Abstract
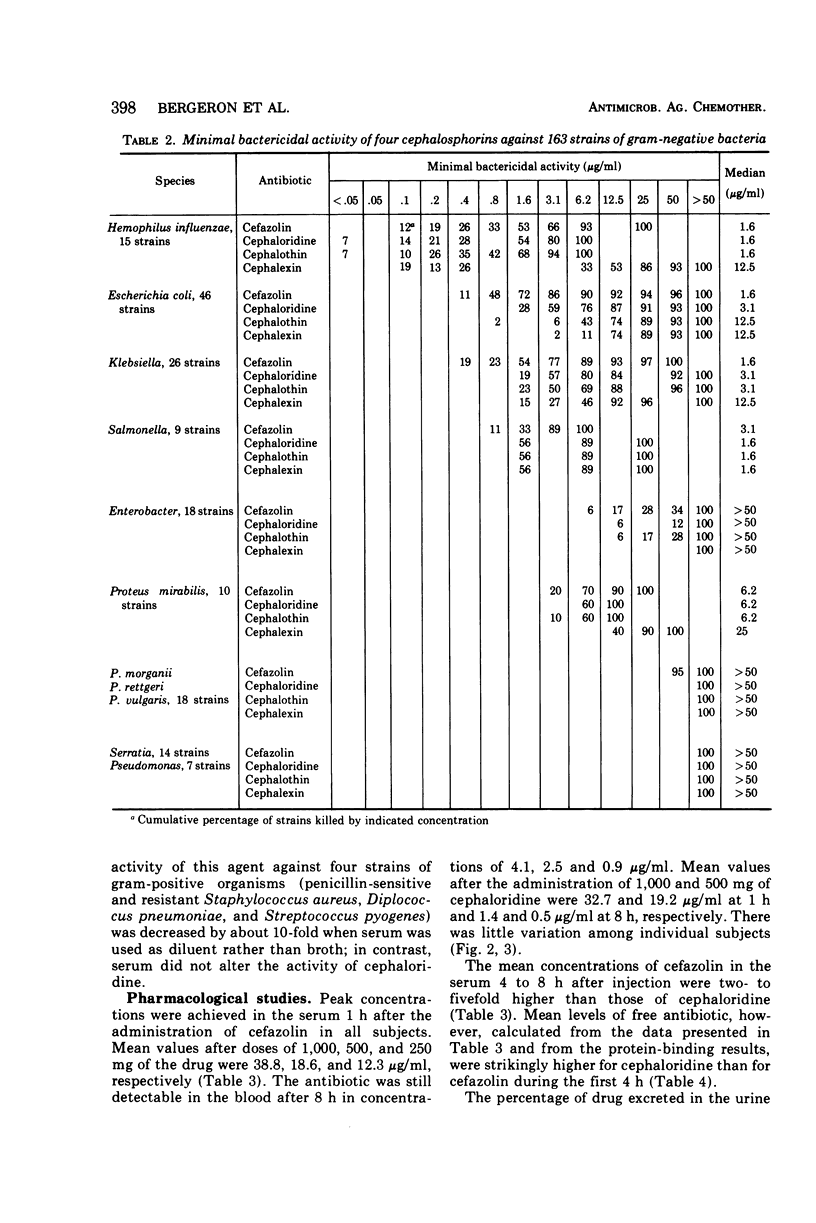
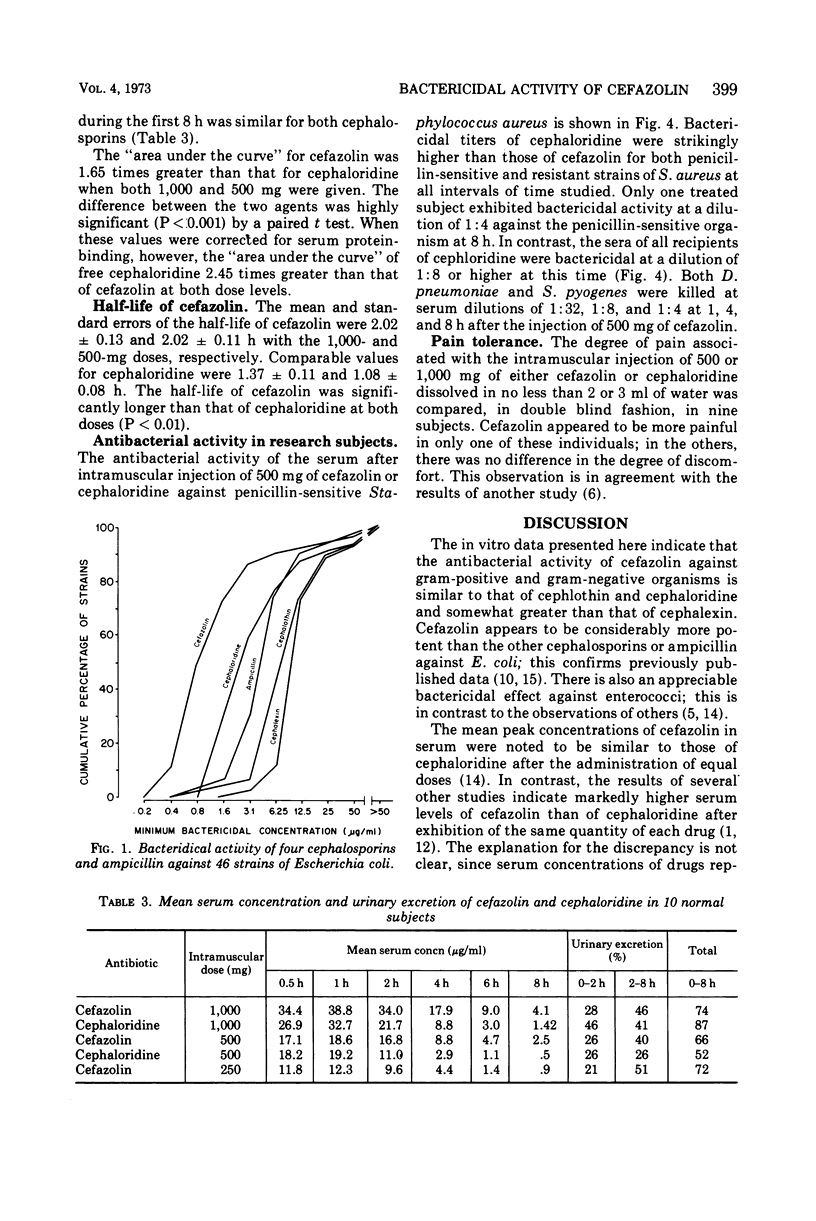
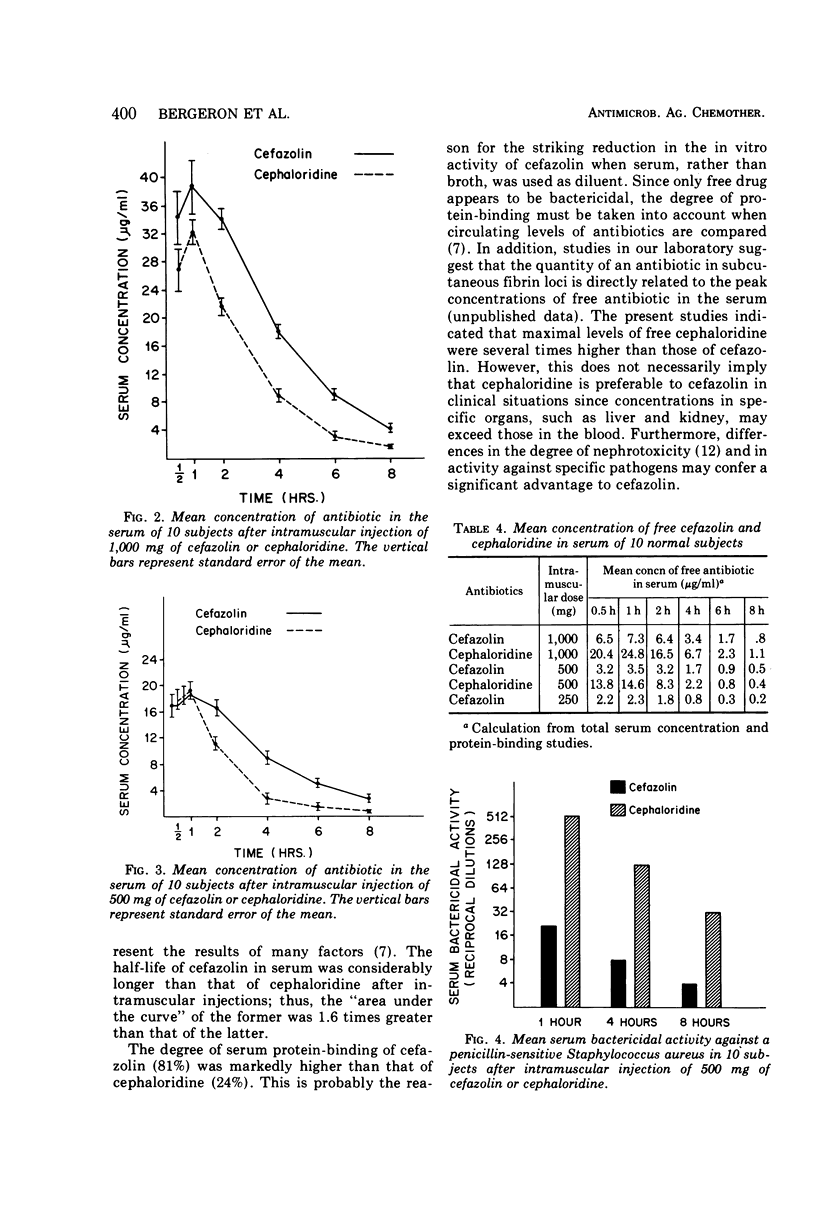
The in vitro bactericidal activity of cefazolin was found to be similar to that of cephaloridine and cephalothin but slightly greater than that of cephalexin against a majority of 233 strains of gram-positive and gram-negative organisms. Cefazolin, however, was two- to eightfold more active than the other two drugs against Escherichia coli and klebsiella. The mean peak concentrations in the serum in 10 normal subjects 1 h after intramuscular injections of 1,000, 500, and 250 mg of cefazolin were 38.8, 18.6, and 12.2 μg/ml, respectively. The antibiotic could still be detected at 8 h. Peak values for a given dose of cephaloridine were comparable. However, blood levels of cefazolin were regularly higher than those of cephaloridine over the first 8 h. The mean half-life of cefazolin in the serum was 2 h, whereas that of cephaloridine was 1.4 h. The degree of serum protein-binding was strikingly higher for cefazolin (81%) than for cephaloridine (24%), suggesting that the antibacterial activity of the former in serum might be less than that of cephaloridine after equal doses. This proved to be the case when the bacterial activity of blood drawn 1, 4, and 8 h after injection of the two drugs was examined.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- De Schepper P., Harvengt C., Vranckx C., Boon B., Lamy F. Pharmacologic study of cefazolin in volunteers. J Clin Pharmacol New Drugs. 1973 Feb-Mar;13(2):83–88. doi: 10.1002/j.1552-4604.1973.tb00257.x. [DOI] [PubMed] [Google Scholar]
