Abstract
Molecular chaperones such as heat shock protein 70 (Hsp70) are crucial for protein folding. Crystal structures of Hsp70 in a complex with the nucleotide exchange factor (NEF) Hsp110 reported in this issue of Cell (Polier et al., 2008) and in Molecular Cell (Schuermann et al., 2008) provide new insights into how NEF action specifies Hsp70 cellular function.
Molecular chaperones of the heat shock protein 70 (Hsp70) class are essential for cellular homeostasis because they facilitate the folding and assembly of nascent polypeptides, the transport of proteins across membranes, and the selection of misfolded proteins for degradation. Hsp70 is an ATP-dependent peptide-binding machine whose diverse cellular functions are specified through interactions with cochaperones and other cellular factors (Cyr et al., 1994). Defective regulation of Hsp70's nucleotide exchange cycle is lethal to the budding yeast Saccharomyces cerevisiae (Raviol et al., 2006) and is associated with certain human neurodegenerative diseases (Senderek et al., 2005). Thus, nucleotide exchange factors (NEFs) for Hsp70 are crucial for Hsp70 function. In two new studies, Schuermann et al. (2008) and Polier et al. (2008) now report structures of bovine and human Hsp70 in complex with the NEF Hsp110 from yeast, called Sse1. These new structures shed light on the mechanism by which NEF action regulates Hsp70 activity.
Prototypical Hsp70 proteins contain a nucleotide-binding domain (NBD) and a peptide-binding domain (PBD) that are connected by a flexible linker region that contributes to allosteric regulation of NBD and PBD activity (Figure 1A; Liu and Hendrickson, 2007). The Hsp70 NBD has two structurally similar lobes (I and II) that comprise four subdomains (IA, IB, IIA, and IIB). Tight nucleotide binding occurs when domains of lobes I and II collapse around an ATP molecule. The PBD comprises a β sandwich subdomain (β-PBD) and an extended α-helical subdomain (α-PBD). Using a groove formed by the β subdomain in the PBD, Hsp70 binds to proteins that have exposed hydrophobic regions with an extended conformation. An ATP hydrolytic cycle regulates polypeptide binding and release by Hsp70. In the ATP-bound state, the Hsp70 PBD binds to substrates with low affinity as the NBD and PBD make extensive contacts with one another (Liu and Hendrickson, 2007). However, ATP hydrolysis to ADP drives a conformational change in the NBD:PBD interface in which the α-PBD closes over the β-PBD. This conformational change results in high affinity substrate binding by Hsp70. Subsequent nucleotide exchange to regenerate Hsp70-ATP promotes a conformational change in the PBD that drives substrate release. It is this cycle of Hsp70-substrate binding and release that prevents substrate aggregation, fosters proper folding, and facilitates the engagement of newly synthesized proteins with their assembly partners.
Figure 1. Nucleotide Exchange Factors and Hsp70.
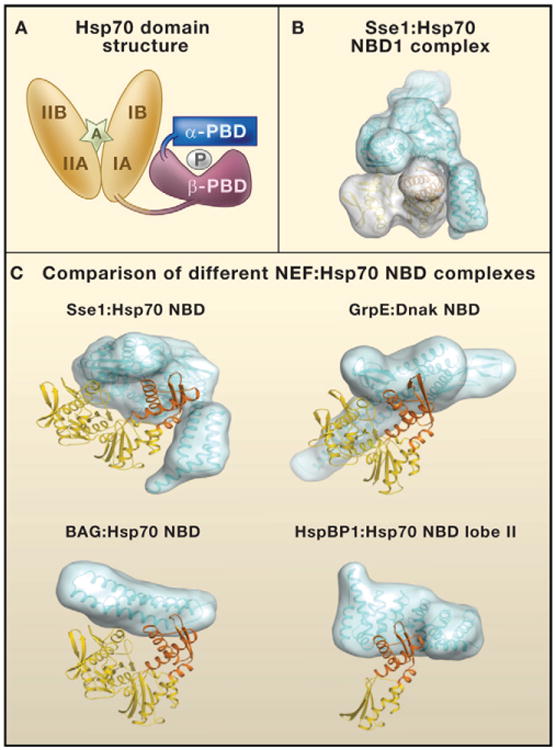
(A) The domain structure of heat shock protein 70 (Hsp70). The nucleotide-binding domain (NBD; orange) consists of two structurally similar lobes (I and II) divided into four subdomains (IA, IB, IIA, and IIB). The peptide-binding domain (PBD) consists of two subdomains: PBD-α (blue) and PBD-β (purple). P denotes a polypeptide in the Hsp70 PBD. An adenosine nucleotide in the Hsp70 NBD is denoted by A.
