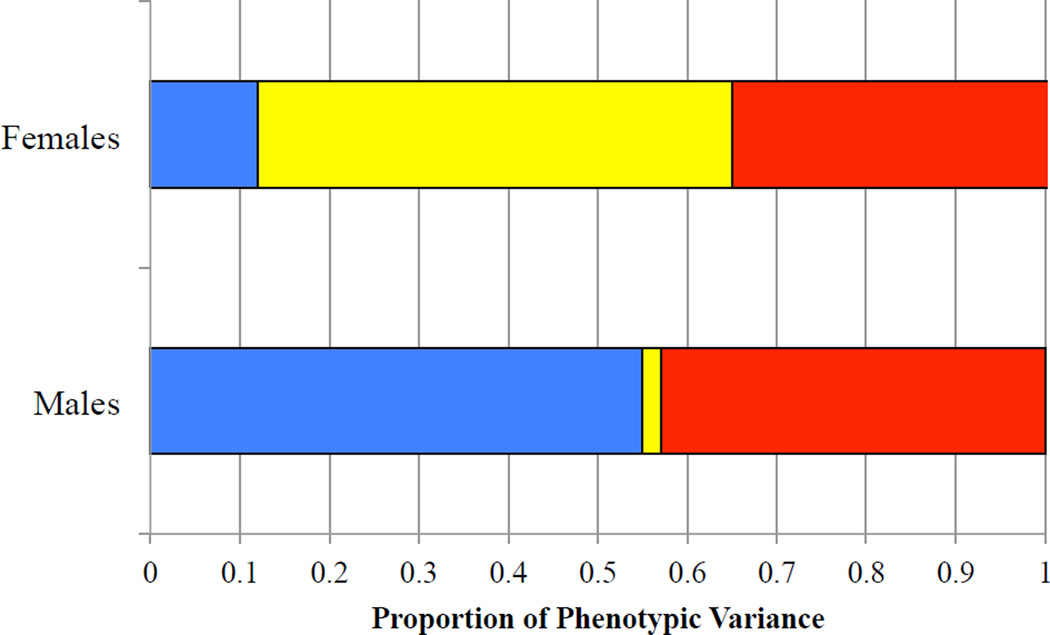
All quantitative genetic models controlled for the sex-specific linear and quadratic effects of age. Comparisons of the sex-differences models are summarized in Table 1. The non-scalar sex-differences model (Model 2), which fixed the correlation between the male A component and the female A component (rA) in opposite-sex DZ twins to 0.5, did not fit significantly worse than a qualitative sex-differences model, which freely estimated rA. In contrast, a no sex-differences model (Model 3) fit significantly worse than the quantitative sex-differences model. The quantitative sex-differences model was therefore selected as the more parsimonious representation of the data. It should be noted, however, that qualitative sex differences may not have been detectable in the current study because of the relatively small number of opposite-sex DZ twin pairs. The parameters of the quantitative sex-differences model are summarized in Table 2 and illustrated in Figure 4. For males, genetic differences accounted for 55% of the variation in testosterone, whereas shared environmental influences were not significantly different than zero. In contrast genetic influences were not significantly different than zero for females; however, the study may have been underpowered to detect small genetic effects. Familial resemblance for testosterone in females was environmental in origin, accounting for 53% of the variance. As a sensitivity analysis, we repeated this model comparison using only same-sex twins (i.e., omitting opposite-sex DZs). Results with same-sex twins also indicated significant sex differences in genetic and environmental influences on testosterone (Δχ2 = 9.14, df = 3, p = .027).
Table 1.
Model Fit Comparisons
| Model Fit Indices | Model Fit Comparison |
|||
|---|---|---|---|---|
| RMSEA | AIC | χ2 | ||
| Model 1: Qualitative Sex Differences in A | .000 | 1001.78 | 54.01 df = 65, P = .83 |
|
| Model 2: Quantitative Sex Differences (Full) | .000 | 1000.01 | 54.24 df = 66, P = .85 |
Model 1 vs. 2: Δχ2 = .23, P = .63 |
| Model 3: No Sex Differences | .000 | 1003.32 | 63.55 df = 69, P = .66 |
Model 2 vs. 3: Δχ2 = 9.31, P = .03 |
Table 2.
