Abstract
Neoadjuvant chemotherapy has practical and theoretical advantages over adjuvant chemotherapy strategy in breast cancer (BC) management. Moreover, metronomic delivery has a more favorable toxicity profile. The present study examined the feasibility of neoadjuvant metronomic chemotherapy in two cohorts [HER2+ (TraQme) and HER2− (TAME)] of locally advanced BC. Twenty patients were prospectively enrolled (TraQme, n=9; TAME, n=11). Both cohorts received weekly paclitaxel at 100 mg/m2 during 8 weeks followed by weekly doxorubicin at 24 mg/m2 for 9 weeks in combination with oral cyclophosphamide at 100 mg/day (fixed dose). The HER2+ cohort received weekly trastuzumab. The study was interrupted because of safety issues. Thirty-six percent of patients in the TAME cohort and all patients from the TraQme cohort had stage III BC. Of note, 33% from the TraQme cohort and 66% from the TAME cohort displayed hormone receptor positivity in tumor tissue. The pathological complete response rates were 55% and 18% among patients enrolled in the TraQme and TAME cohorts, respectively. Patients in the TraQme cohort had more advanced BC stages at diagnosis, higher-grade pathological classification, and more tumors lacking hormone receptor expression, compared to the TAME cohort. The toxicity profile was also different. Two patients in the TraQme cohort developed pneumonitis, and in the TAME cohort we observed more hematological toxicity and hand-foot syndrome. The neoadjuvant metronomic chemotherapy regimen evaluated in this trial was highly effective in achieving a tumor response, especially in the HER2+ cohort. Pneumonitis was a serious, unexpected adverse event observed in this group. Further larger and randomized trials are warranted to evaluate the association between metronomic chemotherapy and trastuzumab treatment.
Keywords: Breast cancer, Metronomic chemotherapy, HER2+
Introduction
Breast cancer (BC) is a highly prevalent disease. Worldwide, it is the most common cancer diagnosed and the leading cause of cancer death in women (1). Among the five major cancer incidence sites, BC is the only malignancy with relatively stable incidence rates from 2005 to 2009, after decreasing by 2% per year from 1999 to 2005 (2).
Although neoadjuvant chemotherapy was initially indicated to convert a nonresectable into a resectable lesion (3-5), it has several theoretical advantages over the adjuvant chemotherapy strategy. A downstaging of operable tumors, allowing for breast-conserving surgery, is also a potential benefit (6). Nevertheless, one of the major appeals of neoadjuvant chemotherapy is the possibility for analyzing the impact of systemic therapy in different BC subtypes, because tumor tissue biopsies can be obtained at different times during treatment, allowing for the development of biomarkers (6).
Also, the pathological complete response (pCR), defined as ‘no invasive and no in situ residual invasive tumor in breast and lymph nodes’, can discriminate between patients with favorable and unfavorable outcomes (7). This was particularly true for estrogen receptor (ER) negative or highly proliferative tumors, such as HER2+ BC subtypes.
There are insufficient data on the efficacy and safety of metronomic chemotherapy in the neoadjuvant setting. The concept of a treatment that is delivered more frequently and with low doses has been proven to target tumor angiogenesis, shifting the tumor vasculature (8,9). Consequently, it would effectively act on angiogenesis, one of the major pathways involved in disease progression and metastasis in BC.
In the metastatic setting, the clinical benefit and safety of metronomic chemotherapy treatment were demonstrated in several trials in BC treatment (10-13). Those trials demonstrated a good toxicity profile from this chemotherapy approach, even in an elderly patient population, combined with hormone therapy (13). In addition, metronomic chemotherapy has been proven to be highly effective in BC. It was reported that the average response rates of complete response (CR) plus partial response (PR) and overall clinical benefit (CR+PR+stable disease [SD] >6 months) of metronomic chemotherapy in BC management reaches 39% (range 12-88%) and 57% (range 24-93%), respectively (14).