(B) Surfaces on the Sse1 (blue):Hsp70 NBD (yellow) complex.
(C) Comparison of different complexes containing nucleotide exchange factors (NEFs; blue) and the Hsp70 NBD domain (yellow; NBD lobe II domain IIB in orange). NEFs are of the four unrelated eukaryotic NEF groups: Hsp110/Hsp170 family (Sse1), GrpE homolog (GrpE), Bcl-2-associated athanogene domain (BAG), and HspBP1 homolog (HspBP1). For Sse1:Hsp70 NBD, the domain with the three α-helical bundles in Sse1 that makes contact with lobe II is the α-PBD. DnaK is the Hsp70 of Escherichia coli. Models were calculated with data in the following PDB fles: 3d2f, 1 dkg, 1hx1, and 1xqs (http://www.rcsb.org/pdb/home/home.do).
The Hsp70-ATP hydrolytic cycle is tightly regulated by Hsp40 cochaperones that stimulate ATP hydrolysis and NEF activity (Shaner and Morano, 2007). Hsp40s are known to specify Hsp70 function by mediating its localization to different cellular microenvironments and by targeting diverse sets of substrates to the Hsp70 PBD (Cyr et al., 1994). More recently, NEFs have also been implicated as important specification factors for Hsp70 (Shaner and Morano, 2007). Hsp70 NEFs promote the exchange of ADP for ATP and exhibit specific nonoverlapping functions that direct Hsp70 to carry out distinct cellular processes (Dragovic et al., 2006; Raviol et al., 2006; Shaner and Morano, 2007). Four unrelated groups of eukaryotic NEFs have been identified: homologs of the protein GrpE from the bacteria Escherichia coli, homologs of the human Hsp70-binding protein 1 (HspBP1), BAG (Bcl-2-associated athanogene) domain proteins, and Hsp110/Hsp170 family members (Shaner and Morano, 2007). The presence of different protein interaction domains helps to determine the specific functions of NEFs. Complexes containing Hsp70, Hsp40, and Hsp110 proteins represent the major protein folding machine of the eukaryotic cytosol (Polier et al., 2008).
The NEFs Hsp110 and glucose-regulated protein 170 (Grp170) are noncanonical Hsp70s that have diverged in amino acid sequence from canonical Hsp70s (Shaner and Morano, 2007). Both the human endoplasmic reticulum (ER) localized Grp170 protein and the cytosolic Hsp110 proteins from human and yeast are known to act as NEFs and chaperones (Steel et al., 2004; Dragovic et al., 2006; Raviol et al., 2006; Shaner and Morano, 2007). How-ever, how these noncanonical Hsp70s work together with Hsp70 to promote protein folding has remained unclear. Is their primary purpose only to provide NEF activity for rapid Hsp70 ATP-ADP cycling between substrate binding and release to enable protein folding? Or do they have NEF activity-independent functions in protein folding? Polier et al. and Schuermann et al. now reveal the mechanism of Hsp110 NEF action through X-ray crystal structures of the cytosolic yeast Hsp110 protein Sse1 alone and in a complex with Hsp70. In addition, they performed mutagenesis analysis of Sse1 and Hsp70 interactions for further insights. Sse1-ATP crystallizes as a homodimer, demonstrating that the NBD and PBD structures of Hsp110s are similar to those of canonical Hsp70s (Liu and Hendrickson, 2007). However, the α-PBD of Sse1 does not form a lid over the β-PBD. Instead, the Sse1 α-PBD makes contact with the NBD of opposing Sse1 monomers in the crystallographic dimer. Thus, contact between the α-PBD of Sse1 and the NBD of Hsp70 might be involved in Hsp110 nucleotide exchange activity. Mutational analysis of Sse1 further demonstrated that the predominant function of Sse1 does seem to be its NEF activity. However, because partial loss of Sse1 NEF activity in vivo did not result in decreased yeast cell viability, it is possible that Sse1 has functions independent of NEF activity that allow it to still promote protein folding in vivo (Polier et al., 2008).
Crystal structures of Sse1:Hsp70 NBD (Polier et al., 2008) and Sse1:Hsp70 (Schuermann et al., 2008) further reveal that the Sse1 NDB and α-PBD contact large areas of lobe I and lobe II of the Hsp70 NBD (Figure 1B). Such contacts would allow the opening of the Hsp70 NBD and would promote nucleotide release. In the Sse1:Hsp70 complex, the Sse1 NBD has a closed conformation, whereas the Hsp70 NBD has an open conformation. Thus, Schuermann et al. proposed that the coupled open-closed isomerization of the Sse1 NBD and Hsp70 NBD reciprocally regulate interactions between Sse1 and Hsp70 (Schuermann et al., 2008).
