Abstract
Maintenance of the proteome is a major homeostatic task of the cell and disregulation of protein homeostasis can be deadly. The accumulation of different forms of misfolded protein can perturb protein homeostasis and cause extensive cell and tissue damage. The cell has various quality control systems to help prevent the accumulation of misfolded proteins and the complexity of the different mechanisms that have evolved is bewildering. The first order of business for all quality control systems is recognition of misfolded proteins, which is followed by a triage decision. In many cases, modular molecular chaperones function in different assemblies with degradatory or folding co-factors to direct a misfolded protein toward continued life or death. Herein, an overview of quality control mechanisms that triage soluble cytosolic proteins, protein aggregates, and ER-associated proteins is presented.
Keywords: Protein quality control, Molecular chaperone, RMA1, Derlin, Proteasome, Hsp70, Endoplasmic reticulum, Autophagy
1. Introduction
The proper folding of proteins is essential to ensure protein function and to prevent the accumulation of toxic protein species. However, protein folding is error-prone and folded proteins exist in an equilibrium between native and non-native states, so the cell is constantly challenged by pools of aggregation prone and potentially toxic protein conformers (1). Improper post-translational modification, disassembly of oligomeric complexes, different types of cellular stress, mutation, and off-pathway folding can cause proteins to misfold. To deal with these situations the cell contains a variety of molecular chaperones that facilitate the folding of newly synthesized polypeptides, refolding of misfolded proteins, and degradation of misfolded clients that cannot fold (2). Some clients escape the action of molecular chaperones and form large aggregates, but must still be degraded (3). Even under normal conditions, up to 30% of the bulk of proteins synthesized can be degraded during or immediately following translation due to the inability to achieve proper folding (4). Due to the timing of protein misfolding and the different conformations of non-native protein species a variety of cellular quality control (QC) systems exist to recognize misfolded proteins and facilitate their refolding or degradation (5). Failure of protein QC systems to manage protein loads can result in protein aggregation and/or formation of toxic protein species. The accumulation of misfolded proteins is the hallmark of a number of diseases including neuro-degeneration, cardiovascular diseases, cataract, and age-related macular degeneration (6). As such there is a great amount of interest in understanding mechanisms for operation of different cellular QC systems.
2. Cytosolic Quality Control
The cytosol is home to an array of molecular chaperones, E3 ubiquitin-ligases, and QC factors that are important for QC of soluble proteins and membrane proteins with cytosolic domains (7, 8) (Fig. 1). The Hsp70 family consists of generally promiscuous chaperones that recognize exposed hydrophobic patches and facilitate refolding via cycles of ATP hydrolysis. Similarly, Hsp90s facilitate the ATP-dependent refolding of proteins that are thought to be in more mature conformations. Both Hsp70 and Hsp90 chaperones utilize co-chaperones to help with substrate recognition and binding. The ATP-independent small heat shock proteins (sHSPs) and Hsp40s recognize and inhibit the aggregation of misfolded proteins (9). Hsp40s and sHSPs then recruit ATP-dependent chaperones to facilitate the folding of substrates.
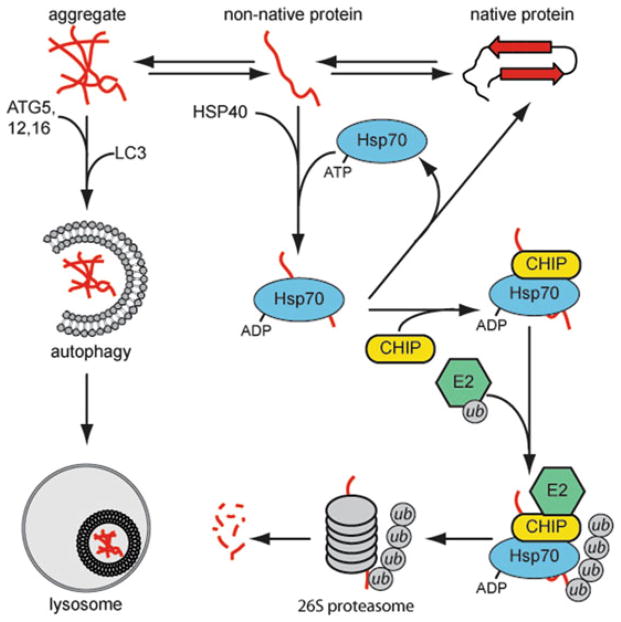
Fig. 1.