Standardized Parameter Estimates from Quantitative Genetic Models of Testosterone
| Additive Genes (h2) |
Shared Environment (c2) |
Non-Shared Environment (e2) |
|
|---|---|---|---|
| Model 1: Qualitative Sex Differences in A | |||
| Male | .34 (.45) | .22 (.41) | .44 (.12)* |
| Female | .14 (.36) | .51 (.30) | .35 (.13)* |
| Model 2: Quantitative Sex Differences (Full) | |||
| Male | .55 (.16)* | .02 (.11) | .43 (.11)* |
| Female | .12 (.40) | .53 (.32) | .36 (.14)* |
| Model 3: No Sex Differences | |||
| Male & Female | .45 (.27) | .14 (.23) | .41 (.09)* |
Note. Standard errors are shown in parentheses. All models controlled for the linear and quadratic effects of age. Model is illustrated in Figure 1.
Significantly different than zero at p < .05.
Figure 4.
Proportion of Variance in Testosterone due to Genetic and Environmental Factors in Adolescent Males and Females
Note. Based on parameter estimates from Model 2, Table 2.
Genetic Influences on Testosterone and Pubertal Status Relative to Age
As reported above, genetic influences on testosterone were only apparent in males, and pubertal status relative to age was significantly associated with testosterone only in males. Quantitative genetic models of the relation between testosterone and pubertal status were therefore conducted in male twin pairs only. Shared environmental influences on pubertal status and testosterone were not significantly different from zero and were dropped from the model. Overall model fit was good (χ2 = 50.23, df = 48, p = .39, RMSEA = .044). Standardized parameter estimates are summarized in Table 3. Approximately 42% of the variation in pubertal status relative to age was attributable to genetic influences, with the remaining 58% due to non-shared environmental influences. Thirty-one percent (31%) of the total variation in testosterone (and 53% of the genetic variance in testosterone) was attributable to genetic influences on pubertal status relative to age. Put differently, 47% of the genetic variance in testosterone was independent of self-reported pubertal development. The regression of testosterone on the E component of pubertal status was not significant, indicating that all of the non-shared environmental influences on testosterone were unique of pubertal development.
Table 3.
Standardized Parameter Estimates from Quantitative Genetic Model of Testosterone and Pubertal Status in Males
| Additive Genetic | Non-Shared Environmental |
|
|---|---|---|
| Pubertal Status | hp = .65 (.11)* | ep = .76 (.10)* |
| Pubertal Status → Testosterone | htc = .56 (.16)* | etc = .02 (.12) |
| Testosterone | htu = .53 (.17) | etu = .64 (.09)* |
Note. Standard errors are in parentheses. Model controlled for the linear and quadratic effects of age. Model is illustrated in Figure 2.
Significantly different from zero at p < .05.
Discussion
In the current paper, we present results from a quantitative genetic study of testosterone levels in adolescent male and female twins. During puberty, activity in the hypothalamic-pituitary-gonadal (HPG) axis stimulates the increased production and release of testosterone by the testes in males. At the same time, the initiation of puberty has been shown (in animal research) to increase the expression of genes necessary for androgen synthesis to adult levels, with additional genes involved in testosterone metabolism showing peak expression during puberty (O’Shaughnessy et al., 2002). This suggests that adolescence is a particularly important developmental period for studying genetic influences on testosterone levels. Compared to the volume of behavioral genetic research on most behavioral phenotypes, the literature regarding genetic influences on testosterone and other individual differences in hormones is actually quite sparse. This is particularly true with regards to the adolescent period. Although adolescence is the time when gonadal hormones reach adult levels and when sex differences in testosterone levels widen markedly, there have only been three previous behavioral genetic studies on gonadal hormones during adolescence.
We found that testosterone levels in male adolescents were substantially heritable, with 55% of the variation attributable to additive genetic influences. This heritability estimate for males is strikingly similar to estimates from the two previous studies of testosterone in adolescent twins (57% by Hoekstra et al., 2006 and 60% by Harris et al., 1998), even though (a) the current study used an ethnically diverse sample of American adolescents, as opposed to predominantly White samples from the Netherlands, and (b) Harris et al. (1998) examined total plasma testosterone from blood samples, whereas the current study and Hoekstra et al. (2006) examined salivary testosterone. A more recent study (Koenis et al., 2013), also using White children from the Netherlands, also produced similar heritability estimates in younger males (64% in 9-year olds and 70% in 12-year olds). In contrast, familial similarity for testosterone in our sample of female adolescents was predominantly environmental in origin, with 53% of the variation in female testosterone attributable to shared environmental influences and negligible heritable variation. Our results differ from previous twin studies, which found significant genetic influences of females, too (Harris et al., 1998; Hoekstra et al., 2006; Koenis et al., 2013).