A previous neoadjuvant trial demonstrated the feasibility of combining oral cyclophosphamide chemotherapy for locally advanced BC treatment. Patients assigned to receive daily cyclophosphamide and weekly doxorubicin had fewer grade 3 to 4 leukopenia and neutropenia and better pCR rates, compared to the group assigned to receive standard doxorubicin and cyclophosphamide, drawing attention to the activity and safety from a regimen in alignment with this concept (15).
Thus, we had great interest in combining two very attractive approaches in BC treatment: neoadjuvant chemotherapy, delivered in a metronomic way. One key concept of this study was the idea that this regimen could be delivered in a simplified manner, without routine growth factor support administration, a distinct approach from the one proposed by SWOG 0012 (15). Furthermore, our study also explored the concept that a highly effective neoadjuvant schedule could eventually allow for shorter trastuzumab treatment duration, a concept that had been successfully explored by another study (16) at the time our study was conceived and that was considered of significant public interest at that time.
This trial was conducted to prospectively evaluate this treatment in locally advanced cohorts of patients: HER2+ and HER2− BC patients. Trastuzumab was added to the metronomic chemotherapy regimen of HER2+ patients. We now report the final results from this trial and the safety data.
Patients and Methods
Patient selection
Patients with untreated unilateral or bilateral primary BC diagnosed at Instituto do Cancer do Estado de São Paulo, Faculdade de Medicina, Universidade de São Paulo (ICESP), were enrolled after giving written informed consent.
Eligibility criteria required histological confirmation of invasive ductal carcinoma by core biopsy. The lesion had to be measurable in two dimensions by clinical examination or an imaging technique, with one diameter of at least 2 cm and/or had a disease staged as more than N1, according to the seventh edition of the TNM staging system. Patients diagnosed with inflammatory BC diagnosis were allowed to enter the study. Patients diagnosed with HER2+ BC, defined by HER2 overexpression or amplification as assessed by immunohistochemical analysis of 3+ or by positive fluorescence in situ hybridization (FISH), were allocated to TraQme, and HER2− BC patients were allocated to TAME (NCT01329640 and NCT01329627).
Patients were excluded if they had evidence of distant metastasis, an Eastern Cooperative Oncology Group (ECOG) performance status of three or more, previous history of malignancy, previous cytotoxic or endocrine therapy, and previous history or echocardiographic evidence of abnormal cardiac function at baseline. Adequate hematological, renal, and hepatic function was required for study admission. There were no violations of the inclusion study criteria.
Study design
Women were enrolled in the study between September 2010 and December 2010. Patients were prospectively treated in a nonrandomized fashion. The study was denoted TraQme for the HER2+ and TAME for the HER2− cohorts of patients.
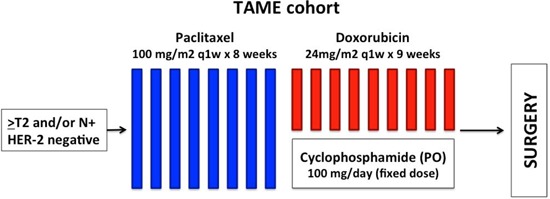
All patients in both groups received weekly paclitaxel at 100 mg/m2 during 8 weeks followed by weekly doxorubicin at 24 mg/m2 for 9 weeks in combination with oral cyclophosphamide at a daily dose of 100 mg. In addition, the HER2+ group received weekly trastuzumab at a loading dose of 4 mg/kg followed by a maintenance dose of 2 mg/kg during the entire chemotherapy treatment. The duration of neoadjuvant chemotherapy was 17 weeks (Figures 1 and 2). Trastuzumab was continued until completion of a total of 1 year of anti-HER2 therapy. The use of granulocyte colony-stimulating factor was allowed per physician choice, but was not routinely recommended as a primary prophylaxis.
Figure 1. TraQme cohort study design.