The triage of misfolded proteins in cytosolic quality control. A denatured protein can spontaneously refold, aggregate, or be recognized by molecular chaperones, such as the Hsp40/Hsp70 co-chaperones. Hsp70 will attempt to refold the substrate protein by cycles of ATP hydrolysis or recruit E3-ubiquitin ligases, such as CHIP, to target the substrate for proteasomal degradation. Aggregated proteins will be cleared by autophagic processes.
E3 ubiquitin-ligases like CHIP (C-terminus of Hsp70 Interacting Protein) work with molecular chaperones to degrade proteins that cannot be refolded (7). CHIP was initially identified as a regulator of Hsc70 function (10). CHIP, which contains a U-box ubiquitin-ligase domain, has been shown to be involved in the degradation of a number of client proteins, including polytopic ER proteins with cytosolic domains (11) and a large number of cytosolic proteins (12–14). The general paradigm for chaperone-assisted degradation of soluble proteins is that misfolded proteins are recognized by an Hsp40 protein (Fig. 1). Hsp70 is then recruited via joint recognition of the Hsp40 and bound client proteins. If refolding does not occur, CHIP is recruited to the Hsp70:polypeptide complex and the misfolded protein is ubiquitylated and targeted for degradation via the proteasome. In addition to CHIP, there are a number of additional E3 ubiquitin-ligases that recognize misfolded proteins (7), so there appears to be a network of QC factors that act in an integrated fashion to degrade misfolded soluble proteins.
3. ER Quality Control
About 30% of total cell protein is synthesized on endoplasmic reticulum (ER)-associated ribosomes, so the cell contains a complex network of ERQC factors that have evolved to mediate QC of a large collection of topologically distinct ER-associated proteins (Fig. 2). Like the cytosol, the ER lumen contains a diverse group of molecular chaperones to aid in the co- and post-translational folding of proteins (5, 15). Modifications to the glycan groups of newly synthesized proteins provide information to specific chaperones about the folding state of the protein. The glycan-dependent chaperones calnexin and calreticulin will associate with a recently synthesized protein and help it to mature. Hsp40s in the ER lumen work with the Hsp70 family member BiP to recognize and bind hydrophobic patches on misfolded proteins. BiP will attempt to refold the protein by rounds of ATP hydrolysis, substrate release, and nucleotide exchange (5). If the protein is unable to fold correctly it will be targeted for proteasomal degradation via the ER-associated degradation (ERAD) pathway (16).
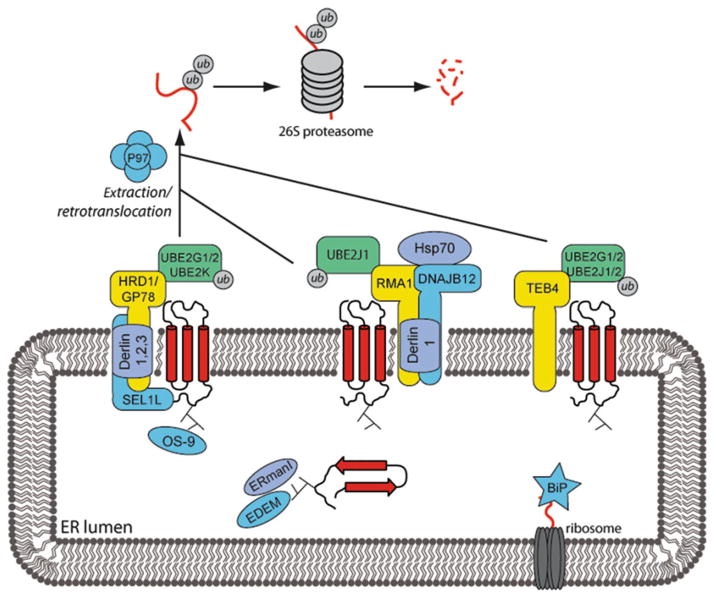
Fig. 2.