Consistent with previous research, testosterone was associated with self-reports of pubertal development (both pubertal status and pubertal status relative to age) in males but not in females. Shirtcliff et al. (2009), for instance, found that 40% of the variation in basal testosterone (aggregated from 32 salivary samples over 5 days) among early adolescent boys (ages 9–14 years) could be accounted for self-reports of pubertal development on the PDS, as opposed to 17% among girls. By comparison, our study used only a single measure of testosterone and a later age range, and we similarly found that 31% of the variance, and 53% of the genetic variance, in testosterone among males was accounted for by self-reported pubertal status. Although self-reports of pubertal development have been criticized as unreliable (Dorn, Dahl, Woodward, & Biro, 2006; Dorn & Biro, 2011), our results suggest that self-reports – at least among adolescent boys – are tapping genetic differences in testosterone. Notably, while nearly half of the variation in testosterone among males was environmental in origin and unique to each twin, this non-shared environmental variance was uncorrelated with pubertal status. That is, comparing within a pair of identical male twins, the twin who reported higher pubertal development than his co-twin did not have higher testosterone than his co-twin.
The age range of the current sample (ages 14–19) must be noted when considering the overlap between self-reported pubertal development and testosterone in the current sample. On the one hand, the sample is older than typical for a study of puberty. All of our participants have initiated puberty and have commenced through many – if not all – of its early stages. This was particularly true for girls; in our sample, less than 5% of girls were pre-menarcheal. Thus relations between self-reported pubertal status and testosterone may have attenuated, particularly among girls, because of restriction of range in pubertal status among older adolescents. On the other hand, there is variation in pubertal status even in a mid-adolescent sample, particularly among boys. This variation gives rise to obvious questions regarding how much of the observed genetic and environmental variation in testosterone is due to differences in the timing of pubertal development – questions that motivated our bivariate quantitative genetic analyses. Moreover, although the hormonal events of puberty are initiated much earlier, testosterone continues to show developmental change throughout middle and late adolescence, especially in boys. The current results therefore contribute to our understanding of developmental change in behaviorally relevant hormones, a process that continues well beyond what is typically thought of as the pubertal period.
Although contrasting with previous twin research, our finding of sex differences in the contribution of genetic variance to testosterone is consistent with emerging results from genome-wide association studies (GWAS). A GWAS of serum testosterone levels identified two polymorphisms at the SHBG (sex hormone-binding globulin) locus and one polymorphism on the X chromosome that were significantly associated with variance in testosterone in adult men (Ohlsson et al., 2011); these associations were replicated in an independent study by Jin et al. (2012). (SHBG binds to sex hormones, including testosterone and estradiol, and regulates their bioavailability.) In contrast, genetic variants at the SHBG locus were not associated with testosterone in a sample of postmenopausal women (Prescott et al., 2012). Moreover, a GWAS meta-analysis of SHBG in over 20,000 men and women from 10 epidemiological studies found evidence for sex-differentiated effects, with three genetic loci showing stronger effects in men than in women (Coviello et al., 2012).
In evaluating the current evidence for sex differences, however, it is important to note that saliva is a peripheral measure of testosterone, which can be considered a reflection of systemic bioactivity to the extent that it is correlated with serum concentrations (Granger, Shirtcliff, Booth, Kivlighan, & Schwartz, 2004). A study of young adults found that salivary testosterone levels were more strongly associated with serum levels for males than for females (Shirtcliff, Granger, & Likos, 2002). Thus sex differences in the validity of peripheral measures of testosterone may have contributed to the sex differences in estimates of genetic influence. Clarifying this issue will require multimethod data (both serum and saliva) on testosterone in twins.