- Gordon C., Regamey C., Kirby W. M. Comparative clinical pharmacology of amoxicillin and ampicillin administered orally. Antimicrob Agents Chemother. 1972 Jun;1(6):504–507. doi: 10.1128/aac.1.6.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiyama S., Nakayama I., Iwamoto H., Iwai S., Okui M. Absorption, tissue concentration, and organ distribution of cefazolin. Antimicrob Agents Chemother (Bethesda) 1970;10:476–480. [PubMed] [Google Scholar]
- KUNIN C. M., FINLAND M. Restrictions imposed on antibiotic therapy by renal failure. Arch Intern Med. 1959 Dec;104:1030–1050. doi: 10.1001/archinte.1959.00270120186021. [DOI] [PubMed] [Google Scholar]
- Kariyone K., Harada H., Kurita M., Takano T. Cefazolin, a new semisynthetic cephalosporin antibiotic. I. Synthesis and chemical properties of cefazolin. J Antibiot (Tokyo) 1970 Mar;23(3):131–136. doi: 10.7164/antibiotics.23.131. [DOI] [PubMed] [Google Scholar]
- Kayser F. H. In vitro activity of cephalosporin antibiotics against Gram-positive bacteria. Postgrad Med J. 1971 Feb;47(Suppl):14–20. [PubMed] [Google Scholar]
- Kozatani J., Okui M., Matsubara T., Nishida M. Cefazolin, a new semisynthetic cephalosporin antibiotic. VI. Excretion and metabolism of cefazolin- 14 C in rats after intramuscular administration. J Antibiot (Tokyo) 1972 Feb;25(2):86–93. [PubMed] [Google Scholar]