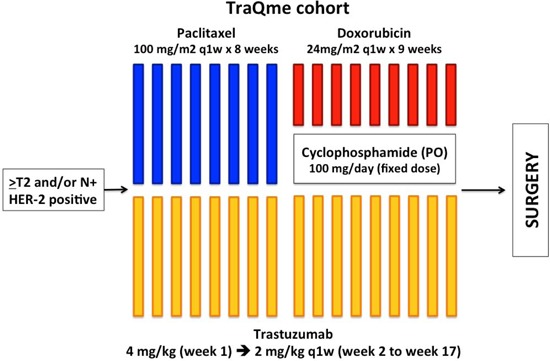
Figure 2. TAME cohort study design.
A clinical oncologist evaluated patients every 3 weeks, and full blood counts were performed every 21 days. If the neutrophil count on the 21st day of the chemotherapy cycle was less than 1.0×109/L or the platelet count was less than 100×109/L, administration of the subsequent pulse of chemotherapy was delayed by 7 days. Hemoglobin levels of less than 9.0 g/L necessitated blood transfusion, although no reduction in the dose of the cytotoxic agents was applied.
Patients were scheduled to undergo surgery approximately 4 weeks after the last chemotherapy cycle. All patients were scheduled to undergo axillary lymph node dissection. Tumor response was assessed clinically on every medical appointment and by ultrasonography at weeks 9 and 17.
For patients in the TraQme cohort, cardiac function reassessment with an echocardiogram was performed at weeks 9 and 17. For patients in the TAME cohort, echocardiogram was performed only at week 17.
Statistical methodology and analysis
The clinical size of the primary breast tumors was determined immediately before administration of each cycle of chemotherapy and before surgery. At each assessment, the product of the two greatest perpendicular diameters was used to quantify the size of the tumors. A reduction of 50% or more in tumor size was categorized as a partial response. An increase of more than 50% in tumor size was categorized as progressive disease. The absence of clinically detected disease in breast and axilla was classified as a clinically complete response. Patients whose response did not meet the previous classifications mentioned above were considered to have stable disease.
As a well-defined nonrandomized feasibility study, our study was primarily concerned with safety data monitoring, including febrile neutropenia and other major toxicities. The study committee was allowed to check safety issues regarding toxicities at any time.
The primary objective was to evaluate the feasibility of these schedules, as defined by a rate of febrile neutropenia lower than 10%, without the use of growth factor support.
The secondary objectives for the study were pCR rates (defined as the absence of microscopic invasive tumors at the primary site and in axillary lymph nodes at the time of surgery); disease-free survival (DFS); the time from registration to disease progression, disease recurrence, or death due to any cause; overall survival (OS); time from registration to death owing to any cause. The Kaplan-Meier method was used to calculate estimated survival by groups. Follow-up from these cohorts of patients is still immature and not statistically significant.
Results
Between September 3 and December 22, 2010, a total of 9 patients were enrolled in the TraQme cohort and 11 in the TAME cohort. The Study Safety Committee performed a single interim analysis on December 22, 2010. This was followed by a recommendation to end the study early because of safety issues (severe pulmonary toxicity).
At that time, four patients from the HER2+ cohort had already finished treatment (1 of them due to cardiac toxicity), while the remaining five were still on chemotherapy. Two of the patients immediately underwent surgery and the other three completed chemotherapy exclusively with paclitaxel and trastuzumab and were then referred to surgery.
Most patients of the TAME group had already undergone all chemotherapy treatment, except for two of them, for which there were 2 weeks of doxorubicin treatment remaining before the end of treatment. Cyclophosphamide was permanently omitted from the last 6 and 5 weeks of doxorubicin treatment in these two patients, respectively.
Efficacy data
TraQme
Table 1 provides the patient characteristics and response to treatment at the time of definitive chemotherapy. Age ranged from 32 to 57 years. All patients had clinical Stage III BC, and a pathology test demonstrated histological grade III invasive ductal carcinoma in all of them. Three patients displayed hormone receptor positivity, with the rest of them being hormone receptor negative (1 patient had unknown hormone receptor status).

Among the nine patients enrolled in the study, we observed five with pCR, resulting in a pCR rate of 55.5%.
At the median follow-up of 33.6 months, five patients were alive, with no evidence of disease recurrence. Three patients had systemic disease recurrence and one of them died due to disease progression. We have been unable to follow up one patient.