The finding of substantial family-level (i.e., shared) environmental influence on testosterone levels in female adolescents is surprising, given that estimates of shared environmental influences on most phenotypes considered in quantitative genetic studies are typically small. Although our sample was racially and ethnically diverse, there were no significant race/ethnic differences in mean levels of testosterone for females, indicating that race/ethnic differences do not account for the family-level environmental variance. The origin of these family-level environmental influences remains unknown. In addition to more proximal influences such as family context, one intriguing possibility is the intrauterine environment, which would be shared by twins and would contribute to greater phenotypic similarity regardless of zygosity. A previous experimental study of rats found sex-specific effects of the prenatal environment on adolescent testosterone levels. Specifically, prenatal nicotine exposure caused elevated testosterone levels in adolescent female offspring, but had no effects on male offspring (Smith et al., 2003). Similarly, in humans, Kandel and Udry (1999) found that maternal smoking was associated with higher maternal testosterone, which in turn accounted for higher levels of testosterone in adult female daughters. This remains an important and exciting area for future study. In addition to their main effects, intrauterine environments, including nicotine exposure, low birth weight, and prenatal androgens, may interact with genetic predispositions and with more proximal environments to shape adolescent testosterone levels.
For both male and female adolescents, nearly half of the variation in testosterone was attributable to environmental influences that are specific to the individual (i.e., the non-shared environment). Because this study assessed testosterone on only one occasion, we cannot evaluate the extent to which the non-shared environmental variation identified in this study represents temporally-stable individual differences versus transient fluctuations in testosterone. Hoekstra et al. (2006), in their study of 12-year old twins, measured testosterone on two consecutive days and found that non-shared environmental influences were unique to measurement occasion. This result can be conceptualized in terms of its implications for within-MZ twin pair differences: The MZ twin who had higher testosterone than his co-twin on day one was not systematically more likely to have higher testosterone than his co-twin on day two. (Genetic influences on testosterone, in contrast, were shared across measurement occasions).This previous result, in conjunction with the finding from the current paper that non-shared environmental influences on testosterone are independent of within-twin pair differences in pubertal development, suggests that environmental influences on male testosterone are more associated with state-related fluctuations. As the sample used in Hoekstra et al. (2006) was younger than the sample used here, it is not yet clear whether environmentally-influenced differences in testosterone become more stable among older adolescents. Additional research incorporating longitudinal follow-up at varying intervals (hours to days versus months to years) is necessary to parse the extent to which twin-specific environmental factors influence state-related fluctuations in testosterone versus chronic testosterone levels.
There are a number of limitations that should be noted. First, as discussed above, testosterone levels were assessed at only one occasion. Second, our sample size is relatively small for a quantitative genetic analysis. In particular, we may be underpowered to detect (1) small genetic influences on testosterone among female adolescents and (2) qualitative sex differences in genetic influence. It should be noted that (1) our sample size is commensurate with previous studies of genetic influence on testosterone, and (2) we had sufficient power to detect significant sex differences in the genetic and shared environmental etiology. Nevertheless, quantitative genetic studies that integrate endocrine measures and that have sufficiently large numbers of twin pairs to allow for the estimation of more complex models (e.g., multivariate analyses of the relation between testosterone and behavioral measures, analyses of genotype × environment interaction) would be an interesting and valuable direction for future research. In particular, we hypothesize that sex differences in the genetic etiology of testosterone emerge over the course of pubertal development, but we did not have sufficient power to test for gene × puberty (or gene × age interactions) in the current data. Similarly, a twin sample covering a broader age range (particularly extending down to middle childhood) will be particularly helpful in elucidating the extent to which genetic and environmental influences on testosterone change across pubertal development. Finally, these findings speak to etiologic influences on testosterone assessed at a single time point. Given the high relevance of changes in state testosterone in response to environmental stressors for predicting social behavior, future research should also employ estimates of testosterone reactivity and recovery in order to better understand how genetic and environmental influences on these aspects of testosterone function may differ from the results reported here.
Testosterone is implicated in a wide variety of social behaviors, including social dominance, aggression, sexual behavior, and risky decision-making. Given these phenotypic associations, and the existence of heritable variation in testosterone levels in males, testosterone is therefore a candidate endophenotype for these more complex behavioral phenotypes (Gottesman & Gould, 2003). Further genetically-informed research would be necessary to test this hypothesis. Specifically, a multivariate, quantitative genetic analysis can test the extent to which genetic influences on more complex behavioral phenotypes are mediated through genetic influences on testosterone. More generally, endocrine functioning is a promising level of analysis for researchers interested in bridging genetic variation with individual differences in behavior.