Misfolded proteins in the ER are subject to the ER-associated degradation (ERAD) pathway. Misfolded proteins are recognized by various ER factors, such as chaperones, and directed toward ER membrane E3 ubiquitin-ligases. The three main ligases identified are RMA1, HRD1, and TEB4. Each ligase is part of a complex with an E2 ubiquitin-conjugating enzyme and other factors. Substrate proteins are ubiquitylated, extracted into the cytoplasm via a p97 AAA + ATPase-dependent process, and degraded by the proteasome.
A variety of mammalian ER-associated E3 ligases have been identified that ubiquitylate terminally misfolded proteins (17). The integral membrane ligase HRD1 (yeast homolog to Hrd1) forms a complex with many adapter proteins including SEL1L (Hrd-3), OS-9 (Yos9), the Derlin proteins (Der1), and the E2 ubiquitin-conjugating enzymes UBE2G1 and UBE2G2 (Ubc6). SEL1L and OS-9 are involved in the recognition of ER luminal folding defects. The Derlin family proteins (Derlin 1–3) are membrane-spanning adapter proteins that are candidates to be involved in the recognition of folding defects in membrane domains (17). Another ligase, GP78, is ~50% homologous to HRD1 and is also responsible for targeting ERAD substrates for degradation. TEB4 (homolog to the yeast Doa10) is a 14-transmembrane domain E3 ligase, whose only known adapter proteins are E2 ubiquitin-conjugating enzymes. While little is known about mammalian TEB4, Doa10 ubiquitylates ERAD substrates with cytosolic folding defects (17). The tail anchored E3 ligase RMA1 (which has no yeast homolog) is also implicated in ERAD (18). RMA1 forms a complex with Derlin-1, the E2 UBE2J1, and DNAJB12. DNAJB12 is a transmembrane Hsp40 chaperone with cytosolic J-domain that co-operates with cytosolic Hsp70 to mediate RMA1-dependent substrate ubiquitylation (19, 20).
Prior to degradation, ERAD substrates must be translocated into the cytosol in a process dependent on ATP and the AAA+ ATPase p97 (21, 22). Membrane-associated ERAD substrates must be extracted from the membrane and ER luminal substrates must cross the membrane into the cytosol. However, the identity of this retrotranslocation/extraction pore remains unknown and little is known about the mechanism for this process. However, there is recent evidence to suggest that transmembrane elements of the Hrd1 may participate in the retrotranslocation process (23).
Interestingly, polytopic membrane proteins with large cytosolic domains are subject to both ERQC and cytosolic quality control (8). For example, there is evidence that the RMA1 E3 complex can sense the assembly status of the N-terminal regions of the substrate CFTR. Whereas, the CHIP/Hsp70 cytosolic QC system appears to act at a checkpoint after RMA1 action (18). Thus, different QC systems can act redundantly and some are able to cooperate to monitor the conformation of different regions of the same protein.