TAME
Table 2 provides patient characteristics and response to treatment at the time of definitive surgery. Age ranged from 34 to 68 years. Thirty-six percent of patients had clinical Stage III BC, and the great majority of them (63%) had histological grade II. Four patients were hormone receptor negative by immunohistochemistry, with the rest of them being hormone receptor positive. We observed only two with pCR (18%) in this cohort.
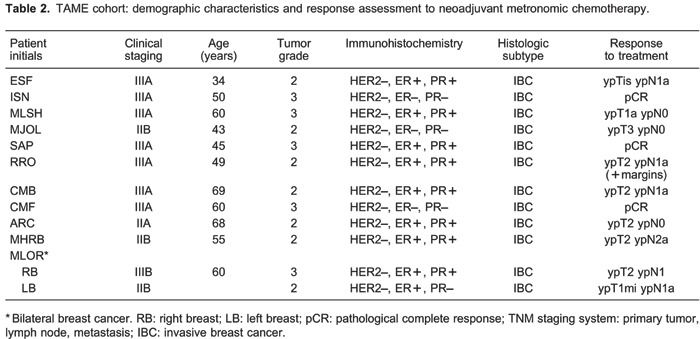
At the median follow-up of 36.1 months, four patients had systemic disease recurrence, whereas two of them had already died (1 due to disease progression and the other due to pulmonary thromboembolism), and the remaining two are being treated with standard palliative regimens for BC management. The remaining seven patients are being followed with no evidence of disease recurrence.
Safety
TraQme
At the time of study discontinuation requirement, four patients had already completed the neoadjuvant treatment and the other five were still receiving it. Hematological toxicity was mild in general, and we did not have any grade 4 event.
Of note, we observed grade 3 pneumonitis development in two patients. They presented with hypoxemia and dyspnea, requiring admission to the Intensive Care Unit. Treatment of this complication included corticosteroids. Mechanical ventilation was not required. One of these patients underwent a transbronchial lung biopsy, which confirmed pneumonitis. In the nonbiopsied patient, clinical presentation, together with the exclusion of other factors, made us infer the same diagnosis. All infectious causes for lung function impairment were excluded.
One patient developed hemorrhagic cystitis, possibly due to oral cyclophosphamide use. There was no neutropenic fever recorded or grade 4 hematologic toxicity.
Cardiac safety
One of the nine patients enrolled in the study developed asymptomatic drop of left ventricular ejection fraction (LVEF). This occurred during the combined paclitaxel and trastuzumab part of treatment. Treatment was suspended and she underwent breast surgery. A cardiologist is still following this patient, and cardiac function did not improve to previous levels, although it showed some improvement since discontinuation of treatment.
TAME
Hand-foot syndrome was the most common adverse reaction in this group, and four patients developed grade 3 palmar-plantar erythrodysesthesia syndrome. In contradiction to the findings from TraQme, hematologic toxicity was an important toxicity issue. Lymphopenia and leucopenia grade 3 occurred in two and four patients, respectively. In addition, three patients had neutropenia grade 4. There were no cases of febrile neutropenia or pneumonitis in this group.
Discussion
In this prospective trial, we demonstrated that the metronomic chemotherapy regimen was effective in achieving a tumor response, but was associated with important toxicity. We divided our patient cohort population into two distinct groups: HER2+ and HER2− patients. Interestingly, the toxic profile from the chemotherapy regimen was quite different when trastuzumab was added.
It is important to emphasize that the two patient populations from TraQme and TAME had distinct tumor characteristics. The HER2+ cohort consisted mainly of advanced BC stages at diagnosis, high-grade pathological classification, and lack of hormone receptor expression. pCR rates in this group were quite high, notably 55%, reflecting the activity of metronomic chemotherapy and trastuzumab in a more proliferate tumor phenotype. On the other hand, the TAME population consisted of a less proliferative tumor profile. In this group, most of the patients expressed hormone receptors and presented with less advanced tumor staging. The pCR rate was 18%, compatible with findings from previous trials in hormone receptor positive patients (17). There was a relatively high rate of disease recurrence events in the HER2+ population, which can be attributable to the advanced stage of disease in this cohort and/or to the shorter duration of adjuvant trastuzumab - the latter has been shown to be potentially detrimental in a recent Phase III trial (18).