Footnotes
Given a 28-day cycle, the first 14 days corresponds, on average, to the follicular phase, or the hormonal nadir; however, menstrual cycles are often irregular among adolescent females, and there is substantial inter-individual variability (Treloar, Boynton, Behn, & Brown, 1967). Without additional hormonal assessments, it was not possible for us to determine the menstrual cycle stage of any individual participant. Twelve female participants (11%) reported that they had not yet begun menstruating (n=5) or that they had not menstruated in the past two months (n=7). Among menstruating females, number of days since last period was not significantly related to testosterone levels [p = .45, R2 = .00]. Ten females (9%) reported taking hormonal contraception. Testosterone levels were significantly higher in females on hormonal contraception (64.1 pg/mL), compared to females not on hormonal contraception (37.6 pg/mL) [p = .01, R2 = .05]. As a post-hoc sensitivity analysis, we estimated models omitting data from females on hormonal contraception, and we found a nearly identical pattern of results.
Consistent with previous research on diurnal variation in testosterone in adolescents (Granger et al., 2003; Matchock et al., 2007), average testosterone was higher for morning samples (0900–1000h; M = 136.10 pg/mL for males, 46.40 pg/mL for females) than for samples from midday (1200–1300h; 116.63 pg/mL for males, 37.11 pg/mL for females) or afternoon (1600–1700h; 119.45 pg/mL for males, 37.77 pg/mL for females). These differences, however, were not statistically significant for males (p = .45) or females (p = .46), and time of day accounted for a trivial amount of the variance in testosterone (R2 = .01 in both sexes). Results from analyses controlling for time of day were nearly identical to the main results presented in this paper.
Controlling for both pubertal status and age would partial variation in testosterone that is associated with being at a higher pubertal status relative to same-aged adolescents. As genetically-influenced differences in testosterone may impact the timing or tempo of puberty, we did not want to partial puberty-related variation in testosterone from our initial heritability estimates. Thus, rather than controlling for pubertal status, we report results of a bivariate examination of the extent to which heritable variation in testosterone is associated with individual differences in pubertal status relative to age.
Contributor Information
K. Paige Harden, Department of Psychology, University of Texas at Austin.
Natalie Kretsch, Department of Psychology, University of Texas at Austin.
Jennifer L. Tackett, Department of Psychology, University of Houston
Elliot M. Tucker-Drob, Department of Psychology, University of Texas at Austin
References
- Akaike H. A new look at the statistical model identification. Automatic Control, IEEE Transactions on. 1974;19(6):716–723. [Google Scholar]
- Archer J, Graham-Kevan N, Davies M. Testosterone and aggression: A reanalysis of Book, Starzyk, and Quinsey's (2001) study. Aggression and violent behavior. 2005;10(2):241–261. doi: http://dx.doi.org/10.1016/j.avb.2004.01.001. [Google Scholar]
- Bateup HS, Booth A, Shirtcliff EA, Granger DA. Testosterone, cortisol, and women's competition. Evolution and Human Behavior. 2002;23(3):181–192. [Google Scholar]
- Biro FM, Lucky AW, Huster GA, Morrison JA. Pubertal staging in boys. Journal of Pediatrics. 1995;127(1):100–102. doi: 10.1016/s0022-3476(95)70265-2. [DOI] [PubMed] [Google Scholar]
- Bogaert V, Taes Y, Konings P, Van Steen K, De Bacquer D, Goemaere S, Kaufman JM. Heritability of blood concentrations of sex-steroids in relation to body composition in young adult male siblings. Clinical Endocrinology (Oxf) 2008;69(1):129–135. doi: 10.1111/j.1365-2265.2008.03173.x. [DOI] [PubMed] [Google Scholar]
- Book AS, Starzyk KB, Quinsey VL. The relationship between testosterone and aggression: a meta-analysis. Aggression and Violent Behavior. 2001;6(6):579–599. doi: http://dx.doi.org/10.1016/S1359-1789(00)00032-X. [Google Scholar]