4. Protein Aggregate Clearance via Autophagy
Despite the evolution of elaborate cytosolic and ERQC systems, unfolded proteins are still able to aggregate during times of stress and these aggregates must be removed from the cell. In some cases, protein aggregates can be resolubilized by molecular chaperones and the proteins in them are then degraded by the ubiquitin–proteasome pathway (24). In other cases, aggregates cannot be disrupted and alternate mechanisms for clearance exist. Autophagy is a process in which cellular material, such as accumulated aggregated or misfolded proteins are engulfed in a double-membrane autopha-gosome (25, 26). Engulfment is initiated when autophagy-related proteins (ATG) such as ATG5, ATG12, and ATG16 are recruited to membranes at an autophagic nucleation site. Next, the recruited ATG proteins facilitate the conjugation of the small ubiquitin-like protein LC3 to the nearby lipids. This induces budding and formation of the autophagosome. The autophagosome fuses with the lysosome or vacuole where the drop in pH changes the conformation of aggregates and endoproteases clip proteins in aggregates to initiate aggregates. Yet, how a detergent insoluble aggregate is dissolved in the lysosome is not entirely clear.
5. Concluding Remarks
There is still much to learn about how the cell deals with protein unfolding and the mechanisms of action for factors are involved in quality control and clearance pathways need to be uncovered. Questions still remain about how the cell partitions unfolded proteins between life and death and how the cell facilitates aggresome and inclusion body assembly is understudy (27). There is much investigation into the identification of factors involved in quality control of different cellular subcompartments and understanding how action of these ERQC machines are integrated in response to stress and disease is a new challenge. Investigators have developed assays to study basic features of protein QC in yeast, cell culture, and in vitro models. The following chapters describe various methods for investigating steps in pathways for cytosolic quality control, ER quality control, and autophagy.
Acknowledgments
DMC is supported by NIH R01GM056981 and Cystic Fibrosis Foundation grant CYRCFF11G0.
References
- 1.Hartl FU, Hayer-Hartl M. Converging concepts of protein folding in vitro and in vivo. Nat Struct Mol Biol. 2009;16:574–581. doi: 10.1038/nsmb.1591. [DOI] [PubMed] [Google Scholar]
- 2.Cyr DM, Hohfeld J, Patterson C. Protein quality control: U-box-containing E3 ubiquitin ligases join the fold. Trends Biochem Sci. 2002;27:368–375. doi: 10.1016/s0968-0004(02)02125-4. [DOI] [PubMed] [Google Scholar]
- 3.Tyedmers J, Mogk A, Bukau B. Cellular strategies for controlling protein aggregation. Nat Rev Mol Cell Biol. 2010;11:777–788. doi: 10.1038/nrm2993. [DOI] [PubMed] [Google Scholar]
- 4.Schubert U, Anton LC, Gibbs J, et al. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature. 2000;404:770–774. doi: 10.1038/35008096. [DOI] [PubMed] [Google Scholar]
- 5.Goeckeler JL, Brodsky JL. Molecular chaperones and substrate ubiquitination control the efficiency of endoplasmic reticulum-associated degradation. Diabetes Obes Metab. 2010;12(Suppl 2):32–38. doi: 10.1111/j.1463-1326.2010.01273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luheshi LM, Dobson CM. Bridging the gap: from protein misfolding to protein misfolding diseases. FEBS Lett. 2009;583:2581–2586. doi: 10.1016/j.febslet.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 7.Heck JW, Cheung SK, Hampton RY. Cytoplasmic protein quality control degradation mediated by parallel actions of the E3 ubiquitin ligases Ubr1 and San1. Proc Natl Acad Sci USA. 2010;107:1106–1111. doi: 10.1073/pnas.0910591107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchberger A, Bukau B, Sommer T. Protein quality control in the cytosol and the endoplasmic reticulum: brothers in arms. Mol Cell. 2010;40:238–252. doi: 10.1016/j.molcel.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Kampinga HH, Craig EA. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol. 2010;11:579–592. doi: 10.1038/nrm2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ballinger CA, Connell P, Wu Y, et al. Identification of CHIP, a novel tetratricopeptide repeat-containing protein that interacts with heat shock proteins and negatively regulates chaperone functions. Mol Cell Biol. 1999;19:4535–4545. doi: 10.1128/mcb.19.6.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meacham GC, Patterson C, Zhang W, et al. The Hsc70 co-chaperone CHIP targets immature CFTR for proteasomal degradation. Nat Cell Biol. 2001;3:100–105. doi: 10.1038/35050509. [DOI] [PubMed] [Google Scholar]