The TraQme cohort was closed untimely due to the unexpected finding of severe pulmonary toxicity. This finding was taken into consideration in the decision also to close the TAME cohort, together with the disclosure of the results of the SWOG 0012 in which a similar metronomic chemotherapy regimen was shown to be potentially inferior to the standard chemotherapy treatment (15).
Biomarkers that might have a predictive value in patients treated with preoperative therapy include the degree of ER expression (19), histological grade (20), and markers of proliferation, such as Ki-67 labeling index (21,22). In the TAME cohort, the great majority of patients did not have these traditional markers of chemosensitivity. Thus, the actual pCR found in our trial would be in accordance with historical controls.
Indeed, the triple-negative BC (TNBC) subtype is a group in which metronomic chemotherapy is being successfully evaluated. Recently, a Phase II study presented at American Society of Clinical Oncology (ASCO) 2013 enrolled patients with TNBC to receive a metronomic neoadjuvant chemotherapy regimen that included weekly paclitaxel in combination with capecitabine and cyclophosphamide, followed by 5-fluorouracil, epirubicin, and cyclophosphamide (FEC). Patients presented with a pCR rate of 54.5%, with an overall response rate of 93.9% (95%CI: 79.8-99.3); and the toxicity profile was manageable (23).
The results of the TraQme study were similar to those results: a high rate of tumor response in a more proliferative tumor profile, in which chemotherapy is a highly active treatment.
There is accumulating evidence for the importance of obtaining a pCR to neoadjuvant chemotherapy in the HER2+ group, especially in the HER2+ nonluminal group (7).
The neoadjuvant treatment response is of great importance for survival outcomes in BC patients. In alignment with this recognition, researchers are seeking to obtain accelerated approval regulation for drugs and regimens on the basis of findings in the neoadjuvant setting. A recent meta-analysis, presented at the 2012 San Antonio Breast Cancer Symposium, included 12,993 patients enrolled in neoadjuvant trials. Patients who achieved a pCR had a 52% reduction in the probability of an event (P<0.001) and a 64% reduction in the probability of death (P<0.001) (24).
A recent study retrospectively evaluated a cohort of HER2+ patients treated with anthracycline-taxane-based chemotherapy and trastuzumab. They categorized patients into two groups: those who achieved a pCR and those who had less than a pCR. Patients in the pCR group had better survival outcomes, including relapse-free survival (RFS), distant relapse-free survival (DRFS), and OS. Failure to achieve a pCR was the strongest independent predictor of recurrence (25).
Metronomic therapy in HER2 patients could theoretically overcome trastuzumab resistance, a phenomenon frequently observed in the metastatic setting (26). In a clinical practice scenario, trastuzumab was previously combined with the traditional metronomic regimen of cyclophosphamide and methotrexate. The combination was effective, with a median time to progression (TTP) of 6 months and mild overall toxicity, in a chemotherapy refractory patient population (27).
The pulmonary toxicity found in the TraQme cohort was unexpected. To date, no patient in the TAME cohort experienced this event. The TraQme and TAME cohorts had the same chemotherapy regimen, and addition of trastuzumab was the only difference between the two groups. Historically, the reported incidence of trastuzumab-induced pneumonitis ranges from 0.4 to 0.6% (28). A few case reports had previously described pneumonitis in patients treated with trastuzumab (29-31). In many of them, the use of trastuzumab was associated with a taxane, as in our study. The taxane itself is also a chemotherapy agent that alone could also lead to pneumonitis (32). We cannot rule out the possibility that the more dose-intensive paclitaxel schedule used in our study has contributed to the increased incidence of noninfectious pneumonitis, although previous studies of dose-dense chemotherapy in combination with trastuzumab have not reported higher rates of pulmonary toxicity (32-34).