- Caramaschi D, Booij L, Petitclerc A, Boivin M, Tremblay RE. Genetic and environmental contributions to saliva testosterone levels in male and female infant twins. Psychoneuroendocrinology. 2012;37(12):1954–1959. doi: 10.1016/j.psyneuen.2012.04.008. doi: http://dx.doi.org/10.1016/j.psyneuen.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Coviello AD, Haring R, Wellons M, Vaidya D, Lehtimaki T, Keildson S, Perry JR. A genome-wide association meta-analysis of circulating sex hormone-binding Genetic Influences on Testosterone globulin reveals multiple Loci implicated in sex steroid hormone regulation. PLoS Genet. 2012;8(7):e1002805. doi: 10.1371/journal.pgen.1002805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabbs JM, Hargrove MF. Age, testosterone, and behavior among female prison inmates. Psychosomatic Medicine. 1997;59(5):477–480. doi: 10.1097/00006842-199709000-00003. [DOI] [PubMed] [Google Scholar]
- Dabbs JM, Carr TS, Frady RL, Riad JK. Testosterone, crime, and misbehavior among 692 male prison inmates. Personal Individual Differences. 1995;18(5):627–633. doi: http://dx.doi.org/10.1016/0191-8869(94)00177-T. [Google Scholar]
- DeSantis AS, Adam EK, Doane LD, Mineka S, Zinbarg RE, Craske MG. Racial/ethnic differences in cortisol diurnal rhythms in a community sample of adolescents. Journal of Adolescent Health. 2007;41:3–13. doi: 10.1016/j.jadohealth.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Denson TF, Mehta PH, Ho Tan D. Endogenous testosterone and cortisol jointly influence reactive aggression in women. Psychoneuroendocrinology. 2012;38(3):416–424. doi: 10.1016/j.psyneuen.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Dorn LD, Biro FM. Puberty and Its Measurement: A Decade in Review. Journal of Research on Adolescence. 2011;21(1):180–195. [Google Scholar]
- Dorn LD, Dahl RE, Woodward HR, Biro F. Defining the boundaries of early adolescence: A user's guide to assessing pubertal status and pubertal timing in research with adolescents. Applied Developmental Science. 2006;10(1):30–56. [Google Scholar]
- Eisenegger C, Haushofer J, Fehr E. The role of testosterone in social interaction. Trends in cognitive sciences. 2011;15(6):263–271. doi: 10.1016/j.tics.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Filaire E, Lac G. Dehydroepiandrosterone (DHEA) rather than testosterone shows saliva androgen responses to exercise in elite female handball players. International Journal of Sports Medicine. 2000;21(1):17–20. doi: 10.1055/s-2000-8851. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. The American Journal of Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Granger DA, Schwartz EB, Booth A, Arentz M. Salivary testosterone determination in studies of child health and development. Hormones and Behavior. 1999;35(1):18–27. doi: 10.1006/hbeh.1998.1492. [DOI] [PubMed] [Google Scholar]
- Granger DA, Shirtcliff EA, Booth A, Kivlighan KT, Schwartz EB. The “trouble” with salivary testosterone. Psychoneuroendocrinology. 2004;29:1229–1240. doi: 10.1016/j.psyneuen.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Granger DA, Shirtcliff EA, Zahn-Waxler C, Usher B, Klimes-Dougan B, Hastings P. Salivary testosterone diurnal variation and psychopathology in adolescent males and females: individual differences and developmental effects. Development and Psychopathology. 2003;15(2):431–449. [PubMed] [Google Scholar]
- Halpern C, Udry R, Suchindran C. Monthly Measures of Salivary Testosterone Predict Sexual Activity in Adolescent Males. Archives of Sexual Behavior. 1998;27(5):445–465. doi: 10.1023/a:1018700529128. [DOI] [PubMed] [Google Scholar]
- Halpern CT, Udry JR, Campbell B, Suchindran C. Testosterone and pubertal development as predictors of sexual activity: a panel analysis of adolescent males. Psychosomatic Medicine. 1993;55(5):436–447. doi: 10.1097/00006842-199309000-00007. [DOI] [PubMed] [Google Scholar]