The major difference between Sse1's interactions with the Hsp70 NBD, as revealed by the new structures, and that of other NEFs such as BAG1 and GrpE is the contacts made by the Sse1 α-PBD. Sse1 is the only NEF that contains a PBD, and its α-PBD makes extensive contacts with lobe II of the Hsp70 NBD (Figures 1B and 1C). Such interactions appear to account for the high stability reported for Hsp70:Sse1 complexes (Shaner and Morano, 2007). Furthermore, in the Hsp70:Sse1 structure, the Sse1 PBD is located in close proximity to the Hsp70 PBD (Schuermann et al., 2008). Thus, Hsp70 and Sse1 may bind simultaneously to certain substrates. Polier et al. propose that polypeptide binding by Sse1 may be important for the folding of large proteins with multiple domains (Polier et al., 2008).
Interactions of Hsp70 with specialized NEFs specify the cellular functions of Hsp70. The cytosol of human cells contains at least eight different types of NEF. The challenge now is to understand the factors that dictate which NEFs interact with which Hsp70 complexes. Conditions for protein folding in the cytosol and cellular compartments such as the ER lumen are markedly different. Thus, an interesting question is whether NEFs such as the ER-specific Grp170 and HspBP1 homolog Sil1/BAP have evolved specialized mechanisms to assist in protein folding in the ER. Sil1/BAP is of particular interest given that its inactivation has been implicated in the autosomal recessive human disease Marinesco-Sjögren syndrome (Senderek et al., 2005). Unfortunately, because HspBP1 binding destabilizes the Hsp70 NBD structure (Shomura et al., 2005), only a crystal structure of HspBP1 in a complex with lobe II of the Hsp70 NBD is available (Figure 1C). The elegant structures of Polier et al. (2008) and Schuermann et al. (2008) not only shed light on the mechanism of Hsp110 action but also may provide fresh leads in understanding the actions of other NEFs such as Grp170 and Sil1/BAP.
Acknowledgments
D.M.C. is supported by the NIH. We thank M. Scully and A. Bracher for helpful discussions and assistance in figure preparation.
References
- Cyr DM, Langer T, Douglas MG. Trends Biochem Sci. 1994;19:176–181. doi: 10.1016/0968-0004(94)90281-x. [DOI] [PubMed] [Google Scholar]
- Dragovic Z, Broadley SA, Shomura Y, Bracher A, Hartl FU. EMBO J. 2006;25:2519–2528. doi: 10.1038/sj.emboj.7601138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Hendrickson WA. Cell. 2007;131:106–120. doi: 10.1016/j.cell.2007.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polier S, Dragovic Z, Hartl FU, Bracher A. Cell. 2008 doi: 10.1016/j.cell.2008.05.022. this issue. [DOI] [PubMed] [Google Scholar]
- Raviol H, Sadlish H, Rodriguez F, Mayer MP, Bukau B. EMBO J. 2006;25:2510–2518. doi: 10.1038/sj.emboj.7601139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuermann JP, Jiang J, Cuellar J, Llorca O, Wang L, Gimenez LE, Jin S, Taylor AB, Demeler B, Morano KA, et al. Mol Cell. 2008 doi: 10.1016/j.molcel.2008.05.006. Published online June 12, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senderek J, Krieger M, Stendel C, Bergmann C, Moser M, Breitbach-Faller N, Rudnik-Schoneborn S, Blaschek A, Wolf NI, Harting I, et al. Nat Genet. 2005;37:1312–1314. doi: 10.1038/ng1678. [DOI] [PubMed] [Google Scholar]
- Shaner L, Morano KA. Cell Stress Chaperones. 2007;12:1–8. doi: 10.1379/CSC-245R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomura Y, Dragovic Z, Chang HC, Tzvetkov N, Young JC, Brodsky JL, Guerriero V, Hartl FU, Bracher A. Mol Cell. 2005;17:367–379. doi: 10.1016/j.molcel.2004.12.023. [DOI] [PubMed] [Google Scholar]
- Steel GJ, Fullerton DM, Tyson JR, Stirling CJ. Science. 2004;303:98–101. doi: 10.1126/science.1092287. [DOI] [PubMed] [Google Scholar]