- 12.Jana NR, Dikshit P, Goswami A, et al. Co-chaperone CHIP associates with expanded polyglutamine protein and promotes their degradation by proteasomes. J Biol Chem. 2005;280:11635–11640. doi: 10.1074/jbc.M412042200. [DOI] [PubMed] [Google Scholar]
- 13.Dickey CA, Koren J, Zhang YJ, et al. Akt and CHIP coregulate tau degradation through coordinated interactions. Proc Natl Acad Sci USA. 2008;105:3622–3627. doi: 10.1073/pnas.0709180105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esser C, Scheffner M, Hohfeld J. The chaperone-associated ubiquitin ligase CHIP is able to target p53 for proteasomal degradation. J Biol Chem. 2005;280:27443–27448. doi: 10.1074/jbc.M501574200. [DOI] [PubMed] [Google Scholar]
- 15.Maattanen P, Gehring K, Bergeron JJ, et al. Protein quality control in the ER: the recognition of misfolded proteins. Semin Cell Dev Biol. 2010;21:500–511. doi: 10.1016/j.semcdb.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Stolz A, Wolf DH. Endoplasmic reticulum associated protein degradation: a chaperone assisted journey to hell. Biochim Biophys Acta. 2010;1803:694–705. doi: 10.1016/j.bbamcr.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Hirsch C, Gauss R, Horn SC, et al. The ubiquitylation machinery of the endoplasmic reticulum. Nature. 2009;458:453–460. doi: 10.1038/nature07962. [DOI] [PubMed] [Google Scholar]
- 18.Younger JM, Chen L, Ren HY, et al. Sequential quality-control checkpoints triage misfolded cystic fibrosis transmembrane conductance regulator. Cell. 2006;126:571–582. doi: 10.1016/j.cell.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto YH, Kimura T, Momohara S, et al. A novel ER J-protein DNAJB12 accelerates ER-associated degradation of membrane proteins including CFTR. Cell Struct Funct. 2010;35:107–116. doi: 10.1247/csf.10023. [DOI] [PubMed] [Google Scholar]
- 20.Grove DE, Fan CY, Ren HY, et al. The endoplasmic reticulum-associated Hsp40 DNAJB12 and Hsc70 cooperate to facilitate RMA1 E3-dependent degradation of nascent CFTR{Delta}F508. Mol Biol Cell. 2011;22:301–314. doi: 10.1091/mbc.E10-09-0760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bagola K, Mehnert M, Jarosch E, et al. Protein dislocation from the ER. Biochim Biophys Acta. 2011;1808:925–936. doi: 10.1016/j.bbamem.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 22.Nakatsukasa K, Brodsky JL. The recognition and retrotranslocation of misfolded proteins from the endoplasmic reticulum. Traffic. 2008;9:861–870. doi: 10.1111/j.1600-0854.2008.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carvalho P, Stanley AM, Rapoport TA. Retrotranslocation of a misfolded luminal ER protein by the ubiquitin-ligase Hrd1p. Cell. 2010;143:579–591. doi: 10.1016/j.cell.2010.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glover JR, Lindquist S. Hsp104, Hsp70, and Hsp40: a novel chaperone system that rescues previously aggregated proteins. Cell. 1998;94:73–82. doi: 10.1016/s0092-8674(00)81223-4. [DOI] [PubMed] [Google Scholar]
- 25.Nakatogawa H, Suzuki K, Kamada Y, et al. Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat Rev Mol Cell Biol. 2009;10:458–467. doi: 10.1038/nrm2708. [DOI] [PubMed] [Google Scholar]
- 26.Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40:280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaganovich D, Kopito R, Frydman J. Misfolded proteins partition between two distinct quality control compartments. Nature. 2008;454:1088–1095. doi: 10.1038/nature07195. [DOI] [PMC free article] [PubMed] [Google Scholar]