- Harden KP, Tucker-Drob EM, Tackett JL. The Texas Twin Project. Twin Research and Human Genetics. 2013;16(Special Issue 01):385–390. doi: 10.1017/thg.2012.97. doi: doi:10.1017/thg.2012.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, Vernon PA, Boomsma DI. The Heritability of Testosterone: A Study of Dutch Adolescent Twins and Their Parents. Behavior Genetics. 1998;28(3):165–171. doi: 10.1023/a:1021466929053. [DOI] [PubMed] [Google Scholar]
- Hiort O. Androgens and Puberty. Best Practice & Research. Clinical Endocrinology & Metabolism. 2002;16(1):31–41. doi: 10.1053/beem.2002.0178. [DOI] [PubMed] [Google Scholar]
- Hoekstra RA, Bartels M, Boomsma DI. Heritability of testosterone levels in 12-year-old twins and its relation to pubertal development. Twin Research and Human Genetics. 2006;9(4):558–565. doi: 10.1375/183242706778025071. [DOI] [PubMed] [Google Scholar]
- Hong Y, Gagnon J, Rice T, Perusse L, Leon AS, Skinner JS, Rao DC. Familial resemblance for free androgens and androgen glucuronides in sedentary black and white individuals: the HERITAGE Family Study. Health, Risk Factors, Exercise Training and Genetics. Journal of Endocrinology. 2001;170(2):485–492. doi: 10.1677/joe.0.1700485. [DOI] [PubMed] [Google Scholar]
- Hu Lt, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal. 1999;6(1):1–55. [Google Scholar]
- Jin G, Sun J, Kim ST, Feng J, Wang Z, Tao S, Xu J. Genome-wide association study identifies a new locus JMJD1C at 10q21 that may influence serum androgen levels in men. Human Molecular Genetics. 2012;21(23):5222–5228. doi: 10.1093/hmg/dds361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs RA, Mehta PH, Carre JM. Gender and social environment modulate the effects of testosterone on social behavior: comment on Eisenegger et al. Trends in Cognitive Science. 2011;15(11):509–510. doi: 10.1016/j.tics.2011.09.002. author reply 510–511. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Udry JR. Prenatal effects of maternal smoking on daughters' smoking: nicotine or testosterone exposure? Am J Public Health. 1999;89(9):1377–1383. doi: 10.2105/ajph.89.9.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivlighan KT, Granger DA, Booth A. Gender differences in testosterone and cortisol response to competition. Psychoneuroendocrinology. 2005;30(1):58–71. doi: 10.1016/j.psyneuen.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Koenis MMG, Brouwer RM, van Baal GCM, van Soelen ILC, Peper JS, van Leeuwen M, Hulshoff Pol HE. Longitudinal Study of Hormonal and Physical Development in Young Twins. Journal of Clinical Endocrinology and Metabolism. 2013;98(3):E518–E527. doi: 10.1210/jc.2012-3361. [DOI] [PubMed] [Google Scholar]
- Legro RS, Lin HM, Demers LM, Lloyd T. Rapid maturation of the reproductive axis during perimenarche independent of body composition. Journal of Clinical Endocrinology and Metabolism. 2000;85(3):1021–1025. doi: 10.1210/jcem.85.3.6423. [DOI] [PubMed] [Google Scholar]
- Matchock RL, Dorn LD, Susman EJ. Diurnal and seasonal cortisol, testosterone, and DHEA rhythms in boys and girls during puberty. Chronobiology International. 2007;24(5):969–990. doi: 10.1080/07420520701649471. [DOI] [PubMed] [Google Scholar]
- Mazur A, Booth A. Testosterone and dominance in men. Behavioral and Brain Sciences. 1998;21(3):353–363. [PubMed] [Google Scholar]
- Mazur A, Susman EJ, Edelbrock S. Sex difference in testosterone response to a video game contest. Evolution and Human Behavior. 1997;18(5):317–326. [Google Scholar]
- Meikle AW, Bishop DT, Stringham JD, West DW. Quantitating genetic and nongenetic factors that determine plasma sex steroid variation in normal male twins. Metabolism. 1986;35(12):1090–1095. doi: 10.1016/0026-0495(86)90020-x. [DOI] [PubMed] [Google Scholar]
- Newman ML, Josephs RA. Testosterone as a personality variable. Journal of Research in Personality. 2009;43(2):258–259. doi: http://dx.doi.org/10.1016/j.jrp.2008.12.028. [Google Scholar]