Hematological toxicity was mild in patients treated in the TraQme cohort, but was more prominent in the TAME cohort. Also, hand-foot syndrome was a clinically relevant adverse event reported in this group. Most previous trials evaluating metronomic chemotherapy in different disease stage scenarios reported these toxicities as a frequent event (35-37). Nonetheless, in many of these trials, neither grade 3 nor grade 4 hematologic toxicity occurred.
In conclusion, the combined analysis from the TraQme and TAME cohorts demonstrated that this metronomic chemotherapy strategy was associated with a reasonable response rate and pCR achievement. Contrary to previous trials that evaluated metronomic chemotherapy in different BC disease scenarios, the regimen was associated with some serious and other non-serious but clinically relevant side effects. Larger prospective randomized trials are warranted to better evaluate metronomic chemotherapy in combination with trastuzumab, with careful attention to the potential toxicity. In HER2− hormone receptor positive BC, metronomic chemotherapy might not warrant further investigation in view of the modest activity, unfavorable toxicity profile, and negative results in a large Phase III trial (15).
Acknowledgments
The primary sponsor of the study was the Instituto do Câncer do Estado de São Paulo, Faculdade de Medicina, Universidade de São Paulo (ICESP). The research was partially supported by CNPq.
Footnotes
First published online.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Danforth DN, Jr, Lippman ME, McDonald H, Bader J, Egan E, Lampert M, et al. Effect of preoperative chemotherapy on mastectomy for locally advanced breast cancer. Am Surg. 1990;56:6–11. [PubMed] [Google Scholar]
- 4.Hortobagyi GN, Ames FC, Buzdar AU, Kau SW, McNeese MD, Paulus D, et al. Management of stage III primary breast cancer with primary chemotherapy, surgery, and radiation therapy. Cancer. 1988;62:2507–2516. doi: 10.1002/1097-0142(19881215)62:12<2507::AID-CNCR2820621210>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz GF, Birchansky CA, Komarnicky LT, Mansfield CM, Cantor RI, Biermann WA, et al. Induction chemotherapy followed by breast conservation for locally advanced carcinoma of the breast. Cancer. 1994;73:362–369. doi: 10.1002/1097-0142(19940115)73:2<362::AID-CNCR2820730221>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 6.Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997;15:2483–2493. doi: 10.1200/JCO.1997.15.7.2483. [DOI] [PubMed] [Google Scholar]
- 7.von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 8.Emmenegger U, Francia G, Shaked Y, Kerbel RS. Metronomic chemotherapy: principles and lessons learned from applications in the treatment of metastatic prostate cancer. Recent Results Cancer Res. 2010;180:165–183. doi: 10.1007/978-3-540-78281-0_10. [DOI] [PubMed] [Google Scholar]
- 9.Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4:423–436. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- 10.Wong NS, Buckman RA, Clemons M, Verma S, Dent S, Trudeau ME, et al. Phase I/II trial of metronomic chemotherapy with daily dalteparin and cyclophosphamide, twice-weekly methotrexate, and daily prednisone as therapy for metastatic breast cancer using vascular endothelial growth factor and soluble vascular endothelial growth factor receptor levels as markers of response. J Clin Oncol. 2010;28:723–730. doi: 10.1200/JCO.2009.24.0143. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Billalabeitia E, Calzas J, Castellano D, Mendiola C, Bezares S, Valentin V, et al. Long-term follow-up of an anthracycline-containing metronomic chemotherapy schedule in advanced breast cancer. Breast J. 2009;15:551–553. doi: 10.1111/j.1524-4741.2009.00783.x. [DOI] [PubMed] [Google Scholar]