- Kunin C. M. Therapeutic implications of serum protein binding of the new semisynthetic penicillins. Antimicrob Agents Chemother (Bethesda) 1965;5:1025–1034. [PubMed] [Google Scholar]
- Molavi A., Barza M., Cole W., Berman H., Weinstein L. In vitro assessment of tobramycin, a new aminoglycoside with anti-Pseudomonas activity. Chemotherapy. 1973;18(1):7–16. doi: 10.1159/000221242. [DOI] [PubMed] [Google Scholar]
- Nishida M., Matsubara T., Murakawa T., Mine Y., Yokota Y. Cefazolin, a new semisynthetic cephalosporin antibiotic. II. In vitro and in vivo antimicrobial activity. J Antibiot (Tokyo) 1970 Mar;23(3):137–148. doi: 10.7164/antibiotics.23.137. [DOI] [PubMed] [Google Scholar]
- Nishida M., Matsubara T., Murakawa T., Mine Y., Yokota Y., Goto S., Kuwahara S. Cefazolin, a new semisynthetic cephalosporin antibiotic. 3. Absorption, excretion and tissue distribution in parenteral administration. J Antibiot (Tokyo) 1970 Apr;23(4):184–194. [PubMed] [Google Scholar]
- Nishida M., Matsubara T., Murakawa T., Mine Y., Yokota Y., Kuwahara S., Goto S. In vitro and in vivo evaluation of cefazolin, a new cephalosporin C derivative. Antimicrob Agents Chemother (Bethesda) 1969;9:236–243. [PubMed] [Google Scholar]
- Ries K., Levison M. E., Kaye D. Clinical and in vitro evaluation of cefazolin, a new cephalosporin antibiotic. Antimicrob Agents Chemother. 1973 Feb;3(2):168–174. doi: 10.1128/aac.3.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata K., Fujii M. Clinical studies of cefazolin in the surgical field. Antimicrob Agents Chemother (Bethesda) 1970;10:467–472. [PubMed] [Google Scholar]