- Ohlsson C, Wallaschofski H, Lunetta KL, Stolk L, Perry JR, Koster A, Haring R. Genetic determinants of serum testosterone concentrations in men. PLoS Genet. 2011;7(10):e1002313. doi: 10.1371/journal.pgen.1002313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shaughnessy PJ, Willerton L, Baker PJ. Changes in Leydig cell gene expression during development in the mouse. Biology of Reproduction. 2002;66(4):966–975. doi: 10.1095/biolreprod66.4.966. [DOI] [PubMed] [Google Scholar]
- Prescott J, Thompson DJ, Kraft P, Chanock SJ, Audley T, Brown J, De Vivo I. Genome-wide association study of circulating estradiol, testosterone, and sex hormone-binding globulin in postmenopausal women. PLoS One. 2012;7(6):e37815. doi: 10.1371/journal.pone.0037815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards RJ, Svec F, Bao W, Srinivasan SR, Berenson GS. Steroid hormones during puberty: racial (black-white) differences in androstenodeione and estradiol – the Bogalusa Heart Study. Journal of Clinical Endocrinology & Metabolism. 1992;75:624–631. doi: 10.1210/jcem.75.2.1639961. [DOI] [PubMed] [Google Scholar]
- Rietveld MJ, van Der Valk JC, Bongers IL, Stroet TM, Slagboom PE, Boomsma DI. Zygosity diagnosis in young twins by parental report. Twin Research. 2000;3(3):134–141. doi: 10.1375/136905200320565409. [DOI] [PubMed] [Google Scholar]
- Ring HZ, Lessov CN, Reed T, Marcus R, Holloway L, Swan GE, Carmelli D. Heritability of Plasma Sex Hormones and Hormone Binding Globulin in Adult Male Twins. Journal of Clinical Endocrinology & Metabolism. 2005;90(6):3653–3658. doi: 10.1210/jc.2004-1025. [DOI] [PubMed] [Google Scholar]
- Rohrmann S, Nelson WG, Rifai N, Brown TR, Dobs A, Kanarek N, Yager JD, Platz EA. Serum estrogen, but not testosterone, levels differ between black and Genetic Influences on Testosterone white men in a nationally representative sample of Americans. Journal of Clinical Endocrinology & Metabolism. 2007;92:2519–2525. doi: 10.1210/jc.2007-0028. [DOI] [PubMed] [Google Scholar]
- Sakai LM, Baker LA, Jacklin CN, Shulman I. Sex steroids at birth: genetic and environmental variation and covariation. Developmental Psychobiology. 1991;24(8):559–570. doi: 10.1002/dev.420240804. [DOI] [PubMed] [Google Scholar]
- Sellers JG, Mehl MR, Josephs RA. Hormones and personality: Testosterone as a marker of individual differences. Journal of Research in Personality. 2007;41(1):126–138. doi: http://dx.doi.org/10.1016/j.jrp.2006.02.004. [Google Scholar]
- Shirtcliff EA, Dahl RE, Pollak SD. Pubertal development: correspondence between hormonal and physical development. Child Development. 2009;80(2):327–337. doi: 10.1111/j.1467-8624.2009.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Likos A. Gender differences in the validity of testosterone measured in saliva by immunoassay. Hormones and Behavior. 2002;42:62–69. doi: 10.1006/hbeh.2002.1798. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol. 2005;26(3–4):163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Smith LM, Cloak CC, Poland RE, Torday J, Ross MG. Prenatal nicotine increases testosterone levels in the fetus and female offspring. Nicotine & Tobacco Research. 2003;5(3):369–374. doi: 10.1080/146222031000094196. [DOI] [PubMed] [Google Scholar]
- Steiger J. Structural Model Evaluation and Modification: An Interval Estimation Approach. Multivariate Behavioral Research. 1990;25(2):173–180. doi: 10.1207/s15327906mbr2502_4. [DOI] [PubMed] [Google Scholar]
- Treloar AE, Boynton RE, Behn BG, Brown BW. Variation of the human menstrual cycle through reproductive life. International Journal Fertility. 1967;12(1 Pt 2):77–126. [PubMed] [Google Scholar]
- Udry JR. Biological Predispositions and Social Control in Adolescent Sexual Behavior. American Sociological Review. 1988;53(5):709–722. [Google Scholar]
- Udry JR, Talbert LM, Morris NM. Biosocial foundations for adolescent female sexuality. Demography. 1986;23(2):217–230. [PubMed] [Google Scholar]
- Winters SJ, Brufsky A, Weissfeld J, Trump DL, Dyky MA, Hadeed V. Testosterone, sex hormone-binding globulin, and body composition in young adult African American and Caucasian men. Metabolism. 2001;50:1242–1247. doi: 10.1053/meta.2001.26714. [DOI] [PubMed] [Google Scholar]