- 12.Colleoni M, Orlando L, Sanna G, Rocca A, Maisonneuve P, Peruzzotti G, et al. Metronomic low-dose oral cyclophosphamide and methotrexate plus or minus thalidomide in metastatic breast cancer: antitumor activity and biological effects. Ann Oncol. 2006;17:232–238. doi: 10.1093/annonc/mdj066. [DOI] [PubMed] [Google Scholar]
- 13.Bottini A, Generali D, Brizzi MP, Fox SB, Bersiga A, Bonardi S, et al. Randomized phase II trial of letrozole and letrozole plus low-dose metronomic oral cyclophosphamide as primary systemic treatment in elderly breast cancer patients. J Clin Oncol. 2006;24:3623–3628. doi: 10.1200/JCO.2005.04.5773. [DOI] [PubMed] [Google Scholar]
- 14.Pasquier E, Kavallaris M, Andre N. Metronomic chemotherapy: new rationale for new directions. Nat Rev Clin Oncol. 2010;7:455–465. doi: 10.1038/nrclinonc.2010.82. [DOI] [PubMed] [Google Scholar]
- 15.Ellis GK, Barlow WE, Gralow JR, Hortobagyi GN, Russell CA, Royce ME, et al. Phase III comparison of standard doxorubicin and cyclophosphamide versus weekly doxorubicin and daily oral cyclophosphamide plus granulocyte colony-stimulating factor as neoadjuvant therapy for inflammatory and locally advanced breast cancer: SWOG 0012. J Clin Oncol. 2011;29:1014–1021. doi: 10.1200/JCO.2009.27.6543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joensuu H, Kellokumpu-Lehtinen PL, Bono P, Alanko T, Kataja V, Asola R, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354:809–820. doi: 10.1056/NEJMoa053028. [DOI] [PubMed] [Google Scholar]
- 17.Colleoni M, Viale G, Zahrieh D, Pruneri G, Gentilini O, Veronesi P, et al. Chemotherapy is more effective in patients with breast cancer not expressing steroid hormone receptors: a study of preoperative treatment. Clin Cancer Res. 2004;10:6622–6628. doi: 10.1158/1078-0432.CCR-04-0380. [DOI] [PubMed] [Google Scholar]
- 18.Pivot X, Romieu G, Debled M, Pierga JY, Kerbrat P, Bachelot T, et al. 6 months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): a randomised phase 3 trial. Lancet Oncol. 2013;14:741–748. doi: 10.1016/S1470-2045(13)70225-0. [DOI] [PubMed] [Google Scholar]
- 19.Colleoni M, Bagnardi V, Rotmensz N, Gelber RD, Viale G, Pruneri G, et al. Increasing steroid hormone receptors expression defines breast cancer subtypes non responsive to preoperative chemotherapy. Breast Cancer Res Treat. 2009;116:359–369. doi: 10.1007/s10549-008-0223-y. [DOI] [PubMed] [Google Scholar]
- 20.Petit T, Wilt M, Velten M, Millon R, Rodier JF, Borel C, et al. Comparative value of tumour grade, hormonal receptors, Ki-67, HER-2 and topoisomerase II alpha status as predictive markers in breast cancer patients treated with neoadjuvant anthracycline-based chemotherapy. Eur J Cancer. 2004;40:205–211. doi: 10.1016/S0959-8049(03)00675-0. [DOI] [PubMed] [Google Scholar]
- 21.Dowsett M, Smith IE, Ebbs SR, Dixon JM, Skene A, Griffith C, et al. Short-term changes in Ki-67 during neoadjuvant treatment of primary breast cancer with anastrozole or tamoxifen alone or combined correlate with recurrence-free survival. Clin Cancer Res. 2005;11:951s–958s. [PubMed] [Google Scholar]
- 22.Colleoni M, Bagnardi V, Rotmensz N, Viale G, Mastropasqua M, Veronesi P, et al. A nomogram based on the expression of Ki-67, steroid hormone receptors status and number of chemotherapy courses to predict pathological complete remission after preoperative chemotherapy for breast cancer. Eur J Cancer. 2010;46:2216–2224. doi: 10.1016/j.ejca.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Higaki K, Masuda N, Takano T, et al. Phase II study of neoadjuvant chemotherapy with a metronomic regimen of paclitaxel + cyclophosphamide + capecitabine followed by 5-fluorouracil + epirubicin + cyclophosphamide in operable triple-negative breast cancer (JBCRG-13 study) ASCO Meeting Abstracts. 2013;31:1048. [Google Scholar]
- 24.Cortazar P, Untch M, et al. Meta-analysis results from the collaborative trials in neoadjuvant breast cancer. San Antonio Breast Cancer Symposium. December 5, 2012. [Google Scholar]
- 25.Kim MM, Allen P, Gonzalez-Angulo AM, Woodward WA, Meric-Bernstam F, Buzdar AU, et al. Pathologic complete response to neoadjuvant chemotherapy with trastuzumab predicts for improved survival in women with HER2-overexpressing breast cancer. Ann Oncol. 2013;24:1999–2004. doi: 10.1093/annonc/mdt131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.du Manoir JM, Francia G, Man S, Mossoba M, Medin JA, Viloria-Petit A, et al. Strategies for delaying or treating in vivo acquired resistance to trastuzumab in human breast cancer xenografts. Clin Cancer Res. 2006;12:904–916. doi: 10.1158/1078-0432.CCR-05-1109. [DOI] [PubMed] [Google Scholar]
- 27.Orlando L, Cardillo A, Ghisini R, Rocca A, Balduzzi A, Torrisi R, et al. Trastuzumab in combination with metronomic cyclophosphamide and methotrexate in patients with HER-2 positive metastatic breast cancer. BMC Cancer. 2006;6:225. doi: 10.1186/1471-2407-6-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vahid B, Marik PE. Pulmonary complications of novel antineoplastic agents for solid tumors. Chest. 2008;133:528–538. doi: 10.1378/chest.07-0851. [DOI] [PubMed] [Google Scholar]
- 29.Abulkhair O, El Melouk W. Delayed Paclitaxel-trastuzumab-induced interstitial pneumonitis in breast cancer. Case Rep Oncol. 2011;4:186–191. doi: 10.1159/000326063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuip E, Muller E. Fatal pneumonitis after treatment with docetaxel and trastuzumab. Neth J Med. 2009;67:237–239. [PubMed] [Google Scholar]
- 31.Vahid B, Mehrotra A. Trastuzumab (Herceptin)-associated lung injury. Respirology. 2006;11:655–658. doi: 10.1111/j.1440-1843.2006.00907.x. [DOI] [PubMed] [Google Scholar]
- 32.Nagata S, Ueda N, Yoshida Y, Matsuda H, Maehara Y. Severe interstitial pneumonitis associated with the administration of taxanes. J Infect Chemother. 2010;16:340–344. doi: 10.1007/s10156-010-0058-4. [DOI] [PubMed] [Google Scholar]
- 33.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 34.Dang C, Fornier M, Sugarman S, Troso-Sandoval T, Lake D, D'Andrea G, et al. The safety of dose-dense doxorubicin and cyclophosphamide followed by paclitaxel with trastuzumab in HER-2/neu overexpressed/amplified breast cancer. J Clin Oncol. 2008;26:1216–1222. doi: 10.1200/JCO.2007.12.0733. [DOI] [PubMed] [Google Scholar]
- 35.Montagna E, Cancello G, Bagnardi V, Pastrello D, Dellapasqua S, Perri G, et al. Metronomic chemotherapy combined with bevacizumab and erlotinib in patients with metastatic HER2-negative breast cancer: clinical and biological activity. Clin Breast Cancer. 2012;12:207–214. doi: 10.1016/j.clbc.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 36.Fedele P, Marino A, Orlando L, Schiavone P, Nacci A, Sponziello F, et al. Efficacy and safety of low-dose metronomic chemotherapy with capecitabine in heavily pretreated patients with metastatic breast cancer. Eur J Cancer. 2012;48:24–29. doi: 10.1016/j.ejca.2011.06.040. [DOI] [PubMed] [Google Scholar]
- 37.Perroud HA, Rico MJ, Alasino CM, Queralt F, Mainetti LE, Pezzotto SM, et al. Safety and therapeutic effect of metronomic chemotherapy with cyclophosphamide and celecoxib in advanced breast cancer patients. Future Oncol. 2013;9:451–462. doi: 10.2217/fon.12.196. [DOI] [PubMed] [Google Scholar]


